Abstract
Some new complexes of mefenamic acid with potentially interesting biological activity are described. The complexes of mefenamic acid [Mn(mef)2(H2O)2], 1, [Co(mef)2(H2O)2], 2, [Ni(mef)2(H2O)2], 3, [Cu(mef)2(H2O)]2, 4 and [Zn(mef)2], 5, were prepared by the reaction of mefenamic acid, a potent anti-inflammatory drug with metal salts. Optical and infrared spectral data of these new complexes are reported. Monomeric six-coordinated species were isolated in the solid state for Mn(II), Ni(II) and Co(II), dimeric five-coordinated for Cu(II) and monomeric four-coordinated for Zn(II). In DMF or CHCl3 solution the coordination number is retained and the coordinated molecules of water are replaced by solvent molecules. The anti-oxidant properties of the complexes were evaluated using the 1,1-diphenyl-2-picrylhydrazyl, DPPH, free radical scavenging assay. The scavenging activities of the complexes were measured and compared with those of the free drug and vitamin C. We have explored their ability to inhibit soybean lipoxygenase, β-glucuronidase and trypsin- induced proteolysis. The complex [Mn(mef)2(H2O)2] exhibits the highest antioxidant activity and the highest inhibitory effect against the soybean lipogygenase (LOX), properties that are not demonstrated by mefenamic acid. Their inhibitory effects on rat paw edema induced by Carrageenan was studied and compared with those of mefenamic acid. The complex [Zn(mef)2] exhibited a strong inhibitory effect at 0.1 mmol/Kg B.W. (81.5 ± 1.3% inhibition), superior to the inhibition induced by mefenamic acid at the same dose (61.5 ± 2.3% inhibition). Mefenamic acid and its metal complexes have been evaluated for antiproliferative activity in vitro against the cells of three human cancer cell lines: MCF-7 (human breast cancer cell line), T24 (bladder cancer cell line), A-549 (non-small cell lung carcinoma) and a mouse fibroblast L-929 cell line. The copper(II) complex displays against T24, MCF-7 and L-929 cancer cell lines, IC50 values in a μM range similar to that of the antitumor drug cis-platin and they are considered for further stages of screening in vitro and/or in vivo as agents with potential antitumor activity.
Introduction
Metal complexes with active drugs as ligands is a research area of increasing interest for inorganic and medicinal chemistry and has concentrated much attention as an approach to new drug development [Citation1,Citation2].
The synthesis of metal complexes with non-steroidal anti-inflammatory drugs (NSAIDs) as ligands has acquired new impetus in the past decade. First, on the basis of pure co-ordination chemistry NSAIDs are very versatile ligands and show a huge variety of ligating modes as function of the metal and the environmental conditions. The information collected from the preparative, structural and reactivity studies have high significance for several fields which span from the bio-sciences to the material sciences. Second, NSAIDs have numerous applications as pharmaceutical agents. Third, for this type of drugs the complex formation with specific metals may improve the activity towards certain diseases and hopefully may increase the activity spectrum. The combination of two or more different species into the same compound may bring to a multi-therapeutic agent which can be expanded by the synergic action of the metal residue once the co-ordination compound dissociate inside the target tissue. The coordination chemistry of non-steroidal anti-inflammatory drugs (NSAIDs) has been studied by several groups worldwide. Some complexes have increased pharmaceutical or biological activity with respect to the drug, or are interesting from a purely chemical point of view Citation3,Citation4,Citation5,Citation6.
Most of the NSAIDs, including aspirin, possess a carboxylate group, which is able to coordinate metal ions. Non-steroidal anti-inflammatory drugs, NSAIDs, from the carboxylic acid family, derivatives of N-phenylanthranilic acid, such as mefenamic acid, tolfenamic acid and sodium diclofenac are widely used in inflammatory and painful diseases of rheumatic and non-rheumatic origin. The anti-inflammatory activity of NSAIDs and most of its other pharmacological effects are related to the inhibition of the conversion of arachidonic acid, AA, to prostaglandins, which are mediators of the inflammatory process. The enzymes cycloxygenase, COX and lipoxygenase, LOX, which catalyze the oxidative metabolism of AA, are useful targets for the design and the development of new drugs that substantially inhibit the generation of the final inflammatory products and the propagation of inflammation [Citation7].
Mefenamic acid is a commonly used NSAID that is a cyclooxygenase-1 and 2 inhibitor with anti-inflammatory properties. It was found to exert neuroprotective effects and improve cognitive impairment in vitro and in vivo Alzheimer's disease models and also neuroprotective activities against neurodegeneration [Citation8].
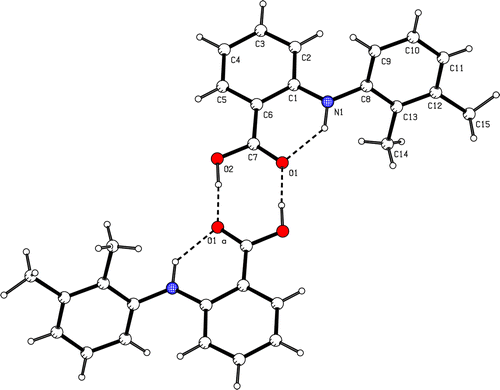
We have prepared some novel complexes of mefenamic acid, , with Mn(II), Co(II), Ni(II), Cu(II) and Zn(II), [Mn(mef)2(H2O)2], [Co(mef)2(H2O)2], [Ni(mef)2(H2O)2], [Cu(mef)2(H2O)]2·2H2O and [Zn(mef)2], in order to obtain information on structure-activity relationships for systems involving metal atoms. Crystal structures of dimeric tetraorganodistannoxane and monomeric triphenyl adducts of mefenamic acid have been already reported by our group [Citation6]. The crystal structures of mefenamic acid and a copper(II) complex, [Cu(mef)2DMSO]2 have been solved, [Citation10].
