Abstract
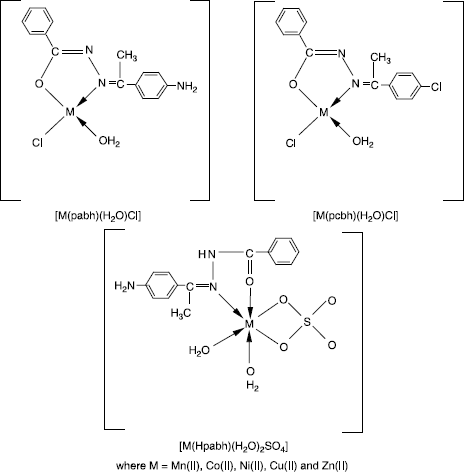
Complexes of the type [M(pabh)(H2O)Cl], [M(pcbh)(H2O)Cl] and [M(Hpabh)(H2O)2 (SO4)] where, M = Mn(II), Co(II), Ni(II), Cu(II) and Zn(II); Hpabh = p-amino acetophenone benzoyl hydrazone and Hpcbh = p-chloro acetophenone benzoyl hydrazone have been synthesized and characterized with the help of elemental analyses, electrical conductance, magnetic susceptibility measurements, electronic, ESR and IR spectra, thermal (TGA & DTA) and X-ray diffraction studies. Co(II), Ni(II) and Cu(II) chloride complexes are square planar, whereas their sulfate complexes have spin-free octahedral geometry. ESR spectra of Cu(II) complexes with Hpabh are axial and suggest ![]() as the ground state. The ligand is bidentate bonding through >C = N − and deprotonated enolate group in all the chloro complexes, whereas, >C = N and >C = O groups in all the sulfato complexes. Thermal studies (TGA & DTA) on [Cu(Hpabh)(H2O)2(SO4)] indicate a multistep decomposition pattern, which are both exothermic and endothermic in nature. X-ray powder diffraction parameters for [Co(pabh)(H2O)Cl] and [Ni(Hpabh)(H2O)2(SO4)] correspond to tetragonal and orthorhombic crystal lattices, respectively. The ligands as well as their complexes show a significant antifungal and antibacterial activity. The metal complexes are more active than the ligand.
as the ground state. The ligand is bidentate bonding through >C = N − and deprotonated enolate group in all the chloro complexes, whereas, >C = N and >C = O groups in all the sulfato complexes. Thermal studies (TGA & DTA) on [Cu(Hpabh)(H2O)2(SO4)] indicate a multistep decomposition pattern, which are both exothermic and endothermic in nature. X-ray powder diffraction parameters for [Co(pabh)(H2O)Cl] and [Ni(Hpabh)(H2O)2(SO4)] correspond to tetragonal and orthorhombic crystal lattices, respectively. The ligands as well as their complexes show a significant antifungal and antibacterial activity. The metal complexes are more active than the ligand.
Introduction
The chemistry of transition metal complexes with multidentate Schiff base ligands has attracted particular attention because these metal ions can exhibit several oxidation states [Citation1]. Such complexes with different oxidation states have a strong role in bio-inorganic chemistry and redox enzyme systems [Citation2,Citation3] and may provide the basis of models for active sites of biological systems [Citation4,Citation5] or act as catalysts Citation6, Citation7, Citation8.
Metal complexes of multidentate acylhydrazone Schiff bases have been extensively studied Citation9, Citation10, Citation11 because such ligands can bind with one, two or more metal centers involving various coordination modes and allow successful synthesis of homo and hetero nuclear metal complexes with interesting stereo-chemistry Citation12, Citation13, Citation14. Since the complexes of acylhydrazones involving bivalent transition metal ions may have their potential use in the biological fields, it is worthwhile to undertake the present study. Accordingly, we have synthesized and characterized a number of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes with p-substituted acetophenone benzoylhydrazone ligand and studied their bio-activity.
Experimental
Materials
All the chemicals used were of BDH or equivalent grade. The precursor benzoyl hydrazine, C6H5CONHNH2 (bh) was prepared by the reported procedure [Citation10] by refluxing methyl benzoate with hydrazine hydrate in 1:1 molar ratio in a RB flask containing 10 mL ethanol for 4 h. The pure product was characterized by its melting point, mp 111°C (lit. 112°C).
