Abstract
In an effort to establish new candidates with improved antimicrobial activities we report here the synthesis and in vitro biological evaluation of various series of compounds (5a-j) and (7a-j) which were evaluated against two Gram positive (S. aureus, B. subtilis), two Gram negative (S. typhosa, E. coli) strains and a yeast-like fungi (C. albicans) using the micro-dilution procedure. Among the synthesized compounds 2-(cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(2-chloro phenyl ureido) s-triazine (7e) and 2-(cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(4-chloro phenyl ureido) s-triazine (7g) proved to be effective with MIC (0.019 mg ML−1) against S. typhosa & E. coli respectively.
Keywords::
Introduction
In recently decades, the problems of multi-drug resistant microorganism have reached on alarming level in many countries around the world [Citation1–3]. A numbers of recent clinical reports describe the increasing occurrence of meticillin-resistant S. aureus and other antibiotic-resistant human pathogenic microorganisms in United State and European countries. Infections caused by those microorganisms pose a serious challenge to the medical community and need for an effective therapy has led to a search for novel antimicrobial agents. In this work, we report the synthesis and biological activity of some 1,3,5-triazinyl urea and thiourea analogues a class of privileged structures that have a wide range of biological properties. Among the compound having good antimicrobial properties, thiourea derivatives constitute an important class of compounds possessing diverse pharmacological activities including broadly active as potent vanilloid receptor agonists and analgesics [Citation4], antibacterial [Citation5], as a new class of anti-allergic agents inhibiting IgE/Fc
RI receptor mediated mast cell leukotriene release [Citation6], antiviral [Citation7] and possible anticonvulsant activities [Citation8]. It also represent a new class of human immuno deficiency virus type (HIV-1), non-nucleoside reverse transcriptase (NNRT) inhibitors [Citation9], found as antagonist of transient receptor potential channel antagonist [Citation10] and high density lipoprotein (HDL) elevating agents [Citation11,Citation12]. The combination the urea derivatives with thiourea lead to inhibitors of DNA topoisomerase [Citation13], antihypertensive activity [Citation14]. Furthermore, the pronounced antimicrobial activity of urea as well as thiourea derivatives [Citation15,Citation16] encouraged us to study the effect of the synthesized compounds against a variety of microorganisms.
Materials and methods
Chemistry
All chemical were of analytical grade and use directly. All melting points were determined in PMP-DM scientific melting point apparatus and are uncorrected. The completion of reaction was monitored by thin-layer chromatography (TLC) using silica gel-G coated Al-plates (0.5 mm thickness, Merck) and spots were visualized under UV radiation. Elemental analyses were done on “Haraeus Rapid Analyser”. Infra Red spectra were recorded on Perkin Elmer-spectrum RX-1 model spectrophotometer using KBr pallets. 1H NMR spectra were acquired on a Bruker Avance-2 model spectrophotometer using CDCl3 as a solvent and TMS as an internal reference (chemical shifts in δ, ppm).
