Abstract
In the eukaryotic cell, phospholipids can be biosynthesized by two pathways, one from choline and the other one from ethanolamine. The functional effectiveness of each pathway depends on the type of the cell. Thiazolium designed-drugs have shown, under in vivo conditions, antiplasmodial and antimalarial activities with inhibition of the phospholipids biosynthesis. This study aimed to discover the pathways involved in the biosynthesis of phospholipids in Plasmodium and deduce the biochemical steps inhibited by T4, a bis-thiazolium bromide drug. We compared the uptake of radiolabeled precursors and their selective incorporation in the phospholipids of cultured Plasmodium-infected and -uninfected erythrocytes which revealed that phosphatidylcholine of Plasmodium is synthesized both from choline and ethanolamine (4.7 vs 1.9 nmol/1010 cells.h−1). T4 has no effect on the biosynthesis of phosphatidylethanolamine but T4 inhibited, in a selective way, the in vitro uptake of choline. However no enzymes in the biosynthesis of phospholipids seem to be inhibited by T4 but rather an inhibition of choline entry into the parasite.
| Abbreviations: | ||
| Cho, | = | choline |
| CDP, | = | cytidine diphosphate |
| CDP | = | choline, cytidine diphosphate choline |
| CDP | = | ethanolamine, cytidine diphosphate ethanolamine |
| CMP, | = | cytidine 5-monophosphate |
| DAG, | = | diacylglycerol |
| Etn, | = | ethanolamine |
| PC, | = | phosphocholine |
| PE, | = | phosphoethanolamine |
| PtdCh, | = | phosphatidylcholine |
| PtdEtn, | = | phosphatidylethanolamine |
| SEM, | = | standard error of mean |
| NA, | = | Nucleic Acid |
Introduction
Plasmodium is the parasite responsible for malaria, the most wild spread infectious disease in the world. P. falciparum is the most deadly species among the four humans plasmodial infectious. Despite the huge efforts to control this disease, malaria still occurs as a public health problem in several countries. The different approaches to control this disease are vector control, vaccination, immunotherapy and chemotherapy. Chemotherapy approach progresses on different pathways and has indicated several potential biochemical targets [Citation1–5]. However, many of these approaches need to be validated as effective and specific antiplasmodial biochemical targets.
Due to drug resistance of some strains, we constantly need to find out new more efficient drugs.
Several ammonium quaternary-derived drugs have shown an in vivo antimalarial activity in Plasmodium-infected rodents or monkeys [Citation3, Citation6]or the mouse [Citation3, Citation7]. Recent extended researches showed potent antimalarial activities with bis- thiazolium drugs [Citation8]. Several bis-thiazolium derived-molecules such as T4, a methoxy-derived bromide bis-thiazolium, have shown an in vivo antimalarial activity. T4 exhibited, with in vitro test against P. falciparum, an IC50 value of 0.65 nM and a good antimalarial property against P. vinckei (ED50 = 0.14 mg/kg) [Citation8]. T16, a structural analogue of T4, shows an inhibitory property on the choline uptake and an inhibition of phosphatidylcholine biosynthesis [Citation9], indicating a potential mechanism of the antimalarial activity of the bis-thiazolium drug.
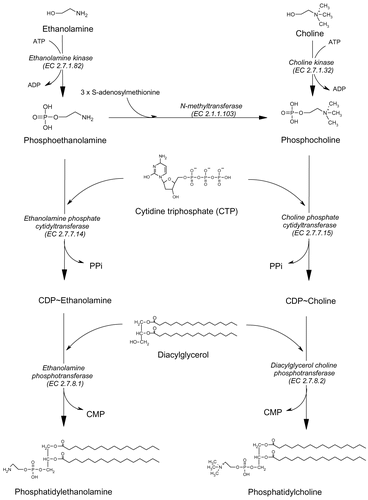
On the biological side, the erythrocyte is not expected to synthesize phospholipids, because of the lack of any machinery so that the growth of Plasmodium inside the erythrocyte requires an active production of phosphatidylcholine and phosphatidylethanolamine, the main compounds for the cytoplasmic membrane of the parasite. Depending on the type of cell, several pathways, from choline or ethanolamine, have been shown to lead to these phospholipids. One of them, well known as the Kennedy pathway, consists in the phosphorylation of choline into phosphocholine (PC), then the transfer of cytidylmonophosphate onto the PC, leading to CDP-Choline. And finally, the CDP-Choline is transferred onto a diacylglycerol (DAG) to get the PtdCho. Another pathway is the tri-methylation of the phosphoethanolamine (PE) into the PC and then conversion via the PtdCho via the CDP-Cho. The last one is the tri-methylation of phosphatidylethanolamine (PtdEtn) into PtdCho (). The involvement of each pathway depends on the cell type. Little is known on the functional metabolic pathways used by Plasmodium to synthesize PtdCho and PtdEtn.