Here mefenamic acid and its metal complexes were tested for their antioxidant and anti-inflammatory activities in the rat carageenin paw edema assay, a model for acute inflammation. Their inhibitory activities on LOX, on β-glucuronidase and on trypsin- induced proteolysis were evaluated.
It has been found that mefenamic acid induces apoptosis in human liver cancer cell-lines through caspase-3 pathway [Citation9a]. Several NSAIDs, such as mefenamic acid, sulindac or indomethacin have been used in combination with a number of cytotoxic drugs cyclophosphamide, melphalanor and carmustine [Citation9b]. The effect on cytotoxicity of clinically important NSAIDs drugs with a variety of chemotherapeutics was studied in different human cancer cells. A specific group of NSAIDs, indomethacin, sulindac, tolmetin, acemetacin, zomepirac and mefenamic, all at non-toxic levels, significantly increased the cytotoxicity of the anthracyclines, doxorubicin, daunorubicin and epirubicin, as well as teniposide, VP-16 and vincristine [Citation9c]. A triphenyltin complex of mefenamic acid was tested for antimycobacterial activity against Mycobacterium tuberculosis H37Rv and was found to be a very good anti-tuberculosis agent [Citation6b]. Here the cytotoxic activities of mefenamic acid and its metal complexes have been evaluated for antiproliferative activity in vitro against the cells of three human cancer cell lines: MCF-7 (human breast cancer cell line), T24 (bladder cancer cell line), A-549 (non-small cell lung carcinoma) and a mouse L-929 (a fibroblast-like cell line cloned from strain L).
Herein, the goal is to define the probability to extend the pharmacological profile of mefenamic acid, in order to discover new properties such as anti-oxidant and anti-cancer activity and to prepare new complexes of mefenamic acid with essential metal ions, which would exhibit improved or different biological behaviour compared to the “parent drug”, mefenamic acid.
Materials and methods
The reagents (Aldrich, Merck) were used as supplied while the solvents were purified according to standard procedures. Mefenamic acid was a gift from “VIANNEX. A.E”. Trypsin (pancreas protease) 200Fip U/g, salicylic acid (SA), N-tosyl-methyl-arginine ester (TAME) 1,1-diphenyl-2-picrylhydrazyl (DPPH), nordihydroguairetic acid (NDGA) were purchased from the Aldrich Chemical Co. Milwaukee, WI, (USA). Soybean Lipoxygenase, linoleic acid sodium salt was obtained from Sigma Chemical, Co. (St. Louis, MO, USA) and carrageenin, type K, commercially available.
Melting points were determined in open capillaries and are uncorrected. C, H, and N analyses were carried out by the microanalytical service of the University of Ioannina. Infrared and far-infrared spectra were recorded on a Nicolet 55XC Fourier transform spectrophotometer using KBr pellets (4000-400 cm− 1) and nujol mulls dispersed between polyethylene disks (400-40 cm− 1). UV spectra were acquired with a JASCO V-570 spectrophotometer UV/VIS/NIR.
Synthesis of [Mn(mef)2(H2O)2] (1)
A solution of MnCl2 (0.0314 g, 0.26 mM) in methanol (3.0 mL) was added to a solution of mefenamic acid (0.1253 g, 0.52 mM) in methanol (1.5 mL). Drops of triethylamin, N(Eth)3, were added till the apparent pH value was ∼7. The reaction mixture was stirred at room temperature for 1 h and cooled to 5°C in a refrigerator for 4 h. After the addition of a few drops of distilled water a pale brown powder was precipitated. The precipitate was collected by filtration, washed with cold water and dried in vacuo to afford (1). [Mn(mef)2(H2O)2] (1): D. p. 160°C. Yield 10%. IR (cm− 1): 3314m, ν(NH); 3067m, 2942m v(CH3); 1612s vasym(COO); 1503s vsym(COO); 1579s vasym(COO); 1456s vsym(COO); 443ms v(Mn-OH2O);, 374 mw, 245ms v(Mn-Ooco). μeff = 5.39 MB. Anal. Calc. for C30H32N2O6Mn: C, 62.59; H, 5.27; N, 4.70. Found: C, 63.05; H,5.64; N, 4.90; %.
Synthesis of [Co(mef)2(H2O)2] (2)
A solution of Co(CH3OO)2·4H2O (0.0314 g, 0.125 mM) in methanol (1.5 mL) was added to a solution of mefenamic acid (0.0627 g, 0.26 mM) in methanol (1.5 mL). Drops of triethylamine, N(Eth)3, were added till the apparent pH value was ∼7. The reaction mixture was stirred at room temperature for 1 h and cooled to 5°C in a refrigerator for 4 h. After the addition of a few drops of distilled water a violet powder was precipitated. The precipitate was collected by filtration, washed with cold water and dried in vacuo to afford (1). [Co(mef)2(H2O)2] (2): D. p. 115°C. Yield 80%. IR (cm− 1): 3552s, 3311s ν(NH); 3062m, 2943m; v(CH3); 1649s, δ(NH); 1613s, vasym(COO); 1505s, vsym(COO); 1579s, vasym(COO); 1455mw, vsym(COO); 429msv(Co-OH2O); 408sh, 230ms v(Co-Ooco). μeff = 4.53 MB. Anal. Calc. for C30H32N2O6Co: C,62.62; H, 5.60; N, 4.87. Found: C, 63.07; H,5.61; N, 4.92; %.