Preparation of p-substituted acetophenone benzoylhydrazone
p-Amino acetophenone benzoylhydrazone (Hpabh), H2NC6H4C(CH3) = NNHCO C6H5 and p-chloro acetophenone benzoylhydrazone (Hpcbh), ClC6H4C(CH3) = NNHCO C6H5 were prepared separately by reacting p-amino acetophenone (10 mmol, 13.5 g) or p-chloro acetophenone (10 mmol, 15.5 g) with bh (10 mmol, 13.6 g) dissolved in 50 mL ethanol. The reaction mixtures were taken in a RB flask and refluxed for 4-5 h and then transferred into a beaker. The products were filtered on a suction pump, washed several times with distilled water and a little ethanol and recrystallized from hot benzene. The pure compounds were dried over anhydrous CaCl2 in a desiccator.
The ligands were characterized by elemental analyses (C, H, N), melting points (), infrared spectra () 1H and 13C NMR spectra.
Table I. Analytical data of the ligands and their complexes.
Hpabh
1H NMR (ppm): –CH3 protons (1.0), -NH2 (4.2), > NH (6.8), aromatic ring protons (7.0-7.9). Hpabh, 13C NMR (ppm): –CH3 (11.4), aromatic ring carbons (118.0-147.1), >C = N- (156.3), >C = O (171.4).
Hpcbh
1H NMR (ppm): –CH3 protons (1.1), >NH (6.6), aromatic ring protons (7.1-7.7). Hpcbh, 13C NMR (ppm): –CH3 (11.6), aromatic ring carbons (128.7-139.3), >C = N- (158.2), >C = O (171.0).
Synthesis of the metal complexes
The metal(II) chloride complexes were synthesized by reacting 50 mL of ethanolic solution containing 10 mmol each of MnCl2.4H2O (1.98 g), CoCl2.6H2O (2.38 g), NiCl2.6H2O (2.38 g), CuCl2.2H2O (1.70 g), ZnCl2.H2O (1.54 g) with 50 mL ligand solution of Hpabh (10 mmol, 2.53 g) or Hpcbh (10 mmol, 2.73 g) in hot ethanol in 1:1 (M:L) molar ratio and were refluxed in a RB flask for ∼2 h. Adding 10 mL diethyl ether after cooling the reaction mixture at room temperature precipitated the complexes. The compounds were filtered in a glass crucible and were purified by washing several times washed with ethanol and finally with diethyl ether and dried in a desiccator over anhydrous CaCl2 at room temperature.
The metal(II) sulfate complexes with Hpabh were prepared by reacting 50 mL of aqueous ethanolic solution (50%, v/v) containing 10 mmol each of MnSO4.4H2O (2.23 g), CoSO4.7H2O (2.81 g), NiSO4.7H2O (2.81 g), CuSO4.5H2O (2.50 g) and ZnSO4.7H2O (2.87 g) at room temperature with hot ethanolic solution of Hpabh (10 mmol, 2.53 g) separately in a beaker in 1:1 (M:L) molar ratio. The complexes were precipitated immediately and were filtered in a glass crucible, washed with aqueous ethanol and finally with diethyl ether and dried in a desiccator.
Analyses of the complexes
Elemental analyses
The complexes were analyzed for metal content gravimetrically by literature procedures [Citation15] after decomposing the organic matter with a mixture of HNO3 and HCl and evaporating the residue to dryness with conc. H2SO4. The chloride content in the complex was determined gravimetrically as AgCl and sulfate as BaSO4.
Carbon, hydrogen and nitrogen were determined microanalytically on a Elementar Vario EL III Carlo Erba 1108 model, microanalyzer.