In this work we aimed to find out the biochemical mechanism and the interference of T4 on PtdCho and PtdEtn metabolisms in Plasmodium.
Materials and methods
Chemical materials
The drug T4, in a bis-thiazolium bromide, was synthesized in-house and the stocks of samples were provided by the research unit UMR CNRS 5810 in France. Labeled precursors, [1-3H]ethan-1-ol-2-amine, [methyl-3H]choline and [3H]hypoxanthine were purchased from Amersham™ corp. The chemical materials were finally used diluted in a solution of RPMI 1640 obtained with Gibco™. When necessary, the organic solvent was previously dried in a nitrogen stream before dissolved in RPMI 1640.
In vitro-cultured P. falciparum
The 3D7 strain of P. falciparum was provided by Dr B. Pradines (IMTSSA in Marseille France). The infected-erythrocytes were incubated in a modified RPMI 1640, freed of choline and ethanolamine, obtained from Gibco™ and supplemented with 25 mM HEPES (pH 7.6). The parasitemia was 12 % with a 2.5 % hematocrit in a total amount of 6.106 infected cells. A 100 μL volume suspension of culture were distributed in microwell plate and incubated at 37°C in a 5 % CO2 atmosphere.
Assessing T4 activity on infected-erythrocyte
We assessed the activity of T4 on the uptake of labeled-radioactive precursors by the infected-erythrocyte. At time 0 min, a ranging concentration of 50 μL of T4 [4.6 x10−9 to 10−5 molar] was added in the suspension of cultured Plasmodium. At time 60 min, were added 50 μL solutions of radioactive [3H]-choline and radioactive [3H]-ethanolamine (in a final concentration of 2.5 μM) each with a specific activity of 2 Ci/mmol. In control test was added [3H]-hypoxanthine (1μCi per well). The final volume of the suspension in each microwell was 200 μL. Metabolism reactions were stopped at time 240 min in a 4°C atmosphere. All the suspensions were passed through a cell harvester and macromolecules were transferred onto glass fiber filters. Radioactive precursors incorporated in macromolecule were measured with a beta scintigraph counter from Beckman™.
The uptake of choline, ethanolamine and hypoxanthine in macromolecule is expressed as percent of control (assay without T4). Each well of the assay was performed in triplicate.
T4 effects on phospholipids biosynthesis
P. falciparum-infected erythrocytes in 1.25 x106 total infected cells were incubated in a final volume of 200 μL of modified RPMI 1640™ choline-free, supplemented with 25 mM HEPES (pH 7.6) and with choline (5 μM; 1.95 Ci/mmol), ethanolamine (2 μM; 2.29 Ci/mmol) and hypoxanthine (1 μCi/sample-test). Solution of T4 was added in ranging concentrations as described above. Suspension of cells was incubated for 4 h at 37 °C in a 5 % CO2 atmosphere. Reactions were stopped at 4 °C and cultures were washed with NaCl (0.9%). Phospholipids were extracted according to the Folch protocol [Citation10]. The organic phase and water phase were separately fractionated by thin-layer chromatography. Migrated spots were revealed with ninhydrine and iodine vapor. Fractionated spots were identified with known control samples and were scraped off. Then the radiolabeled silica were measured with a liquid scintillation counter from Beckman™. Results are expressed as percentage of control infected erythrocytes without T4. Results are means of duplicate tests.
