Abstract
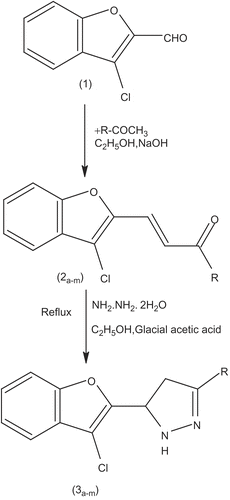
3-Chlorobenzofuran-2-carbaldehyde was condensed with substituted acetophenone by using the Claisen-Schmidt condensation to obtain 3-(3-chlorobenzofuran-2-yl)-1-(substituted phenyl)-2-propen-1-one (2a-m) which upon further treatment with hydrazine hydrate gave 2-[3-(substituted phenyl)-4,5-dihydro-1H-5- pyrazolyl)benzofuran-3-yl chloride derivatives (3a-m). All the newly synthesized derivatives were evaluated in vitro for cytotoxicity and antiviral activity in Crandell-Rees Feline Kidney cell, human embryonic lung (HEL) cell, HeLa cell and Vero cell cultures against different viruses. Several compounds, i.e. 2f, 2g, 2i, 2m, 3b, 3d, 3g, 3h and 3m proved quite cytotoxic to the host cells (minimum cytotoxic concentration: 1-10 μg/mL). No specific antiviral activity [50% antivirally effective concentration (EC50) ≥ 5-fold lower than the minimum cytototoxic concentration] was observed for any of the compounds.
Introduction
In view of the high occurrence of viral infections, increasing number of relapses and narrow antiviral spectrum of existing drugs, antiviral drug development programs have been intensified. Similarity in the metabolism of virus-infected as compared to uninfected cells makes it difficult to destroy a virus without affecting the host cell; and, hence cytotoxicity is commonly associated with antiviral agents. Attempts are being made to develop more efficient drugs that allow greater inhibition of viruses, greater selectivity for virus-specific functions, and fewer side effects, and may avoid emergence of drug-resistant mutants.
In continuation of our work on pyrazolines (Citation1–4) these compounds have been accredited with a variety of activities viz. antidepressant (Citation5), antimycobacterial (Citation6), anti-inflammatory (Citation7), antibacterial and anti-fungal (Citation8), anticonvulsant (Citation9), MAO inhibiting (Citation10) and anti-androgenic (Citation11). Here we report the synthesis and biological properties of some 3-chloro benzofuran-2-carbaxaldehyde derived chalcones and their pyrazolines.
3-(3-chlorobenzofuran-2-yl)-1-(substituted phenyl)-2-propen-1-one (2a-m), obtained by Claisen Schmidt reaction of 3-chlorobenzofuran-2-carbaldehyde and appropriately substituted acetophenone in ethanolic solution of NaOH, were subjected to cyclo-condensation with hydrazine hydrate to obtain the title compounds (3a-m).
Materials
All the chemicals used were laboratory grade and provided by E. Merck (Germany) and S.D. Fine Chemicals (India). Melting points were determined by open tube capillary method and are uncorrected. Thin layer chromatography (TLC) plates, (silica gel G) were used to confirm the purity of the commercial reagents used, the compounds synthesized and to monitor the reactions as well. Two different solvent systems; toluene: ethyl acetate: formic acid (5:4:1) and petroleum ether: toluene: acetic acid (5:4:1), were used to run the TLC. The spots were visualized under iodine vapors/UV light. IR spectra were obtained on a Perkin-Elmer 1720 FT-IR spectrometer (KBr Pellets). 1H NMR spectra were recorded on Bruker AC 400 MHz, spectrometer using TMS as internal standard in CDCl3 / DMSO-d6. The FAB mass spectra were recorded on a JEOL SX 102/DA-6000 Mass Spectrometer.
Methods
Chemistry
General procedure for synthesis of chalcones (2a-m)
To 0.01mole ethanolic solution (25mL) of 3-chlorobenzofuran-2-carbaldehyde, an appropriately substituted acetophenone (0.01mole) & 5mL 10% aqueous solution of NaOH was added. The reaction mixture was stirred at 25°C for 2-3 h. After completion of the reaction, the contents were poured onto the crushed ice, and neutralized with dilute HCl. The precipitates so obtained were filtered, washed with water, dried and re-crystallized from ethanol to get the desired product (2a-m).