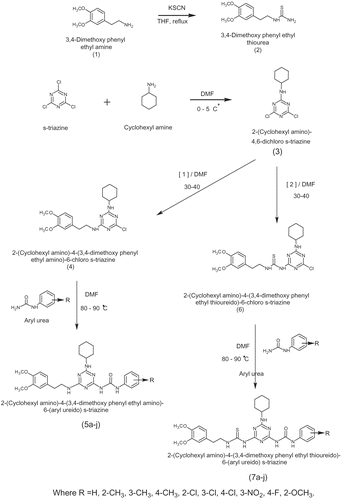
2-(Cyclohexyl amino)-4,6-(dichloro) s-triazine (3)
To a stirred solution of cyclohexyl amine (0.1 mol, 10 ML) was added in cyanuric chloride (0.1 mol, 18.4g) in D.M.F. (92 ML) at 0-5°C and pH was maintained neutral by the addition of 10% sodium carbonate solution. The stirring was continued at 0-5°C for 4h. After the completion of reaction the stirring was stopped and the solution was treated with crushed ice. The solid product obtained was filtered and dried. The crude product was purified by crystallization from absolute alcohol. m.p. 165 °C, yield 85%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(chloro) s-triazine (4)
To a stirred solution of (3) (0.1 mol, 24.7 g) in D.M.F. (92 ML) at 0-5°C, the solution of 3,4-dimethoxy phenyl ethyl amine (1) (0.1 mol, 17.93ML) in D.M.F. (25 ML) was added drop wise maintaining the temperature 35°C. The pH was adjusted neutral by the addition of 10% sodium carbonate solution. The temperature was gradually raised to 45°C during 2h. After the completion of reaction, the resultant content was poured into ice cold water. The solid product obtained was filtered and dried. The crude product was purified by crystallization from absolute alcohol. m.p. 147 °C, yield 68%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(phenyl ureido) s-triazine (5a)
A mixture of (4) (0.01 mol, 4.50 g) and phenyl urea (0.01 mol, 1.36 g) in acetone (20 ML) was refluxed in a water bath. The temperature was gradually raised to 80-90°C during 3h. The pH was adjusted neutral by the addition of 10% sodium carbonate solution. After the completion of reaction, the refluxed content was added to cold water. The solid product obtained was filtered and dried. The crude product was purified by crystallization from absolute alcohol. m. p. 202-204 °C and yield 60%. IR (KBr): 1567 (s), 2944 (s), 1607 (w), 2993 (s), 1471 (m), 1231 (s), 1515(s), 3280 (s), 2838 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.74 (s, 3H, -OCH3), 3.84 (s, 3H, -OCH3), 3.33 (t, 2H, -CH2), 3.24 (t, 2H, -CH2), 10.21 (s, 1H, -NH), 7.2-8.25(m 8H, Ar-H), 9.24 (s, 1H, -NH), 4.60 (s, 1H, -NH), 4.55 (s, 1H, -NH), 2.81 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2). Anal. Found: C 63.50, H 6.74, N 19.93%. Calcd for C26H33N7O3: C 63.52, H 6.77, N 19.95%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(2-methyl phenyl ureido) s-triazine (5b)
m. p. 230-231 °C and yield 65%. IR (KBr): 1560 (s), 2949 (s), 1632 (w), 2989 (s), 1465 (m), 1222 (s), 1512(s), 3276 (s), 2848 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.69 (s, 3H, -OCH3), 3.74 (s, 3H, -OCH3), 2.83(s, 3H, -CH3), 3.29 (t, 2H, -CH2), 3.21 (t, 2H, -CH2), 10.09 (s, 1H, -NH), 7.21-8.25(m 7H, Ar-H), 9.29 (s, 1H, -NH), 4.67 (s, 1H, -NH), 4.51 (s, 1H, -NH), 2.83 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2). Anal. Found: C 64.12, H 6.95, N 19.37%. Calcd for C27H35N7O3: C 64.14, H 6.98, N 19.39%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(3-methyl phenyl ureido) s-triazine (5c)
m. p. 202-203 °C and yield 80%. IR (KBr): 1555 (s), 2958 (s), 1641 (w), 769 (s), 2994 (s), 1460 (m), 1230 (s), 1519(s), 3272 (s), 2854 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.61 (s, 3H, -OCH3), 3.69 (s, 3H, -OCH3), 2.33(s, 3H, -CH3), 3.21 (t, 2H, -CH2), 3.29 (t, 2H, -CH2), 9.98 (s, 1H, -NH), 7.12-8.25(m 7H, Ar-H), 9.33 (s, 1H, -NH), 4.57 (s, 1H, -NH), 4.54 (s, 1H, -NH), 2.78 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2). Anal. Found: C 64.11, H 6.96, N 19.36%. Calcd for C27H35N7O3: C 64.14, H 6.98, N 19.39%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(4-methyl phenyl ureido) s-triazine (5d)
