Abstract
Fifty-four cyanobacterial strains of the genus Nostoc from different habitats were screened for acetylcholinesterase inhibitory activity. Water-methanolic extracts from freeze-dried biomasses were tested for inhibitory activity using Ellman's spectrophotometric method. Acetylcholinesterase inhibitory activity higher than 90% was found in the crude extracts of Nostoc sp. str. Lukešová 27/97 and Nostoc ellipsosporum Rabenh. str. Lukešová 51/91. Extracts from Nostoc ellipsosporum str. Lukešová 52/91 and Nostoc linckia f. muscorum (Ag.) Elenk. str. Gromov, 1988, CALU-980 inhibited AChE activity by 84.9% and 65.3% respectively. Moderate AChE inhibitory activity (29.1–37.5%) was found in extracts of Nostoc linckia Roth. str. Gromov, 1962/10, CALU-129, Nostoc muscorum Ag. str. Lukešová 127/97, Nostoc sp. str. Lhotsky, CALU-327 and Nostoc sp. str. Gromov, CALU-998. Extracts from another seven strains showed weak anti-AChE activities.
The active component responsible for acetylcholinesterase inhibition was identified in a crude extract of Nostoc sp. str. Lukešová 27/97 using HPLC and found to occur in one single peak.
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder of the central nervous system. 10–15% of elderly people suffer from different forms of dementia, 60–70% of these have AD. Clinical symptoms are typically memory malfunctions, deteriorative cognitive functions or changes in emotive behaviour. These problems are caused by changes in the patient's brain, mainly due to the formation of β-amyloid plaques and neurofibrillary tangles [Citation1]. Formation of these constituents initiates other processes such as the production of reactive oxygen radicals or inflammatory reactions. All these processes lead to the loss of synaptions and degradation of the brain neurons, mainly in the cholinergic system. The exact cause of AD is still unknown, therefore only palliative treatment of AD is possible. So far, the only approved medication for the treatment of the symptoms of AD are acetylcholinesterase (AChE, EC 3.1.1.7) inhibitors and memantine [Citation2]. Examples of these are donepezil (ARICEPT®), galanthamin (Reminyl®), huperzine A and rivastigmin (Exelon®).
Until the causes of AD will fully understood there is a requirement for new, AChE inhibitors. Currently the search for new compounds is focused on screening both natural and synthetic sources. Until now the search to find new natural AChE inhibitors has focused on screening crude extracts prepared from various parts of higher plants Citation3, Citation4, Citation5, Citation6. Once identified, further studies are required to isolate, determine the structure and kinetics of the active compounds. Many new interesting compounds belonging to the alkaloid family have been found, e.g. the pregnane type alkaloids isolated from Sarcococca saligna [Citation7], non-competitive sterane-alkaloid inhibitors produced by Haloxylon recurvum [Citation8] and triterpenoid alkaloides isolated from Buxus papillosa [Citation9]. Non-alkaloid inhibitors with the skeleton of terpenoids [Citation10], coumarins [Citation11], xanthones [Citation12] or pregnane glycosides [Citation13] have also been found in plant material. Only a few studies have investigated microorganisms, mainly fungi and bacteria. Screening of more than 7000 microorganisms resulted in the isolation of two meroterpenoids with selective anti-AChE activity compared to butyrylcholinesterase (BChE, EC 3.1.1.8) [Citation14]. Streptomyces antibioticus and Streptomyces levandulae produce cyclic organophosphates with AChE inhibitory activity [Citation15,Citation16]. Anabaena flos-aquae and Nostoc 78-12A are the only known cyanobacteria to produce cholinesterase inhibitors. Anabaena flos-aquae is a producer of the irreversible AChE inhibitor, anatoxin-a(s) [Citation17]. Becher et al. have isolated nostocarboline, quarternary β-carboline alkaloid, from the biomass of Nostoc 78-12A [Citation18]. Unfortunately, nostocarboline is known only to inhibit the activity of BChE and not AChE.
Cyanobacteria produce various secondary metabolites, which differ in both chemical structures and biological activities. These have a wide range of bioactive properties (e.g. toxicity, alleopathy, antibacterial, antifungal or enzyme inhibitory activities) which makes some of these compounds potentially useful in pharmacology or agriculture, even though their actual functions in cyanobacteria are often not understood.
Considering the wide variety of natural sources that have been screened for anti-AChE activity, it is surprising that so few cyanobacteria have been considered. The objective of this work was to screen more than fifty strains of the genus Nostoc for AChE inhibitory activity.
