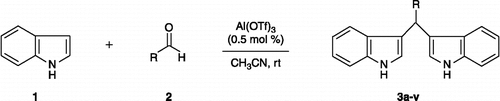
Abstract
A versatile and efficient method has been developed for the synthesis of bis(indolyl)methanes by using aluminium triflate (0.5 mol%) as a novel catalyst. Further, some of the synthesized compounds were evaluated for their efficacy as antibacterial and antifungal activities. Most of the compounds have shown moderate to good inhibitory activity.
Introduction
Indole derivatives are found to exhibit a wide spectrum of pharmacological activities such as cytotoxic [Citation1, Citation2], antitumour [Citation3], antiviral [Citation3], antimicrobial [Citation4] and anti-inflammatory activities [Citation5]. Bis(indolyl)methanes (BIMs), which contain two indole or substituted indole units in a molecule, are found in bioactive metabolites of terrestrial and marine origin [Citation6–8]. Over the past few years, much attention has been given for the search of specific bis(indole) secondary metabolites in view of their novel structural feature and broad spectrum of biological activities [Citation9]. Recently, some bis(indole) alkaloids have been isolated from plants (I and II) and sponges (III and IV) wherein, two indole units are joined through different heterocyclic moieties. Further, such molecules have been also designed and synthesized that exhibit promising antibacterial, as well as antifungal activities ([Citation10–13]; ).
Figure 1. Chemical structures of bis(indole) alkaloids, I. , II isolated from Aspidosperma ramiflorum and III, IV from sponge Spongosorites sp., nifuroxazide (V) and nitrofuryl BIM (3q).
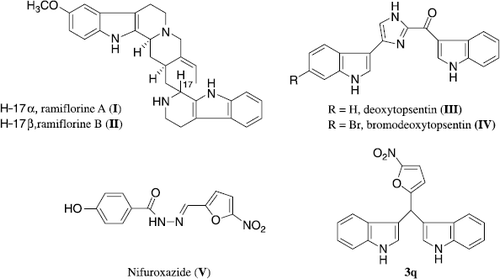
Nitrofurans and isosteric nitrothiophenes are heterocyclic compounds that have been well documented as potential antimicrobial agents Citation14–18. In spite of extensive research work that has been carried out in this class of compounds, there is still scope for the development of new molecules based on this type of heterocyclic systems [Citation19–23]. It is well established that the activity of such nitroheterocycles is mainly due to the metabolic reduction of their nitro functionality by nitroreductase enzyme [Citation24]. Moreover, a number of other furan derivatives have also exhibited antibacterial activity [Citation25,Citation26]. Therefore, in view of these findings, we have designed some new bisindoles by employing a new methodology. Furthermore, these compounds have been evaluated for their efficacy as antibacterial and antifungal agents.
It is well known in the literature that the reaction of indole with aromatic or aliphatic aldehydes and ketones produce azafulvenium salts, and this can undergo further addition with the second indole molecule to afford BIM [Citation27]. Several other methods have been reported in the literature for the synthesis of BIMs using protic acid (e.g. HCl; [Citation28–30]) or Lewis acids (e.g. AlCl3, BF3; [Citation31,Citation32]). These reactions have also been investigated using various triflates and chlorides Citation33–36]. More recently, Bhuyan and co-workers [Citation37] have reported the synthesis of BIMs in protic solvents like methanol and water. However, this method requires longer reaction time (1–20 h) and heterocyclic moieties like furfural 2-aldehyde, thiophene 2-aldehyde and aliphatic aldehydes did not react with indole in aqueous media, whereas in methanol low yields (16–65%) have been obtained. In today's context, if water can be replaced instead of organic solvents, it is significant from the environmental concerns point of view. However, this replacement is difficult for mainly two reasons; firstly, most of the organic substances are insoluble in water and secondly, many reactive substrates, reagents as well as catalysts are decomposed or deactivated by water. In recent years, aluminium triflouromethanesulfonate has been used for various transformations in organic synthesis [Citation38–42]. Therefore, based on the versatility and usefulness of this catalyst, herein, we report a new and efficient method for the synthesis of BIMs catalyzed by 0.5 mol% Al(OTf)3. Further, these compounds have been evaluated for their antimicrobial activity against various antibacterial and antifungal strains. Most of these compounds have shown good antibacterial and antifungal activities.
