Abstract
2,4,5-trimethoxycinnamic acid (TMC), the major and non toxic metabolite of α-asarone (2,4,5-trimethoxy-1-propenyl benzene), retains most of the pharmacological properties of α-asarone, since both substances, administered to hypercholesterolemic rats at 80 mg/kg body wt, decreased total serum cholesterol, lowered LDL-cholesterol levels and kept unaffected HDL-cholesterol levels. In addition, both substances increased bile flow, especially in hypercholesterolemic rats, by rising the secretion of bile salts, phospholipids and bile cholesterol. These drugs also reduced cholesterol levels of gallbladder bile, whereas phospholipids and bile salts concentrations were increased, decreasing the cholesterol saturation index (CSI). We also found that α-asarone was 20 times better inhibitor of HMG-CoA reductase than TMC. This effect on HMG-CoA reductase was the only property highly reduced in TMC in comparison with α-asarone, while the other pharmacological properties of α-asarone were retained by TMC. These experiments strongly suggest that TMC can be further studied as a possible hypocholesterolemic and cholelitholytic agent.
Introduction
Yumel (Guatteria gaumeri, Greenman, Annonaceae) is a native plant from Yucatán, México that has been used as a bark infusion in traditional medicine for the treatment of gallstones [Citation1]. It was found that the active principle of G. gaumeri was α-asarone, (2,4,5-trimethoxy-1-propenylbenzene), since this substance isolated from the dried ground bark of this plant was able to decrease rat and human serum levels of cholesterol [Citation2]. The hypocholesterolemic and the cholelitholytic effects of α-asarone were confirmed by others [Citation3]. Furthermore, in previous investigations, we found that α-asarone inhibited hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase), the rate controlling enzyme in cholesterol biosynthesis, lowered serum LDL-cholesterol levels, leaving serum HDL-cholesterol levels unaffected; increased bile flow and reduced biliary cholesterol saturation index (CSI) in hypercholesterolemic rats [Citation4]. These findings could explain the hypocholesterolemic and the cholelitholytic properties of α-asarone. It has also been demonstrated that α-asarone possess genotoxic [Citation5,Citation6] and hepatocarcinogenic properties in rodents [Citation7]. Therefore, α-asarone cannot be used in clinical trials. On the contrary, 2,4,5-trimethoxycinnamic acid (TMC), the major metabolite of α-asarone, does not have the above mentioned toxicological effects [Citation6].
It is well known that many of the metabolic biotransformation reactions leading to inactive metabolites of drugs can also generate biologically active metabolites [Citation8]. Therefore, in this investigation, we comparatively studied the effect of TMC and α-asarone on the activity of hepatic HMG-CoA reductase and on the balance between circulating lipoprotein-cholesterol levels and bile secretion, as well as on the levels of cholesterol, phospholipids and bile salts secreted in bile, in order to find out if TMC retains some of the pharmacological properties of α-asarone.
Materials and methods
Materials
Cholesterol, 2,4,5-trimethoxycinnamic acid, α-asarone, sodium cholate, sodium pentobarbital, NADPH, NAD, cholestyramine, HMG-CoA, EDTA, dithiothreitol, bovine serum albumin, Tris-HCl, and 3-α-hydroxysteroid dehydrogenase were obtained from Sigma-Aldrich. All other chemicals used were of the highest commercially available grade.
Animals and diet
Seventy male Wistar rats (15 week-old rats, raised in our own breeding unit), divided into seven groups, and were placed at random in metal cages (5 rats per cage) under normal lighting conditions, with free access to food and water. Three groups received standard pellet diet and three groups received a hypercholesterolemic diet (standard pelled diet supplemented with 1% cholesterol, 0.2% sodium cholate and 5% olive oil). Six experimental groups were constituted (10 rats per group): 1) normolipemic, 2) normolipemic/TMC-treated, 3) normolipemic/α-asarone-treated, 4) hypercholesterolemic, 5) hypercholesterolemic/α-asarone-treated and 6) hypercholesterolemic/TMC-treated group. The seventh group (10 rats) was used for the isolation of HMG-CoA reductase from liver. These rats were acclimated to an alternate 12-h light-dark cycle for a period of 2-3 weeks and were fed rat chow ad libitum, containing 5% cholestyramine, for 7 days prior to sacrifice at the mid-dark period, which is the diurnal high point of HMGCo A reductase activity of rat liver microsomes.