Physico-chemical measurements
The molar conductance of the complexes was determined by preparing 10− 3 M solutions of the complexes in distilled water at room temperature and measured on a Systronic Conductivity meter model-306. Thermal studies (TGA and DTA) of some of the complexes were carried out on a Perkin-Elmer Thermal Analyzer between room temperature to 800°C. Room temperature magnetic susceptibility measurements were carried out on a Faraday balance using Hg[Co(SCN)4] as calibrant and corrected for diamagnetism [Citation16]. The electronic spectra of the complexes were recorded in nujol on a Perkin-Elmer Lambda-2 spectrophotometer in the range 1100-200 nm. Infrared spectra of the complexes and parent ligands were recorded on Vector-22 spectrophotometer in the range 4000-500 cm− 1 in KBr medium. 1H and 13C NMR spectra of the ligands were recorded in DMSO on a JEOL AL 300 FT NMR Spectrometer. The X-band ESR spectra of copper(II) complexes were recorded on a EMX 1444 EPR spectrometer at room temperature (298 K) in solid state using DPPH as g marker (g = 2.0023). Powder X-ray diffraction patterns of a few complexes were recorded on Iso Debye Flex 2002 apparatus using CuKα radiation. The analytical and physico-chemical data are given in .
Table II. Magnetic moments and electronic spectral data of the complexes.
Table III. Important IR spectral bands (cm−1) and their assignments.
Table IV. ESR spectral parameters of Cu(II) complexes in solid state at room temperature (298 K).
Table V. Thermal decomposition of [Cu(Hpabh)(H2O)2(SO4)] complex.
Table VI. Observed and calculated Q and hkl values.
Biological activity
Antifungal activity
The ligands as well as their complexes were screened for their antifungal activity against various fungi viz. Alternaria sp., Rizoctonia sp., Stemphylium sp. and Penicillium sp. These species were isolated from the infected organs of the host plants on potato dextrose agar (potato 250 g+ dextrose 20 g+ agar 20 g) medium. The cultures of the fungi were purified by single spore isolation technique.
The solution in different concentrations 0.5, 1 and 1.5 mg/mL of each compound in water were prepared for testing against spore germination. A drop of the solution of each concentration was kept separately on glass slides. The conidia, fungal reproducing spores (approx. 200) lifted with the help of an inoculating needle, were mixed in every drop of each compound separately. Each treatment was replicated thrice and a parallel water solvent control set was run concurrently on separate glass slides. All the slides were incubated in humid chambers at 25 ± 2°C for 24 h. Each slide was observed under the microscope for spore germination and percent germination was finally calculated. The results were also compared with a standard antifungal drug miconazole at the same concentrations.
Antibacterial activity
The antibacterial activity of the ligands and their complexes was studied against Clostridium sp. and Pseudomonas sp. bacteria. Each of the compounds was dissolved in water and solutions of the concentration 1 mg/mL and 2 mg/mL were prepared separately. Paper discs of Whatman filter paper (No. 42) of uniform diameter (2 cm) were cut and sterilized in an autoclave. The paper discs soaked in the desired concentration of the complex solutions were placed aseptically in the petridishes containing nutrient agar media (agar 20 g+ beef extract 3 g+ peptone 5 g) seeded with Clostridium sp. and Pseudomonas sp. bacteria separately. The petridishes were incubated at 37°C and the inhibition zones were recorded after 24 h of incubation. Each treatment was replicated 9 times.
The antibacterial activity of a common standard antibiotic Ampicillin was also recorded using the same procedure as above at the same concentrations and solvent. The % Activity Index for the complex was calculated by the formula as under:
Determination of minimum inhibitory concentration (MIC) value
The antibacterial screening concentrations of the compounds to be used were estimated from the minimum inhibitory concentration (MIC) value. The MIC was determined using the disc diffusion technique by preparing discs containing 0.1 to 1.0 mg/mL of each compound against both the bacteria and applying the protocol. All the compounds were more effective at 1.0 and 2.0 mg/mL concentrations. Consequently all the compounds were screened at these concentrations against both the bacteria. The results of MIC values (mg/mL) are given in .
Results and discussion
Analysis of complexes
The analytical data of the complexes () show that the ligands p-amino acetophenone benzoylhydrazone (Hpabh) and p-chloro acetophenone benzoyl hydrazone (Hpabh) react with metal(II) salts in 1:1 (M:L) molar ratio to give complexes of general compositions [M(pabh)(H2O)Cl], [M(pcbh)(H2O)Cl] and [M(Hpabh)(H2O)2(SO4)]. It also appears from the analytical data that both the ligands Hpabh and Hpcbh enolize and deprotonate during complexation with metal(II) chloride. The reactions may have proceeded as follows:
where, M = Mn(II), Co(II), Ni(II), Cu(II) and Zn(II)
The complexes are insoluble in benzene, carbon tetrachloride, chloroform, diethyl ether and n-hexane but soluble in water, hot ethanol, DMF and DMSO. They are light yellow, pink and brown to dark green in color. The complexes melt with decomposition between 188-270°C and are non-electrolytes [Citation17] as indicated by their low molar conductance values of 10− 3 M solutions of the complexes in water at room temperature (2.08-9.02 Ω− 1 cm2 mol− 1).