3-(3-chlorobenzofuran-2-yl)-1-phenyl-2-propen-1-one (2a)
IR (KBr, cm−1): 1685 (C=O), 1606 (C=C), 755 (C-Cl). 1H-NMR (DMSO-d6, δ, ppm): 6.80 (1H, d, Hα, J = 8.4 Hz), 7.26-7.66 (9H, m, Ar-H), 7.68 (1H, d, Hβ, J = 8.8 Hz). Anal. Calcd. for C17H11ClO2: C, 72.22; H, 3.92. Found: C, 72.06; H, 3.93%.
3-(3-chlorobenzofuran-2-yl)-1-(4-chlorophenyl)-2-propen-1-one (2b)
IR (KBr, cm−1):1682 (C=O), 1605 (C=C), 755 (C-Cl). 1H-NMR (DMSO-d6, , ppm): 6.86 (1H, d, Hα, J = 8.0 Hz), 6.90 (2H, d, Ar-H, J = 8 Hz), 7.26 (4H, m, Ar-H), 7.66 (2H, d, Ar-H, J = 8.8 Hz), 7.96 (1H, d, Hβ, J = 8.4 Hz). Anal. Calcd. for C17H10Cl2O2: C, 64.38; H, 3.18. Found: C, 64.16; H, 3.19%.
3-(3-chlorobenzofuran-2-yl)-1-(4-methylphenyl)-2-propen-1-one (2c)
IR (KBr, cm−1): 1690 (C=O), 1601 (C=C), 755 (C-Cl). 1H-NMR (DMSO-d6, δ, ppm): 2.46 (3H, s, -CH3), 6.72 (1H, d, Hα, J = 8.0 Hz), 7.25-7.39 (5H, m, Ar-H), 7.48 (1H, d, Ar-H, J = 6.4 Hz), 7.66 (2H, d, Ar-H, J = 8.4 Hz), 7.86 (1H, d, Hβ, J = 8.0 Hz). Anal. Calcd. for C18H13ClO2: C, 72.85; H, 4.42. Found: C, 72.96; H, 4.41%.
3-(3-chlorobenzofuran-2-yl)-1-(4-nitrophenyl)-2-propen-1-one (2d)
IR (KBr, cm−1): 1680 (C=O), 1608 (C=C), 1523 & 1377 (NO2), 747 (C-Cl). 1H-NMR (DMSO-d6, δ, ppm): 6.79 (1H, d, Hα, J = 8.8 Hz), 6.95(2H, d, Ar-H, J = 8.4 Hz) 7.32-7.56 (4H, m, Ar-H), 7.60 (2H, d, Ar-H, J = 8.4 Hz), 7.65 (1H, d, Hβ, J = 8.8 Hz). Anal. Calcd. for C17H10ClNO4: C, 62.30; H, 3.08; N, 4.27. Found: C, 62.22; H, 3.08; N, 4.28%.
3-(3-chlorobenzofuran-2-yl)- 1-(4-aminophenyl)-2-propen-1-one (2e)
IR (KBr, cm−1): 1685 (C=O), 1606 (C=C), 755 (C-Cl). 1H-NMR (DMSO-d6, δ, ppm): 3.23 (2H, s, NH2), 6.86 (1H, d, Hα, J = 8.4 Hz), 7.26-7.48 (4H, m, Ar-H), 7.49 (1H, d, Ar-H, J = 6.4 Hz ), 7.51 (1H, d, Ar-H, J = 6.4 Hz), 7.55 (2H, d, Ar-H, J = 8.4 Hz), 7.85 (1H, d, Hβ, J = 8.8 Hz). Anal. Calcd. for C17H12ClNO2: C, 68.58; H, 4.06; N, 4.70. Found: C, 68.46; H, 4.08; N, 4.72%.
3-(3-chlorobenzofuran-2-yl)-1-(4-hydroxyphenyl)-2- propen-1-one (2f)
IR (KBr, cm−1): 3320 (OH), 1685 (C=O), 1606 (C=C), 755 (C-Cl). 1H-NMR (DMSO-d6, δ, ppm): 6.81 (1H, d, Hα, J = 8.4 Hz), 7.22-7.64 (8H, m, Ar-H), 7.87 (1H, d, Hβ, J = 8.8 Hz), 9.90 (1H, s, OH). Mass m/z: 299 (M+1). Anal. Calcd. for C17H11ClO3: C, 68.35; H, 3.71. Found: C, 68.38; H, 3.72%.
