Abstract
Some 2,6-diarylpiperidin/tetrahydrothiopyran/tetrahydropyran-4-one oximes were synthesized in dry media under microwave irradiation and were evaluated for their in vitro antibacterial activity against clinically isolated bacterial strains i.e. S.aureus, β-H.Streptococcus, E.coli, P.aeruginosa, S.typhii and in vitro antifungal activities against fungal strains i.e. C.albicans, Rhizopus, A.niger and A.flavus. Structure-activity relationships for the synthesized compounds showed that compounds 12 and 15 exerted excellent antibacterial activity against all the tested bacterial strains except 15 against S.aureus and β-H.streptococcus. Against C.albicans and A.flavus, compound 15 exerted potent antifungal activities while against Rhizopus, compound 16 showed promising activity.
Introduction
Organic reactions under solvent-free conditions involving easily separable solid catalysts are receiving considerable attention in recent times, particularly from the point of view of green chemistry. Solvent- free solid state synthetic methods have been shown to be very efficient and advantageously coupled with microwave (MW) activation [Citation1] and many organic reactions have been carried out using “microwave induced organic reaction enhancement” (MORE) technique delivering high yields in a short reaction time compared with the conventional heating mode performed in preheated thermostat oil bath [Citation2]. The use of supported reagents has attracted much attention because of the selectivity, reactivity and associated ease of manipulation [Citation3]. Microwave induced chemical reactions [Citation4] especially on solid supports and those conducted in solvent less systems, [Citation5] have gained popularity. Now-a-days, bioactive heterocyclic ring systems having 2,6-diarylpiperidin-4-one and their analogous thiopyran and pyran nuclei have aroused great interest due to their wide variety of biological properties such as antiviral, antitumour [Citation6,Citation7], central nervous system [Citation8], local anesthetic [Citation9], anticancer [Citation10], and antimicrobial activity [Citation11] and their derivative piperidine are also biologically important and act as neurokinin receptor antagonists [Citation12], analgesic and anti-hypertensive agents [Citation13]. Oximes of various substituted piperidones were also reported to exhibit antimicrobial, analgesic, local anesthetic and antifungal activities [Citation14].
In the course of broad programme in developing biologically active molecules, we have recently reported the synthesis of 2,6-diarylpiperidin-4-one derivatives and evaluated their biological importance Citation15,Citation16,Citation17. Consequently it was planned to synthesize 2,6-diarylpiperidin-4-one oximes, their analogous 2,6-diaryltetrahydrothiopyran-4-one oximes and 2,6-diaryltetrahydropyran-4-one oximes. In turn to extend our knowledge in structure-activity relationship, all the synthesized compounds are tested for their in vitro antibacterial and antifungal activities and the influence of some structural variations by varying the substituents at the phenyl ring in the synthesized compounds towards their biological activities is evaluated.
Experimental
Chemistry
Performing TLC assessed the reactions and the purity of the products. All the reported melting points were taken in open capillaries and were uncorrected. A conventional (unmodified) domestic microwave oven equipped with a turntable (LG, MG-395 WA, 230V ∼ 50 Hz, 760 W) was used for the irradiation. IR spectra were recorded in KBr (pellet forms) on a Nicolet-Avatar–330 FT-IR spectrophotometer and note worthy absorption values (cm− 1) alone are listed. 1H and 13C NMR spectra were recorded at 400 MHz and 100 MHz respectively on Bruker AMX 400 NMR spectrometer using CDCl3 as solvent. The ESI + ve MS spectra were recorded on a Bruker Daltonics LC-MS spectrometer and satisfactory microanalysis was obtained on a Carlo Erba 1106 CHN analyzer ().
Table I. Physical and analytical data of 2,6-diarylpiperidin/tetrahydrothiopyran and tetrahydropyran-4-one oximes 10-18.
2,6-diarylpiperidin-4-ones 1-3, 2,6-diaryltetrahydrothiopyran-4-ones 4-6 and 2,6-diaryltetrahydropyran-4-ones 7-9 were respectively prepared according to literature procedures previously described Citation18,Citation19,Citation20.