Results
Because of the lack of its own machinery, the erythrocyte is not expected to synthesize macromolecule like phospholipids or DNA. The Plasmodium which infects the erythrocyte has to synthesize macromolecules for its own growth. We assessed the activity of T4 on phospholipids metabolism by measuring the uptakes of choline and ethanolamine and their respective incorporation in the macromolecules of Plasmodium.
T4 activity
We performed assays with asynchronous culture of Plasmodium, containing merozoites, trophozoites, young and later schizontes. The uptake of Etn in control test (15.9 nmol/1010 cells) is 6 times superior to the uptake of Cho (2.6 nmol/1010 cells).
Compared with control, T4 inhibits the uptake of choline in Plasmodium with a dose-response effect. The concentration of T4 which inhibits 50 % of choline uptake [IC50(Cho)] is about 5.7 x10−7 M. Whereas, the uptake of ethanolamine is about 100 % of control test and evolves as a plateau whatever is the concentration of T4. This means that T4 has no inhibitory effect on the uptake of ethanolamine. In the same way, the uptake of hypoxanthine is constant and about 100 % of the control test uptake whatever is the T4 concentration. The shape of the curves from the T4 concentration 1.23 x10−7 M, testifies to a selective action of the drug on the phospholipids metabolism ().
Figure 2. Effects of T4 on choline, ethanolamine and hypoxanthine uptakes in Plasmodium.
P. falciparum-infected erythrocytes, 3D7 strain, (12 % parasitemia, 2.5 % hematocrit in 6 x106 total infected cells) were incubated in a final volume of 200 μL of modified RPMI 1640 choline-free supplemented with 25 mM Hepes (pH 7.6) and the indicated concentration of the compounds T4 (at time 0). Suspensions were incubated at 37°C in a 5 % CO2 incubator. Incorporation of choline, ethanolamine and hypoxanthine were assessed by adding [3H]-Choline (2.5 μM, 2 Ci/mmol), [3H]-ethanolamine (2.5 μM, 2 Ci/mmol) and [3H]-Hypoxanthine (1μCi per sample test) at time 60 min for 3 h. Reactions were stopped at 4°C and radioactive incorporation was measured after filtration using cell harvester. Incorporations of the labeled precursors are expressed as percent of control (without drugs) and are means of triplicate values ± SEM. (Choline and ethanolamine incorporation in control-test were respectively: 2.6 and 15.9 nmol/1010 cells; The T4 inhibitory concentration of 50% choline uptake is 5.7 × 10−7 M).
![Figure 2. Effects of T4 on choline, ethanolamine and hypoxanthine uptakes in Plasmodium.P. falciparum-infected erythrocytes, 3D7 strain, (12 % parasitemia, 2.5 % hematocrit in 6 x106 total infected cells) were incubated in a final volume of 200 μL of modified RPMI 1640 choline-free supplemented with 25 mM Hepes (pH 7.6) and the indicated concentration of the compounds T4 (at time 0). Suspensions were incubated at 37°C in a 5 % CO2 incubator. Incorporation of choline, ethanolamine and hypoxanthine were assessed by adding [3H]-Choline (2.5 μM, 2 Ci/mmol), [3H]-ethanolamine (2.5 μM, 2 Ci/mmol) and [3H]-Hypoxanthine (1μCi per sample test) at time 60 min for 3 h. Reactions were stopped at 4°C and radioactive incorporation was measured after filtration using cell harvester. Incorporations of the labeled precursors are expressed as percent of control (without drugs) and are means of triplicate values ± SEM. (Choline and ethanolamine incorporation in control-test were respectively: 2.6 and 15.9 nmol/1010 cells; The T4 inhibitory concentration of 50% choline uptake is 5.7 × 10−7 M).](/cms/asset/d1f92dea-99fe-4d87-a327-5428247881dc/ienz_a_344965_f0002_b.gif)
Incorporation of precursors in phospholipids
These assays were performed in order to emphasize the nature of the phospholipids in which precursors are incorporated and also to find out the biochemical pathway which is targeted by T4, in the metabolism of phospholipids. In this metabolism only the Kennedy pathway is known to lead choline to phosphatidylcholine. But ethanolamine is expected to be synthesized into PtdEtn or PtdCho.