Experimental procedures
In the present study, the changes produced by TMC and α-asarone in various biochemical parameters affecting serum cholesterol-lipoproteins levels were determined in normal and hypercholesterolemic rats. The TMC, dissolved in sodium bicarbonate solution and α-asarone, dissolved in corn oil, were injected subcutaneously at a dose of 80 mg/kg body wt/day for the treated groups, over 8 days at 8:00 h, after the rats were weighed. At the end of the treatment, all rats were anesthetized with sodium pentobarbital (5 mg/100 g body wt). The abdomen was opened by a midline incision and bile was collected from the common bile duct cannulated with polyethylene tubing (Clay Adams No. 10). Bile was collected for 60 min and the bile flow, as well as the amount of bile acids, phospholipids, and cholesterol secretions in the bile were determined. In addition, blood was obtained by abdominal aorta puncture and allowed to clot, and serum was collected from all the animals to determine total cholesterol and cholesterol-lipoproteins. These animals were fasted overnight prior to blood collection to allow proper evaluation of cholesterol and lipoproteins. Finally, these animals were killed by decapitation and their livers were removed immediately and weighed. This investigation was performed according to the Guide for the care and use of laboratory animals published by the US National Institute of Health [Citation9].
Serum analysis
Serum cholesterol and lipoproteins were measured using enzymatic procedures and commercial kits. Serum cholesterol levels and cholesterol in HDL were determined using kits from Lakeside (Monotest cholesterol CHOD-PAP). Cholesterol in LDL was determined using kits from Boehringer Mannheim (Cholesterol-LDL method PSV).
Bile analysis and cholesterol saturation index (CSI)
Since the CSI depends on the total and relative concentration of bile lipids, total bile acids concentration was estimated by an enzymatic method using 3-α-hydroxysteroid dehydrogenase [Citation10]. Cholesterol concentrations in bile were measured using the abo ve mentioned commercial kit. Phospholipid concentrations were determined via an enzymatic method using a commercial kit from BioMérieux. Then the bile CSI was determined using Carey's critical tables [Citation11].
Isolation and assay of HMG-CoA reductase
Microsomes were prepared from livers of the rats maintained on rat chow containing 5% cholestyramine for 7 days. HMG-CoA reductase was solubilized from the microsomes and purified through a second ammonium sulfate precipitation step as described by Kleinsek et al. [Citation12]. The enzyme preparation was stored at − 80°C in 100 μL aliquots. Prior to use, the enzyme was activated at 37°C for 30 min. The assay was similar to that described by Beg et al. [Citation13].
Statistical analysis
Data from these studies were statistically analyzed using Student's t-test. Values of lipid concentrations and of LDL- and HDL-cholesterol concentrations were expressed as mean ± SE.
Results
Effects of 2,4,5-trimethoxycinnamic acid and α-asarone on the levels of serum total cholesterol
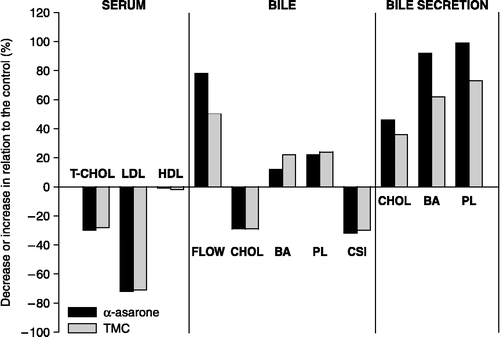
In keeping with previous observation [Citation4,Citation14] and after feeding the rats for 8 days with hypercholesterolemic diet, mean serum total cholesterol increased from 104.5 ± 5.42 mg/dL in rats with normal pelleted diet, to 176.7 ± 7.52 mg/dL () in hypercholesterolemic control rats. Under our experimental conditions, TMC and α-asarone treatment had no effect on the levels of cholesterol in normolipemic rats. In contrast, in rats with high cholesterolemia, TMC and α-asarone reduced this cholesterolemia significantly. Thus, the serum levels of cholesterol were 25.5% lower in the TMC treated hypercholesterolemic rats and 30% lower in the α-asarone treated hypercholesterolemic rats than in the untreated hypercholesterolemic rats (, ).