Materials and methods
Chemistry
1H and 13C NMRs were recorded on a Bruker UXNMR/XWIN-NMR (200 MHz) or Varian VXR-Unity (400 MHz) with TMS (0 ppm) as an internal standard. Coupling constants are reported in Hertz. EI mass spectra were recorded on a VG-7070H Micromass mass spectrometer at 200°C, 70 eV, with a trap current of 200 μA and 4 kV of acceleration voltage. LC–MS were recorded on instrument LC-MSD-Trap-SL and ESI MS on Micro mass, Quattro LC using ESI+ software with capillary voltage 3.98 kV. Melting points were determined with an Electrothermal melting point apparatus and are reported uncorrected. IR spectra (KBr) were measured with a Thermo Nicolet Nexus 670 Spectrometer (νmax in cm− 1). Analytical TLC of all reactions was performed on Merck prepared plates (silica gel 60 F-254 on glass). Column chromatography was performed using Acme silica gel. Starting materials were purchased from Sigma-Aldrich (Poole, UK). MeCN was distilled from CaH2 and dried over 4 Å molecular sieves. Micro analytical data (C, H and N) agreed with the proposed structures within ± 0.4% of the theoretical values. All the standard organisms were obtained from IMTECH Chandigarh, and nutrient agar and potato dextrose agar (PDA) were procured from Himedia Laboratories, Mumbai, India.
General procedure for the synthesis of bis(indolyl) methanes (3a–v)
To a mixture of indole (217 mg, 1 mmol), substituted aldehydes (0.5 mmol) in acetonitrile (5 ml), aluminium triflate (0.5 mol%) was added and stirred at room temperature for the appropriate time (Tables and ). After completion of the reaction as monitored by TLC, water was added to the reaction mixture and products were extracted into ethyl acetate (3 × 20 ml). The combined organic layer was washed with brine, dried over anhydrous Na2SO4, concentrated in vacuum and purified by column chromatography, by using ethyl acetate–petroleum ether mixture as eluent to afford the desired products.
Table I. Al(OTf)3 catalysed synthesis of BIM derivatives (3a. –v) by the condensation of indole with various aldehydes.
Table II. Antibacterial activitya of BIMs expressed in zone of inhibition in millimetres.
Spectral data for selected compounds
3,3′-Bis-indolyl phenylmethane (3a)
mp 148–150°C (lit.17 150–152°C); 1H NMR (200 MHz, CDCl3 + DMSO-d6): δ 7.77 (bs, 2H, NH), 7.15–7.35 (m, 9H), 7.10 (t, J = 8.13 Hz, 2H), 6.94 (t, J = 8.31 Hz, 2H), 6.57 (d, J = 2.26 Hz, 2H), 5.83 (s, 1H); 13C NMR (50 MHz, CDCl3 + DMSO-d6): δ 136.0, 133.8, 130.1, 129.7, 129.7, 129.4, 126.8, 124.5, 122.6, 119.5, 119.0, 113.2, 39.3; LC-MS m/z = 321.0 (M − 1)+, 345.1 (M + Na)+; IR (KBr) (νmax/cm− 1): 3395 (NH), 3050, 2924, 1596, 1455, 1088, 1004, 746. Anal. Calcd for C23H18N2: C, 85.68; H, 5.63; N, 8.69. Found: C, 85.92; H, 5.54; N, 8.42.
3,3′-Bis-indolyl(4-nitrophenyl)methane (3g)
mp 219–221 (lit.17e 217–219°C); 1H NMR (200 MHz, CDCl3 + DMSO-d6): δ 10.50 (bs, 2H, NH), 8.15 (d, J = 8.56 Hz, 2H), 7.57 (d, J = 8.56 Hz, 2H), 7.39 (d, J = 7.81 Hz, 2H), 7.28 (d, J = 7.81 Hz, 2H), 7.09 (t, J = 7.81 Hz, 2H), 6.91 (t, J = 7.81 Hz, 2H), 6.73 (d, J = 2.34 Hz, 2H), 6.00 (s, 1H); 13C NMR (50 MHz, CDCl3 + DMSO-d6): δ 136.3, 134.2, 129.4, 129.0, 128.5, 124.1, 123.2, 122.4, 121.5, 118.4, 118.2, 112.5, 39.2; LC-MS m/z = 366 (M − 1)+; IR (KBr) (νmax/cm− 1): 3420 (NH), 3050, 2923, 1594, 1505, 1454, 1340, 1242, 1095, 1008, 743. Anal. Calcd for C23H17N3: C, 75.19; H, 4.66; N, 11.44. Found: C, 75.44; H, 4.54; N, 11.68.