Synthesis of [Ni(mef)2(H2O)2] (3)
A solution of NiCl2·6H2O (0.0297 g, 0.125 mM) in methanol (1.5 mL) was added to a solution of mefenamic acid (0.0627 g, 0.26 mM) in methanol (1.5 mL). Drops of triethylamine N(Eth)3 were added till the apparent pH value was ∼7. The reaction mixture was stirred at room temperature for 1 h and a light green powder was precipitated. The solution was cooled to 5°C in a refrigerator for 4 h. The precipitate was collected by filtration, washed with cold methanol and dried in vacuo to afford (3). [Ni(mef)2(H2O)2] (3): D. p. 270°C. Yield 45%. IR (cm− 1): 3400br, ν(NH); 3067m 2943m; v(CH3); 1613s vasym(COO); 1503, vsym(COO); 1579s vasym(COO); 1455 vsym(COO); 450mv(Ni-OH2O); 379mw, 252ms v(Ni-Ooco). μeff = 3.13 MB. Anal. Calc. for C30H32N2O6Co: C, 62.64; H, 5.60; N, 4.87. Found: C, 62.07; H,6.03; N, 5.02; %.
Synthesis of [Cu(mef)2(H2O)]2·2H2O (4)
A solution of Cu(CH3COO)2·H2O (0.02499 g, 0. 25 mM) in methanol (2.0 mL) was added to a solution of mefenamic acid (0.1253 g, 0.52 mM) in methanol (2.0 mL). Drops of triethylamine, N(Eth)3, were added till the apparent pH value was ∼7. The reaction mixture was stirred at room temperature for 1 h and a dark green powder was precipitated. The solution was cooled to 5°C in a refrigerator for 4 h. The precipitate was collected by filtration, washed with cold methanol and dried in vacuo to afford (4). [Cu(mef)2(H2O)]2·2H2O (4): D. p. 155°C. Yield 52%. IR (cm− 1): 3341s, ν(NH); 3067m 2943m; v(CH3); 1616s vasym(COO); 1455m vsym(COO); 429m v(Cu-OH2O); 348m, 277ms v(Cu-Ooco). μeff = 2.02 MB. Anal. Calc. for C30H34N2O7Cu: C, 60.25; H, 5.72; N, 4.68. Found: C, 60.22; H, 5.58; N, 4.25%.
Synthesis of [Zn(mef)2] (5)
A solution of ZnCI2 (0.05 g, 0.37 mM) in methanol (3.0 mL) was added to a solution of mefenamic acid (0.169 g, 0.7 mM) in methanol (3.0 mL). Drops of a methanolic solution 1 N NaOH were added till the apparent pH value was ∼7. The reaction mixture was stirred at room temperature for 1 h. After the addition of a few drops of distilled water a yellow powder was precipitated. The solution was cooled to 5°C in a refrigerator for 4 h. The precipitate was collected by filtration, washed with cold methanol/H2O, 5:1 and dried in vacuo to afford (5). [Zn(mef)2] (5): D. p. 115°C. Yield 20%. IR (cm− 1): 3341s ν(NH); 3068m, 2942m; v(CH3); 1614s vasym(COO); 1503s vasym(COO); 1579s vasym(COO); 1461s vsym(COO); 456ms, 434sh, v(M-Ooco). Anal. Calc. for C30H28N2O4Zn: C, 66.01; H, 5.17; N, 5.13. Found: C, 66.06; H,5.30; N, 5.00; %.
Biological assays
In vitro and in vivo [Citation11]
In the in vitro assays each experiment was performed at least in triplicate and the standard deviation of absorbance was less than 10% of the mean.
Determination of the reducing activity of the stable radical 1,1-diphenyl-picrylhydrazyl (DPPH) [Citation11]
To a solution of DPPH (0.1 and 0.5 mM) in absolute ethanol an equal volume of the compounds dissolved in ethanol was added. As control, solution ethanol was used. The concentrations of the solutions of the compounds were 0.1 and 0.5 mM. After 20 and 60 min at room temperature the absorbance was recorded at 517 nm.
Competition of the tested compounds with DMSO for hydroxyl radicals [Citation11]
The hydroxyl radicals generated by the Fe3 + /ascorbic acid system, were detected according to Nash, by the determination of formaldehyde produced from the oxidation of DMSO. The reaction mixture contained EDTA (0.1 mM), Fe3 + (167 μM), DMSO (0.1 and 6.6 mM) in phosphate buffer (50 mM, pH 7.4), the tested compounds (concentration 1 mM) and ascorbic acid (10 mM). After 30 min of incubation (37°C) the reaction was stopped with CCl3COOH (17% w/v).
Soybean lipoxygenase inhibition study in vitro [Citation11]
In vitro study was evaluated as reported previously. The tested compounds (0.033 and 0.333 mM) dissolved in ethanol were incubated at room temperature with sodium linoleate (0.1 mM) and 0.2 mL of enzyme solution soybean lipoxygenase, dissolved in 0.9% NaCl solution [250 u/mL]. The conversion of sodium linoleate to 13-hydroperoxylinoleic acid at 234 nm was recorded during 5 min and compared with the appropriate standard inhibitor (nordihydroguaiaretic acid 0.1 mM 83.7% inhibition; 1 mM 94.7% inhibition).
Inhibition on β-glucuronidase [Citation11]
Compounds in acetate buffer (0.1 mM, pH 7.4) were tested against β-glucuronidase (0.1 mL of 1 U/mL) with 2.5 mM p-nitrophenyl-β-D-glucopyranosiduronic acid. After incubation at 37°C for 30 min, 2 mL of 0.5 N NaOH solution was added to the mixture and the absorbance of the mixture was measured at 410 nm.
Inhibition on proteolysis [Citation11]
Tosyl arginine methyl ester (TAME) was used as substrate for trypsin. The reaction mixture consisted of 0.75 mL buffer (0.1 M tris-HCl, pH 7.8 in 50% methanol v/v) and 0.14 mL TAME (0.01 M in 50% v/v methanol). Compounds dissolved in 50% methanol were added (0.3 mM). The reaction was started by addition of 0.1 mL trypsin (1 mg/mL 0.001 N HCl). The increase in the absorbance at 256 nm was determined in the next 4 min.