Table I. Serum total cholesterol and lipoproteins-cholesterol distribution in rats.
Figure 1. Comparative effect of α-asarone and 2,4,5-trimethoxycinnamic acid on several biochemical and physiological parameters related to cholesterol metabolism in hypercholesterolemic rats. Total serum cholesterol (T-CHOL), LDL, HDL, cholesterol (CHOL), bile acids (BA), phospholipids (PL), bile flow (FLOW), bile cholesterol saturation index (CSI).
Effects of 2,4,5-trimethoxycinnamic acid and α-asarone on levels of serum lipoproteins, LDL-cholesterol and HDL-cholesterol
After TMC and α-asarone-treatment, no changes in the serum levels of LDL-cholesterol and HDL-cholesterol were observed in treated normolipemic rats versus untreated control. On the contrary, in the hypercholesterolemic rats, mean serum LDL-cholesterol levels declined 73.3% and 74.7% for TMC and α-asarone, respectively; whereas no significant changes were detected in HDL-cholesterol (). In consequence, the average value of the ratio LDL/HDL was 74%, for the TMC and α-asarone treated groups, indicating that the most important hypocholesterolemic effect of α-asarone and TMC concerned the LDL-cholesterol more than the HDL-cholesterol levels ().
Effects of 2,4,5-trimethoxycinnamic acid and α-asarone on bile flow, and biliary lipids secretion
The TMC and α-asarone were injected daily into normolipemic and hypercholesterolemic rats and after 8 days of treatment the rats were anesthetized with sodium pentobarbital and equipped with catheters in the bile duct. Bile was then collected for 60 min and the bile flow and the amount of bile acids, phospholipids, and cholesterol secreted in bile were determined. We found that bile flow was increased significantly in the TMC treated hypercholesterolemic rats, from 1.00 ± 0.05 to 1.50 ± 0.06 μL/min/g liver (50%) and in the α-asarone treated hypercholesterolemic rats, from 1.00 ± 0.05 to 1.79 ± 0.07 μL/min/g liver (79%) (, ). These increases in bile flow consequently increased biliary lipids secretion in hypercholesterolemic rats.
Table II. Effect of TMC and α-asarone on biliary lipids concentration and CSI.
Biliary lipid concentration and cholesterol saturation index
Subsequently, we also investigated whether administration of TMC and α-asarone influenced biliary lipid concentration and CSI (, ). We found that the biliary concentration of cholesterol in the TMC-treated and α-asarone-treated hypercholesterolemic rats decreased in comparison with that of the hypercholesterolemic control rats (0.82 ± 0.18 versus 0.60 ± 0.09 mM [27%] for TMC and 0.60 ± 0.10 mM [27%] for α-asarone). In contrast, the concentration of bile acids in the bile was increased significantly from 11.90 ± 0.75 to 14.70 ± 0.81 mM (23%) in the TMC-treated hypercholesterolemic rats and to 13.60 ± 0.80 mM (14.2%) in the α-asarone-treated hypercholesterolemic rats. Whereas the concentration of phospholipids in the bile was increased significantly from 2.90 ± 0.38 to 3.70 ± 0.36 mM (27%) in the TMC-treated hypercholesterolemic rats and to 3.60 ± 0.50 mM (24%) in the α-asarone-treated hypercholesterolemic rats, leading to a reduction of the CSI from 1.07 ± 0.02 in the hypercholesterolemic control group to 0.75 ± 0.03 (30%) in the TMC-treated hypercholesterolemic group and to 0.73 ± 0.04 (32%) in the α-asarone-treated hypercholesterolemic group. In normolipemic rats this short-term administration of α-asarone and TMC did not significantly change the biliary lipids concentrations or the CSI.