3,3′-Bis-indolyl(4-methoxyphenyl)methane (3i)
mp 183–185°C (lit.17e 187–189°C); 1H NMR (300 MHz, CDCl3): δ 7.88 (bs, 2H, NH), 7.35 (q, J = 7.55 Hz, 4H), 7.23 (d, J = 8.30 Hz, 2H), 7.15 (t, J = 7.55 Hz, 2H), 6.98 (t, J = 7.55 Hz, 2H), 6.80 (d, J = 8.30 Hz, 2H), 6.62 (d, J = 2.26 Hz, 2H), 5.82 (s, 1H), 3.78 (s, 3H, –OCH3); 13C NMR (75 MHz, CDCl3): δ 143.7, 136.2, 133.6, 130.8, 130.2, 128.7, 126.8, 124.5, 122.6, 120.2, 119.6, 114.8, 55.4, 39.6; LC-MS m/z = 353.1 (M + 1)+, 375.0 (M + Na)+; IR (Neat) (νmax/cm− 1): 3394 (NH), 3054, 2925, 1608, 1507, 1554, 1336, 1241, 1090, 1025, 742. Anal. Calcd for C24H20N2O: C, 81.79; H, 5.72; N, 7.95. Found: C, 81.92; H, 5.81; N, 7.60.
3,3′-Bis-indolyl furfurylmethane (3p)
mp > 300°C (lit.17>300°C); 1H NMR (200 MHz, CDCl3 + DMSO-d6): δ 10.23 (bs, 2H, NH), 7.33 (q, J = 7.03 Hz, 5H), 7.02 (t, J = 7.03 Hz, 2H), 6.80–6.95 (m, 4H), 6.25 (q, J = 1.56 Hz, 1H), 6.00 (d, J = 3.12 Hz, 1H), 5.84 (s, 1H); 13C NMR (50 MHz, CDCl3 + DMSO-d6): δ 157.0, 140.5, 136.1, 126.1, 122.8, 120.8, 118.8, 118.2, 115.8, 110.9, 109.6, 105.7, 33.5; ESI MS m/z = 311.27 (M − 1)+; IR (KBr) (νmax/cm− 1): 3406 (NH), 3052, 2924, 1614, 1453, 1336, 1091, 1006, 739. Anal. Calcd for C21H16N2O: C, 80.95; H, 5.26; N, 8.97. Found: C, 80.61; H, 5.17; N, 9.19.
3,3′-Bis-indolyl(5-nitrofurfuryl)methane (3q)
mp 97°C (charred); 1H NMR (200 MHz, CDCl3 + DMSO-d6): δ 10.60 (bs, 2H, NH), 7.27–7.42 (m, 5H), 7.05 (t, J = 7.03 Hz, 2H), 6.85–6.99 (m, 4H), 6.32 (d, J = 3.90 Hz, 1H), 5.96 (s, 1H); 13C NMR (50 MHz, CDCl3): δ 161.5, 150.4, 136.0, 126.9, 125.4, 123.1, 120.7, 118.2, 118.0, 112.8, 110.9, 109.9, 33.9; LC-MS m/z = 380 (M + Na)+; IR (KBr) (νmax/cm− 1): 3407 (NH), 3053, 2926, 1721, 1485, 1354, 1239, 1094, 1015, 741. Anal. Calcd for C21H15N3O3: C, 70.58; H, 4.23; N, 11.76. Found: C, 70.32; H, 4.11; N, 11.79.