Antiproliferative assay in vitro [Citation12]
Compounds
Test solutions of the compounds tested (1 mg/ml) were prepared by dissolving the substance in 100 μL of DMSO completed with 900 μL of tissue culture medium. Afterwards, the tested compounds were diluted in culture medium to reach the final concentrations of 100, 10, 1 and 0.1 μg/mL. The solvent (DMSO) in the highest concentration used in test did not reveal any cytotoxic activity.
Cells
The established in vitro human cancer cell line MCF-7 (human breast cancer cell line), T24 (bladder cancer cell line), A-549 (non-small cell lung carcinoma) and a mouse L-929 (a fibroblast-like cell line cloned from strain L) were applied: The cell lines are maintained in the Cell Culture Collection of the University of Ioannina. Twenty-four hours before addition of the tested agents, the cells were plated in 96-well plates at a density of 104 cells per well. The T-24 and MCF-7 cells were cultured in the D-MEM (Modified Eagle's Medium) medium supplemented with 1% antibiotic and 10% fetal calf serum. L-929 cells were grown in Hepes-buffered RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin (50 U/mL) and streptomycin (50 mg/mL). A-549 cells were grown in F-12K Ham's medium supplemented with 1% glutamine, 1% antibiotic/antimycotic, 2% NaHCO3 and 10% fetal calf serum. The cell cultures were maintained at 37°C in a humid atmosphere saturated with 5% CO2. Cell number was counted by the Trypan blue dye exclusion method. MCF-7, L-929 and A-549 cells were determined by the sulforhodamine B assay [Citation13], and T24 cells by the MTT assay [Citation12].
SRB assay
The details of this technique were described by Skekhan et al. [Citation13]. The cytotoxicity assay was performed after 72-h exposure of the cultured cells to varying concentrations (from 0.1 to 100 μg/mL) of the tested agents. The cells attached to the plastic were fixed by gently layering cold 50% trichloroacetic acid (TCA) on the top of the culture medium in each well. The plates were incubated at 4°C for 1 h and then washed five times with tap water. The background optical density was measured in the wells filled with culture medium, without the cells. The cellular material fixed with TCA was stained for 30 min with 0.4% sulforhodamine B dissolved in 1% acetic acid. Unbound dye was removed by rinsing with 1% acetic acid. The protein-bound dye was extracted with 10 mM unbuffered Tris base for determination of optical density (at 540 nm) in a computer-interfaced, 96-well microtiter plate.
MTT
This technique was applied for cytotoxicity screening against T-24 cells growing in suspension culture. An assay was performed after 72 h exposure to varying concentrations (from 0.1 to100 μg/mL) of the tested agents. For the last 3-4 h of incubation 20 μL of MTT solution was added to each well [MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; Sigma); stock solution: 5 mg/mL]. The mitochondria of viable cells reduce a pale yellow MTT to a navy blue formazan, so if more viable cells are present in the well, more MTT will be reduced to formazan. When the incubation time was completed (4 h), 80 μL of the lysing mixture was added to each well (lysing mixture: 225 mL dimethylformamide, 67.5 g sodium dodecylsulfate (both from Sigma) and 275 mL of distilled water). After 24 h, when formazan crystals had dissolved, the optical densities of the samples were read on an Elisa spectra max 190 photometer at 570 nm wavelength. Each compound at a given concentration was tested in triplicate in each experiment, which was repeated three times.
Inhibition of the carrageenin-induced edema [Citation11]
Edema was induced in the right hind paw of Fisher 344 rats (150-200 g) by the intradermal injection of 0.1 mL 2% carrageenin in water. Both sexes were used. Pregnant females were excluded. Each group was composed of 6-15 animals. The animals, which have been bred in our laboratory, were housed under standard conditions and received a diet of commercial food pellets and water ad libitum during the maintenance but they were entirely fasted during the experiment period. Our studies were in accordance with recognised guidelines on animal experimentation.
The tested compounds 0.01 or 0.1 mmol/kg body weight, were suspended in water, with few drops of Tween 80 and ground in a mortar before use or dissolved in water and were given intraperitoneally simultaneously. The rats were euthanized 3.5 h after carrageenin injection. The difference between the weight of the injected and uninjected paws was calculated for each animal. The change in paw weight was compared with that in control animals (treated with water) and expressed as a percent inhibition of the edema CPE % values . Values CPE % are the mean from two different experiments with a standard error of the mean less than 10%.
Results and discussion
Synthetic aspects
We have prepared complexes of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) with mefenamic acid, 2-[(2,3-dimethylphenyl)amino]benzoic acid, HMef, in order to obtain information on structure-activity relationships for systems involving nickel(II), copper(II) and zinc(II) atoms. The complexes [Mn(mef)2·(H2O)2] (1), [Co(mef)2·(H2O)2] (2), [Ni(mef)2·(H2O)2] (3), [Cu(mef)2·H2O]2·2H2O (4) and [Zn(mef)2] (5), were synthesized according to the reactions 1,2,3,4,5, in methanolic and aqueous solutions.
The complexes are microcrystalline or powder-like and stable in atmospheric conditions. All the complexes are insoluble in water, except of 1 which is slight soluble in water, in pentane and cyclohexane and all are soluble in the solvents Me2CO, CHCl3, CH2Cl2, EtOH, DMSO, DMF, benzene and toluene. The elemental analyses confirm their stoichiometry. The μeff values of the complexes, show that these are all high spin; the large and small orbital contribution in the 2 and 3, ![]() eg2 Co(II) and
eg2 Co(II) and ![]() eg2 Ni(II), respectively, indicate six-coordinate structures.
eg2 Ni(II), respectively, indicate six-coordinate structures.
