Abstract
This report shows that 30 nM PFK-1 and 30 nM AK were both affected by the presence of NH4+, Na+, and K+ salts but with opposite consequences. Low concentrations of PFK-1 lose about half of its activity as a result of dilution and become susceptible to further activity losses owing to the presence of monovalent salts. On the other hand low concentrations of AK lose about 75 percent of its activity but regains activity losses owing to the presence of monovalent salts. It was determined that regain of AK activity did not appear to be a reflection of a major effect on the Km value of either AMP or ATP. Dilution to 30 nM AK resulted in no increase Km values compared to Km values at 140 nM AK. Dilution caused major decreases in the maximum velocities, Vmax, when ATP or fructose 6-phosphate was the variable substrate. It was shown in earlier reports that these same low concentrations of PFK-1 and AK were susceptible inhibitions by ascorbate. These attributes are discussed as they may relate to the role of ascorbate facilitation glycogen synthesis in resting muscle and the role that the cytoskeleton infrastructure scaffold may play is also discussed.
| Abbreviations: | ||
| = | F 6-P denotes fructose 6-phosphate and F 1,6-BP denotes fructose 1,6 bisphosphate. In kinetic studies, Kmatp and Kmf6p are Km values when ATP (adenosine triphosphate) and F 6-P are the variable substrate, respectively; Vmatp and Vmf6p are the corresponding maximum velocity values | |
Introduction
That vitamin C (ascorbate) functions in glycogen synthesis by inhibiting glycolysis via the inhibition of LDH (lactate dehydrogenase), PFK-1 (phosphofructokinase-1) and AK (adenylate kinase) was presented earlier [Citation1]. Previous studies [Citation1–4] showed that ascorbate specifically inhibited rabbit muscle isozymes AK, LDH, and PFK-1. A working hypothesis was developed that in resting muscle ascorbate inhibits glycolysis to facilitate glycogen synthesis; in active muscle, these isozymes form complexes with specific muscle proteins that reverse and/or prevent ascorbate inhibition. It was also shown that rabbit muscle isozymes AK [Citation3] and PFK-1 [Citation4], but not LDH [Citation2], lose activity at concentrations below 100 nmolar and became increasingly sensitive to ascorbate inhibition. These losses of activity were interpreted [4] as dissociations to protomers or lower molecular weight oligomers with lower activities and increased sensitivity to ascorbate inhibition.
It was also shown [Citation3,Citation4] that G-actin prevented activity losses of at low AK and PFK-1 concentrations, but while G-actin only stabilizes PFK-1 activity losses, aldolase prevents and restores some of AK activity loss due to low concentration. These present studies investigate AK and PFK-1 at concentrations as low as 20 nmolar and find other characteristics differing distinctly from concentrations above 100 nmolar and differing from one another. For example, we found that the ammonium, potassium, and sodium salts inhibited low PFK-1 concentrations but stimulated low AK concentrations under similar conditions. The results of these studies are discussed relative to the working hypothesis and the binding of enzymes and other proteins to intracellular infrastructures.
Material and methods
Materials
Biologicals.
These studies used rabbit enzymes unless stated otherwise. Sigma-Aldrich Co was the source of enzymes used in assay systems. Rabbit muscle G-actin (A 2522) was found to have no aldolase, LDH or AK activity. Rabbit muscle aldolase (A 8811) was found to be free of AK and LDH activity under our conditions.
Phosphofructokinase-1.
Rabbit muscle PFK-1 and bovine muscle PFK-1 (BPFK-1) used in these experiments was prepared by our laboratory from frozen tissue according to the method of Kemp [Citation5]. All rabbit muscle PFK-1 samples used in these experiences were shown to have a single band in SDS PAGE, as given below, and were devoid of AK, LDH and aldolase activities. All BPFK-1 were shown to be devoid of AK, LDH and aldolase activities.
Criteria of purity.
The purity of proteins was determined using SDS (sodium dodecyl sulfate) polyacrylamide gel electrophoresis (PAGE, not shown). SDS PAGE was run with 12 percent cross-linked gels and the Bio-Rad Mini-Protean II Cell assembly and gels were silver stained for proteins using the procedure of Morrissey [Citation6].