3,3′-Bis-indolyl thienylmethane (3r)
mp 155–157°C (lit.18 150–153°C); 1H NMR (200 MHz, CDCl3 + DMSO-d6): δ 10.21 (bs, 2H, NH), 7.35 (t, J = 8.57 Hz, 4H), 7.10–7.15 (m, 1H), 7.05 (dt, J = 7.85, 1.43 Hz, 2H), 6.85–6.95 (m, 6H), 6.10 (s, 1H); 13C NMR (50 MHz, CDCl3 + DMSO-d6): δ 143.2, 132.0, 126.2, 121.6, 121.2, 119.8, 118.6, 117.5, 115.3, 114.5, 106.5, 33.6; ESI MS m/z = 327.20 (M − 1)+; IR (KBr) (νmax/cm− 1): 3403 (NH), 3077, 2925, 1616, 1454, 1333, 1213, 1086, 1002, 744. Anal. Calcd for C21H16N2S: C, 76.80; H, 4.91; N, 8.53. Found: C, 76.56; H, 4.74; N, 8.76.
3,3′-Bis-indolyl(5-nitrothienyl)methane (3s)
mp 181°C (charred); 1H NMR (200 MHz, CDCl3 + DMSO-d6): δ 10.60 (bs, 2H, NH), 7.80 (d, J = 4.68 Hz, 1H), 7.39 (d, J = 8.59 Hz, 4H), 7.10 (t, J = 7.81 Hz, 2H), 6.90–7.02 (m, 5H), 6.14 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 15.9, 148.1, 135.7, 127.9, 125.1, 123.9, 122.7, 120.5, 117.9, 117.8, 115.4, 110.7, 35.2; LC-MS m/z = 396.9 (M + Na)+; IR (KBr) (νmax/cm− 1): 3444 (NH), 3056, 2922, 1525, 1458, 1335, 1212, 1090, 737. Anal. Calcd for C21H15N3SO2: C, 67.54; H, 4.05; N, 11.25. Found: C, 67.28; H, 4.12; N, 11.38.
3,3′-Bis-indolyl ethane (3t)
mp 153–155°C (lit.17 148–150°C); 1H NMR (200 MHz, CDCl3 + DMSO-d6): δ 9.95 (bs, 2H, NH), 7.45 (d, J = 8.01 Hz, 2H), 7.28 (d, J = 8.01 Hz, 2H), 7.00 (t, J = 8.01 Hz, 2H), 6.91 (d, J = 2.18 Hz, 2H), 6.85 (d, J = 7.28 Hz, 2H), 4.60 (q, J = 6.55 Hz, 1H), 1.77 (d, J = 6.55 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 135.7, 125.5, 120.3, 119.7, 119.4, 118.0, 116.9, 110.2, 27.0, 20.0; ESI MS m/z = 259.20 (M − 1)+; IR (KBr) (νmax/cm− 1): 3413 (NH), 3075, 2957, 1622, 1455, 1336, 1094, 1011, 737. Anal. Calcd for C18H16N2: C, 83.05; H, 6.19; N, 10.76. Found: C, 83.40; H, 6.30; N, 10.62.
3,3′-Bis-indolyl propane (3u)
Semi solid; 1H NMR (200 MHz, CDCl3 + DMSO-d6): δ 10.00 (bs, 2H, NH), 7.46 (d, J = 7.81 Hz, 2H), 7.27 (d, J = 7.81 Hz, 2H), 6.92–7.06 (m, 4H), 6.86 (t, J = 7.03 Hz, 2H), 4.29 (t, J = 7.03 Hz, 1H), 2.20 (p, J = 7.03 Hz, 2H), 0.98 (t, J = 7.03 Hz, 3H); 13C NMR (50 MHz, CDCl3 + DMSO-d6): δ 135.9, 127.4, 122.3, 120.6, 120.5, 116.0, 115.2, 109.3, 30.6, 24.8, 8.2; LC-MS m/z = 273.1 (M − 1)+; IR (KBr) (νmax/cm− 1): 3414 (NH), 3053, 2926, 2865, 1615, 1456, 1340, 1093, 1012, 744. Anal. Calcd for C19H18N2: C, 83.18; H, 6.61; N, 10.21. Found: C, 83.01; H, 6.80; N, 10.33.