Synthesis of 2,6-diphenylpiperidin-4-one oxime 10
2,6-diphenylpiperidin-4-one 1 (0.001 mol) and hydroxylamine hydrochloride (0.001 mol) were mixed thoroughly with CaO (50 mg) in an alumina bath. The mixture was placed inside a microwave oven and irradiated for 5-10 min. (monitored by TLC) and the alumina bath containing the reaction mixture was taken out from the oven, cooled and CHCl3 (15 mL) was added to the crude mixture in order to obtain a suspension with the catalyst. The catalyst was removed by simple filtration. The CHCl3 layer was dried over anhydrous sodium sulphate and distilled off under reduced pressure yielded 2,6-diphenylpiperidin-4-one oxime 10. IR (KBr) (cm− 1): 3250, 3031, 2917, 2801, 1674, 1600, 754, 700; 1H NMR (δ ppm): 2.00 (s, 1H, H1), 3.94 (dd, 1H, H2a, J2a,3a = 11.4 Hz), 3.88 (dd, 1H, H6a, J6a,5a = 11.6Hz), 3.54 (t, 1H, H5e, J6a,5e = 2.98 Hz), 2.56 (t, 1H, H3e, J2a,3e = 2.92 Hz), 2.36-2.40 (m, 1H, H3a), 2.01-2.03 (m, 1H, H5a), 7.48-7.18 (m, 10H, Harom), 8.00 (s, 1H, N-OH).
The compounds 11-18 were synthesized similarly.
2,6-bis(4-methylphenyl)piperidine-4-one oxime
11 IR (KBr) (cm− 1): 3248, 3028, 2914, 2841, 2804, 1671, 1604, 748, 696; 1H NMR (δ ppm): 1.98 (s, 1H, H1), 3.92 (dd, 1H, H2a, J2a,3a = 11.30 Hz), 3.86 (dd, 1H, H6a, J6a,5a = 11.41 Hz), 3.51 (t, 1H, H5e, J6a,5e = 2.95 Hz), 2.54 (t, 1H, H3e, J2a,3e = 2.90 Hz), 2.32 (s, 6H, CH3 at phenyl ring); 2.35-2.37 (m, 1H, H3a), 2.03-2.05 (m, 1H, H5a), 7.52-7.09 (m, 8H, Harom), 8.03 (s, 1H, N-OH).
2,61-bis(4-chlorophenyl)piperidine-4-one oxime
12 IR (KBr) (cm− 1): 3253, 3035, 2921, 1677, 1598, 762, 705; 1H NMR (δ ppm): 2.02 (s, 1H, H1), 3.96 (dd, 1H, H2a, J2a,3a = 11.7 Hz), 3.89 (dd, 1H, H6a, J6a,5a = 11.8 Hz), 3.56 (t, 1H, H5e, J6a,5e = 2.94 Hz), 2.58 (t, 1H, H3e, J2a,3e = 2.92 Hz), 2.39-2.42 (m, 1H, H3a), 2.07-2.09 (m, 1H, H5a), 7.52-7.24 (m, 8H, Harom), 8.06 (s, 1H, N-OH).
2,6-diphenyltetrahydrothiopyran-4-one oxime
13 IR (KBr) (cm− 1): 3172, 3060, 2900, 1649, 748, 696; 1H NMR (δ ppm): 4.20 (dd, 1H, H2a, J2a,3a = 12.16 Hz), 4.14 (dd, 1H, H6a, J6a,5a = 12.4 Hz), 3.96 (dd, 1H, H5e, J6a,5e = 2.44 Hz), 2.93 (dd, 1H, H3e, J2a,3e = 2.72 Hz), 2.77 (dd, 1H, H3a, J3a,3e = 13.52 Hz), 2.37 (dd, 1H, H5a, J5a,5e = 13.62 Hz), 7.40-7.29 (m, 10H, Harom), 7.55 (s, 1H, N-OH).
2,6-bis(4-methylphenyl)tetrahydrothiopyran-4-one oxime
14 IR (KBr) (cm− 1): 3170, 3057, 2896, 1647, 744, 693; 1H NMR (δ ppm): 4.17 (dd, 1H, H2a, J2a,3a = 12.16 Hz), 4.12 (dd, 1H, H6a, J6a,5a = 12.30 Hz), 3.94 (dd, 1H, H5e, J6a,5e = 2.43 Hz), 2.91 (dd, 1H, H3e, J2a,3e = 2.70 Hz), 2.75 (dd, 1H, H3a, J3a,3e = 13.54 Hz), 2.30 (s, 6H, CH3 at phenyl ring); 2.35 (dd, 1H, H5a, J5a,5e = 13.60 Hz), 7.37-7.26 (m, 8H, Harom), 7.51 (s, 1H, N-OH).