Spectroscopy
Infrared spectroscopy
As the carboxyl hydrogen is more acidic than the amino hydrogen the deprotonation occurs in the carboxylic group. This is confrmed by the IR spectra of the complexes, showing the characteristic bands for the secondary amino groups and for the coordinated carboxylato group. A broad absorption at 3500 cm− 1 in the spectra of the complexes was attributed to the presence of coordinated water. The absence of large systematic shifts of the v(NH) and δ(NH) bands in the spectra of the complexes compared with those of the ligand indicates that there is no interaction between the NH group and the metal ions. The νas(COO) and νsym(COO) bands of the prepared complexes are at 1615-1580 and at 1500-1450 cm− 1 respectively; the difference, Δ [νas(COO) - νsym(COO)] between these frequencies for 1-3 and 5 is significantly less than the ionic value (for sodium mefenamic the Δ value is 180 cm− 1), as expected for the bidentate chelating mode of carboxylate ligation [Citation7a,Citation14]. The difference, Δ [νas(COO) − νsym(COO)] between these frequencies for 4 is close and less (161 cm− 1) to that for sodium mefenamic, as expected for bridging bidentate carboxylato groups, [Citation15] supporting a structure analogous to [Cu(mef)2(DMSO)]2, [Citation10b] and [Cu(diclof)2(DMF)]2 [Citation15]. The four carboxylate groups from four ligands are in a bidentate syn, syn η1:η1:μ2 bridging mode. The square pyramid geometry with a dimethylformamide oxygen or water occupying both apical positions as was established by single crystal X-ray study [Citation10b,Citation15]. The compounds, 1-5 gave bands at ∼3300 cm− 1 attributable to intramolecular hydrogen bonds NH…O. The medium bands at ∼400 cm− 1 is attributed to the v(M-OH2O) stretching mode, while the bands at 250-210 cm− 1 to the v(M-Ooco) stretching mode Citation14,Citation15,Citation16.
Electronic spectroscopy
The electronic spectra were recorded in DMF and CHCl3 solution and absorption maxima in the uv-visible region are listed in along with suggested assignments Citation14,Citation15,Citation16. The electronic spectra of the Mn(II) complex, 1, can be assigned six coordinate stereochemistry [Citation14]. The absorption of the organic ligand tailing into the visible region obscure the very weak d-d absorption bands of the manganese (II) complexes Citation14,Citation15,Citation16,Citation17. The d-d spectra of the solvated 1-3 can be assigned to transitions in pseudo-octahedral structures or six-coordinated tetragonally distorted stereochemistries. The band frequencies and the 10Dq value in 3 are characteristic of a Ni(II)O6 chromophore, which do not cause a pronounced nephelauxetic effect [Citation11,Citation16]. The solution spectral data indicate the complexes 1-3 are all solvated with two solvent molecules coordinating in a pseudo-octahedral arrangement.
Table I. Spectral data (UV-vis) and μeff values for the prepared complexes and the parent drug.
Where M is Mn(II) or Ni(II) or Co(II) and solvate is DMF or CHCl3.
The solution spectra of 4 shows two bands having maxima at ∼14,000-14,900 (band I) and at 22,500-25,000 cm− 1 (band II), typical for square pyramidal species with CuO5 chromophore. It is recognized that band I is assigned to a ligand field transition dxz,dyz → dx2 − y2 and band II to a charge transfer from carboxylato-oxygen atoms to the metal ion, without ruling out a transition of the type dz2 → dx2 − y2. The same pattern of spectrum is shown when compound 4 is dissolved in CHCl3 and DMF, suggesting that these axial ligands exchanged with solvent molecules according to the reaction [Citation14].
Biological studies
In vitro biological studies
In this investigation all compounds were studied in order to gain insight into their biological response. The metal complexes and the parent drug were studied with regard to their antioxidant ability as well as to the inhibition of a) soybean lipoxygenase, b) β-glucuronidase and c) of trypsin- induced proteolysis.
Nowadays, antioxidants that exhibit DPPH radical scavenging activity is increasingly receiving attention. They have been reported to have interesting anticancer, anti-aging, and anti-inflammatory activities. Consequently, compounds with antioxidant properties could be expected to offer protection in rheumatoid arthritis and inflammation, and to lead to potentially effective drugs. In fact, many non-steroidal anti-inflammatory drugs have been reported to act either as inhibitors of free radical production or as radical scavengers. The model of the scavenging of the stable DPPH radical is extensively used to evaluate antioxidant activities in less time than other methods. DPPH is a stable free radical that can accept an electron or hydrogen radical and thus be converted into a stable, diamagnetic molecule. DPPH has an odd electron and so has a strong absorption band at 517 nm. When this electron becomes paired off, the absorption decreases stoichiometrically with respect to the number of electrons taken up. Such a change in the absorbance produced in this reaction has been widely applied to test the capacity of numerous molecules to act as free radical scavengers. The reducing abilities of the complexes, 1-5 were evaluated using the 1,1-diphenyl-1-picrylhydrazyl, DPPH, free radical scavenging assay [Citation11] in an iron-free system. This interaction expresses the reducing ability of the compounds. The scavenging activities of the complexes were measured and compared with those of the free drug and of vitamin C.
Not all the tested compounds were found to interact with the stable free radical DPPH, (). The mefenamic acid and complexes 2, 3 and 5 present very low interactions (8,6.2 and 3.8%). It seems that interaction increases with the time and the concentration. Complex 4 possess mild interaction. Complex [Mn(mef)2·(H2O)2] (1), presents the highest interaction with DPPH, which is time and concentration dependent.
Table II. Interaction % with DPPH- Reducing ability (RA %).