3,3′-Bis-indolyl pentane (3v)
Semi solid; 1H NMR (200 MHz, CDCl3 + DMSO-d6): δ 7.60 (bs, 2H, NH), 7.52 (d, J = 7.55 Hz, 2H), 7.16–7.23 (m, 2H), 7.07 (dt, J = 6.79, 1.51 Hz, 2H), 6.97 (dt, J = 6.79, 1.51 Hz, 2H), 6.0 (dd, J = 2.66, 0.75 Hz, 2H), 4.40 (t, J = 7.55 Hz, 1H), 2.17 (q, J = 7.55 Hz, 2H), 1.20–1.40 (m, 4H), 0.87 (t, J = 6.79 Hz, 3H); 13C NMR (50 MHz, CDCl3 + DMSO-d6): δ 136.8, 126.2, 123.1, 119.7, 119.8, 117.0, 116.0, 109.5, 34.2, 31.5, 26.7, 22.2, 12.2; LC-MS m/z = 301.1 (M − 1)+; IR (KBr) (νmax/cm− 1): 3413 (NH), 3054, 2927, 2858, 1617, 1456, 1339, 1094, 1011, 742. Anal. Calcd for C21H22N2: C, 83.40; H, 7.33; N, 9.26. Found: C, 83.16; H, 7.42; N, 9.39.
Antibacterial assay
The antibacterial activity of the synthesized compounds was determined by the well diffusion method according to Linday [Citation43]. Three to five identical colonies from each agar plate were lifted with a sterile wire loop and transferred into a tube containing 5 ml of nutrient agar. The turbidity of each bacterial suspension was adjusted to reach an optical comparison to that of a 0.5 McFarland standard; resulting in a suspension containing approximately 1–2 × 108 CFU/ml. Nutrient agar plates were inoculated by streaking the swab over the entire sterile agar surface. This procedure was repeated by streaking two more times, rotating the plate approximately 60° each time to ensure uniform distribution of the inoculum. As a final step, the rim of the agar was also swabbed. After allowing the inoculum to dry at room temperature, 6 mm diameter wells were prepared in the agar with the help of sterilized cork borer. The different concentrations of the test compounds (100–150 μg/ml) were prepared by dissolving in dimethyl sulfoxide (DMSO) and introduced into duplicate wells. The plates were incubated at 37°C for 24 h. Subsequently, the plates were examined for bacterial growth inhibition and the inhibition zone diameter measured to the nearest millimeter. The standard antibiotic (streptomycin 50 μg/well) was used as positive control, whereas, the equivalent amount of solvent (DMSO) did not exhibit any activity in the assay.
Antifungal assay
The method followed for antifungal bioassay is similar to that followed for antibacterial assay, where the medium is PDA 39 g/l. All the test compounds were studied for their antifungal activity at concentration 100–150 μg/ml using DMSO as a solvent. The solvent did not exhibit any activity at the concentrations used. The treated and the controls were kept in an incubator at 28 ± 2°C for 48 h and inhibition zones were measured to the nearest millimeter. Three replicates were maintained for each treatment. Amphotericin-B (50 μg/ml) was used as positive control.
Results and discussion
Chemistry
BIMs were obtained by the condensation of indole (1) and various aldehydes (2a–v) in acetonitirle at room temperature in presence of 0.5 mol% Al(OTf)3 in high yields in shorter reaction time (Scheme , ). Aromatic aldehydes with electron deficient substituents required longer reaction times to produce comparable yields than those of their simple and electron-rich counterparts. Electron rich aldehydes like anisaldehyde, reacted rapidly using indole within 12 min (compound 3i), whereas, aliphatic aldehydes gave good yields of their corresponding products in 25–40 min (compounds 3t–v). To best of our knowledge, this is the first report for the synthesis of BIMs using this catalyst which possess more advantages over earlier methods like mild reaction conditions, cleaner reactions, shorter reaction times, high yield of products and low catalytic amount.