2,6-bis(4-chlorophenyl)tetrahydrothiopyran-4-one oxime
15 IR (KBr) (cm− 1): 3175, 3066, 2905, 1652, 756, 690; 1H NMR (δ ppm): 4.22 (dd, 1H, H2a, J2a,3a = 12.18 Hz), 4.16 (dd, 1H, H6a, J6a,5a = 12.41 Hz), 3.97 (dd, 1H, H5e, J6a,5e = 2.40 Hz), 2.95 (dd, 1H, H3e, J2a,3e = 2.71 Hz), 2.78 (dd, 1H, H3a, J3a,3e = 13.55 Hz), 2.39 (dd, 1H, H5a, J5a,5e = 13.64 Hz), 7.45-7.31 (m, 8H, Harom), 7.58 (s, 1H, N-OH).
2,6-diphenyltetrahydropyran-4-one oxime
16 IR (KBr) (cm− 1): 3226, 3030, 2896, 1661, 736, 690; 1H NMR (δ ppm): 4.78 (dd, 1H, H2a, J2a,3a = 10.30 Hz), 5.13 (dd, 1H, H6a, J6a,5a = 12.53 Hz), 3.33-3.35 (m, 1H, H5e), 2.68-2.72 (m, 2H, H3), 2.83-2.85 (m, 1H, H5a), 7.62-7.14 (m, 10H, Harom), 7.90 (s, 1H, N-OH).
2,6-bis(4-methylphenyl)tetrahydropyran-4-one oxime
17 IR (KBr) (cm− 1): 3223, 3027, 2891, 1658, 733, 688; 1H NMR (δ ppm): 4.77 (dd, 1H, H2a, J2a,3a = 10.29 Hz), 5.10 (dd, 1H, H6a, J6a,5a = 12.54 Hz), 3.31-3.33 (m, 1H, H5e), 2.28 (s, 6H, CH3 at phenyl ring); 2.65-2.69 (m, 2H, H3), 2.80-2.82 (m, 1H, H5a), 7.58-7.11 (m, 8H, Harom), 7.87 (s, 1H, N-OH).
2,6-bis(4-chlorophenyl)tetrahydropyran-4-one oxime
18 IR (KBr) (cm− 1): 3229, 3035, 2899, 1665, 739, 696; 1H NMR (δ ppm): 4.79 (dd, 1H, H2a, J2a,3a = 10.29 Hz), 5.15 (dd, 1H, H6a, J6a,5a = 12.55 Hz), 3.36-3.38 (m, 1H, H5e), 2.71-2.75 (m, 2H, H3), 2.87-2.89 (m, 1H, H5a), 7.68-7.19 (m, 8H, Harom), 7.92 (s, 1H, N-OH).
Microbiology
Materials
All the clinically isolated bacterial strains namely Staphylococcus aureus, β-Heamolytic streptococcus, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhii and clinically isolated fungal strains namely Candida albicans, Rhizopus, Aspergillus niger and Aspergillus flavus are obtained from Faculty of Medicine, Annamalai University, Annamalainagar-608 002, Tamil Nadu, India.
In vitro antibacterial and antifungal activity
The in vitro activities of the compounds were tested in Sabouraud dextrose broth (SDB) (Hi-media, Mumbai) for fungi and nutrient broth (NB) (Hi-media, Mumbai) for bacteria by two-fold serial dilution method [Citation21]. The respective test compounds 10-18 were dissolved in dimethylsulfoxide to obtain 1 mg mL− 1 stock solution. Seeded broth (broth containing microbial spores) was prepared in NB from 24 h old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C while fungal spores from 1 to 7 days old Sabouraud agar (Hi-media, Mumbai) slant cultures were suspended in SDB. The colony forming units (cfu) of the seeded broth were determined by plating technique and adjusted in the range of 104-105 cfu/mL. The final inoculums size was 105cfu/mL for antibacterial assay and 1.1-1.5 × 102 cfu/mL for antifungal assay. Testing was performed at pH 7.4 ± 0.2 for bacteria (NB) and at a pH 5.6 for fungi (SDB). Exactly 0.4 mL of the solution of test compound was added to 1.6 mL of seeded broth to form the first dilution. One milliliter of this was diluted with a further 1 mL of seeded broth to give the second dilution and so on till six such dilutions were obtained. A set of assay tubes containing only seeded broth was kept as control. The tubes were incubated in BOD incubators at 37 ± 1°C for bacteria and 28 ± 1°C for fungi. The minimum inhibitory concentrations (MICs) were recorded by visual observations after 24 h (for bacteria) and 72-96 h (for fungi) of incubation. Ciprofloxacin was used as standard for bacteria studies and Amphotericin-B was used as standard for fungal studies.