Magnetic moments
Where spin–spin coupling between unpaired electrons belonging to different copper ions is absent, μeff varies between 1.75 and 2.20 B.M., depending on the geometries of the complexes due to difference in orbital contribution. The magnetic moments of the three Cu(II) complexes correspond to μeff values of one unpaired electron (1.70, 1.80, 1.82 B.M.) suggesting a square planar or distorted octahedral geometry for the complexes. The effective magnetic moments reported for high spin octahedral Ni(II) complexes are in the range 2.9-3.4 B.M., while for the tetrahedral nickel(II) complexes, the values range from 3.5-4.0 B.M. [Citation18]. Ni(II) sulfate complex in this study, shows μeff value 2.91 B.M. corresponding to two unpaired electrons in octahedral environment, whereas Ni(II) chloride complexes are diamagnetic suggesting square planar geometry for the complex ().
Cobalt(II) tetrahedral complexes generally show magnetic moments between 4.0-4.6 B.M. while the octahedral complexes show between 4.7-5.2 B.M. because of the orbital contribution [Citation16]. The μeff value 4.71 B.M. observed for Co(II) sulfate complex is fairly close to those reported for three unpaired electrons in an octahedral environment. However, Co(II) chloride complexes show a low μeff value (2.10, 2.21 B.M.) corresponding to one unpaired electron suggests square planar geometry for the complexes. The Mn(II) complexes have their μeff values 5.90, 5.92 and 5.88 B.M. corresponding to five unpaired electrons in tetrahedral or octahedral environment.
Electronic spectra (Table II)
[Cu(pabh)(H2O)Cl] and [Cu(pcbh)(H2O)Cl] show a broad band centered at 16,600 and 16,770 cm− 1, respectively, indicating a square planar geometry for both the complexes, similar to [Cu(NH3)4]2 + (16,660 cm1) [Citation19]. The other complex in this study [Cu(Hpabh)(H2O)2(SO4)] shows two bands at 11,235 cm− 1 and 14,700 cm− 1, which may be assigned to 2B1 g → 2B2 g and → 2A1 g suggesting a tetragonally distorted octahedral geometry for the complex.
Nickel(II) complexes generally show three bands in octahedral environment corresponding to the transitions 3A2 g (F) → 3T2 g (F) (v1), → 3T1g (F) (v2) and → 3T1 g (P) (v3) [Citation18]. In the spectra of [Ni(NH3)6]2 + , these bands have been reported at 10,700, 17,540 and 28,170 cm1, respectively. [Ni(Hpabh)(H2O)2(SO4)] complex also shows above three transitions at 10,630 cm− 1 (v1), 16,520 cm− 1 (v2) and 26850 cm− 1 (v3) suggesting an octahedral geometry for the complex. Ligand field parameters (10 Dq, B, β and β°) have also been calculated by the procedure laid down by Lever [Citation19]. Energy of the first absorption band is taken to be equal to 10 Dq. The low value of Racah parameter, B (765 cm− 1) compared to the free ion value of 1041 cm− 1 indicates significant covalent character in the complex. The nephelauxetic ratio β (0.735) and percent covalency β° (26.50) also support some covalent character in the metal-ligand bond. The electronic spectra of [Ni(pabh)(H2O)Cl] and [Ni(pcbh)(H2O)Cl] complexes suggest a square planar geometry similar to that reported for Ni(II) acetylacetone bis-acylhydrazones [Citation9] and many other nickel(II) complexes [Citation20].