3-(3-chlorobenzofuran-2-yl)-1-(2-hydroxyphenyl)-2-propen-1-one (2g)
IR (KBr, cm−1): 3528 (OH), 1682 (C=O), 1614 (C=C), 743 (C-Cl). 1H-NMR (DMSO-d6, δ, ppm): 6.94 (1H, d, Hα, J = 8.0 Hz), 7.23-7.81 (8H, m, Ar-H), 7.92 (1H, d, Hβ, J = 8.0 Hz), 10.11 (1H, s, OH). Anal. Calcd. for C17H11ClO3: C, 68.35; H, 3.71. Found: C, 68.28; H, 3.70%.
3-(3-chlorobenzofuran-2-yl)-1-(4-methoxyphenyl)-2-propen-1-one (2h)
IR (KBr, cm−1): 1685 (C=O), 1645 (C=C), 750 (C–Cl).; 1H-NMR (DMSO-d6, δ, ppm): 3.52 (3H, s, OCH3), 6.82 (1H, d, Hα, J = 8.4 Hz), 6.91 (2H, d, Ar-H, J = 8 Hz), 6.99-7.67 (5H, m, Ar-H), 7.69 (1H, d, Ar-H, J = 6.8 Hz), 7.78 (1H, d, Hβ, J = 8.8 Hz). Anal. Calcd. for C18H13ClO3: C, 69.13; H, 4.19. Found: C, 69.26; H, 4.20%.
3-(3-chlorobenzofuran-2-yl)-1-(2,4-dihydroxyphenyl)-2-propen-1-one (2i)
IR (KBr, cm−1): 3530 (OH), 1682 (C=O), 1606 (C=C), 755 (C-Cl). 1H-NMR (DMSO-d6, δ, ppm): 6.83 (1H, d, Hα, J = 8.8 Hz), 7.26-7.67 (7H, m, Ar-H), 7.72 (1H, d, Hβ, J = 8.8 Hz) 10.23 (2H, s, 2 × OH). Anal. Calcd. for C17H11ClO4: C, 64.88; H, 3.52. Found: C, 64.90; H, 3.53%.
3-(3-chlorobenzofuran-2-yl)-1-(2,4-dimethoxyphenyl)-2-propen-1-one (2j)
IR (KBr, cm−1): 1685 (C=O), 1653 (C=C), 735 (C-Cl). 1H-NMR (DMSO-d6, δ, ppm): 3.66 (6H, s, 2 × OCH3), 6.92 (1H, d, Hα, J = 8.0 Hz), 7.24-7.84 (7H, m, Ar-H), 7.89 (1H, d, Hβ, J = 8.4 Hz). Anal. Calcd. for C19H15ClO4: C, 66.58; H, 4.41. Found: C, 66.66; H, 4.40%.
3-(3-chlorobenzofuran-2-yl)-1-(3,4-dimethoxyphenyl)-2-propen-1-one(2k)
IR (KBr, cm−1): 1685 (C=O), 1602 (C=C), 765 (C-Cl). 1H-NMR (DMSO-d6, δ, ppm): 3.68 (6H, s, 2 × OCH3), 6.82 (1H, d, Hα, J = 8.4 Hz), 6.89-7.68 (7H, m, Ar-H), 7.29 (1H, d, Hβ, J = 8.0 Hz). Anal. Calcd. for C19H15ClO4: C, 66.58; H, 4.41. Found: C, 66.48; H, 4.42%.
3-(3-chlorobenzofuran-2-yl)-1-(4-hydroxy-3-methylphenyl)-2-propen-1-one (2l)
IR (KBr, cm−1): 3530 (OH), 1685 (C=O), 1606 (C=C), 756 (C-Cl). 1H-NMR (DMSO-d6, δ, ppm): 2.26 (3H, s, CH3), 6.76 (1H, d, Hα, J = 8.8 Hz), 6.95-7.62 (7H, m, Ar-H), 7.68 (1H, d, Hβ, J = 8.8 Hz) 8.83 (1H, s, OH). Anal. Calcd. for C18H13ClO3: C, 69.13; H, 4.19. Found: C, 69.26; H, 4.22%.