Results and discussion
Chemistry
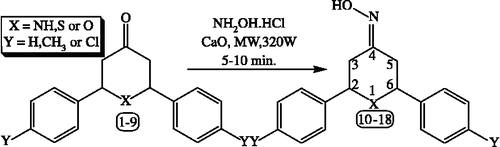
The oximes [Citation22] of aldehyde and ketone served as protecting, selective activating groups [Citation23] and intermediates for many reactions such as the preparation of amides by Beckmann rearrangement. Further, oximes are used for the purification of carbonyl compounds. The oximes can be prepared by the addition of hydroxylamine to aldehydes and ketones. The formation of oximes is usually catalyzed by acids [Citation24]. The preparations of oximes from aldehydes or ketones using hydroxylamine as reactant and sulphuric acid as catalyst [Citation24] have many disadvantages. This procedure is not applicable for acid-sensitive compounds, the yields of the oximes are pH dependent [Citation25] and it requires costly solvent like pure ethanol. Cyclohexanone oxime is synthesized by liquid-phase ammoximation of cyclohexanone using ammonia, hydrogen peroxide as the oxidizing agent and titanium silicate as the catalyst. The ammoximation reaction is suitable for the synthesis of several oximes [Citation26] but use of H2O2 and titanium silicate increased the cost of production. To avoid liquid phase oximation reactions, we use CaO as a solid catalyst for the above reactions. In continuation of our interest in synthesizing pharmacologically important compounds in ‘dry media’ [Citation15,Citation16,Citation27], we wish to report CaO as an efficient catalyst for oximation by microwave irradiation under solvent free conditions. Target molecules, 2,6-diarylpiperidin/tetrahydrothiopyran/tetrahydropyran-4-one oximes 10-18 are synthesized as a result of single-step solid-state synthetic strategy. In a typical experiment, appropriate 2,6-diaryl-piperidin/tetrahydrothiopyran/tetrahydropyran-4-ones 1-9 was mixed with CaO and hydroxylamine hydrochloride in an alumina bath and the mixture was irradiated in a microwave oven with a power level of 320W for 5 to 10 min. yield the title compounds in high yields with shorter time period in dry media under MW irradiation than the classical method [Citation22], which require a longer reaction time using ethanol as solvent medium and the use of column chromatographic technique to purify the products. CaO catalyst was shown to be one of the most efficient MW absorber with a very high specificity to MW heating. It was able to reach a temperature of 90°C after 3 minutes of irradiation in a domestic oven (320 W). 50 mg of CaO to 0.001 moles of substrates is the most acceptable ratio in terms of efficiency and safety; a power level of 320 watts is the most suitable one. The importance of the title compounds is due to their diverse potential, broad-spectrum biological activity. The schematic representation and the analytical data of compounds 10-18 are given in and , respectively. The structure of the newly synthesized compounds 10-18 is confirmed by melting point, elemental analysis, MS, FT-IR, one-dimensional 1H NMR spectroscopic data.
Antibacterial activity
Biolabile 2,6-diarylpiperidin/tetrahydrothiopyran and tetrahydropyran-4-one oximes 10-18 were tested for their antibacterial activity in vitro against S.aureus, β-H.Streptococcus, E.coli, P.aeruginosa and S.typhii. Ciprofloxacin was used as standard drug. Minimum inhibitory concentration (MIC) in μg/mL values is reproduced in . All the synthesized oxime derivatives 10-18 exhibit a wide range of antibacterial potency against the tested strains except compounds 10,11 and 16, which did not show activity against E.coli, S.typhii and β-H.Streptococcus. Among the compounds having no substitution at the para position of phenyl rings (i.e., compounds 10, 13 and 16) only exerted moderate activities against all the used bacterial strains. Structure-activity relationship results for the synthesized compounds have shown that piperidone oxime with electron with drawing chloro functional group at the para position 12 and tetrahydrothiopyran oxime with electron with drawing chloro functional group at the para position 15 exerted excellent antibacterial activity against all the tested bacterial strains except 15 against S.aureus and β-H.Streptococcus.