The incorporation of Cho into PtdCho is superior to the incorporation of Etn into PtdCho (4.7 vs 1.9 nmol/1010 cells.h−1). But the incorporation of Etn into PtdEtn is more superior (14.3 nmol/1010 cells.h−1) ().
Table 1. Precursors incorporation in phospholipids of infected-erythrocyte for control-test (without T4). (Experiment performed according the protocol reported in legend).
As seen above, T4 inhibits the uptake of choline and its incorporation in the phospholipids PtdCho and PtdEtn. The activity of T4 mainly concerns inhibition of metabolized Cho into PtdCho and metabolized Etn into PtdCho. Compared with the pathway of PtdCho from Etn, a lower concentration of T4 can inhibit the pathway of PtdCho from Cho (). The Kennedy pathway seems to be more sensitive to T4: the 50 % inhibitory concentration of PtdCho from Cho (IC50) is 7.8 × 10−7 M vs 7.3 × 10−5 M for PtdCho from Etn. T4 shows an inhibitory activity on the metabolism of PtdCho with a selective activity on the PtdCho synthesized from Cho.
Figure 3. Effects of T4 on phospholipids biosynthesis and hypoxanthine incorporation by Plasmodium.
P. falciparum-infected erythrocytes (3D7 strain) (10 % parasitemia, 2.5 % hematocrit in 5x106 total infected cells) were incubated in a final volume of 200 μL of modified RPMI 1640 choline-free supplemented with 25 mM Hepes (pH 7.6) and [3H]-choline (20 μM; 0.5 Ci/mmol) or [3H]-ethanolamine (2.2 μM; 2.2 Ci/mmol). Suspension was incubated for 4 h at 37°C in a 5 % CO2 incubator. Reactions were stopped at 4°C and wash with NaCl (0.9%). Phospholipids were extracted according Folch process (chloroform / methanol / water : 8/4/3) and fractionated by thin-layer chromatography. Identified fractionated spots were sp. and radiolabeled metabolites were measured by scintillation counting. Results are means of triplicate values ± SEM.
![Figure 3. Effects of T4 on phospholipids biosynthesis and hypoxanthine incorporation by Plasmodium.P. falciparum-infected erythrocytes (3D7 strain) (10 % parasitemia, 2.5 % hematocrit in 5x106 total infected cells) were incubated in a final volume of 200 μL of modified RPMI 1640 choline-free supplemented with 25 mM Hepes (pH 7.6) and [3H]-choline (20 μM; 0.5 Ci/mmol) or [3H]-ethanolamine (2.2 μM; 2.2 Ci/mmol). Suspension was incubated for 4 h at 37°C in a 5 % CO2 incubator. Reactions were stopped at 4°C and wash with NaCl (0.9%). Phospholipids were extracted according Folch process (chloroform / methanol / water : 8/4/3) and fractionated by thin-layer chromatography. Identified fractionated spots were sp. and radiolabeled metabolites were measured by scintillation counting. Results are means of triplicate values ± SEM.](/cms/asset/49f3da98-d1a1-49b2-bfba-4a6046fc0ace/ienz_a_344965_f0003_b.gif)
T4 has no inhibitory activity on the uptake of hypoxanthine and neither on its incorporation into nucleic acid. In the same way, the uptake of Etn is not influenced by T4. The amount of Etn incorporated in PtdEtn is significantly superior to 100 % of the control test without drug.
Incorporation of precursors in the metabolites of phospholipids synthesis
In order to find out the biochemical target of T4 in PtdCho and PtdEtn metabolism, we performed the following assays. We assessed the synthesis of all intermediates and final molecules in the metabolism of phospholipids i.e. the water-soluble molecules and organic-soluble ones from the Kennedy pathway (intracellular Cho, PC, CDP-Cho, and PtdCho) and those from the pathways starting with Etn and leading to PtdEtn or PtdCho (intracellular Etn, PE, PC, CDP-Etn, CDP-Cho). We expected to observe an increased synthesis of molecules on the upstream side of the eventual blocked pathway. On the contrary, we could expect a decrease on the backing side of the same blocked pathway. The amount of each synthesized molecules species was assessed after fractionation and quantification of the radioactivity.