Inhibition of HMG-CoA reductase by 2,4,5-trimethoxycinnamic acid and α-asarone
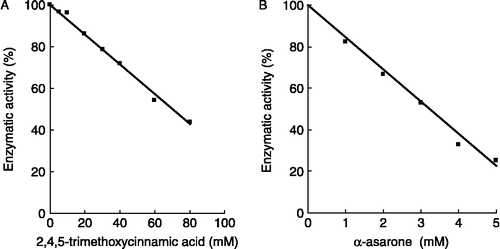
The effects of TMC and α-asarone on the activity of HMG-CoA reductase, the rate-limiting enzyme in cholesterol biosynthesis were also investigated. As shown in , both substances inhibited the HMG-CoA reductase activity and the concentration required for 50% inhibition (IC50) of the enzymatic activity was 60 mM for TMC and 3 mM for α-asarone.
Figure 2. Inhibition of HMG-CoA reductase by TMC (A) and α-asarone (B). Experiments were carried out as described in materials and methods. The results are expressed as percentage of mevalonolactone of the control HMG-CoA reductase activity without inhibitor; control value was 0.122 nmol/min/mg protein.
Discussion
High blood cholesterol level is a major risk factor for the development of atherosclerosis [Citation15]. In humans, most of the cholesterol in the body is synthesized de novo, mainly in the liver; so the search for drugs that inhibit cholesterol biosynthesis has long been pursued as a means to lower cholesterol plasma level that could help in the prevention and treatment of atherosclerosis [Citation16,Citation17,Citation18].
The low density lipoproteins (LDL) are responsible for the transport and delivery of cholesterol to extrahepatic tissues; in circulation these LDL are taken up by a LDL receptor-mediated process. This process involves the binding of LDL to specific, high-affinity binding sites located on the surface of plasma membranes. Once binding has occurred, the LDL-receptor complex is internalized by endocytosis and digested by lysosomal enzymes to liberate free cholesterol. This cholesterol is utilized as an important structural component for cell membranes and, in several tissues, as the precursor for the synthesis of steroid hormones. This hepatic LDL receptor pathway is the dominant mechanism for controlling plasma LDL levels in humans and the liver is, therefore, the key organ in the maintenance of whole body cholesterol homeostasis in most mammalians, including human beings [Citation19].
High levels of serum LDL, the major vehicle for cholesterol transport in humans, are correlated with an increase occurrence of atherosclerosis [Citation20]. Therefore, therapy for hypercholesterolemia is mainly focused on the induction of LDL receptors in the liver, leading to an enhanced clearance and thus catabolism of LDL from blood stream [Citation19,Citation21]. This process is accomplished through inhibition of cholesterol synthesis or by stimulation of biliary cholesterol excretion [Citation19,Citation22].
In previous investigations, we found that α-asarone inhibited HMGCoA reductase, lowered serum LDL-cholesterol levels, increased bile flow and stimulated biliary cholesterol excretion in hypercholesterolemic rats [Citation4]. In this research, we comparatively studied the effect of 2,4,5-trimethoxycinnamic acid (TMC) and α-asarone, in order to find if TMC, the major metabolite of α-asarone [Citation6], retains some of the above mentioned pharmacological properties. Therefore, we evaluated the effect of both substances on several biochemical parameters related to serum cholesterol level in normal and hypercholesterolemic rats. These drugs were administered (80 mg/kg) over a short period of time (8 days) that was adequate for the study of possible changes in HDL-cholesterol and LDL-cholesterol metabolism [Citation4]. We focused on the inhibition of HMG-CoA reductase, the rate-controlling enzyme in cholesterol biosynthesis, as well on the balance between circulating lipoproteins-cholesterol levels, bile flow and on cholesterol, phospholipids and bile salts levels secreted in bile.