Methods
All operations were at 25 °C unless otherwise stated. Buffers used in these studies were 0.1 mM potassium phosphate buffer, pH 8.0 for AK solutions and and 0.1 mM Tris-phosphate pH 8 for PFK-1 solutions unless stated otherwise.
Phosphofructokinase-1 assay.
We measured PFK-1 activity, F 6-P + ATP = F 1,6-BP, with a modification of the method by Anderson et al. [Citation7]. A 1 mL assay mixture contained 2 mM fructose 6-phosphate; 1 mM ATP (A 7699); 3.0 mM MgCl2; 0.13 mM NADH (N 1161); 1.7 eu/mL glyceraldehyde 3- phosphate dehydrogenase (G 0763); 18 eu/mL triose phosphate isomerase (G 1881); 1.3 eu/mL aldolase (A 8811); and 100 mM Tris-phosphate buffer, pH 8.0 as final concentrations.
Assay components above were concentrated 10 times the final assay concentrations into 0.1 mL. This allowed PFK-1 samples up to 0.9 mL and more accurate measurements of PFK-1 solutions at low concentrations for rates below 0.05 absorbancy unit/min in 100 μL of sample. A molar absorptivity value of 6,220 was used to convert NADH absorbance changes to μmoles of product formed. One PFK-1 enzyme unit (eu) of activity is defined as 1 μmole of NAD+ formed per minute.
Adenylate kinase (AK) assay.
We measured AK activity, AMP + MgATP = ADP + MgADP, according to Adam [Citation8]. A 1 mL assay mixture contained the final concentrations 0.3 mM phosphoenolpyruvate; 0.4 mM NADH; 8.0 mM ATP and 8 mM AMP; 8.1 mM MgCl2; and 20 mM potassium phosphate buffer, pH 8.
Assay components above were concentrated 10 times the final assay concentrations into 0.1 mL. This allowed AK samples up to 0.9 mL and more accurate measurements of AK solutions at low concentrations with rates below 0.05 absorbancy unit/min in 100 μL of sample. A molar absorptivity value of 6,220 was used to convert NADH absorbance changes to μmoles of product formed. One PFK-1 enzyme unit (eu) of activity is defined as 1 μmole of NAD+ formed per minute.
Aldolase assay.
Reagents for the measurement of aldolase activity was the same as for PFK-1 assay except that 2 mM fructose 6-phosphate and 1 mM ATP were omitted and replaced by 2 mM fructose 1,6-bisphosphate. One enzyme unit (eu) of aldolase activity is defined as 1 μmole of NAD+ formed per minute.
Dilutions of PFK-1 and AK to low concentrations.
Standard procedures for preparing all low concentrations of PFK-1 were as follows unless stated otherwise. A solution of 3 μM PFK-1 (3.0 eu/mL) in 0.1 M Tris-phosphate, pH 8 was diluted with the same buffer to final concentrations, usually 30 nM, 70 nM, 140 nM or 200 nM PFK-1, unless stated otherwise, and allowed to stand at 25 °C for at least 1 h (hour) to allow activity losses due to low PFK-1 concentrations to stabilize, remaining constant for more than 2 h [Citation3, Citation4] under these conditions. After 1 h standing, additions were made. Additions to PFK-1 diluted samples were usually accomplished by adding 1/20 volume of test sample at a 20-fold concentration of desired final concentrations. Test samples were then incubated at 25 °C for an additional 1 h and the PFK-1 activity then determined.
Dilutions of AK samples and incubations were the same as those described above for PFK-1, except that 0.02 M potassium phosphate, pH 8 was used to dilute 3 μM AK. Activities were measured both shortly after the dilution and at 1 h to assure that activity loss due to dilution had stabilized [Citation3].
shows average stabilized activity losses after 1 h incubation and the estimated activities based upon dilutions. The values have a standard deviation of less than ± 10 percent. Both AK and PFK-1 begin to show losses of activity due to dilution below 100 nmolar.