Cobalt(II) complexes give rise to three absorption bands in the visible region under the influence of the octahedral field by the excitation of the electron from the ground state 4T1 g (F) to the excited states 4T2 g (F), 4A2 g (F) and 4T1 g (P). Incase of [Co(H2O)6]2 + , three transition are observed at 8,130, 17,540 and 21,980 cm− 1, respectively. In[Co(Hpabh)(H2O)2(SO4)] complex, only two bands are observed at 9,260 cm− 1 (v1) and 19,880 cm− 1 (v3) indicating octahedral geometry for the complex. The v2 transition was not observed due to very week intensity [Citation19]. The various ligand field parameters 10 Dq (10,415 cm− 1), B (785 cm− 1), β (0.808) and β° (19.20) were also calculated for this complex. The two bands observed for [Co(pabh)(H2O)Cl] and [Co(pcbh)(H2O)Cl] complexes are in good agreement with the bands reported for cobalt(II) square planar complexes [Citation21].
The intensities of octahedral manganese(II) complexes are extremely low as a consequence of their doubly forbidden nature. For both tetrahedral and octahedral fields the ground state of Mn(II) being unaffected by the crystal field will be the same [Citation19]. It has already been proven that the octahedral field energy level sequence for a dn configuration is the same as the tetrahedral field energy level sequence for the d10 − n configuration. It follows therefore that for a d5 ion, which is its own hole equivalent, the same energy level diagram may be used for octahedral or tetrahedral fields. The weak bands observed for [Mn(pabh)(H2O)Cl] and [Mn(pcbh)(H2O)Cl] complexes suggest a tetrahedral geometry whereas, [Mn(Hpabh)(H2O)2(SO4)] shows an octahedral geometry.
IR spectra (Table III)
The ligands Hpabh and Hpcbh show a broad band in the region 3290 and 3282 cm− 1 respectively, due to v(NH/NH2). In the metal complexes, this band occurs either at the same wave number as in the parent ligands or at slightly shifted wave numbers, indicating non-involvement of >NH or –NH2 groups in bonding. A broad band in the same region (centered between 3380-3410 cm1) in all the metal complexes may also indicate v(OH) due to presence of H2O molecule in these complexes.
A shift to lower wave numbers in amide I, v(C = O) (15-20 cm− 1) and amide(II) (13-21 cm− 1) and a shift to higher wave numbers in the amide(III) band (5-14 cm− 1) in spectra of all the metal(II) sulfate complexes () compared to that of the parent ligands indicates coordination through carbonyl oxygen [Citation22]. The disappearance of v(C = O) band in all the metal(II) chloride complexes and appearance of v(N = C − O) and v(C − O) in the ranges 1470-1490 cm− 1 and 1320-1330 cm1, respectively, suggest bonding to metal through deprotonated C − O group [Citation23]. In all the complexes v(C = N) band appears at the lower frequency (18-30 cm− 1) than that observed in the ligands, indicating the coordination through nitrogen atom of the azomethine ( >C = N − ) group to the metal [Citation9]. v(N − N) observed at 990 and 985 cm− 1 in the ligands Hpabh and Hpcbh respectively, shifts to higher frequency by 12-32 cm− 1 in the complexes, indicating the coordination of one of the nitrogen atom of the N-N group [Citation24].
All the metal complexes also show weak bands in the 901-910, 752-760 and 638-649 cm− 1 ranges due to coordinated water [Citation25]. The bands observed near 1200-1220, 1160-1175 and 1040-1060 cm− 1 ranges suggest the presence of bidentate chelating sulfate groups in all the metal(II) sulfate complexes. A non-ligand band observed in the region 570-590 cm− 1 has been assigned to v(M-O).
ESR spectra (Table IV)
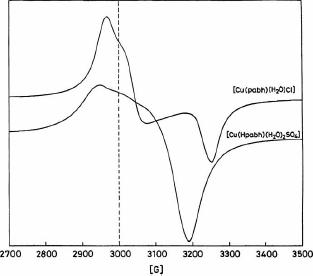
ESR spectra of powdered samples of the Cu(II) complexes at room temperature exhibit an axial signal with two g values (). The axial signals were analyzed by the procedure given by Hathaway and Billing [Citation26]. The g‖ and g⊥ values are >2.04 (), consistent with copper(II) in axial symmetry with an elongated tetragonally distorted octahedral stereochemistry for copper(II) sulfate complex and a square planar stereochemistry for copper(II) chloride complex. The G factor, defined as G = (g‖-2)/(g⊥ - 2), is greater than 4.00, suggests that the local tetragonal axes are only slightly misaligned and that the exchange interactions between copper(II) centers in the solid state are negligible [Citation26]. Moreover, the observation that g‖ > g⊥ > ge (2.0023), shows that the unpaired electron is in ![]() orbital of the copper(II) ion [Citation27,Citation28].
orbital of the copper(II) ion [Citation27,Citation28].