3-(3-chlorobenzofuran-2-yl)-1-(4-hydroxy-2-methylphenyl)-2-propen-1-one (2m)
IR (KBr, cm−1): 3530 (OH), 1686 (C=O), 1606 (C=C), 754 (C-Cl).; 1H-NMR (DMSO-d6, δ, ppm): 2.42 (3H, s, CH3), 6.83 (1H, d, Hα, J = 8 Hz), 7.27-7.67 (7H, m, Ar-H), 7.68 (1H, d, Hβ, J = 8.4 Hz), 8.93 (1H, s, OH). Anal. Calcd. for C18H13ClO3: C, 69.13; H, 4.19. Found: C, 69.12; H, 4.22%.
General procedure for synthesis of compound (3a-m)
To an ethanolic solution of 2a-m (0.01 mol; 20mL), a solution of hydrazine hydrate(0.015mol) in 5mL glacial acetic acid was added, the reaction mixture was allowed to reflux for 3-6 h in presence of 100mg of 5A × 1.5mm molecular sieves. After completion of the reaction, the contents were concentrated and poured onto 100 g crushed ice, and neutralized with ammonia. Solid mass was filtered, washed with plenty of cold water and recrystallized with hydrated ethanol to get the pure product (3a-m).
2-(3-phenyl-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (3a)
IR (KBr, cm−1): 3580 (N-H), 1596 (C=N), 1324 (C-N), 757 (C-Cl). 1H -NMR (DMSO-d6, , ppm): 3.49 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.73 (1H, dd, Hb, J = 12.0, 12.4 Hz), 5.87 (1H, dd, Hx, J = 5.6, 5.6 Hz), 7.2-7.8 (9H, m, Ar-H). 8.5 (1H, s, NH). Anal. Calcd. for C17H13ClN2O: C, 68.81; H, 4.42; N, 9.44. Found: C, 68.72; H, 4.41; N, 9.45%.
2-[3-(4-chlorophenyl)-4,5-dihydro-1H-5-pyrazolyl] benzofuran-3-yl chloride (3b)
IR (KBr, cm−1): 3569 (N-H), 1596 (C=N), 1322 (C-N), 747 (C-Cl). 1H-NMR (CDCl3-d6, , ppm): 3.53 (1H, dd, Ha, J = 5.2, 5.6 Hz), 4.24 (1H, dd, Hb, J = 12, 12 Hz), 5.72 (1H, dd, Hx, J = 5.6, 5.6 Hz), 6.96 (1H, d, Ar-H, J = 6.4 Hz ), 7.34 (2H, m, Ar-H and NH pyrazoline), 7.52 (1H, d, Ar-H, J = 6.4 Hz), 7.58 (2H, d, Ar-H, J = 7.6 Hz), 7.80 (2H, d, Ar-H, J = 8 Hz). Anal. Calcd. for C17H12Cl2N2O: C, 61.65; H, 3.65; N, 8.46. Found: C, 61.54; H, 3.64; N, 8.45%.
2-[3-(4-methylphenyl)-4,5-dihydro-1H-5-pyrazolyl] benzofuran-3-yl chloride (3c)
IR (KBr, cm−1): 3560 (N-H), 1590 (C=N), 1326 (C-N), 747 (C-Cl). 1H -NMR (CDCl3-d6, , ppm): 2.40 (3H, s, -CH3), 3.41 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.65 (1H, dd, Hb, J = 12, 12.4 Hz), 5.82 (1H, dd, Hx, J = 5.6, 5.6 Hz), 7.25 (2H, d, Ar-H, J = 8 Hz ), 7.34 (1H, d, Ar-H, J = 7.6 Hz) 7.48 (2H, m, Ar-H and NH pyrazoline), 7.52 (1H, d, Ar-H, J = 6.8 Hz), 7.65 (2H, d, Ar-H, J = 8 Hz). Anal. Calcd. for C18H15ClN2O: C, 69.57; H, 4.86; N, 9.01. Found: C, 69.68; H, 4.85; N, 9.00%.