Table 1. Activities of 30 nM, 70 nM and 140 nM PFK-1 and AK.
Time course of inhibitors.
Activity losses due to dilution were separated from losses due to inhibitors by first performing dilutions and 1 h incubations to stabilize activities as described above; PFK-1 or AK were then mixed with inhibitors and activities measured at indicated times. Under our conditions, it was determined that no significant changes in inhibition levels occurred after 0.5 h of the additions and were stable beyond 2 hours.
Inhibitor titrations against a constant PFK-1 or AK concentrations.
After 1 h incubations as described above, fixed PFK-1 or AK concentrations with added varied monovalent salt concentrations were incubated at least an additional 1 h before activities were determined.
PFK-1 or AK titrations against a constant inhibitor.
After the usual 1 h incubations of AK and PFK-1 following dilutions as described above, fixed monovalent salt concentrations with varying PFK-1 or AK concentrations were incubated an additional 1 h before activities were determined.
Determinations of Km and Vm values.
Km and Vm values were determined from graphic extrapolations of Lineweaver-Burk plots [Citation9], Initial velocities were determined at a constant concentration of 2 mM ATP.Mg or 2 mM AMP while the other was varied. Other conditions were similar to those given in PFK-1 Assay and AK Assay described above.
Measurements of protein concentration.
PFK-1 protein concentrations during purification procedures were determined using the following formula: mg protein/mL = 1.55 A280 – 0.76 A260, where A280 and A260 are absorbencies at 280 nm and 260 nm, respectively [Citation10]. The spectrophotometric protein determinations were comparable to the Bradford method [Citation11].
Results
Effect of ammonium salt and LDH on PFK-1 activity
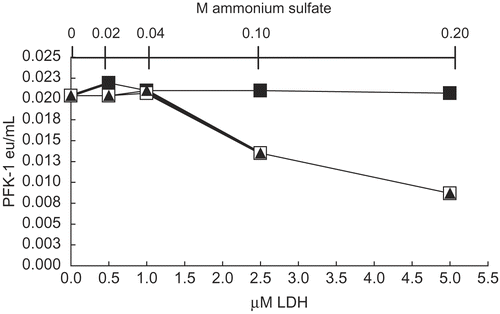
Previous studies [Citation2–4] showed that the proteins G-actin and aldolase protected AK and PFK-1 from losses of activity due to dilution and also protected AK, PFK-1, and LDH from inhibition by ascorbate. We considered the possibility that a protein might act as an inhibitor of these enzymes, especially when preliminary studies showed that the addition of commercial samples of LDH inhibited low concentrations of PFK-1. shows that addition of commercial LDH, suspended in 3.2 M ammonium sulfate, inhibited 30 nM PFK-1, resulting in a more than 50 percent inhibition of PFK-1 activity (□) at 5 μM LDH and 0.2 M ammonium sulfate after dilution with in 0.1 M Tris-phosphate, pH 8 buffer. The addition of ammonium sulfate alone, at the same concentrations as that in commercial LDH, produced identical degrees of inhibition (▴). Conversely, addition of LDH in the absence ammonium sulfate (▪) resulted in no losses of activity other than the usual losses due to dilution to 30 nM PFK-1 and 1 h incubation (see Methods). It can also be shown that 5 μM PFK-1 had no measurable effect on LDH activities. The unexpected inhibition of PFK-1 by ammonium sulfate suggested a study of the effect of the monovalent salts of Na+ and K+ on the rabbit muscle isozymes of interest, LDH, PFK-1, and AK. Though not shown, neither the Na+ nor K+ salts at the NH4+ concentrations shown in had any detectable effect on LDH activity.
Figure 1. Effects of LDH and ammonium sulfate on 30 nM PFK-1 activity. Solutions were prepared as given in Methods. After 1h dilution to 30 nM PFK-1, salt-free LDH (▪) was added at the final concentrations indicated and activities determined after 1h. Commercial LDH as a suspension in 3.2M ammonium sulfate (□) was added to 30 nM PFK-1 at the same final concentrations as salt-free LDH. Ammonium sulfate alone was added (▴) at the same final concentrations occurring in commercial LDH (shown in the secondary X axis).