General structures are proposed for the metal complexes on the basis of above discussion ().
Thermal analyses (TGA & DTA)
Thermal studies on [Cu(Hpabh)(H2O)2(SO4)] complex () indicate that the complex is highly stable and shows no weight loss upto 154°C. The complex looses weight at 155°C corresponding to the removal of two water molecules. This indicates that both the water molecules are coordinated to the metal. The complex further looses weight appreciably near 250°C due to partial decomposition of organic ligand. The DTA study shows significant heat liberation during ligand decomposition. The weight of the complex observed at 360°C corresponds to CuSO4 as a result of complete decomposition of organic ligand.
X-ray diffraction studies
The X-ray powder diffraction patterns for [Co(pabh)(H2O)Cl] and [Ni(Hpabh)(H2O)2(SO4)] complexes were recorded and successfully indexed () by using Ito's method [Citation29] and the lattice constants calculated as follows:
The above constants indicate a tetragonal crystal lattice for the former and an orthorhombic crystal lattice for the latter complex.
Antifungal activity
The antifungal experimental data () indicate that the complexes show a fair degree of activity against Alternaria sp., Rizoctonia sp., Stemphylium sp. and Penicillium sp. at 0.5, 1.0 and 1.5 mg/mL concentration. Comparative analysis shows a higher antifungal activity for the metal complexes than the free ligands [Citation30]. The activity is appreciably enhanced at the higher concentration of the compounds. Both the ligands Hpabh and Hpcbh show better activity against Stemphylium sp. [Cu(pabh)(H2O)Cl] shows the highest activity (95, 97, 90 and 98%) against Alternaira sp., Rizoctonia sp., Stemphylium sp. and Penicillium sp., respectively, among all the complexes at the concentration of 1.5 mg/mL. The complexes generally vary in their antifungal activity in the following order of fungal species;
Table VIIA. Antifungal activity of the ligands and their complexes.
All the metal complexes exhibited better antifungal activities against Penicillium sp. as compared to the standard drug Miconazole. They are also more effective against Alternaria sp. than the standard except the Ni(II) and Zn(II) complexes. Cu(II), Co(II) and Ni(II) sulfate complexes exhibited greater activity against Rizoctonia sp. than the standard drug. The ligand Hpabh and its complexes are more effective as compared to ligand Hpcbh and its complexes.
The increase in antimicrobial activity of metal complexes may be explained on the basis of chelation theory. On chelation, the polarity of the metal ion will be reduced to a greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups [Citation31]. Further, the mode of action of the compounds may involve the formation of a hydrogen bond through the azomethine group with the active centre of cell constituents, resulting in interference with the normal cell process [Citation32]. The toxicity of the complexes can be related to the strength of the metal-ligand bond, besides other factors such as size of the cation [Citation33] receptor sites, diffusion and a combined effect of the metal and ligands for inactivation of the biomolecules [Citation34].
Antibacterial activity
The metal complexes, ligands, standard drug Ampicillin and control were screened separately for their antibacterial activity against Pseudomonas sp. (Gram − ve) and Clostridium sp (Gram + ve). The activity generally increases with increasing the concentration of the complexes [Citation35]. The metal complexes are more effective against Pseudomonas sp. than Clostridium sp. The activity of the complexes has been compared with the activity of a common standard antibiotic Ampicillin and % Activity Index has been calculated for the complexes. The antibacterial results suggest that the ligands and their complexes () show a moderate activity against both the bacteria [Citation36,Citation37] as compared to the standard drug (Ampicillin). The metal complexes show higher antibacterial activity than the parent ligands. The % Activity Index data indicate the highest activity (89 %) for [Cu(pabh)(H2O)Cl], [Cu(Hpabh)(H2O)2(SO4)] and [Ni(Hpabh)(H2O)2(SO4)] complexes against Pseudomonas sp. at the concentration of 2.0 mg/mL.