During the inflammatory process, phagocytes generate the superoxide anion radical at the inflammed site, and this is connected to other oxidizing species such as HO√. Hydroxyl radicals are produced by reactions which are depended on transition metals, particularly iron. The competition of the compounds (0.1 mM) with DMSO for HO√ generated by the Fe3 + /ascorbic acid system, expressed as the inhibition of formaldehyde production, was used for the evaluation of their hydroxyl radical scavenging activity. In these experiments, (), mefenamic acid exhibits the highest competition, compounds 2-5 show potent inhibition, while complex 1 is totally inactive. Trolox was used as a reference compound.
Table III. Competition % with DMSO (0.1 mM) for hydroxyl radical HO·, inhibition of LOX and antiproteolytic activity of mefenamic acid and its metal complexes.
In this investigation all complexes were studied in order to gain insight their LOX-inhibition. Most of the recognised LOX inhibitors are antioxidants or free radical scavengers since lipoxygenation occurs via a carbon centered radical. The activity of the metal complexes and the “parent drug” to inhibit the enzyme soybean lipoxygenase has been studied. Mefenamic acid did not exhibit inhibition of the enzyme LOX. Perusal of the % inhibition values, that complex [Mn(mef)2·(H2O)2] (1), presents the higher activity, while complex 4 is totally inactive, ().
The role played by proteases in the early stage of inflammatory process is well documented. Some anti-inflammatory agents have been reported to exhibit antiproteolytic activity [Citation11]. The antiproteolytic activity of the metal complexes and the parent drug was studied. Complexes [Co(mef)2(H2O)2] (2) and [Ni(mef)2(H2O)2] (3) were found to inhibit significantly high β-glucuronidase, while complexes 1, 4 and 5 did not present any effect against this enzyme under the reported experimental conditions. Complexes 1 (47.8%) and 2 (58.2%), were found to inhibit trypsin in vitro. Mefenamic acid does not present any effect and complexes 3-5 (23-34%) were also found to be inactive under the reported experimental conditions, ().
Antiproliferative activity in vitro [Citation18]
The results of cytotoxic activity in vitro are expressed as IC50 – the concentration of compound (in μM) that inhibits a proliferation rate of the tumor cells by 50% as compared to control untreated cells, (). Mefenamic acid and the metal complexes 1-5 were tested for their antiproliferative activity in vitro against the cells of three human cancer cell lines: MCF-7 (human breast cancer cell line), T24 (bladder cancer cell line), A-549 (non-small cell lung carcinoma) and a mouse fibroblast L-929 cell line and the results are compared with the known chemotherapeutic cis-platin.
Table IV. The antiproliferative activity in vitro of mefenamic acid its metal complexes (expressed as IC50 (M) against MCF-7, T-24, A-549 and L-929 cancer cell lines.
The IC50 value for for the complex [Cu(mef)2(H2O)]2 against the T24 cell line is 7.77 × 10− 6 M. The IC50 values shown by [Cu(mef)2(H2O)]2 against MCF-7 and L-929 cancer cell lines are in a μM range similar to cis-platin 2.8 times and 8.0 times less cytotoxic against L-929 and MCF-7 cancer cell lines respectively.
These results indicate that coupling of mefenamic acid to Cu(II) metal center result in a metallic complex with important biological properties. The cytotoxic activity shown by [Cu(mef)2(H2O)]2 against T24, MCF-7 and L-929 cancer cell lines indicates that coupling of mefenamic acid to Cu(II) metal center result in metallic complexes with important biological properties since it displays IC50 values in a M range similar or better to that of the antitumor drug cis-platin.
In vivo biological studies
The in vivo anti-inflammatory effects of the tested metal complexes and of their parent drug were assessed by using the functional model of carrageenin-induced rat paw edema and are presented in as percentage of weight increase at the right hind paw in comparison to the uninjected left hind paw. Carrageenin- induced edema is a non-specific inflammation resulting from a complex of diverse mediators [Citation19]. Since oedemas of this type are highly sensitive to non-steroidal anti-inflammatory drugs (NSAIDs), carrageenin has been accepted as a useful agent for studying new anti-inflammatory drugs [Citation20]. This model reliably predicts the anti-inflammatory efficacy of the NSAIDs and during the second phase it detects compounds that are anti-inflammatory agents as a result of inhibition of prostaglandin amplification [Citation21]. In , is shown the protection observed against carrageenin –induced paw edema, by the investigated complexes (0.01 mmol/kg). The reference drug, mefenamic acid induced 61.5% protection at an equivalent concentration. The low solubility of [Cu(mef)2·H2O]2 prevents the measurement of CPE activity.
Table V. Inhibition % of induced carrageenin rat paw edema (CPE %) at 0.1 mmol/kg.
Actually the [Ni(mef)2·(H2O)2] was the most potent presenting toxic effects at 0.1 mmol/kg body weight. Thus, this compound was tested in lower concentration (0.01 mmol/kg). The inhibition caused by the complex [Ni(mef)2·(H2O)2] was found to be 17.5 ± 0.4% at 0.01 mmol/kg body weight BW. The complex [Zn(mef)2] exhibited a strong inhibitory effect at 0.1 mm/Kg B.W. (81.5 ± 1.3% inhibition), superior of the inhibition induced by mefenamic acid at the same dose (61.5 ± 2.3% inhibition). The observed differences in activity seem to depend on the nature of the metal.
Conclusions
The complexes of mefenamic acid [Mn(mef)2(H2O)2], [Co(mef)2(H2O)2], [Ni(mef)2(H2O)2], [Cu(mef)2(H2O)]2 and [Zn(mef)2] were synthesized and characterized. Spectral studies reveal monomeric six-coordinated species for Mn(II), Ni(II) and Co(II), dimeric five-coordinated for Cu(II) and monomeric four-coordinated for Zn(II). In DMF or CHCl3 solution the coordination number is retained and the coordinated molecules of water are replaced by solvent molecules.