Effect of 0.2 M ammonium ion at various AK concentrations
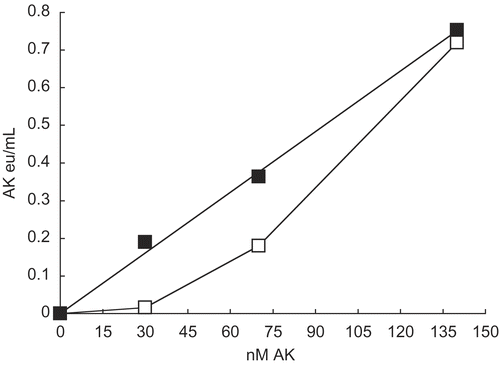
shows that the presence of ammonium sulfate restored the linear relationship between activity and low AK concentrations in a manner similar to the effect of G-actin on low concentrations of AK [Citation3]. Deviations from linearity for AK and PFK-1 are attributed to dissociations of dimeric AK and tetrameric PFK-1 to monomers [Citation3] and dimers [Citation4], respectively, that have lower activities than their oligomers.
Figure 2. Effect of 0.2M ammonium sulfate on activity at 30 nM, 70 nM, and 140 nM AK. As given in Methods, AK was diluted with 0.02 M potassium phosphate, pH 8 to concentrations shown and allowed to stand 1 h as given in Methods. No additions (□) and 0.2 M ammonium sulfate (▪) were then made, incubated for 1 h and AK activities were then determined.
Effect of NH4+, Na+, and K+sulfates on the activities of various concentrations of AK and PFK-1
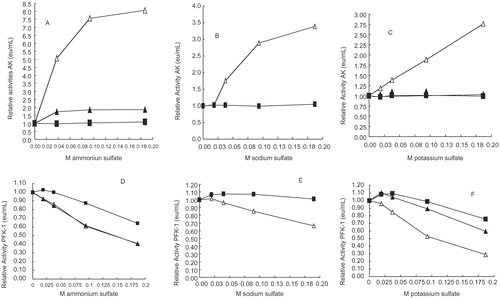
compares differences between AK and PFK-1 at various concentrations and the effects of NH4+, Na+, and K+ sulfate concentrations on their activities. shows that all three salts restore AK some activity losses resulting from dilutions. NH4+ has the greatest effect and K+ the least. By contrast, show that all of the sulfate salts result in PFK-1 activity losses additional to activity losses incurred by dilution; K+ have the greatest effect and Na+ the least. E shows that Na+ have little or no effect at 140 nM PFK. Though not shown, the effects of Na+ on 70 nM PFK-1 was similar to that shown for 140 nM PFK-1.
Figures 3 A-F. Relative activities of 30 nM (△), 70 nM (▴), and 140 nM (▪) PFK-1 and AK versus NH4+, Na+, and K+ion concentrations. The PFK-1 concentrations were derived from dilution of a 3 μM PFK-1stock solution and the preparation of samples is given in the Methods section. shows that NH4+, Na+, and K+ salts in decreasing order of effectiveness, resulted in recovery of some AK activity losses due to its low concentration. PFK-1 was treated in the same manner as AK and show that the presence of these salts results in losses of PFK-1 activities; K+, NH4+, and Na+ salts inhibited PFK-1 in decreasing order of effectiveness.
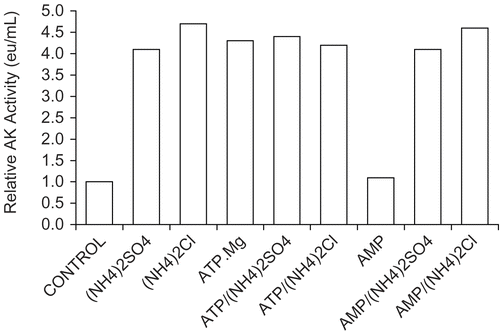
Consideration was made that effects show in A-F might be due to the sulf ateanion. shows that chloride of the monovalent ions were as equally effective in stimulating the activity of 30 nM AK, as were the sulfates; also shows that the substrate ATP.Mg was also equally effective in restoring activity while the other substrate AMP had no effect.