2-[3-(4-nitrophenyl)-4,5-dihydro-1H-5-pyrazolyl] benzofuran-3-yl chloride (3d)
IR (KBr, cm−1): 3569 (N-H), 1596 (C=N), 1322 (C-N), 747 (C-Cl).; 1H -NMR (CDCl3-d6, , ppm): 3.22 (1H, dd, Ha, J = 5.6, 5.6 Hz), 4.10 (1H, dd, Hb, J = 12.0, 12.0 Hz), 5.52 (1H, dd, Hx, J = 5.6, 5.2 Hz), 6.95(2H, d, Ar-H, J = 8.4 Hz), 7.32-7.43 (4H, m, Ar-H and pyrazoline), 7.90 (2H, d, Ar-H, J = 8.4 Hz). Anal. Calcd. for C17H12ClN3O3: C, 59.75; H, 3.54; N, 12.30. Found: C, 59.64; H, 3.56; N, 12.28%.
2-[3-(4-aminophenyl)-4,5-dihydro-1H-5-pyrazolyl] benzofuran-3-yl chloride (3e)
IR (KBr, cm−1): 3280 (N-H), 1596 (C=N), 1324 (C-N), 757 (C-Cl). 1H -NMR (CDCl3, , ppm): 2.38 (2H, s, NH2), 3.40 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.64 (1H, dd, Hb, J = 12.0, 12.8 Hz), 5.82 (1H, dd, Hx, J = 5.6, 5.6 Hz), 7.26 (2H, m, Ar-H), 7.34 (1H, d, Ar-H, J = 6.8 Hz ), 7.43 (1H, s, NH pyrazoline), 7.51 (1H, d, Ar-H, J = 6.8 Hz), 7.58 (2H, d, Ar-H, J = 7.6 Hz), 7.60 (2H, d, Ar-H, J = 8 Hz). Anal. Calcd. for C17H14ClN3O: C, 65.49; H, 4.53; N, 13.48. Found: C, 65.48; H, 4.52; N, 13.50%.
2-[3-(4-hydroxyphenyl)-4,5-dihydro-1H-5-pyrazolyl] benzofuran-3-yl chloride (3f)
IR (KBr, cm−1): 3532 (OH), 3285 (N-H), 1594 (C=N), 1324 (C-N), 756 (C-Cl).; 1H-NMR (DMSO-d6, , ppm): 3.79 (1H, dd, Ha, J = 5.2, 4.8 Hz), 4.07 (1H, dd, Hb, J = 12.4, 12.8 Hz), 6.07 (1H, dd, Hx, J = 5.6, 5.6Hz), 7.27(2H, d, Ar-H, J = 8 Hz), 7.64-7.71 (3H, m, Ar-H and NH pyrazoline), 7.76(1H, d, Ar-H, J = 7.2 Hz), 8.00 (2H, d, Ar-H, J = 8 Hz), 9.91 (1H, s, OH). Mass m/z: 312 (M+). Anal. Calcd. for C17H13ClN2O2: C, 65.29; H, 4.19; N, 8.96. Found: C, 65.30; H, 4.20; N, 8.97%.
2-[3-(2-hydroxyphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (3g)
IR (KBr, cm−1): 3538 (OH), 3295 (N-H), 1596 (C=N), 1324 (C-N), 754 (C-Cl). 1H -NMR (DMSO-d6, , ppm): 3.62 (1H, dd, Ha, J = 5.6, 5.6 Hz), 4.68 (1H, dd, Hb, J = 12.4, 12.4 Hz), 6.01 (1H, dd, Hx, J = 5.6, 5.2 Hz), 7.26-7.73 (6H, m, Ar-H and NH pyrazoline), 7.74 (1H, d, Ar-H, J = 6.8 Hz), 7.78 (1H, d, Ar-H, J = 6.8 Hz), 8.92 (1H, s, OH). Anal. Calcd. for C17H13ClN2O2: C, 65.29; H, 4.19; N, 8.96. Found: C, 65.38; H, 4.20; N, 8.95%.
2-[3-(4-methoxyphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (3h)
IR (KBr, cm−1): 3560 (N-H), 1590 (C=N), 1326 (C-N), 750 (C–Cl). 1H -NMR (DMSO-d6, , ppm): 3.39 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.63 (1H, dd, Hb, J = 12.4, 12.0 Hz), 3.85 (3H, s, -OCH3), 5.81 (1H, dd, Hx, J = 5.6, 5.6 Hz), 6.94 (2H, d, Ar-H, J = 8.4 Hz), 7.26-7.32 (2H, m, Ar-H and NH pyrazoline), 7.34 (1H, d, Ar-H, J = 8.0 Hz), 7.51 (1H, d, Ar-H, J = 6.4 Hz), 7.70 (2H, d, Ar-H, J = 8.8 Hz). Anal. Calcd. for C18H15ClN2O2: C, 66.16; H, 4.63; N, 8.57. Found: C, 66.20; H, 4.64; N, 8.55%.