Table VIIB. Antibacterial activity of the ligands and their complexes.
Acknowledgements
The authors thank the Head, Department of Chemistry, Indian Institute of Technology, Kanpur for recording UV-Vis, IR and ESR spectra, Dr. Nand Lal, Department of Life Sciences, C. S. J. M. University, Kanpur for help in biological screening.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- A Berkessel, M Bolte, T Neumann, and L Seidel. (1996). Synthesis and X-ray crystal structure of the first mononuclear nickel(II) alkane thiolate complex with a mixed (S,N,N,O) ligand field. Chem Ber 129:1183–1189.
- JR Lancaster. The Bioinorganic Chemistry of Nickel. New York: Wiley-VCH; (1988).
- AF Kolodziej. (1994). The chemistry of nickel containing enzymes. Prog Inorg Chem 41:493–597.
- RK Parashar, RC Sharma, A Kumar, and G Mohan. (1988). Stability studies in relation to IR data of some Schiff base complexes of transition metals and their biological and pharmacological studies. Inorg Chim Acta 151:201–208.
- DX West, H Gebremedhin, RJ Butcher, JP Jasinski, and AE Liberta. (1993). Structures of nickel(II) and copper(II) complexes of 2-acetylpyridine azacyclothiosemicarba zones. Polyhedron 12:2489–2497.
- M Beley, JP Collin, R Rupert, and JP Sauvage. (1986). Electrocatalytic reduction of CO2 by Ni(cyclam)2+ in water. Study of the factors affecting the efficiency and the selectivity of the process. J Am Chem Soc 108:7461–7467.
- E Fujita, BS Brunschwig, T Ogata, and S Yanagida. (1994). Toward photochemical carbon dioxide activation by transition metal complexes. Coord Chem Rev 132:195–200.
- E Kimura, S Wada, M Shionoya, and Y Okazaki. (1994). New series of multi functionalized nickel(II)-cyclam complexes. Application to the photoreduction of carbon dioxide. Inorg Chem 33:770–778.
- KK Narang, and VP Singh. (1993). Synthesis and characterization of cobalt(II), nickel(II), copper(II) and zinc(II) complexes with acetylacetone bis-benzoyl hydrazone and acetylacetone bis-isonicotinoyl hydrazone. Transition Met Chem 18:287–290.
- B Singh, R Srivastava, KK Narang, and VP Singh. (1999). Synthesis and spectral studies of copper(II) sulfate complexes with some acetophenone acylhydrazones. Synth React Inorg Met-Org Chem 29:1867–1881.
- A Bacchi, M Carcelli, P Pelagatti, G Pellizi, C Salati, and P Sgarabotto. (1999). Nickel(II) and palladium(II) complexes with acylhydrazone ligands of alpha diketones: the electronic and steric factors affecting the formation of The dimeric palladium(II) complexes. Inorg Chim Acta 295:171–179.
- P Zanello, S Tamburini, PA Vigato, and GA Mazzocchin. (1987). Synthesis, structure and electrochemical characterization of homodinuclear and heterodinuclear copper complexes with compartmental ligands. Coord Chem Rev 77:165–273.
- Y Ikawa, T Nagata, and K Maruyama. (1993). Synthesis and electrochemical properties of dinuclear manganese(II) complexes with octadentate Schiff base macrocycles. Chem Lett 6:1049–1052.
- T Aono, H Wada, Y Aratake, N Matsumoto, H Okawa, and Y Matsuda. (1996). Crystal structure and spin doublet electron spin resonance of a magnetically coupled di (mu-phenoxo) copper(II) nickel(II) complex. J Chem Soc Dalton Trans 25–29.
- AI Vogel. Vogel's text book of Quantitative Chemical Analysis. 5th ed.Amsterdam: Longman; (1989).
- RL Dutta, and A Syamal. Elements of Magnetochemistry. 2nd ed.New Delhi: Affiliated East-West Press Pvt. Ltd; (1993).
- WJ Geary. (1971). The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord Chem Rev 7:81.
- FA Cotton, G Wilkinson, CA Murillo, and M Bochmann. Advanced Inorganic Chemistry. 6th ed. New York: John Wiley & Sons Inc. (2003).