Figure 4. Comparison of effects on 30 nM AK activity by sulfates and chlorides of NH4+, K+, and Na+ and the effect of substrates. A 30 nM AK solution was prepared as described in Methods (see ) and solutions were made 0.2 molar with respect to each monovalent salts and 1 mmolar with respect to AMP or ATP.Mg when present. A 1 h incubation period followed and activities were then determined. The 30 nM AK control had an activity of 0.021 ± 0.002 eu/mL from 4 determinations.
Effect of aldolase on the inhibition of PFK-1 by ammonium ion
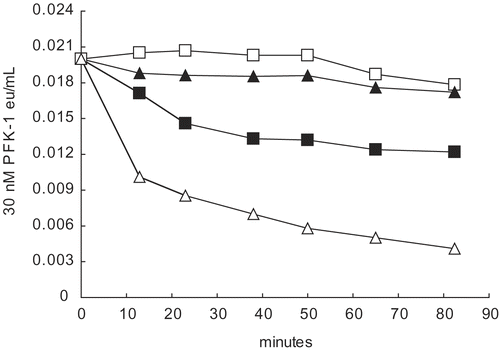
Previous studies [Citation12–14] showed that commercial aldolase containing ammonium sulfate prevented PFK-1 losses of activity due to dilution but did not restore activity. Our observations that ammonium sulfate restored some activity losses of dilute 30 nM AK (see ) prompted us to consider that restoration of dilute PFK-1 activity losses by aldolase might have been masked [Citation14] by the inhibiting presence of ammonium sulfate in the commercial aldolase (see ). shows that aldolase alone (□) stabilized 30 nM PFK-1 from losses of activity due the dilution but did not restore activity. Aldolase also prevented activity losses due to the presence of ammonium ion (▴) with stabilization of activity at a low PFK-1 concentration. Similar aldolase protection of PFK-1 from inhibitions by Na+ and K+ can be shown. The results of these experiments show that ammonium sulfate does not mask any capability of aldolase to restore activity due to PFK-1 dilution [Citation14].
Bovine muscle phosphofructokinase-1 (BPFK-1)
Dilution of BPFK-1 to 30 nM PFK-1 resulted in activity losses similar to rabbit muscle PFK-1, resulted in sensitivity to inhibition by ascorbate, and resulted in sensitivity to inhibition by NH4+, Na+, and K+ salts. Similar to rabbit muscle PFK-1, these losses of BPFK-1 activity were prevented by 5 μM rabbit muscle aldolase [Citation4]. Consistent with evidence for evolutionary conservation of muscle PFK-1 [Citation15] and muscle aldolase [Citation16], there were no discernible differences between BPFK-1 and rabbit muscle PFK-1 in these studies.
Determination the Km and Vm values of AK substrates in the presence of monovalent salts
In an effort to understand the ability of monovalent salts to enhance 30 nM AK activity (), an examination of the effect of monovalent salts on Km and Vm values of AK substrates was undertaken at 30 nM and 140 nM AK and shown in
. At 30 nM AK, 0.2 molar salts resulted in about 50 percent increase in Kmatp and an 80 percent increase in Km amp compared to their Controls. At 140 nM AK, the presence of 0.2 molar monovalent salts showed no Km values changes for the substrates compared to their Controls.
Table 2. The effect of 0.2 M sulfates of ammonium, potassium, and sodium on Km and Vm values of 30 nM and 140 mM AK.
The monovalent salts had major effects on the Vm values. At 30 nM AK, the Vm values in show that the presence of monovalent salts resulted in a 2.8 fold increase in the Vm atp and a 5.7 fold increase in the Vm amp, resulting in a recovery of some of the activity losses due to dilution (). At 140 nM AK, the presence of monovalent salts resulted in Vmatp and Vmamp values that were lower by 0.7 and 0.6 of the Control values, respectively. These differences between 30 nM AK and 140 nM AK in all probability reflect differences in the physical and kinetic properties of the AK monomers and dimers.