Antibacterial activity
Amongst the synthesized compounds 3a, 3g, 3i and 3p–v were screened for their antibacterial activity against Escherichia coli (MTCC 443), Pseudomonas aeruginosa (MTCC 1688), Pseudomonas oleovorans (MTCC 617), Klebsiella pneumoniae (MTCC 618) as Gram-negative bacteria, Bacillus subtilis (MTCC 441), Staphylococcus aureus (MTCC 96), as Gram-positive bacteria and the antifungal activity was evaluated against yeast Candida albicans (MTCC 227) and filamentous fungi Rhizopus oryzae (MTCC-262), Aspergillus niger (MTCC 1344). The inhibitory zones (in mm) were determined by using agar well method (cup plate method). Antibiotics, streptomycin and amphotericin-B were used as positive controls against bacteria and fungi, respectively. In all determinations, tests were performed in duplicate and results were reported as mean of at least three determinations. showed that all the tested compounds exhibited moderated to good antibacterial activity. Compounds 3q, 3t and 3u showed significant inhibition against all the bacteria tested and were not strain dependent. In compounds 3a, 3g and 3i, there was no effect of activating or deactivating substituents on the aryl ring in their inhibitory activity and found to be less potent than the other compounds tested. Moreover, these compounds were inactive against K. pneumoniae at all the concentrations used. The incorporation of 5-nitro furfuroyl group in BIM (3q) enhanced the activity in all the strains except P. aeruginosa than the corresponding furfuroyl derivative (3p) and activity reduced against S. aureus. Similarly, 5-nitrothiophenyl derivative (3s) was found to be more potent than the corresponding thiophenyl derivative (3r). The significant inhibition exhibited by the compounds 3q and 3s might be due to the presence of nitro group on the fifth position of their corresponding hetero aryl rings. Among the heteroaryl methyl(bisindoles) the activity is in the order 3q>3s>3p>3r. The replacement of aryl group with alkyl chain on the 3-methyl of bisindoles (3t–v) also enhanced the significant inhibitory activity. Compound 3u was found to be the most active from this series against all the bacterial strains tested.
Antifungal activity
The investigation of antifungal screening data from revealed that all the tested compound showed moderate to good fungal inhibition. Compounds 3q and 3s–u were active against all the fungal species. The 5-nitro heteroaryl derivatives (3q and 3s) exhibited better inhibitory activity against all the three fungal strains than the corresponding heteroaryl derivatives, whereas, compound 3p was mild active only against R. oryzae and 3r active only against C. albicans. Compound 3t was found to be the most active against C. albicans and R. oryzae and 3u was the most active against A. niger.
Table III. Antifungal activity of BIMs expressed in zone of inhibition in millimetres.
Conclusions
In conclusion, we have developed an efficient synthetic protocol for the synthesis of BIMs from various aldehydes and indoles using 0.5 mol% Al(OTf)3. Furthermore, the synthesized compounds using this method have been evaluated for their antibacterial and antifungal activities. Almost all the compounds showed moderate to good antibacterial and antifungal activities. 5-Nitro heteroaryl containing BIM (3q and 3s) have exhibited significant antibacterial activity. Compound 3u was found to be the most active from the tested compound in this series. Compounds 3s–u demonstrated significant antifungal activity. Further research in this area is in progress in our laboratory.
Acknowledgements
The authors (M.N.A.K., K.S.R. and S.K.A.) thank CSIR and the author (Y.V.V.S) thanks UGC, New Delhi, for the award of research fellowship.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- S Sakemi, and HH Sun. (1991). Nortopsentins A, B, and C. Cytotoxic and antifungal imidazolediyl bis[indoles] from the sponge Spongosorites ruetzleri. J Org Chem 56:4304–4307.
- B Bao, Q Sun, X Yao, J Hong, CO Lee, CJ Sim, KS Im, and JH Jung. (2005). Cytotoxic bisindole alkaloids from a marine sponge Spongosorites sp. J Nat Prod 68:711–715.
- AE Wright, SA Pomponi, SS Ctross, and PJ McCarthy. (1992). A new bis-(indole) alkaloid from a deep-water marine sponge of the genus Spongosorites. J Org Chem 57:4772–4775.
- SP Gunasekera, PJ McCarthy, and M Kelly-Borges. (1994). Hamacanthins A and B, new antifungal bis indole alkaloids from the deep-water marine sponge, Hamacantha Sp. J Nat Prod 57:1437–1441.
- I Mancini, G Guella, F Pietra, C Debitus, and J Waikedre. (1996). From inactive nortopsentin D, a novel bis(indole) alkaloid isolated from the axinellid sponge Dragmacidon sp. from deep waters south of new caledonia, to a strongly cytotoxic derivative. Helv Chim Acta 79:2075–2082.
- E Fahy, BCM Potts, DJ Faulkner, and K Smith. (1991). 6-Bromotryptamine derivatives from the Gulf of California tunicate Didemnum candidum. J Nat Prod 54:564–569.