- , and Lever ABP, and Inorganic Electronic Spectroscopy. 2nd ed.New York: Elsevier; (1984).
- F Bigoli, P Cassaux, P Deplano, ML Mercuri, MA Pellighelli, G Pintus, A Serpe, and EF Trogu. (2000). Synthesis, structure and properties of new unsymmetrical nickel dithiolene complexes useful as near-infrared dyes. J Chem Soc Dalton Trans 24:4639–4644.
- Y Nishida, and S Kida. (1979). Splitting of d-orbitals in square planar complexes of copper(II), nickel(II) and cobalt(II). Coord Chem Rev 27:275–298.
- SA Shama, and H Omara. (2001). Synthesis and characterization of some transition metal complexes with Schiff bases derived from 2-hydroxy acetylacetophenone. Spectroscopy Lett 34:49.
- RJ Butcher, J Jasinski, GM Mockler, and E Sinn. (1976). Synthesis and crystal structure of bis-MU-(5-chloro 2-hydroxy N-methyl alpha-phenyl benzylideneiminato-N,O)-bis[ethanol (nitrato-O-O′) nickel(II)] New type of nickel(II) dimmer. J Chem Soc 2:1099–1102.
- ZH Chohan, MA Farooq, A Scozzafava, and CT Supuran. (2002). Antibacterial Schiff bases of oxalyl-hydrazine/diamide incorporating pyrolyl and salicylyl moieties and of their zinc(II) complexes. J Enz Inhib Med Chem 17:1.
- KK Narang, and VP Singh. (1996). ESR studies on acylhydrazine and hydrazone copper(II) sulfate complexes. Transition Met Chem 21:507–511.
- BJ Hathaway, and DE Billing. (1970). Electronic properties and stereochemistry of mono-nuclear complexes of copper(II) ion. Coord Chem Rev 5:143.
- P Bindu, MRP Kurup, and TR Satyakeerty. (1999). EPR, cyclic voltametric and biological activities of copper(II) complexes of salicylaldehyde N(4)-substituted thiosemicarbazone and heterocyclic bases. Polyhedron 18:321–331.
- OI Singh, M Damayanti, NR Singh, RKH Singh, M Mohapatra, and RM Kadam. (2005). Synthesis, EPR and biological activities of bis(1-n-butyl amidino-o-alkylurea) copper(II) chloride complexes. Polyhedron 24:909–916.
- VP Singh, and P Gupta. (2006). Synthesis, characterization and biocidal activities of some metal(II) complexes with diacetyl salicylaldehyde acyldihydrazone. J Coord Chem 59:1483–1494.
- R Nagar. (1990). Synthesis, characterization and microbial activity of some transition metal complexes involving potentially active O-donor and N-donor heterocyclic ligands. J Inorg Biochem 40:349–356.
- SU Rehman, ZH Chohan, F Gulnaz, and CT Supuran. (2005). In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. J Enz Inhib Med Chem 20:333–340.
- N Dharmaraj, P Viswanathamurthi, and K Natarajan. (2001). Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Transition Met Chem 26:105–109.
- KN Thimmaiah, GT Chandrappa, and Rangaswamy Jayarama. (1984). Structural studies of biologically active complexes of zinc(II), cadmium(II), mercury(II) and copper(II) with p-anisaldehyde thiosemicarbazone. Polyhedron 3:1237–1239.
- RB Johari, and RC Sharma. (1988). Synthetic and biocidal studies of some bivalent metal complexes of benzaldehyde salicyloylhydrazone. J Indian Chem Soc 65:793–794.
- ZH Abd El-Wahab, and MR El-Sarrag. (2004). Derivatives of phosphate Schiff base transition metal complexes: Synthesis, studies and biological activity. Spectrochim Acta 60A:271–277.
- PK Panchal, HM Parekh, and MN Patel. (2005). Preparation, characterization and toxic activity of oxovanadium(IV) mixed-ligand complexes. Toxicol Env Chem 87:313.
- K Deepa, and KK Aravindakshan. (2005). Synthesis, characterization and antifungal studies of metal complexes of benzoyl- and salicylylhydrazones of omega-bromoacetanilide. Synth React Inorg Met-Org Nano-Met Chem 35:409–416.