Discussion
When diluted to 30 nmolar, rabbit muscle AK and rabbit muscle PFK-1 show the following similar properties: losses of activity due to dilution (), attributed to AK dissociations from dimers to monomers [Citation3,Citation4] and PFK-1 dissociations from tetramers to dimers [Citation4,Citation12–14]; ascorbate inhibition imposes additional losses and rabbit muscle aldolase (,) or rabbit muscle G-actin protects both AK and PFK-1 from these activity losses [Citation3,Citation4]. Protections by aldolase and G-actin prompted a search for a protein inhibitor(s). The search uncovered a stimulation of 30 nM AK () activity and an inhibition of 30 nM PFK-1 () by NH4+ salts. Further investigation showed that Na+ and K+ salts also stimulated 30 nM AK () and under similar conditions inhibited 30 nM PFK-1 (), contrary to early reports under different conditions that K+ and NH4+ salts were activators of PFK-1 [Citation17, Citation18]. As mentioned earlier in the Results section but not shown, rabbit muscle LDH was not affected by the presence of monovalent salts under conditions given here.
Figure 5. Effect of 0.2 M ammonium sulfate on 30 nM PFK-1 in the presence and absence of 5 μM aldolase. Dilutions to 30 nM PFK-1 (▪) were made with 0.1 M Tris-phosphate buffer, pH 8.0. The following were in 30 nM PFK-1 as final concentrations: 5 μM aldolase (□); 5 μM aldolase and 0.2 M ammonium sulfate (▴); and ammonium sulfate (△). The initial reading was at 13 minutes The 0-time value was based upon the estimated eu/mL values based on dilution shown in , Methods. Differences between aldolase alone (□) and aldolase and 0.2 M ammonium sulfate (▴) could not be shown to be significantly difference (p<0.01).
We tend to view inhibition of PFK-1 by ascorbate and by K+ salt () and the stimulation of AK activity by K+ salt (), as support for facilitation of glycogen synthesis owing to inhibition of glycolysis in resting muscle. We are currently investigating whether the enhancement of AK activity by K+ salt is due to a conformational change [Citation19] or a shift towards dimer formation [Citation4]. Some support for this view occurs in studies of the nucleotide levels in active and resting frog muscle [Citation20]. Due initially to creatine kinase and then to AK activity, concentrations of ATP in active muscle vary little from resting and active muscle while AMP concentrations vary hundreds of times above resting levels. It was shown in active frog muscle [Citation20], for example, a decrease in ATP concentration of less than 5 percent is accompanied by a 200 percent increase in AMP concentration owing in large part to AK activity [Citation20]. In active muscle AK converts ADP, the product of contraction, to ATP and to the positive allosteric effector of PFK-1, AMP. PFK-1, the reputed controlling enzyme of glycolysis, is not only of limited concentration but it is also sensitive to allosteric control by a number of metabolic factors [Citation21]. When frog muscle rests, AMP, ADP and ATP return to resting concentrations, again due in large part to AK activity [Citation20].
Among other factors effecting enzyme activity is evidence for the structural organization of glycolytic enzymes at several levels and spatial segregations [Citation15,Citation16,Citation27, Citation30, Citation31]. There is evidence, for example, that muscle PFK-1 and other glycolytic enzymes engage and disengage with the large, negatively charged filamentous scaffold of the cell [Citation22–Citation30, Citation35]. With respect to PFK-1, components of cytoskeleton infrastructure can have opposite effects on activity – actin prevents inhibition by ascorbate [Citation4] and monovalent salts (not shown) while microtubules inhibit PFK-1 [Citation26]. In concert with the energy status of a cell, these infrastructures and their effects on PFK-1 activity invite speculation that glycolytic enzyme transfers from one infrastructure to another is accompanied by dramatic alterations of enzyme activities. With regard to these studies, we posit that the demonstrated PFK-1 inhibitions by ascorbate and by K+, coupled with K+ activation of AK and protection from inhibition by aldolase (), are supplementary to PFK-1 infrastructure transfers during muscle transitions from active to rest conditions and the reverse.