The metal complexes and the “parent drug” have been studied with regard to their antioxidant ability as well as their inhibition of LOX, of β-glucuronidase and of trypsin- induced proteolysis. The complex [Mn(mef)2(H2O)2] exhibits the highest antioxidant activity and the highest inhibitory effect against the soybean lipogygenase (LOX), properties that are not demonstrated by mefenamic acid. Their antiinflammatory effects on rat paw edema induced by Carrageenan was studied and compared with those of mefenamic acid. The complex [Zn(mef)2] exhibited a strong in vivo inhibitory effect at 0.1 mm/Kg B.W. (81.5 ± 1.3%), superior than the inhibition induced by mefenamic acid at the same molar dose (61.5 ± 2.3%). These compounds may prove useful for treating a variety of inflammatory diseases and may lead to the development of new drugs. Mefenamic acid and [Cu(mef)2(H2O)]2 are considered as agents with potential antitumor activity, and can therefore be candidates for further stages of screening in vitro and/or in vivo.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsiblefor the content and writing of the paper.
References
- R Cini. Anti-Inflammatory compounds as ligands in metal complexes as revealed in X-ray structural studies. Comments Inorg Chem 2000;22:151–186. and references therein
- D Kovala-Demertzi. Transition metal complexes of diclofenac with potentially interesting anti-inflammatory activity. J Inorg Biochem 2000;79 (1–4):153–157.
- (a) S Ramadan, TW Hambley, BJ Kennedy, PA Lay. NMR spectroscopic characterization of copper(II) and zinc(II) complexes of indomethacin. Inorg Chem 2004;43 (9):2943–2946, (b) MA Méndez-Rojas, F Cordova-Lozano, G Gojon-Zorrilla, E González-Vergara, MA Quiroz. Direct electrosynthesis of Cu, Cd, Zn complexes of piroxicam (4-hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazine-3-carboxamide-1,1,-dioxide) and isoxicam (4-hydroxy-2-methyl-N-(5-methyl-3-isoxazolyl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide) in nonaqueous media by in-situ generation of supporting electrolyte. Polyhedron 1999;18:2651–2658.
- (a) A Theodorou, MA Demertzis, D Kovala-Demertzi, EE Lioliou, AA Pantazaki, DA Kyriakidis. Copper(II) complexes of diclofenac: Spectroscopic studies and DNA strand breakage. Biometals 1999;12:167–172, (b) M Konstandinidou, A Kourounaki, L Hadjipetrou, D Kovala-Demertzi, S Hadjikakou, MA Demertzis. Anti-inflammatory properties of diclofenac transition metalloelement complexes. J Inorg Biochem 1998;70(1):63–69; (c) D Kovala-Demertzi, A Galani, MA Demertzis, S Skoulika, C Kotoglou. Binuclear copper(II) complexes of tolfenamic: Synthesis, crystal structure, spectroscopy and superoxide dismutase activity. J Inorg Bιοchem 2004;98:358–364; (d) V Dokorou, D Kovala-Demertzi, JP Jasinski, A Galani, MA Demertzis. Synthesis, spectroscopic studies, and crystal structures of phenylorganotin derivatives with [bis(2,6-dimethylphenyl)amino]benzoic acid: Novel antituberculosis agents. Helv Chim Acta 2004;87:1940–1950.
- (a) AL Abuhijleh, C Woods. Mononuclear copper (II) salicylate imidazole complexes derived from copper (II) aspirinate. Crystallographic determination of three copper geometries in a unit cell. Inorg Chem Commun 2001;4:119–123, (b) R Bucci, AD Magri, AL Magri, A Napoli. Spectroscopic characteristics and thermal properties of divalent metal complexes of diclofenac. Polyhedron 2000;19:2515–2520 (c) A Fini, G. Feroci, G Fazio. Interaction between indomethacin and heavy metal ions in aqueous solution. Eur J Pharm Sci 2001;13:213–217.
- (a) D Kovala-Demertzi. Recent advances on non-steroidal anti-inflammatory drugs, NSAIDs: Organotin complexes of NSAIDs. J Organom Chem 2006;691:1767–1774, (b) D Kovala-Demertzi, V Dokorou, Z Ciunik, N Kourkoumelis, MA Demertzis. Organotin mefenamic complexes - preparations, spectroscopic studies and crystal structure of a triphenyltin ester of mefenamic acid: Novel anti-tuberculosis agents. Appl Organomet Chem 2002;16:360–368; (c) V Dokorou, Z Ciunik, U Russo, D Kovala-Demertzi. Synthesis, crystal structures and spectroscopic studies of diorganotin derivatives with mefenamic acid. Crystal and molecular structures of 1,2:3,4-di-μ2-2-[(2,3-dimethylphenyl)amino]-benzoato-O, O-1,3-bis-2-[(2,3-dimethylphenyl)amino]benzoato-O-1,2, 4:2,3,4-di-μ3-oxo-tetrakis[di-methyltin(IV)] and 1,2:3,4-di- μ2-2-[(2,3-dimethylphenyl)amino]-benzoato-O,O-1,3-bis- 2-[(2,3-dimethylphenyl)amino]benzoato-O-1,2,4:2,3,4-di- μ3-oxo-tetrakis[di-n-butyltin(IV)]. J Organomet Chem 2001;630:205–214; (d) V Dokorou. Ph. D. Theses, Ioannina, 2000.
- (a) PA Insel. Goodman and Gilman's the pharmacological basis of therapeutics. New-York: McGraw-Hill; 1996. ch 27; (b) JEF Reynolds, K Parfitt, AV Parsons, SC Sweetman. Martindale, The extra pharmacopoeia, 30th ed., London, 1993; p. 15, 23; (c) M Zheng, Z Zhang, W Zhu, H Liu, X Luo, K Chen, H Jiang. Essential structural profile of a dual functional inhibitor against cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX): Molecular docking and 3D-QSAR analyses on DHDMBF analogues. Bioorg Med Chem 2006;14:3428–3437; (d) JY Gauthier. Synthesis and biological evaluation of 2,3-diarylthiophenes as selective cox-2 inhibitors. part II: Replacing the heterocycle Bioorg Med Chem Let 1996;6(1):87–92.