T4 inhibited all the intermediate molecules of the metabolism of PtdCho from Cho. Intracellular Cho, PC, CDP-Cho and PtdCho decreased according the increase of T4 concentration (). On the contrary, T4 has no effect onto the incorporation of Etn in phospholipids intermediate molecules. PE, PC, PtdEtn and PtdCho are not influenced by T4 whatever its concentration ().
Figure 4. Effects of T4 on choline metabolism pathway.
P. falciparum-infected erythrocytes, 3D7 strain, 2.7 x107 total infected cells were incubated in a final volume of 200 μL of modified RPMI 1640 choline-free supplemented with 25 mM Hepes (pH 7.6) and [3H]-choline (32 μM; 2.95 Ci/mmol). T4 was added at the indicated concentrations. Suspension was incubated for 2 h at 37°C in a 5 % CO2 atmosphere. Reactions were stopped at 4°C. Phospholipids and water-soluble metabolites were separated according the Folch extracting protocol. A thinlayer-chromatography was performed to fractionate the phospholipids and the aqueous metabolites, respectively in the organic phase and the supernatant aqueous phase. Revealed spots with iodine vapour and ninhydrine were identified with standards. Each spots were sp. off and measured by scintillation counting. Results are means of 4 values ± SEM.
![Figure 4. Effects of T4 on choline metabolism pathway.P. falciparum-infected erythrocytes, 3D7 strain, 2.7 x107 total infected cells were incubated in a final volume of 200 μL of modified RPMI 1640 choline-free supplemented with 25 mM Hepes (pH 7.6) and [3H]-choline (32 μM; 2.95 Ci/mmol). T4 was added at the indicated concentrations. Suspension was incubated for 2 h at 37°C in a 5 % CO2 atmosphere. Reactions were stopped at 4°C. Phospholipids and water-soluble metabolites were separated according the Folch extracting protocol. A thinlayer-chromatography was performed to fractionate the phospholipids and the aqueous metabolites, respectively in the organic phase and the supernatant aqueous phase. Revealed spots with iodine vapour and ninhydrine were identified with standards. Each spots were sp. off and measured by scintillation counting. Results are means of 4 values ± SEM.](/cms/asset/1f3adf56-71af-48dc-b3ee-542a588cb84f/ienz_a_344965_f0004_b.gif)
Figure 5. Effect of T4 on the ethanolamine metabolism pathway.
P. falciparum-infected erythrocytes (3D7 strain), hematocrit 1.5% and 2.7 × 107 total infected cells obtained with the Vario Macs™ concentration protocol were incubated in a final volume of 200 μL RPMI 1640 supplemented with 25 mM Hepes (pH 7.6) and [3H]-ethanolamine (3.2 μM; 1.30 μCi). T4 was added at the indicated concentrations. Suspension was incubated for 2 h at 37°C in a 5 % CO2 atmosphere. Reactions were stopped at 4°C and water-soluble metabolites were obtained from the aqueous supernatant according the Folch protocol. A thin-layer-chromatography was performed to fractionate the aqueous metabolites. Spots revealed with iodine vapour and ninhydrine were identified by compared standards. They were sp. off and measured by scintillation counting. Results are means of duplicate values ± SEM.