In conclusion, this report showed that PFK-1 and AK were affected by the dominant intracellular K+ in a manner that could serve to facilitate glycogen synthesis. By supplementing inhibition of PFK-1 by ascorbate, K+ would assist in inhibiting glycolysis in resting muscle. At the same time, the K+ effect of activating AK maintains nucleotides levels in resting muscle and increases AMP concentration, a major positive allosteric effector of PFK-1, when muscle is active. The effect of K+ increasing the activity of 30 nM AK () does not appear to be an effect on the Km values of its substrates. On the other hand, the decrease in activity of 30 nM AK due to dilution () is accompanied by a 3-fold decrease of the Km value at 140 nM AK in the presence of K+. These finding are consistent with the hypothesis that ascorbate facilitates glycogen synthesis [Citation1–4] when the muscle is at rest by inhibiting glycolysis.
Two questions about concentrations may be raised related to this work. One is whether concentrations of potassium salts used in these studies bear a relation to intracellular concentrations and therefore to a meaningful consequence. The usual citations for muscle intracellular potassium concentrations are about 150 mmolar [Citation32,Citation33, Citation34]; a concentration that is near the upper limit of our studies (see ). The other relevant more complex question relates to intracellular PFK-1 concentrations. Several studies indicate that PFK-1 and other glycolytic enzymes associate with cytoskeleton infrastructures [Citation22–35], primarily actin and microtubules, with the former activating PFK-1 [Citation12, Citation25] and the latter inhibiting [Citation12, Citation13, Citation26, Citation29]. Associations with the infrastructures necessarily decrease and limit concentrations of soluble intracellular PFK-1. Other studies [Citation1,Citation3, Citation4] and the present ones suggest that any soluble PFK-1 at low concentrations would be greatly inhibited by ascorbate and potassium salts and serve additionally to routes that limit PFK-1 activity under resting muscle cell conditions.
Acknowledgements
The project described was partially supported by Grant Number P60 MD00220, from the San Diego EXPORT National Center of Minority Health and Health Disparities, National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The authors also appreciate the following for support of this work: HCOE Grant 5 D34 MB02060; CURE Grant P30 CA23100-23S2; HCOP Grant D 18 HP03977; and Bridges to the Future Grant 1 R25 GM73590.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Russell PJ, Williams A, Todd A, Inhibition of rabbit muscle isozymes by vitamin C. J Enz Inhib 2000; 15:283–296.
- Russell PJ, Williams A, Amador X, Vargas R. Aldolase and actin protect rabbit muscle lactate dehydrogenase from ascorbate inhibition. J Enz Inhib Med. Chem 2004: 19:91–98.
- Russell PJ, Williams A, Abbott A, DeRosales B, Vargas R, Characteristics of rabbit muscle adenylate kinase inhibition by ascorbate. J Enz Inhib Med Chem 2006; 21:61–67.
- Russell PJ, Williams A, Marquez K, Tahir Z, Lam K, Hosseinian B. Some Characteristics of rabbit muscle phosphofructokinase-1 inhibition by ascorbate. J. Enz Inhib Med Chem 2008; 23:411–417.
- Kemp RG, Woods W, Eds. Phosphofructokinase from rabbit skeletal muscle. Methods in Enzymology 1975; 42C:71–77.
- Morrissey JH. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem Analyt Biochem 1981; 117:307–310.
- Anderson RL, Hanson TE, Sapico VL. D-Fructose-1-phosphate kinase: A. aerogenes. J Biol Chem 1969; 244:6280–6288.
- Adam H. Methods of Enzymatic Analysis, New York, Academic Press; 1963: 145–152.
- Lineweaver H, Burk D. Determination of enzyme dissociation constants. J Amer Chem Soc 1934; 56:658–666.
- Wilson K, Walker J. Principles and Techniques of Practical Biochemistry, 5th ed. Cambridge: Cambridge University Press 2001; p 230.
- Bradford MM. Determination of protein concentration in the manufacture and characterization of biopharmaceuticals. Analyt Biochem 1976: 72:248–254.