We found that TMC and α-asarone treatment had no effect on the levels of cholesterol in normolipemic rats. This results are in agreement with those reported previously by others [Citation23] suggesting that rats have a strong ability to maintain serum cholesterol level. In contrast, in rats with high cholesterolemia, TMC and α-asarone reduced this cholesterolemia significantly: serum cholesterol level was 25.5% lower in the TMC treated hypercholesterolemic rats and 30% lower in the α-asarone treated hypercholesterolemic rats than in untreated hypercholesterolemic rats. These treatments affected mainly the serum levels of LDL-cholesterol, while the serum HDL-cholesterol levels were unaffected. Since there was an extensive lowering of serum LDL-cholesterol, 73.3% for TMC and 74.4% for α-asarone, without effect on the absolute amount of HDL-cholesterol, the net result was a decrease in LDL/HDL ratio of 74%. These findings strongly suggest that the LDL-receptor pathway is also operating in these hypercholesterolemic rats. We observed a higher decrease in serum LDL-cholesterol levels, because in rats, LDL-cholesterol is not the predominant serum lipoprotein. Therefore, further studies must be performed to investigate the hypocholesterolemic activity of α-asarone and TMC in animals more comparable to humans in terms of lipoproteins profile, in which the predominant serum lipoproteins are the LDL-cholesterol [Citation20]. In humans, the LDL normally accounts for two thirds of plasma cholesterol content.
The major route of excretion of cholesterol is its conversion to bile acids (cholate and chenodesoxycholate) which occurs only in the liver. Hence, we investigated whether administration of α-asarone and TMC influenced biliary secretion. We found that bile flow was increased significantly in the TMC (50%) and α-asarone (75%) treated hypercholesterolemic rats, whereas in normolipemic rats, the stimulation of bile flow was only 20%. The drugs significantly stimulated the secretion of bile acids, bile phospholipids and bile cholesterol. These effects could be understood on the basis of an increment of intrahepatic cholesterol, due to the enhanced clearance of LDL-cholesterol from bloodstream, induced by α-asarone and TMC treatment. The excess of hepatic cholesterol is diverted to bile secretion, probably due to the a direct stimulation of 7-α-hydroxylase, the rate-limiting enzyme for bile acid synthesis [Citation24], by cholesterol, as it was demonstrated in rats [Citation25], or to the induction of this enzyme by cholesterol at the transcriptional level, also demonstrated in rats [Citation26].
Bile is a micellar solution and the predominant lipids of these micelles are bile acids, the phospholipid lecithin and cholesterol. The fatty acids chains of lecithin which form the center of these micelles acts as a solvent for cholesterol which is completely insoluble in water [Citation27]. Since the cholesterol saturation index (CSI) depend on the total and relative concentrations of cholesterol, bile acids and lecithin, we also determined the biliary concentration of these lipids and with these data bile CSI was determined using Carey's critical tables [Citation11].
When we tested the effect of α-asarone and TMC on bile composition, we found that these drugs reduced the cholesterol level of gallbladder bile, whereas the concentration of bile salts and phospholipids (lecithin) were increased, leading to a decrease in the bile cholesterol saturation index (CSI) of hypercholesterolemic rats. Since bile saturated or supersaturated with cholesterol is considered as an important first event in the pathogenesis of cholesterol gallstones [Citation28], those drugs that increase bile flow and decrease CSI can be also be used as drugs for treatment of cholesterol gallstones [Citation29]. Because the increase in bile flow induced by these drugs will ensure continual bathing of gallstones with unsaturated bile, and according to Danzinger et al. [Citation30], if unsaturated bile enters the gallbladder and rapidly takes up cholesterol from the liquid phase of stones until saturation, the stones should be dissolved in a few weeks. This cholelitholytic effect was already demonstrated for α-asarone [Citation31] and remains to be demonstrated for TMC.
The effects of TMC and α-asarone on the activity of HMG-CoA reductase, the rate limiting enzyme in cholesterol biosynthesis were also investigated. We found that both substances inhibited the HMG-CoA reductase activity and the concentration required for 50% inhibition of the enzymatic activity (IC50) was 60 mM for TMC and 3 mM for α-asarone. The poor inhibitory effect of TMC in comparison to α-asarone may be explained in terms of their polarity. While α-asarone is an hydrophobic substance, TMC is polar in nature. Therefore, α-asarone will have more affinity for the HMG-CoA reductase, an hydrophobic enzyme inserted in the membrane of the endoplasmic reticulum, whereas TMC will have less affinity for this membranal enzyme and consequently less inhibitory activity.
The poor inhibitory effect of TMC on HMG-CoA reductase cannot fully explain the hypocholesterolemic effect of this substance. However, since the synthesis of cholesterol from acetates is catalyzed by soluble cytosol enzymes and by enzymes bound to endoplasmic reticulum membranes, like HMG-CoA reductase, we cannot discard the possibility that the polar TMC will also inhibit some of these soluble cytosol enzymes, and this could account for the hypocholesterolemic effect of TMC.