Materials and methods
Cyanobacterial origin and cultivation
Cyanobacterial strains were obtained from the culture collection of soil algae and cyanobacteria of the Institute of Soil Biology of the Academy of Sciences of the Czech Republic and from the culture collection of the Department of Microbiology - St. Petersburg State University in Russia. The majority of strains used in this study were originally isolated from soils collected from different types of habitat and geographical areas including: Africa (Egypt, Guinea), Europe (Czech Republic, Russia), North and South America (Canadian Arctic, Brazil), Asia (Russia), Cuba and Antarctica.
All cyanobacterial strains were grown using a batch system. Cultivations were carried out in glass tubes containing 400 mL of Allen & Arnon medium [Citation19]. The media was stirred using a flow of mixed air and CO2 (98:2; V/V) at a constant temperature of 30 ± 0.5°C and with continuous illumination (234 μmol m− 1 s− 1). The biomass was harvested by centrifugation after depletion of nitrates from the cultivation medium and lyophilized before subsequent assays.
Analysis of nitrates
A DIONEX ICS-90 Ion Chromatography System with Chromeleon Client, 6.50 SP3 Build 980 software was used for the analysis of nitrates. Samples of the media were centrifuged and 10 μL of the supernatant injected onto an IonPac® AS9-HC (4 × 250 mm) analytical column and eluted with a mobile phase of 9 mM Na2CO3 at a flow rate of 1 mL min− 1. A bipolar heated conductivity cell detector was used to detect the nitrates.
Extract preparation
200 mg of the freeze-dried biomass was homogenized with sea sand and extracted with 6 mL of methanol-water mixture (7:3, V/V) for 1 h. This mixture was then centrifuged, evaporated to dryness and redissolved in the same solvent to obtain concentration of 3 mg mL− 1 for the AChE inhibitory test.
Preparation of analytical solutions
Acetylthiocholine iodide (ATCI), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) and AChE from electric eel (type VI-S) were obtained from Sigma-Aldrich (Czech Republic). All the other chemicals were analytical grade.
Acetylcholinesterase was dissolved in phosphate buffer pH 8.0 to produce a 20 U mL− 1 stock solution and stored at − 27°C. Enzymatic activity was adjusted to 0.4 U mL− 1 before use. Acetylthiocholine iodide was dissolved in phosphate buffer pH 8.0 to produce a 6.2 mM solution. 5,5′-dithiobis(2-nitrobenzoic acid) was dissolved in phosphate buffer pH 7.0 to make a 7.6 mM solution. All solutions were kept at 5°C. Acetylthiocholine iodide and DTNB solutions were prepared fresh each day.
Acetylcholinesterase inhibition assay
Ellman's method [Citation20] was optimized for 96-well microplates. 125 μL of 0.1 M phosphate buffer pH 8.0, 50 μL of 0.4 U mL− 1 AChE, 25 μL of 7.6 mM DTNB and 20 μL of crude extract were used in the reaction mixture. For the blank, 50 μL of 0.4 U mL− 1 AChE was replaced by the same volume of 0.1 M phosphate buffer pH 8.0. After 30 min incubation at 30°C the reaction was started by the addition of 30 μL of 6.2 mM ATCI. The increase in absorbance at 412 nm was measured every 11 s for 220 s (TECAN SunriseTM absorbance reader, XFluor4 software). Reaction velocities were determined from the slope of the linear part of the graph of time versus concentration of the reaction product (5-thio-2-nitrobenzoate). Inhibitory activities were recalculated from reaction velocities as a percentage compared to an assay using 20 μL of methanol-water (7:3, V/V) instead of crude extract. All assays were done in triplicate.
Analysis of extracts
HPLC/MS analysis was used to separate and identify the chemical components of the Nostoc (sp. str. Lukešová 27/97) extract. The system consisted of an Agilent 1100 Series LC/MSD Trap, ChemStation for LC 3D and LC/MSD Trap software (V4.1). 20 μL aliquots of the sample were injected onto a ZORBAX Eclipse XDB-C8 column (150 × 4.6 mm, 5 μm) and eluted with the mixture of methanol:water:formic acid (30:70:0.1, V/V/V) at a flowrate of 0.6 mL min− 1 and methanol gradient of 30–85% (0–26.5 min), 85–100% (26.5–27.5 min), 100% (27.5–37.0 min). UV detection (280 nm) was used with parameters of ESI-MS, positive ion polarity; dry temp., 325°C; nebulizer, 50 psi (345 kPa); dry gas, 10 L min− 1 and capillary current, 59.8 nA.