- R Bell, S Carmeli, and N Sar. (1994). Vibrindole A, a metabolite of the marine bacterium, Vibrio parahaemolyticus, isolated from the toxic mucus of the boxfish Ostracion cubicus. J Nat Prod 57:1587–1590.
- TR Garbe, M Kobayashi, N Shimizu, N Takesue, M Ozawa, and H Yukawa. (2000). Indolyl carboxylic acids by condensation of indoles with α-keto acids. J Nat Prod 63:596–598.
- DJ Faulkner. (2002). Marine natural products. Nat Prod Rep 19:1–49.
- JCA Tanaka, CC da Silva, AJB de Oliveria, CV Nakamura, and BPD Filho. (2006). Antibacterial activity of indole alkaloids from Aspidosperma ramiflorum. Braz J Med Bio Res 39:387–391.
- K-B Oh, W Mar, S Kim, J-Y Kim, T-H Lee, J-G Kim, D Shin, CJ Sim, and J Shin. (2006). Antimicrobial activity and cytotoxicity of bis(indole) alkaloids from the sponge Spongosorites sp. Biol Pharm Bull 29:570–573.
- M Prudhomme, M Sancelme, A Bonnefoy, D Fabbro, and T Meyer. (1999). Synthesis and antimicrobial activities of monoindolyl- and bisindolyloximes. Eur J Med Chem 34:161–165.
- RK Tiwari, D Singh, J Singh, V Yadav, AK Pathak, R Dabur, AK Chhillar, R Singh, GL Sharma, R Chandra, and AK Verma. (2006). Synthesis and antibacterial activity of substituted 1,2,3,4-tetrahydropyrazino[1,2-a]indoles. Bioorg Med Chem Lett 16:413–416.
- E Szarvasi, L Fontaine, and A Betbeder-Matibet. (1973). Antimicrobials. New nitrofuran derivatives. J Med Chem 16:281–287.
- W Hoyle, GP Roberts, and O Meth-Cohn. (1973). Potential antimicrobial furans. J Med Chem 16:709–710.
- PJ Islip, and MR Johnson. (1973). Nitrofuryl heterocyclics. 3. J Med Chem 16:1308–1310.
- HA Burch, LE Benjamin, HE Russell, and R Freedman. (1974). Nitrofurfuryl heterocycles. 12. 4-Amino-6-(5-nitro-2-furyl)isoxazolo[5,4-d]pyrimidines and 4-amino-2-(5-nitro-2-furyl) pyrimido[4,5-d]pyrimidines. J Med Chem 17:451–453.
- CY Wang, CW Chiu, K Muraoka, PD Michie, and GT Bryan. (1975). Antibacterial activity of nitropyrroles, nitrothiophenes, and aminothiophenes in vitro. Antimicrob Agents Chemother 8:216–219.
- A Masunari, and LC Tavares. (2007). A new class of nifuroxazide analogues: Synthesis of 5-nitrothiophene derivatives with antimicrobial activity against multidrug-resistant Staphylococcus aureus. Bioorg Med Chem 15:4229–4236.
- A Foroumadi, S Mansouri, Z Kiani, and A Rahmani. (2003). Synthesis and in vitro antibacterial evaluation of N-[5-(5-nitro-2-thienyl)-1,3,4-thiadiazole-2-yl] piperazinyl quinolones. Eur J Med Chem 38:851–854.
- JR Pires, C Saito, SL Gomes, AM Giesbrecht, and AT Amaral. (2001). Investigation of 5-nitrofuran derivatives: Synthesis, antibacterial activity, and quantitative structure–activity relationships. J Med Chem 44:3673–3681.
- M Monasterios, M Escorche, and M Avendano. (2005). Conformational analysis, electronic properties and molecular electrostatic potential of nitrofurans derivatives with antibacterial activity. J Mol Structure 748:49–55.
- BS Holla, PM Akberali, and MK Shivananda. (2001). Studies on nitrophenylfuran derivatives: Part XII. Synthesis, characterization, antibacterial and antiviral activities of some nitrophenylfurfurylidene-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines. II Farmaco 56:919–927.
- GL Kedderis, and GT Miwa. (1988). The metabolic activation of nitroheterocyclic therapeutic agents. Drug Metab Rev 19:33–62.