![Figure 5. Effect of T4 on the ethanolamine metabolism pathway.P. falciparum-infected erythrocytes (3D7 strain), hematocrit 1.5% and 2.7 × 107 total infected cells obtained with the Vario Macs™ concentration protocol were incubated in a final volume of 200 μL RPMI 1640 supplemented with 25 mM Hepes (pH 7.6) and [3H]-ethanolamine (3.2 μM; 1.30 μCi). T4 was added at the indicated concentrations. Suspension was incubated for 2 h at 37°C in a 5 % CO2 atmosphere. Reactions were stopped at 4°C and water-soluble metabolites were obtained from the aqueous supernatant according the Folch protocol. A thin-layer-chromatography was performed to fractionate the aqueous metabolites. Spots revealed with iodine vapour and ninhydrine were identified by compared standards. They were sp. off and measured by scintillation counting. Results are means of duplicate values ± SEM.](/cms/asset/deb5a8f4-15a1-4295-8471-b3f0512c20b8/ienz_a_344965_f0005_b.gif)
Discussion
The modified medium, RPMI 1640 choline and ethanolamine-freed, was labeled by adding a known amount of radioactive [3H]-choline or [3H]-ethanolamine so as to assess the accurate amount of choline and ethanolamine uptake as suggested by Elabbadi [Citation11]. Incubation time, concentrations of choline and ethanolamine were chosen as to enable the biosynthesis of phospholipids. Therefore all the experimental conditions allowed an optimal growth of the parasites in a blank test [Citation12]. The uptake of parasite is the uptake of the infected-erythrocyte complex deduced from the one of an uninfected-erythrocyte.
T4 is a bis-thiazolium derived molecule. T16 and T3 have shown antiplasmodial and potent antimalarial activities [Citation9, Citation13] so we expected T4, a structural analogue of T16, to show a similar activity.
The other known metabolites of choline are acetylcholine and betaine. They are known to be synthesized by superior mammalian cells, but Plasmodium is not expected to synthesize these two small molecules [Citation14]. Whatever acetylcholine, betaine or other small derived molecules would have been synthesized, only the macromolecules like nucleic acid or phospholipids are expected to be retained on the fiber glass and therefore capable of measurement.
The known phospholipids in the cell structure of Plasmodium are mainly found in the cytoplasmic and the vacuole membranes. Therefore, the extracted lipid in the organic phase is expected to represent all the phospholipids from the cytoplasmic membrane of the parasite and the parasitophorous vacuole membrane. The water-soluble phase contains the non-lipid metabolites like intracellular Cho, PE, PC, CDP-Cho and CDP-Etn.
Since the experiments were performed on an asynchronous culture of Plasmodium, it means different levels of metabolism. Therefore, our results can be seen as the mean intensity of the metabolism in one parasite life cycle.
The metabolism of phospholipids is complex. It involves Cho, Etn and serine. Several pathways had been identified and their enzymes characterized [Citation15–18]. These pathways are different according to the cell species. The tri-methylation of PtdEtn into PtdCho is known to occur in mammalian cells and yeast [Citation19–21]. This pathway had been evoked in Plasmodium but had not been conclusively shown [Citation14, Citation22]. Recently, Witola localized the phosphoethanolamine N-methyl transferase in the Plasmodium cell and therefore showed the coupling of Etn metabolism with the Kennedy pathway [Citation23].
The IC50(Cho) of T4 is within the range of chloroquine or quinine IC50 [Citation24]. This is in favor of the potential biochemical target of T4 on the choline metabolism.
Hypoxanthine was used to assess the metabolism of nucleic acid. The biosynthesis of nucleic acid is assumed to be molecular evidence of the parasite livelihood. But nothing is known about the delay between the stop of nucleic acid biosynthesis and its outcome on cellular growth. When T4 inhibits the uptake of Cho with a dose-response effect, the parasites still incorporate hypoxanthine for the synthesis of DNA even though this DNA is not proved to be functional. At this average concentration of T4, Etn is still incorporated for the biosynthesis of phospholipids with a mean rate over 100 % (). This means a selective action of the drug. This selective inhibition on Cho metabolism does not exclude any other activity on another vital metabolism of the parasite, (ie: heme detoxication). Therefore, a potential specific action of T4 needs to be clarified.
At the trophozoite and schizonte stage of Plasmodium, the phospholipids of the cytoplasmic membrane are predominantly PtdCho. But the incorporation of precursors into PtdEtn appears to be superior to their incorporation into PtdCho. This could mean a more active turnover of PtdEtn or a possible tri-methylation of PtdEtn into PtdCho as suggested by Vial [Citation22].
T4 has a dose-response effect on the PtdCho coming from the Cho suggesting that the Kennedy pathway could be inhibited by the drug.