It has been demonstrated that α-asarone possess hypocholesterolemic [Citation2,Citation3,Citation4] and hypolipidemic properties [Citation3] due to the inhibition of HMG-CoA reductase [Citation4], however α-asarone cannot be used in clinical trials due to its genotoxic and hepatocarcinogenic properties in rodents [Citation5,Citation6]. On the contrary, TMC, the major metabolite of α-asarone, does not have the above mentioned toxicological effects [Citation6]. Therefore, in this investigation, we comparatively studied the effect of TMC and α-asarone on pharmacological properties related to the metabolism of cholesterol.
The results of this study (summarized in ) indicated that TMC, the major and non-toxic metabolite of α-asarone, retains most of the pharmacological properties of α-asarone, since both substances reduced total serum cholesterol, lowered serum LDL-cholesterol levels leaving serum HDL-cholesterol levels unaffected, increased bile flow, bile acids and phospholipids, and reduced bile cholesterol and biliary cholesterol saturation index (CSI) in hypercholesterolemic rats. The drugs also stimulated the secretion of bile acids, bile phospholipids and bile cholesterol as a consequence of the increase in bile flow. The inhibitory effect on HMG-CoA reductase was the only property highly reduced in TMC, in comparison with α-asarone.
These experiments strongly suggest that TMC can be further studied as a possible hypocholesterolemic and cholelitholytic agent.
Acknowledgements
This work was partially supported by research grants from the Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-IPN), México. C.W., L.R.P. and I.B. are fellows of the COFAA-IPN and of the SNI-CONACYT. J.A.S, F.H.D., M.A.V. and S.I.R. are fellows of the CONACYT and PIFI-IPN.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- M Martínez. The medicial plants of Mexico 1967. p 125–127, ed. Ed. Botas. México.
- JJ Mandoki, C Krumm-Heller, J Vega-Noverola, C Wong-Ramírez, C Arriaga, R Roa, C Rubio, N Mendoza-Patiño. Isolation of α-asarone from the bark of Guatteria gaumeri (Elemuy) and the study of its hypocholesterolemic effect 1980., IV National Congress of Pharmacology, University of Yucatán, Mérida, Yucatán, México.
- J Poplawski, B Lozowicka, AT Dubis, B Lachowska, S Witkowski, D Siluk, J Petrusewic, R Kaliszan, J Cybulski, M Strzalkowska, Z Chilmoneczyk. Synthesis and hypolipidemic and antiplatelet activities of alpha-asarone isomers in humans (in vitro), mice (in vivo), and rats (in vivo). J Med Chem 2000;43:3671–3676.
- L Rodriguez-Paez, M Juarez-Sanchez, J Antunez-Solis, I Baeza, C Wong. Alpha-asarone inhibits HMG-CoA reductase, lowers serum LDL-cholesterol levels and reduces biliary CSI in hypercholesterolemic rats. Phytomedicine 2003;10:397–404.
- AJ Howes, VS Chan, J Caldwell. Structure-specificity of the genotoxicity of some naturally occurring alkenil benzenes determined by the unscheduled DNA synthesis assay in rat hepatocytes. Food Chem Toxicol 1990;28:537–542.
- E Hasheminejad, J Caldwell. Genotoxicity of the alkenylbenzenes alpha- and beta-asarone, myristicin and elimicin as determined by the UDS assay in cultured rat hepatocytes. Food Chem Toxicol 1994;32:223–231.
- RW Wiseman, EC Miller, JA Miller, A Liem. Structure–activity studies of the hepatocarcinogenicities of alkenylbenzene derivatives related to estragole and safrole on administration to preweanling male C57BL/6J × C3H/HeJ F1 mice. Cancer Res 1987;47:2275–2283.
- GR Wilkinson. Pharmacokinetics: The dynamics of drug absorption, distribution, and elimination. In: JG Hardman, LE Limbird. Goodman & Gilman's the pharmacological basis of therapeutics. New York: Mc Graw-Hill; 2001. p. 12–16.