- Vértessy BG, Orosz F, Kovács J, and Ovádi J. Alternative binding of two sequential glycolytic enzyme to microtubules. J Biol Chem 1997: 272:25542–25546.
- Orosz F, Christova TY, Ovadi J. Aldolase decreases the dissociation-induced inactivation of muscle phosphofructokinase. Biochem Biophys Res Commun 1987; 147:1121–1128.
- Räis B, Ortega F, Puigjaner J, Comin B, Orosz F, Ovádi J, Cascante M. Quantitative characterization of homo- and heteroassociations of muscle phosphofructokinase with aldolase. Biochim Biophys Acta 2000; 1479:303–314.
- Masters CJ. Interaction between soluble and insoluble enzymes and subcellular structure. CRC Crit. Rev. Biochem 1981; 11:105–43.
- Janmey P. Cell membranes and the cytoskeleton, in Structure and Dynamics of Membranes. Amsterdam: Elsevier 1995: p 805.
- Bloxham DP, Lardy HA. Phosphofructokinase, Enzymes 1973; 8:239–278.
- Passonneau JV, Lowry, OH. The role of phosphofructokinase in metabolic regulation. Adv Enzyme Regul 1964; 2: 265–274.
- Russell PJ, Chinn E, Williams A, David-DiMarino C, Taulane JP, Lopez R. Evidence for conformers of rabbit muscle adenylate kinase. J Biol Chem 1990; 265:11804–11809.
- Krause U, Wegener G. Exercise and recovery in frog muscle: metabolism of PCr, nucleotides, and related compounds. Am J Regulatory Integrative Comp Physiol 1996; 270:811–820.
- Li Y, Rivera D, Ru W, Gunasekera D, Kemp RG. Identification of allosteric sites in rabbit phosphofructo-1-kinase. Biochemistry 1999; 38; 16407–16412.
- Arnold DH, Pette D. Binding of aldolase and triosphosphate dehydrogenase to F-actin and modification of catalytic properties of aldolase. Eur J Biochem 1970; 15:360–366.
- Clarke FM, Shaw RD, Morton DJ. Effect of electrical stimulation post mortem bovine muscle on the binding of glycolytic enzymes. Biochem J 1980; 186:105–109.
- Masters C. Interactions between glycolytic enzymes and components of the cytomatrix. J Cell Biol 1984; 99:222s–225s.
- Roberts SJ, Somero GN. Binding of phosphofructokinase to filamentous actin, Biochemistry 1987; 26:3437–3442.
- Lehotzky A, Telegdi M, Liliom K, Ovadi J. Interaction of phosphofructokinase with tubulin and microtubules. Quantitative evaluation of the mutual effects. J Biol Chem 1993; 268:10888–10894.
- MacRae TH. Microtubule organization by cross-linking bundling proteins. Biochim Biophys Acta 1992; 1160:145–155.
- Tang JX, Janmey PA. The polyelectrolyte nature of f-actin and the mechanism of actin bundle formation with which can form complexes. J Biol Chem 1996; 271:8556–8563.
- Vértessy BG, Orosz F, Kovács J, Ovádi J. Alternative binding of two sequential glycolytic enzymes to microtubules. J Biol Chem 1997; 272:25542–25546.
- Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Revs 1998; 78:763–781.
- Tang JX, Janmey P. The polyelectric nature of F-actin and the mechanism of actin bundle formation. J Biol Chem 1996; 271:8556–8563.
- Sejersted OM, Sjogaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev 2000; 80:1412–1481.
- McDonough AA, Thompson C B, Youn J H. Skeletal muscle regulates extracellular potassium. Am J Physiol Renal Physiol 2001; 282:967–974.
- Resnick LM, Barbagallo M, Dominguez LJ, Veniero JM, Nicholson JP, Gupta RK. Relation of Cellular Potassium to Other Mineral Ions in Hypertension and Diabetes. Hypertension 2001; 38:709–712.
- Schindler R, Weiichselsdorfer E, Wagner O, Beriter-Hahn J. Aldolase-localization in cultured cells: Cell-type and substrate-specific regulation of cytoskeletal associations. Biochem Cell Biol, 2001; 79:719–728.