A significant amount of PC coming from labeled Etn means that the Etn pathway is coupled with the Kennedy pathway probably by the methylation of PE into PC as suggested by recent molecular biology studies [Citation25, Citation26]. This coupling pathway, starting with Etn and leading to PtdCho, was not inhibited by T4 when the experiment was performed with a normal culture, medium containing Cho (). On the contrary, T4 inhibited the biosynthesis of PtdCho coming from Etn when the experiment was performed in a modified medium freed from Cho (). It means different effects of T4 on PtdEtn biosynthesis. Choline seems to play a role in the regulation of the PtdCho biosynthesis coming from Etn as suggested by Witola et al [Citation27]. So the decrease of synthesized PtdCho from Etn observed in could be a combined effect of T4 and Cho deficiency. Besides this observation, the non inhibition of PtdCho coming from Etn means that the inhibitory activity of T4 does not come from a hypothetical blocking of CCT (EC 2.7.7.15) or CPT (EC 2.7.8.2). These two enzymes catalyze respectively the transformation of PC into CDP-Cho and the CDP-Cho into PtdCho.
To conclude, T4 shows a selective inhibition on the biosynthesis of PtdCho. There’s a selective action on the Cho and the Etn pathways. Nevertheless, none of the enzymes catalyzing the biosynthesis of the phospholipids inside the parasite seem to be inhibited by the drug. But our results are in favor of a coupled pathways of Cho and Etn with a mutual interference on the biosynthesis of phospholipids.
Funding
SCAC grant from the Cooperation Department of the French Ministry of Foreign Affairs.
Acknowledgement
The authors acknowledge S Wein, M Maynadier, F Boudou for their technical assistances. We thank S Herbute K, Wengelnick and H Vial at the Laboratory of “Dynamique des Interactions Membranaires Normales et Pathologiques” (CNRS UMR 5235) University of Montpellier II for their technical assistance and helpful advice concerning the protocol of experiments.
We acknowledge M. Calas at the Laboratory of “Amino-acides Peptides et Protéines” (CNRS UMR 5810) for providing stocks of T4.
Declaration of interest: The authors report no conflicts of interest.
References
- Tripathi RP, Mishra RC, Dwivedi N, Tewari N, Verma SS. Current status of malaria control. Curr Med Chem 2005; 12: 2643–2659.
- Gornicki P. Apicoplast fatty acid biosynthesis as a target for medical intervention in apicomplexan parasites. Int J Parasitol 2003; 33: 885–896.
- Ancelin ML, Calas M, Vidal-Sailhan V, Herbute S, Ringwald P, Vial HJ. Potent inhibitors of Plasmodium phospholipid metabolism with a broad spectrum of in vitro antimalarial activities. Antimicrob Agents Chemother 2003; 47: 2590–2597.
- Mitamura T, Palacpac NM. Lipid metabolism in Plasmodium falciparum-infected erythrocytes: possible new targets for malaria chemotherapy. Microbes Infect 2003; 5: 545–552.
- Roth EF, Jr., Calvin MC, Max-Audit I, Rosa J, Rosa R. The enzymes of the glycolytic pathway in erythrocytes infected with Plasmodium falciparum malaria parasites. Blood 1988; 72: 1922–1925.
- Ancelin ML, Vial HJ. Quaternary ammonium compounds efficiently inhibit Plasmodium falciparum growth in vitro by impairment of choline transport. Antimicrob Agents Chemother 1986; 29: 814–820.
- Wengelnik K, Vidal V, Ancelin ML, Cathiard AM, Morgat JL, Kocken CH, Calas M, Herrera S, Thomas AW, Vial HJ. A class of potent antimalarials and their specific accumulation in infected erythrocytes. Science 2002; 295: 1311–1314.
- Hamze A, Rubi E, Arnal P, Boisbrun M, Carcel C, Salom-Roig X, Maynadier M, Wein S, Vial H, Calas M. Mono- and bis-thiazolium salts have potent antimalarial activity. J Med Chem 2005; 48: 3639–3643.