Fractionation of the crude extract
HPLC was used to fractionate the crude Nostoc (sp. str. Lukešová 27/97) extract to enable identification of the active component. 60 μl of the sample was injected onto the column using the same conditions described above. 10 fractions were collected at following time intervals: 1. 0–20.7 min, 2. 20.7–21.1 min, 3. 21.1–22.3 min, 4. 22.3–23.0 min, 5. 23.0–23.5 min, 6. 23.5–24.1 min, 7. 24.1–27.2 min, 8. 27.2–27,6 min, 9. 27,6–28,4 min and 10. 28.4–41.0 min. Fractions were evaporated to dryness at 40°C and 25 mBar (2.5 kPa), dissolved in 100 μL of methanol and tested for AChE inhibitory activity again.
Results
Inhibitory activities of the crude extracts from lyophilized biomasses of cyanobacterial strains are summarized in . All percentage inhibition values represent the mean of three measurements. The majority of the tested extracts exhibited no inhibitory activity against AChE. Only fifteen extracts were found active against AChE. Highly significant inhibitory activity was found in four extracts prepared from biomasses of Nostoc sp. str. Lukešová 27/97, Nostoc ellipsosporum Rabenh. str. Lukešová 51/91, Nostoc ellipsosporum str. Lukešová 52/91 and Nostoc linckia f. muscorum (Ag.) Elenk. str. Gromov, 1988, CALU-980, that decreased AChE activity by 96.5%, 96.6%, 84.9% and 65.3% respectively. Extracts from Nostoc muscorum Ag. str. Lukešová 127/97, Nostoc linckia Roth. str. Gromov, 1962/10, CALU-129, Nostoc sp. str. Lhotsky, CALU-327 and Nostoc sp. str. Gromov, CALU-998 showed moderate inhibitory activity of between 29% and 38%. Low anti-AChE activity was observed in seven extracts from biomasses of Nostoc sp. str. Lukešová 18/89, Nostoc calcicola Bréb. str. Lukešová 2/89, Nostoc muscorum str. Lukešová 31/87, Nostoc muscorum str. Lukešová 2/91, Nostoc muscorum str., CALU-526, Nostoc linckia f. muscorum str. Gromov, 1988, CALU-981 and Nostoc linckia f. muscorum str., CALU-993.
Table I. Acetylcholinesterase inhibitory activity of the extracts from the cyanobacterial biomasses.
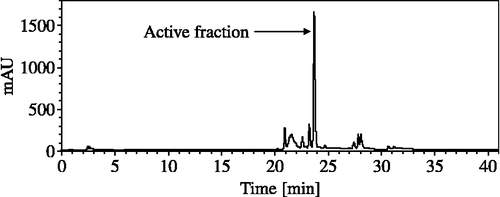
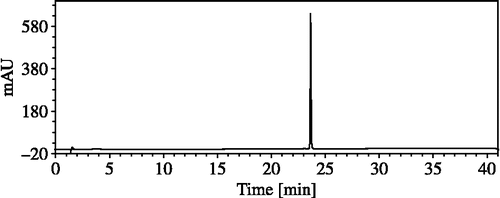
HPLC analysis of the crude extract of Nostoc sp. str. Lukešová 27/97 is shown in . The crude extract was separated into 10 fractions, however significant anti-AChE activity of 97.8% was only found in the sixth fraction corresponding to the peak with a Rt = 23.7 min. Mass spectrum of the sixth fraction showed three peaks only, a prominent molecular ion [M + H]+ at m/z = 799.4 (rel. int. = 100.0%), and two peaks of adducts [M + Na]+ = 821.4 (rel. int. = 9.3%) and [M + K]+ = 837.4 (rel. int. = 4.6%). HPLC analysis of the sixth fraction is shown in .
Discussion
Acetylcholinesterase inhibitors are important medicines for the treatment of Alzheimer's disease. Despite the availability of several commercial products, there is still a pressing need for new substances. To date, the majority of studies that have been successful in isolating new AChE inhibition compounds from natural sources have done so from higher plants and a few from fungi [Citation21]. Cyanobacteria are also a rich source of secondary metabolites. They are well known for their production of various toxins, eg. microcystins, nodularins, saxitoxins [Citation22,Citation23]. Anatoxin-a(s) is the only known cynobacterial secondary metabolite with anti-AChE activity. Toxicity of anatoxin-a(s), produced by Anabaena flos-aquae, is due to the formation of a covalent bond to AChE that results in an irreversible complex of inhibitor and enzyme [Citation17].