Table II. In vitro antibacterial activity (MIC) values for compounds 10-18.
Antifungal activity
The in vitro antifungal activity of the synthesized novel heterocyclic oximes, 10-18 was studied against the fungal strains viz., C.albicans, Rhizopus, A.niger and A.flavus. Amphotericin-B was used as a standard drug. Minimum inhibitory concentration (MIC) in μg/mL values is reproduced in . Compound 10 did not exerted antifungal activity against C.albicans, A.niger and A.flavus. Further introduction of electron donating methyl functional group at the para position of phenyl ring in 11 also did not promote activity against C.albicans even at 200 μg/mL while against A.flavus, it has registered maximum activity at 50 μg/mL. Also, this methyl group modification has marked antifungal activity against A.niger too. Against C.albicans and A.flavus, compound 15 exerted potent antifungal activities while against Rhizopus, compound 16 showed promisable activities.
Table III. In vitro antifungal activity (MIC) values for compounds 10-18.
Conclusion
The present work describes the synthesis of 2,6-diarylpiperidin-4-one oximes 10-12; 2,6-diphenyltetrahydrothiopyran-4-one oximes 13-15; 2,6-diphenyltetrahydropyran-4-one oximes 16-18 and the results of their screening for antibacterial and antifungal activities. Several compounds display antimicrobial activity similar and us potent as that of Ciprofloxacin and Amphotericin-B. Results of the present investigation prompted us to broaden our investigation of structure-activity relationships in this piperidin-4-one/tetrahydrothiopyran and tetrahydropyran ring system so as to discover the factors underlying the biological activity and this aspect suggest molecules with optimum structural features for maximum activity, is currently under investigation by our research group.
M. Gopalakrishnan, J. Thanusu, & V. Kanagarajan
A facile solid-state synthesis and in vitro antimicrobial activities of some 2,6-diarylpiperidin/tetrahydrothiopyran and tetrahydropyran-4-one oximes
669–675
Acknowledgements
Authors are thankful to NMR Research Centre, Indian Institute of Science, Bangalore for recording the spectra. Two of our authors namely J.Thanusu and V.Kanagarajan are highly thankful for Annamalai University authorities for providing financial support in the form of Research Fellowship.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- RS Varma. Solvent-free organic syntheses using supported reagents and microwave irradiation. Green Chem 1999; 1:43–56.
- S Caddick. Microwave assisted organic reactions. Tetrahedron 1995; 51:10403–10432.
- M Balogh, P Laszlo. Organic Chemistry Using Clays. Berlin: Springer Verlag; 1993., b) JH Clark. Catalysis of Organic Reactions by Supported Organic Reagents VCH Publishers Inc.; New York. 1994.
- JS Yadav, BVS Reddy, G Satheesh, PN Lakshimi, AC Kunvar. Rapid and efficient synthesis of optically active pyrazoles under solvent-free conditions. Tetrahedron Lett 2003; 45:8587–8590.
- H Rodriguez, M Suarez, R Perez, A Petit, A Loupy. Solvent-free synthesis of 4-aryl substituted 5-alkoxycarbonyl-6-methyl-3,4-dihydropyridones under microwave irradiation. Tetrahedron Lett 2003; 44:3709–3712.
- HI El-Subbagh, SM Abu-Zaid, MA Mahran, FA Badria, AM Alofaid. Synthesis and biological evaluation of certain α, β-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J Med Chem 2000; 43:2915–2921.
- AA Watson, GWJ Fleet, N Asano, RJ Molyneux, RJ Nugh. Polyhydroxylated alkaloids. Natural occurrence and therapeutic applications. Phytochemistry 2001; 56:265–295.
- CR Ganellin, RG Spickett. Compounds affecting the central nervous system. 1,4-piperidones and related compounds. J Med Chem 1965; 8:619–625.
- RE Hagenbach, H Gysin. Piperidones as potential antitumour agents. Experimentia 1952; 8:184–187.
- B Ileana, V Dobre, I Nicluescu-Duvaz. Spin labeled nitrosoureas. Potential anticancer agents. J Prakt Chem 1985; 327:667–674.