- (a) Y Joo, HS Kim, RS Woo, CH Park, KY Shin, JP Lee, KA Chang, S Kim, YH Suh. Mefenamic acid shows neuroprotective effects and improves cognitive impairment in in vitro and in vivo alzheimer's disease models. Mol Pharmacol 2006;69 (1):76–84, (b) M Asanuma, S Nishibayashi-Asanuma, I Miyazaki, M Kohno, N Ogawa. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J Neurochem 2001;76(6):1895–1904.
- DH Woo, IS Han, G Jung. Mefenamic acid-induced apoptosis in human liver cancer cell-lines through caspase-3 pathway. Life Sci 2004;75:2439–2449, (b) BA Thicher, TT Korbut, K Menon, SA Holden, G Ara. Cyclooxygenase and lipoxygenase inhibitors as modulators of cancer therapies Cancer Chemother Pharmacol 1994;33:515–522; (c) CP Duffy, CJ Elliott, RA O'Connor, MM Heenan, S Coyle, IM Cleary, K Kavanagh, S Verhaegen, CM O'Loughlin, R NicAmhlaoibh, M Clynes. Enhancement of chemotherapeutic drug toxicity to human tumor cells in vitro by a subset of non-steroidal anti-inflammatory drugs (NSAIDs). Europ J Canc 1998;8:1250–1259.
- (a) V Dhanaraj, M Vijayan. Structural studies of analgesics and their interactions. XII. Structure and interactions of anti-inflammatory fenamates: A concerted crystallographic and theoretical conformational study. Act Crystallogr Section B 1998;44:406–412, (b) G Facchin, MH Torre, E Kremer, OE Piro, EJ Baran. Crystal structure and spectroscopic behavior of a binuclear copper(II) complex of mefenamic acid and dimethylsulfoxide. Zeitsc Naturforsc B 1998;53(8):871–874.
- C Kontogiorgis, D Hadjipavlou-Litina. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidants agents. J Enz Inhib Med Chem 2003;18:63–69.
- (a) M Ohno, T Abe. Rapid colorimetric assay for the quantification of leukemia inhibitory factor (LIF) and interleukin-6 (IL-6). J Immunol Meth 1991;145:199–203, (b) TJ Sabo, VM Dinovic, GN Kaluderovic, TP Stanojkovic, GA Bogdanovic, ZD Juranic. Syntheses and activity of some platinum(IV) complexes with N-methyl derivative of glycine and halogeno ligands against HeLa, K562 cell lines and human PBMC. Inorg Chim Acta 2005;358:2239–2245; (c) T Mosman. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
- P Skehan, R Storeng, D Storeng, A Monks, J McMahon, D Vistica, JT Warren, H Bokesch, S Kenney, MR Boyd. New colorimetric cytotoxicity assay for anticancer-drug screening. Natl Cancer Inst 1990;82:1107–1112.
- D Kovala-Demertzi, SK Hadjikakou, MA Demertzis, Y Deligiannakis. Metal ion-drug interactions. Preparation and properties of manganese(II), cobalt(II) and nickel(II) complexes of diclofenac with potentially interesting anti-inflammatory activity: Behavior in the oxidation of 3,5-di-tert-butyl-o-catechol. J Inorg Biochem 1998;69:223–229.
- D Kovala-Demertzi, A Theodorou, MA Demertzis, CP Raptopoulou, A Terzis. Synthesis and characterization of tetrakis-μ-2-[(2,6-dichlorophenyl)amino]benzeneacetodiaquodicopper(II) dihydrate and tetrakis-μ-2-[(2,6-dichlorophenyl)amino]benzeneacetodimethylformamidodicopper(II). J Inorg Biochem 1997;65:151–157.
- (a) K Nakamoto. Infrared and Raman spectra of inorganic and coordination compounds. 4th ed. New York: Wiley; 1986, (b) B Szymanska, D Skrzypek, D Kovala-Demertzi, M Staninska, MA Demertzis. Synthesis and spectroscopic study of copper(II) and manganese(II) complexes with pipemidic acid. Spectrochimica Acta Part A 2006;63:518–523.
- ABP Lever. Inorganic electronic spectroscopy. 2nd ed. Amsterdan: Elsevier; 1984. p 480–505.
- D Kovala-Demertzi, A Galani, N Kourkoumelis, JR Miller, MA Demertzis. Polyhedron 2007;26:2871, (b) D Kovala-Demertzi, P Nath Yadav, J Wiecek, S Skoulika, T Varadinova, MA Demertzis. J Inorg Biochem 2006, 100/9, 1558.
- TY Shen. Non-steroidal anti-inflammatory agents. In: ME Wolf. Burger's medicinal chemistry. New York: John Wiley and Sons; 1980. p 1217–1219.
- CA Winter. Non-steroidal anti-inflammatory drugs. In: S Garattini, MNG Dukes, editors. Amsterdam: Excepta Medica; 1965. p 190.
- T Kuroda, F Suzuki, T Tamura, K Ohmori, H Hosoe. A synthesis and potent anti-inflammatory activity of 4-hydroxy-2-(H)-oxo-1-phenyl-1,8-naphthylyridine-3-carboxamides. J Med Chem 1992;53:1130–1136.

![Figure 2. The structure of [Cu(mef)2(DMSO)]210b.](/cms/asset/f6dde2c8-77ed-4211-97c1-d771f690e191/ienz_a_336325_f0002_b.gif)