Until now, no other cyanobacteria with AChE inhibitory activity have been published. Of the fifty-four cyanobacterial strains of the genus Nostoc in this study, four strains have proved to be promising; Nostoc sp. str. Lukešová 27/97, Nostoc ellipsosporum Rabenh. str. Lukešová 51/91, Nostoc ellipsosporum str. Lukešová 52/91 and Nostoc linckia f. muscorum (Ag.) Elenk. str. Gromov, 1988, CALU-980. The first three strains originate from soils of the Czech Republic (CR). Nostoc sp. str. Lukešová 27/97 was isolated from a dump after coal mining and both strains of Nostoc ellipsosporum were isolated from an arable field. Nostoc linckia f. muscorum (Ag.) Elenk. str. Gromov CALU-980 was isolated from soil in Guinea (Africa). The most active Nostoc ellipsosporum Rabenh. str. Lukešová 51/91 has been sequenced by Rajaniemi et al. [Citation24] under the name Nostoc ellipsosporum V and the sequences are available in the GenBank. Other strains with moderate anti-AChE activity originate mostly from the CR and Guinea soils and belong mostly to Nostoc muscorum ().
It would be interesting to determine whether this inhibition activity is random or whether there is a correlation (relationship) between strain toxicity, species and locality from where the strain was isolated. However, this can only be achieved after correct species identification, which is extremely difficult in the Nostoc genus, and with very detailed information on the collection site. This information is normally available for strains stored in culture collections making them the most appropriate source of material.
AChE inhibition activity of crude extracts of organisms provides basic information on their potential for the production of bioactive compounds. Crude extracts are very complex matrixes and their AChE inhibitory activities indicates the presence of one or more active compounds. HPLC fractionation of the crude extract from Nostoc sp. str. Lukešová 27/97, which was one of the most active strains in this study, revealed that only one fraction was responsible for anti-AChE activity. This fraction conforms to the only compound identified as a single peak in the HPLC chromatogram of the crude extract from Nostoc sp. str. Lukešová 27/97. Mass spectrometry of this peak identified a molecular ion [M + H]+ and relevant adducts of [M + Na]+ and [M + K]+. Thus, AChE inhibitory activity found in Nostoc sp. str. Lukešová 27/97 was assigned to this compound with a relative molecular weight of 798.4.
However, this first stage is an important step in selecting the strains which are likely to be worthy of further study. Unfortunately, it is impossible to predict whether the AChE inhibitory activity of the crude extract is due to the presence of a highly active compound at very low concentration or a less potent inhibitor at a higher concentration.
The cyanobacterial strains with high anti-AChE activity identified in this study are interesting from both a pharmaceutical and toxicological point of view. The next step will be to isolate the active fractions of the crude extracts from these strains and to determine their type of inhibition of AChE. Following this, their half-inhibition concentration (IC50) can then be compared with known AChE inhibitors. If the product proves to be a reversible inhibitor with comparable IC50 to that of already approved medicines for AD, it will have to undergo a number of other important studies before it could considered to provide the basis of a valuable new drug.
Aquatic cyanobacteria are considered to be the most important toxicologically. Where excessive growth occurs they form unwanted water blooms and make water management problematic. As a consequence they have been the subject of many studies and are known to produce a wide variety of secondary metabolites [Citation25,Citation26]. Many of these toxins are responsible for acute or subchronic poisoning of animals and humans. The AChE inhibitory activity of the tested strains from soil suggest that these may also produce toxins.
In contrast to the aquatic cyanobacteria, Nostoc strains used in this screening originated from soils and are considered to be less important from a toxological point of view. Further studies of the Nostoc strains with the highest AChE inhibitory activity will lead to an evaluation of whether these strains could be a new sources of potential medicines for AD and/or if they are important from a toxicological point of view.
Acknowledgements
This research was supported by project ME 874 of the Ministry of Education, Youth and Sports of the Czech Republic.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- H Vinters, and J Felix. Alzheimer's disease. In: JL Cummings, editor. The neuropsychiatry of Alzheimer's disease and related dementias. London: Martin Dunitz Ltd; (2003). p 57–116.
- G Waldemar, and B Vellas. Memantine in moderate to severe Alzheimer's disease. In: B Vellas, LJ Fitten, H Feldman, E Giacobini, M Grundman, B Winblad, and A Kurz, editor. Research and practise in Alzheimer's disease 8. Severe dementia. Paris: Serdi Publisher; (2003). p 83–92.