- BS Holla, BS Rao, K Shridhara, and PM Akberali. (2000). Studies on arylfuran derivatives: Part XI. Synthesis, characterisation and biological studies on some mannich bases carrying 2,4-dichlorophenylfurfural moiety. II Farmaco 55:338–344.
- BS Holla, PM Akberali, and MK Shivananda. (2000). Studies on arylfuran derivatives: Part X. Synthesis and antibacterial properties of arylfuryl-Δ2-pyrazolines. II Farmaco 55:256–263.
- JS Yadav, BVS Reddy, CVSR Murthy, GM Kumar, and Ch Madan. (2001). Lithium perchlorate catalyzed reactions of indoles: An expeditious synthesis of bis(indolyl)methanes. Synthesis 5:783–787.
- BV Gregorovich, K Liang, M Clugston, and S Macdonald. (1968). Reductive C-alkylation. Can J Chem 46:3291–3300.
- M Roomi, and S Macdonald. (1970). Cyclizative condensation. I. 2-methylindole with acetone and methyl ethyl ketone. Can J Chem 48:139–143.
- M Auria. (1991). Photochemical synthesis of diindolylmethanes. Tetrahedron 47:9225–9230.
- A Chatterjee, S Manna, J Benerji, C Pascard, T Prange, and J Shoolery. (1980). Lewis-acid-induced electrophilic substitution in indoles with acetone. Part 2. J Chem Soc Perkin Trans 1:553–555.
- WE Noland, MR Venkiteswaran, and CG Richards. (1961). Cyclizative condensations. I. 2-Methylindole with acetone and methyl ethyl ketone. J Org Chem 26:4241–4248.
- DP Chen, LB Yu, and PG Wang. (1996). Lewis acid-catalyzed reactions in protic media. Lanthanide-catalyzed reactions of indoles with aldehydes or ketones. Tetrahedron Lett 37:4467–4470.
- R Nagarajan, and PT Perumal. (2002). InCl3 and In(OTf)3 catalyzed reactions: Synthesis of 3-acetyl indoles, bis-indolylmethane and indolylquinoline derivatives. Tetrahedron 58:1229–1232.
- XL Mi, SZ Luo, JQ He, and JP Chen. (2004). Dy(OTf)3 in ionic liquid: An efficient catalytic system for reactions of indole with aldehydes/ketones or imines. Tetrahedron Lett 45:4567–4570.
- SJ Ji, SY Wang, Y Zhang, and TT Loh. (2004). Facile synthesis of bis(indolyl)methanes using catalytic amount of iodine at room temperature under solvent-free conditions. Tetrahedron 60:2051–2055.
- JP Bhuyan, and ML Deb. (2006). An efficient and clean synthesis of bis(indolyl)methanes in a protic solvent at room temperature. Tetrahedron Lett 47:1441–1443.
- GA Olah, O Farooq, SMF Farnia, and JA Olah. (1988). Friedel–Crafts chemistry. 11. Boron, aluminum, and gallium tris(trifluoromethanesulfonate) (triflate): Effective new Friedel–Crafts catalysts. J Am Chem Soc 110:2560–2565.
- A Kamal, MNA Khan, KS Reddy, YVV Srikanth, and T Krishnaji. (2007). Al(OTf)3 as a highly efficient catalyst for the rapid acetylation of alcohols, phenols and thiophenols under solvent-free conditions. Tetrahedron Lett 48:3813–3818.
- H Firouzabadi, N Iranpoor, and G Kohmareh. (2003). Aluminium trifluromethanesulfonate [Al(OTf)3] as a highly efficient and chemoselective catalyst for thioacetalization of carbonyl compounds under mild conditions. Synth Commun 33:167–173.
- DBG Williams, and M Lawton. (2005). Aluminium triflate: A remarkable Lewis acid catalyst for the ring opening of epoxides by alcohols. Org Biomol Chem 3:3269–3272.
- DBG Williams, and M Lawton. (2006). Aluminium triflate: An efficient recyclable Lewis acid catalyst for the aminolysis of epoxides. Tetrahedron Lett 47:6557–6560.
- ME Linday. Practical introduction to microbiology. London: E & F.N. Spon Ltd; (1962). p 177.