2-[3-(2,4-dihydroxyphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (3i)
IR (KBr, cm−1): 3530 (OH), 3280 (N-H), 1596 (C=N), 1324 (C-N), 757 (C-Cl). 1H -NMR (DMSO-d6, , ppm): 3.45 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.98 (1H, dd, Hb, J = 12, 12 Hz), 6.11 (1H, dd, Hx, J = 5.6, 5.6 Hz), 6.98-7.42 (3H, m, Ar-H), 7.45 (1H, d, Ar-H, J = 6.8 Hz), 7.61-7.72 (3H, m, Ar-H and NH pyrazoline), 10.2 (2H, s, 2 × OH). Anal. Calcd. for C17H13ClN2O3: C, 62.11; H, 3.99; N, 8.52. Found: C, 62.10; H, 4.00; N, 8.51%.
2-[3-(2,4-dimethoxyphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride(3j)
IR (KBr, cm−1): 3280 (N-H), 1590 (C=N), 1324 (C-N), 755 (C-Cl). 1H -NMR (CDCl3-d6, , ppm): 3.36 (1H, dd, Ha, J = 5.6, 5.6 Hz), 3.62 (1H, dd, Hb, J = 12, 12 Hz), 3.86 (6H, s, 2 × OCH3), 5.82 (1H, dd, Hx, J = 5.6, 5.6 Hz), 7.26-7.83 (7H, m, Ar-H and NH pyrazoline). Anal. Calcd. for C19H17ClN2O3: C, 63.96; H, 4.80; N, 7.85. Found: C, 64.05; H, 4.81; N, 7.84%.
2-[3-(3,4-dimethoxyphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (3k)
IR (KBr, cm−1): 3280 (N-H), 1596 (C=N), 1324 (C-N), 757 (C-Cl). 1H -NMR (CDCl3-d6, , ppm): 3.38 (1H, dd, Ha, J = 5.2, 5.2 Hz), 3.59 (1H, dd, Hb, J = 12.4, 12.4 Hz), 3.89 (6H, s, 2 × OCH3), 6.17 (1H, dd, Hx, J = 5.2, 5.2 Hz), 6.98 (1H, s, Ar-H), 7.06-7.13 (3H, m, Ar-H), 7.17 (1H, bs, NH), 7.50-7.58 (3H, m, Ar-H). Anal. Calcd. for C19H17ClN2O3: C, 63.96; H, 4.80; N, 7.85. Found: C, 64.08; H, 4.80; N, 7.84%.
2-[3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (3l)
IR (KBr, cm−1): 3536 (OH), 3279 (N-H), 1590 (C=N), 1324 (C-N), 755 (C-Cl). 1H -NMR (DMSO-d6, , ppm): 2.38 (2H,d,CH2 pyrazoline), 2.43 (3H, s, CH3), 4.52 (1H,t,CH), 7.23-7.72 (7H, m, Ar-H and NH), 9.92 (1H, s, OH). Anal. Calcd. for C18H15ClN2O2: C, 66.16; H, 4.63; N, 8.57. Found: C, 66.15; H, 4.62; N, 8.58%.
2-[3-(4-hydroxy-2-methylphenyl)-4,5-dihydro-1H-5-pyrazolyl]benzofuran-3-yl chloride (3m)
IR (KBr, cm−1): 3535 (OH), 3285(N-H), 1594 (C=N), 1327 (C-N), 754 (C-Cl). 1H -NMR (DMSO-d6, , ppm): 2.36 (2H,d,CH2 pyrazoline), 2.69 (3H, s, CH3), 4.52 (1H,t,CH), 7.32-7.82 (7H, m, Ar-H and NH), 10.21 (1H, s, OH). Anal. Calcd. for C18H15ClN2O2: C, 66.16; H, 4.63; N, 8.57. Found: C, 66.29; H, 4.64; N, 8.56%.