- K Ingkaninan, P Temkitthawon, K Chuenchom, T Yuyaem, and W Thongnoi. (2003). Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol 89:261–264.
- I Orhan, B Şener, MI Choudhary, and A Khalid. (2004). Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J Ethnopharmacol 91:57–60.
- A Adsersen, B Gauguin, L Gudiksen, and AK Jäger. (2006). Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity. J Ethnopharmacol 104:418–422.
- A Ferreira, C Proença, MLM Serralheiro, and MEM Araújo. (2006). The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol 108:31–37.
- Atta-ur-Rahman, Zaheer-ul-Haq, A Khalid, S Anjum, MR Khan, and MI Choudhary. (2002). Pregnane-type steroidal alkaloids of Sarcococca saligna: A new class of cholinesterase inhibitors. Helv Chim Acta 85:678–688.
- E Ahmed, SA Nawaz, A Malik, and MI Choudhary. (2006). Isolation and cholinesterase-inhibition studies of sterols from Haloxylon recurvum. Bioorg Med Chem Lett 16:573–580.
- Atta-ur-Rahman, S Parveen, A Khalid, A Farooq, and MI Choudhary. (2001). Acetyl and butyrylcholinesterase-inhibiting triterpenoid alkaloids from Buxus papillosa. Phytochemistry 58:963–968.
- M Miyazawa, H Watanabe, and H Kameoka. (1997). Inhibition of acetylcholinesterase activity by monoterpenoids with a p-menthane skeleton. J Agric Food Chem 45:677–679.
- SY Kang, KY Lee, SH Sung, MJ Park, and YC Kim. (2001). Coumarins isolated from Angelica gigas inhibit acetylcholinesterase: Structure-activity relationships. J Nat Prod 64:683–685.
- C Brühlmann, A Marston, K Hostettmann, PA Carrupt, and B Testa. (2004). Screening of non-alkaloidal natural compounds as acetylcholinesterase inhibitors. Chem Biodivers 1:819–829.
- KY Lee, SH Sung, and YC Kim. (2003). New acetylcholinesterase-inhibitory pregnane glycosides of Cynanchum atratum roots. Helv Chim Acta 86:474–483.
- K Otoguro, F Kuno, and S Ōmura. (1997). Arisugacins, selective acetylcholinesterase inhibitors of microbial origin. Pharmacol Ther 76:45–54.
- K Ogata, K Ueda, T Nagasawa, and Y Tani. (1974). A cholinesterase inhibitor produced by Aspergillus terreus. J Antibiot 27:343–345.
- R Neumann, and HH Peter. (1987). Insecticidal organophosphates: Nature made them first. Cell Mol Life Sci 43:1235–1237.
- NA Mahmood, and WW Carmichael. (1987). Anatoxin-a(s), an anticholinesterase from the cyanobacterium Anabaena flos-aquae NRC-525-17. Toxicon 25:1221–1227.
- PG Becher, J Beuchat, K Gademann, and F Jüttner. (2005). Nostocarboline: Isolation and synthesis of a new cholinesterase inhibitor from Nostoc 78-12A. J Nat Prod 68:1793–1795.
- MB Allen, and DI Arnon. (1955). Studies on nitrogen-fixing blue green algae: I. Growth and nitrogen fixation by Anabaena cylindrica. Lemm Plant Physiol 30:366–372.
- GL Ellman, KD Courtney, V Andres, and RM Featherstone. (1961). A new rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95.
- K Hostettmann, A Borloz, A Urbain, and A Marston. (2006). Natural product inhibitors of acetylcholinesterase. Curr Org Chem 10:825–847.
- RM Dawson. (1998). The toxicology of microcystins. Toxicon 36:953–962.
- ME Apeldoorn, HP Egmond, GJA Speijers, and GJI Bakker. (2007). Toxins of cyanobacteria. Mol Nutr Food Res 51:7–60.
- P Rajaniemi, P Hrouzek, K Kaštovská, R Willame, A Rantala, L Hoffman, J Komárek, and K Sivonen. (2005). Phylogenetic and morphological evaluation of the genera Anabaena, Aphanizomenon, Trichormus and Nostoc (Nostocales, Cyanobacteria). Int J Syst Evol Microbiol 55:11–26.
- Y Shimizu. (1996). Microalgal metabolites: A new perspective. Annu Rev Microbiol 50:431–465.
- AM Burja, B Banaigs, E Abou-Mansour, JG Burgess, and PC Wright. (2001). Marine cyanobacteria – a prolific source of natural products. Tetrahedron 57:9347–9377.