- NIH. Guide for the care and use of laboratory animals Office of Sciences and Health Report DRR/NIH DHEW Publication 1985. p 85–123.
- SD Turley, JM Dietschy. Re-evaluation of the 3-α-hidroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res 1978;19:924–928.
- MC Carey. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res 1978;19:998–1026.
- A Kleinsek, S Ranganathan, JW Porter. Purification of 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Proc Natl Acad Sci U S A 1977;74:1431–1435.
- ZH Beg, JA Stonik, HB Brewer. Purification and characterization of 3-hydroxy-3-methylglutaryl coenzyme A reductase from chicken liver. FEBS Lett 1977;80:123–129.
- SJ Kim, SH Bok, MK Lee, YB Park, HJ Kim, MS Choi. Lipid-lowering efficacy of 3,4-di(OH)-phenylpropionic l-leucine in high-cholesterol fed rats. J Biochem Mol Toxicol 2005;19:25–31.
- CA Gleissner, N Leitinger, K Ley. Effects of native and modified low-density lipoproteins on monocyte recruitment in atherosclerosis. Hypertension 2007;50:276–283.
- JC Fruchart, P Duriez. Anti-cholesterol agents, new therapeutic approaches. Ann Pharm Fr 2004;62:3–18.
- ES Istvan, J Deisenhofer. Structural mechanism for statin Inhibition of HMG-CoA reductase. Science 2001;29:1160–1164.
- SD Turley. Cholesterol metabolism and therapeutic targets, rationale for targeting multiple metabolic pathways. Clin Cardiol 2004;27:III16–III21.
- MS Brown, JL Goldstein. A receptor-mediated pathway for cholesterol homeostasis. Science 1986;232:34–47.
- AJ Lusis. Atherosclerosis. Nature 2000;407:233–241.
- T Clerc, M Jomier, M Chautan, H Portugal, M Senft, A Pauli, C Laruelle, O Morel, H Lafont, F Chanussot. Mechanism of action in the liver of crilvastatin, a new hydroxy-methylglutaryl-coenzyme A reductase inhibitor. Eur J Pharm 1993;235:59–68.
- AS Wierzbicki. Lipid-altering therapies and the progression of atherosclerotic disease. Cardiovasc Intervent Radiol 2007;30:155–160.
- PD Roach, S Balasubramaniam, F Hirata, M Abbey, A Szanto, LA Simons, PJ Nestel. The low-density lipoprotein receptor and cholesterol synthesis are affected differently by dietary cholesterol in the rat. Biochim Biophys Acta 1993;1170:165–172.
- ZR Vlahcevic, DM Heuman, PB Hylemon. Regulation of bile acid synthesis. Hepatology 1991;13:590–600.
- MS Straka, LH Junker, L Zacarro, DR Zogg, S Dueland, GT Everson, RAJ Davis. Substrate stimulation of 7-alpha-hydroxylase, an enzyme located in the cholesterol-poor endoplasmic reticulum. J Biol Chem 1990;265:7145–7149.
- MP Jones, WM Pandak, DM Heuman, JY Chang, PB Hylemon, ZR Vlahcevic. Cholesterol 7-alpha-hydroxylase, evidence for transcriptional regulation by cholesterol or metabolic products of cholesterol in the rat. J Lipid Res 1992;34:385–392.
- CM Carey, DM Small. The characteristics of mixed micellar solution with particular reference to bile. Am J Med 1970;49:590–608.
- DM Small. The formation of gallstones. Adv Intern Med 1970;16:243–264.
- OJ Smith, DW Erkelens, GP Von Berger Henegorwen. Successful dissolution of cholesterol gallstones during treatment with pravastatin. Gastroenterology 1992;103:1068–1070.
- RG Danzinger, AF Hofmann, LJ Schoenfield, JI Thistle. Dissolution of cholesterol gallstones by chenodeoxycholic acid. N Engl J Med 1972;286:1–8.
- C Gómez, G Chamorro, MA Chávez, G Martínez, M Salazar, N Pages. Effect of α-asarone on hypercholesterolemia and on experimental. cholelitiasis. Plant Med Phytother 1987;21:279–284.