Antiviral activity assays
Confluent cell cultures in micro titer trays were inoculated with 100 CCID50 (1 CCID50 corresponding to the virus stock dilution that proved infective for 50% of the cell cultures). After 1 h of virus adsorption to the cells, residual virus was removed and replaced by cell culture medium (Eagle’s minimal essential medium) containing 3% foetal calf serum and various concentrations of the test compounds. Viral cytopathogenicity was recorded as soon as it reached completion in the untreated virus-infected cell cultures, i.e., at 1 to 2 days for vesicular stomatitis; at 2 days for Coxsackie and herpes simplex virus types 1 and 2 and Sindbis virus; and 6 to 7 days for reovirus and parainfluenza viruses. The antiviral activity of the compounds is expressed as the concentration required to inhibit viral cytopathogenicity by 50%.
Cytotoxicity assays
Cytotoxicity was monitored by direct microscopical inspection of the cell monolayers, which had not been infected but were treated by the compounds at the same concentration as used in the antiviral activity assays.
Results and discussion
Chemistry
The physical constants of chalcones (2a-m) and pyrazoline derivatives (3a-m) are shown in , and the reaction sequence for the synthesis is outlined in . The desired chalcones were obtained by Claisen-Schmidt condensation, by reacting 3-chlorobenzofuran-2-carbaldehyde (Citation1) with the appropriate acetophenone in the presence of a base. It was interesting to report that overall reaction time recorded was considerably less as compared to our earlier reported reactions (Citation1–4). Reaction of 1 with anisaldehyde to get 2h may be considered as prototype of the series with a minimum time (2 h) and maximum yield (98%). Reaction between ethanolic solution of chalcones and hydrazine hydrate in the presence of glacial acetic acid and molecular sieves afforded the titled pyrazolines (3a-m). TLC and elemental analyses were done to confirm the purity of the compounds. Analytical and spectral data [IR & 1H-NMR] of all the synthesized compounds were found in full agreement with the proposed structures, which was further confirmed by mass of selected compounds. In general, the IR spectra of chalcone (2h) revealed C=O, C=C, and C–Cl stretching at 1685, 1645 and 750 cm−1 whereas pyrazoline (3h) revealed N-H, C=N, C-N, and C–Cl band at 3560, 1590, 1326 and 750 cm−1 respectively. In the 1H-NMR spectra the signals of the respective protons of the compounds were verified on the basis of their chemical shifts, multiplicities and coupling constants. The 1H-NMR spectra of chalcones showed two separate characteristic doublets at δ6.81 & 7.86ppm (2f) with trans coupling. The pyrazolines were characterized by disappearance of this (chalcone) doublets in 1H NMR and appearance of C4 (Ha & Hb) and C5 (Hx) protons in the aliphatic zone separately as three distinct double doublets at δ3.79 & 4.07 ppm (for Ha & Hb) and at δ6.07 ppm (for Hx) (3f). It is interesting to report that in some cases (3l & 3m) C4-methylene group and C5 proton of pyrazoline appears as two protons doublet and one proton triplet at a shift of δ2.36 ppm and 4.52 ppm respectively. The results of elemental analysis were found within ±0.4% of the theoretical values.
Table 1. Physical constants of the compound.
Antiviral activity
Antiviral activity was tested against feline corona virus (FIPV) and feline herpes virus (in Crandell-Rees Feline Kidney (CRFK) cell cultures); herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), vaccinia virus, vesicular stomatitis virus, herpes simplex virus-1 TK− KOS ACVr (in HEL cell cultures); vesicular stomatitis virus, Coxsackie virus B4, respiratory syncytial virus (in HeLa cells); parainfluenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus (in Vero cell cultures). For each compound the 50% (antivirally) effective concentration (EC50) and minimum cytotoxic concentration were determined. Ribavirin, brivudin, acyclovir, ganciclovir, dextran sulfate (DS-5000) and (S)-DHPA were used as the reference compounds.
The results of the antiviral assays are shown in . Cytotoxicity towards uninfected host cells was determined microscopically under same conditions as the antiviral activity. The criterion for specific antiviral activity was taken as, the inhibition of virus-induced cytopathogenicity at a concentration that was at least 5-fold lower than the cytotoxic, or concentration required to alter the morphology of the uninfected host cells.
Table 2. Cytotoxicity and antiviral activity of compounds in Crandell-Rees Feline Kidney (CRFK) cell cultures.