- Richier E, Biagini GA, Wein S, Boudou F, Bray PG, Ward SA, Precigout E, Calas M, Dubremetz JF, Vial HJ. Potent antihematozoan activity of novel bisthiazolium drug T16: evidence for inhibition of phosphatidylcholine metabolism in erythrocytes infected with Babesia and Plasmodium spp. Antimicrob Agents Chemother 2006; 50: 3381–3388.
- Lebaron FN, Folch J. The effect of pH and salt concentration on aqueous extraction of brain proteins and lipoproteins. J Neurochem 1959; 4: 1–8.
- Elabbadi N, Ancelin ML, Vial HJ. Use of radioactive ethanolamine incorporation into phospholipids to assess in vitro antimalarial activity by the semiautomated microdilution technique. Antimicrob Agents Chemother 1992; 36: 50–55.
- Vial HJ, Ancelin ML, Thuet MJ, Philippot JR. Phospholipid metabolism in Plasmodium-infected erythrocytes: guidelines for further studies using radioactive precursor incorporation. Parasitology 1989; 98 Pt 3: 351–357.
- Nicolas O, Margout D, Taudon N, Wein S, Calas M, Vial HJ, Bressolle FM. Pharmacological properties of a new antimalarial bisthiazolium salt, T3, and a corresponding prodrug, TE3. Antimicrob Agents Chemother 2005; 49: 3631–3639.
- Ancelin ML, Vial HJ. Regulation of phosphatidylcholine biosynthesis in Plasmodium-infected erythrocytes. Biochim Biophys Acta 1989; 1001: 82–89.
- Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem Cell Biol 2004; 82: 113–128.
- Kent C. Eukaryotic phospholipid biosynthesis. Annu Rev Biochem 1995; 64: 315–343.
- Carman GM, Kersting MC. Phospholipid synthesis in yeast: regulation by phosphorylation. Biochem Cell Biol 2004; 82: 62–70.
- Vial HJ, Eldin P, Tielens AG, van Hellemond JJ. Phospholipids in parasitic protozoa. Mol Biochem Parasitol 2003; 126: 143–154.
- Blusztajn JK, Wurtman RJ. Choline biosynthesis by a preparation enriched in synaptosomes from rat brain. Nature 1981; 290: 417–418.
- Blusztajn JK, Zeisel SH, Wurtman RJ. Synthesis of lecithin (phosphatidyl choline) from phosphatidylethanolamine in bovine brain. Brain Res 1979; 179: 319–327.
- Kanipes MI, Henry SA. The phospholipid methyltransferases in yeast. Biochim Biophys Acta 1997; 1348: 134–141.
- Vial HJ, Thuet MJ, Broussal JL, Philippot JR. Phospholipid biosynthesis by Plasmodium knowlesi-infected erythrocytes: the incorporation of phospohlipid precursors and the identification of previously undetected metabolic pathways. J Parasitol 1982; 68: 379–391.
- Witola WH, Pessi G, El Bissati K, Reynolds JM, Mamoun CB. Localization of the phosphoethanolamine methyltransferase of the human malaria parasite Plasmodium falciparum to the Golgi apparatus. J Biol Chem 2006; 281: 21305–21311.
- Pradines B, Fusai T, Daries W, Laloge V, Rogier C, Millet P, Panconi E, Kombila M, Parzy D. Ferrocene-chloroquine analogues as antimalarial agents: in vitro activity of ferrochloroquine against 103 Gabonese isolates of Plasmodium falciparum. J Antimicrob Chemother 2001; 48: 179–184.
- Pessi G, Kociubinski G, Mamoun CB. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. PNAS 2004; 101: 6206–6211.
- Pessi G, Choi J-Y, Reynolds JM, Voelker DR, Mamoun CB. In Vivo Evidence for the Specificity of Plasmodium falciparum Phosphoethanolamine Methyltransferase and Its Coupling to the Kennedy Pathway. J. Biol. Chem. 2005; 280: 12461–12466.
- Witola WH, Ben Mamoun C. Choline induces transcriptional repression and proteasomal degradation of the malarial phosphoethanolamine methyltransferase. Eukaryot Cell 2007; 6: 1618–1624.