- IG Mokio, AT Soldatenkov, VO Federov, EA Ageev, ND Sergeeva, S Lin, EE Stashenku, NS Prostakov, EL Andreeva. Antimicrobial activity of aliphatic-aromatic ketones, β-ketols and α-glycols. Khim Farm Zh 1989; 23:421–427.
- JR Dimmock, P Kumar. Anticancer and cytotoxic properties of mannich bases. Curr Med Chem 1997; 4:1–22.
- H Kubota, A Kakefuda, Y Okamoto, M Fujii, O Yamamoto, Y Yamgiwa, M Orita, K Ikeda, M Takenchi, T Shibanuma, Y Fsomura. Synthesis of ( ± )-N-[2-(3,4-dichlorophenyl)-4-(spiro-substituted piperidin-1′-yl)butyl]-N-methylbenzamides and evaluation of NK[1]-NK[2] dual antagonistic activities. Chem Pharm Bull 1998; 46:1538–1544.
- N Rameshkumar, A Veena, R Ilavarasu, M Adiraj, P Shanmugapandian, SK Sridhar. Synthesis and biological activities of 2,6-diaryl-4-piperidone derivatives. Biol Pharm Bull 2003; 26:188–193.
- M Gopalakrishnan, P Sureshkumar, J Thanusu, V Kanagarajan, R Govindaraju, G Jayasri. A convenient ‘one-pot’ synthesis and in vitro microbiological evaluation of novel 2,7-diaryl-[1,4]-diazepan-5-ones. J Enz Inhib Med Chem 2007; 22:709–715.
- M Gopalakrishnan, P Sureshkumar, J Thanusu, C Prabhu, V Kanagarajan. One-pot conversion of piperidine-4-ones to [1,4]-diazepan-5-ones under microwave irradiation using silica gel supported NaHSO4 catalyst. J Chem Res 2007; 2:80–81.
- M Gopalakrishnan, P Sureshkumar, J Thanusu, V Kanagarajan. Design, synthesis, characterization, antibacterial and antifungal activities of novel class of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-selenadiazoles. J Enz Inhib Med Chem 2008; 23:347–351.
- CR Noller, V Baliah. The preparation of some piperidine derivatives by the Mannich reaction. J Am Chem Soc 1948; 70:3853–3854.
- F Arndt, P Nachtway, J Pusch. The preparation of some thiopyran derivatives. Chem Ber 1925; 58:1637–1638.
- P Petrenko-Kritschenko. Synthesis of pyran compounds. Chem Ber 1877; 30:2802–2803.
- MH Dhar, MM Dhar, BN Dhawan, BN Mehrotra, C Ray. Screening of Indian plants biological activity. Part I. Ind J Exp Biol 1968; 6:232–247.
- TW Greene, PGM Wuts. Protective Group in Organic Synthesis. 2nd ed. New York: John Wiley and Sons; 1991. p 175–178.
- JK Whitesell, MA Whitesell. Alkylation of ketones and aldehydes via., their nitrogen derivatives. Synthesis 1983. 517–536.
- K Weissermer, HJ Arpe. Industrial Organic Chemistry. Berlin: Springer Verlag; 1978. p 222–225.
- WP Jencks. Studies on the mechanism of oxime and semicarbazone formation. J Am Chem Soc 1959; 81:475–481.
- Z Tvaruzkova, K Habersberger, N ZilkovoJiru. Role of surface complexes on titanium silicate in the ammoximation of cyclohexanone with hydrogen peroxide. Appl Catal A 1991; 79:105–109.
- M Gopalakrishnan, P Sureshkumar, V Kanagarajan, J Thanusu. Design, one-pot synthesis, characterization, antibacterial and antifungal activities of novel 6-aryl-1,2,4,5-tetrazinane-3-thiones in dry media. J Sulf Chem 2007; 28:383–392.
- K Pandiarajan, RT Sabapathymohan, MU Hasan. 13C and 1H NMR spectral studies of some piperidin-4-one oximes. Magn Reson Chem 1986; 24:312–316.
- V Gurumani, K Pandiarajan, M Swaminathan. Conformational studies of some 1-hetera-2,6-diphenylcyclohexan-4-one oximes using NMR spectra-evidence for boat form contributions to trans-2,6-diphenyl systems. Bull Chem Soc Jpn 1997; 70:29–35.