Table 3. Cytotoxicity and antiviral activity of compounds in human embryonic lung (HEL) cell cultures.
Table 4. Cytotoxicity and antiviral activity of compounds in HeLa cell cultures.
As per this criterion, none of the compounds tested showed any specific antiviral activity (). Several compounds, i.e. 2f, 2g, 2i, 2m, 3b, 3d, 3g, 3h and 3m, proved quite cytotoxic to the host cells (minimum cytotoxic concentration: 1-10 μg/mL).
Table 5. Cytotoxicity and antiviral activity of compounds in Vero cell cultures.
Acknowledgements
Of the authors, Prof. Erik De Clercq is most thankful to Mrs. Leentje Persoons (Rega Institute for Medical Research) for excellent technical assistance with the antiviral and cytotoxic assays.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ali MA, Shaharyar M, De Clercq E. Synthesis of 5-(4-hydroxy-3-methylphenyl)-5-(substituted phenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone derivatives with anti-viral activity. J Enz Inhib Med Chem 2007; 22: 702–708.
- Shaharyar M, Siddiqui AA, Ali MA, Murugan V, Raghu C. Synthesis and Cytotoxic activity of novel pyrazoline derivatives against human lung tumor cell line. J Chin Chem Soc 2007; 54: 81–86.
- Ali MA, Shaharyar M. Antitubercular activity of novel substituted 4,5-dihydro-1H-1-pyrazolylmethanethiones. J Enz Inhib Med Chem 2007; 22: 183–189.
- Shaharyar M, Siddiqui AA, Ali MA, Sriram D, Yogeshwari P. Synthesis and in-vitro antimycobacterial activity of N1- nicotinoyl-3-(4_-hydroxy-3_-methylphenyl )-5-[( sub) phenyl]-2-pyrazolines. Bioorg Med Chem Lett 2006; 16: 3947–3949.
- Rajendra Prasad Y, Lakshmana Rao A, Prasoona L, Murali K, Ravi Kumar P. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2”-hydroxynaphthalen-1”-yl)-1,5-diphenyl-2-pyrazolines. Bioorg Med Chem Lett 2005; 15: 5030–5034.
- Kucukguzel SG, Rollas S. Synthesis, characterization of novel coupling products and 4-arylhydrazono-2-pyrazoline-5-ones as potential antimycobacterial agents. Farmaco 2002; 57: 583–587.
- Bekhit AA, Ashour HMA, Guemei AA. Novel pyrazole derivatives as potential promising anti-inflammatory antimicrobial agents. Arch Pharm (Weinheim) 2005; 338: 167–174.
- Turan-Zitouni G, Ozdemir A, Guven K. Synthesis of some 1-[(N,N-disubstituted thiocarbamoylthio)acetyl]-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives and investigation of their antibacterial and antifungal activities. Arch Pharm (Weinheim) 2005; 338: 96–104.
- Ruhoglu O, Ozdemir Z, Calis U, Gumusel B, Bilgin AA. Synthesis of and pharmacological studies on the antidepressant and anticonvulsant activities of some 1,3,5-trisubstituted pyrazolines. Arzneimittel-Forschung 2005; 55: 431–436.
- Ucar G, Gokhan N, Yesilada A, Bilgin AA. 1-N-Substituted thiocarbamoyl-3-phenyl-5-thienyl-2-pyrazolines: a novel cholinesterase and selective monoamine oxidase B inhibitors for the treatment of Parkinson’s and Alzheimer’s diseases. Neurosci Lett 2005; 15: 327–331.
- Amr AE., Abdel-Latif NA, Abdalla MM. Synthesis and antiandrogenic activity of some new 3-substituted androstano-[17,16-c]-5’-aryl-pyrazoline and their derivatives. Bioorg Med Chem 2006; 14: 373–384.
- De Clercq E, Holý A, Rosenberg I, Sakuma T, Balzarini J, Maudgal PC. A novel selective broad-spectrum anti-DNA virus agent. Nature 1986; 323: 464–467.
- Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J. Rapid and automated tetrazoliumbased colorimetric assay for the detection of anti-HIV compounds. J Virol Meth 1988; 20: 309–321.
- De Clercq E. Antiviral and antimetabolic activities of neplanocins. Antimicrob Agents Chemother 1985; 28: 84–89.