m. p. 198-201 °C and yield 68%. IR (KBr): 1561 (s), 2952 (s), 1650 (w), 2994 (s), 1456 (m), 1225 (s), 1519(s), 3266 (s), 2855 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.70 (s, 3H, -OCH3), 3.63 (s, 3H, -OCH3), 2.35(s, 3H, -CH3), 3.27 (t, 2H, -CH2), 3.24 (t, 2H, -CH2), 9.95 (s, 1H, -NH), 7.12-8.25(m 7H, Ar-H), 9.28 (s, 1H, -NH), 4.51 (s, 1H, -NH), 4.47 (s, 1H, -NH), 2.71 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2). Anal. Found: C 64.12, H 6.97, N 19.37%. Calcd for C27H35N7O3: C 64.14, H 6.98, N 19.39%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(2-chloro phenyl ureido) s-triazine (5e)
m. p. 157-158 °C and yield 70%. IR (KBr): 1561 (s), 2952 (s), 1650 (w), 2994 (s), 1456 (m), 1225 (s), 838(s), 1519(s), 3266 (s), 2855 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.75 (s, 3H, -OCH3), 3.83 (s, 3H, -OCH3), 3.21 (t, 2H, -CH2), 3.29 (t, 2H, -CH2), 9.87 (s, 1H, -NH), 7.12-8.25(m 7H, Ar-H), 9.06 (s, 1H, -NH), 4.52 (s, 1H, -NH), 4.55 (s, 1H, -NH), 2.69 (m, 1H, -CH), 0.81-1.94 (m, 10H, -CH2). Anal. Found: C 59.34, H 6.11, N 18.62%. Calcd for C26H32ClN7O3: C 59.37, H 6.13, N 18.64%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(3-chloro phenyl ureido) s-triazine (5f)
m. p. 140-141 °C and yield 62%. IR (KBr): 1569 (s), 2959 (s), 1640 (w), 2984 (s), 767(s), 1453 (m), 1235 (s), 838(s), 1523(s), 3261 (s), 2842 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.85 (s, 3H, -OCH3), 3.92 (s, 3H, -OCH3), 3.29 (t, 2H, -CH2), 3.22 (t, 2H, -CH2), 9.81 (s, 1H, -NH), 7.12-8.25(m 7H, Ar-H), 8.90 (s, 1H, -NH), 4.59 (s, 1H, -NH), 4.49 (s, 1H, -NH), 2.56 (m, 1H, -CH), 0.81-1.93 (m, 10H, -CH2). Anal. Found: C 59.36, H 6.10, N 18.61%. Calcd for C26H32ClN7O3: C 59.37, H 6.13, N 18.64%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(4-chloro phenyl ureido) s-triazine (5g)
m. p. 170-172 °C and yield 76%. IR (KBr): 1556 (s), 2969 (s), 1647 (w), 2977 (s), 1459 (m), 1230 (s), 829(s), 1537(s), 3269 (s), 2842 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.89 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 3.23 (t, 2H, -CH2), 3.14 (t, 2H, -CH2), 9.92 (s, 1H, -NH), 7.12-8.24(m 7H, Ar-H), 8.83 (s, 1H, -NH), 4.51 (s, 1H, -NH), 4.43 (s, 1H, -NH), 2.52 (m, 1H, -CH), 0.84-1.95 (m, 10H, -CH2). Anal. Found: C 59.34, H 6.09, N 18.63%. Calcd for C26H32ClN7O3: C 59.37, H 6.13, N 18.64%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(3-nitro phenyl ureido) s-triazine (5h)
m. p. 176-177 °C and yield 72%. IR (KBr): 1561 (s), 765(s), 2976 (s), 1651 (w), 2981 (s), 1463 (m), 1236 (s), 1531(s), 3262 (s), 2849 (s) cm−1. 1H NMR (DMSO –d6, δ): 4.0 (s, 3H, -OCH3), 3.96 (s, 3H, -OCH3), 3.33 (t, 2H, -CH2), 3.19 (t, 2H, -CH2), 9.88 (s, 1H, -NH), 7.12-8.24(m 7H, Ar-H), 8.81 (s, 1H, -NH), 4.45 (s, 1H, -NH), 4.53 (s, 1H, -NH), 2.42 (m, 1H, -CH), 0.89-1.91 (m, 10H, -CH2). Anal. Found: C 58.18, H 5.98, N 20.80%. Calcd for C26H32N8O5: C 58.20, H 6.01, N 20.88%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(4-fluoro phenyl ureido) s-triazine (5i)
m. p. 170-172 °C and yield 76%. IR (KBr): 1560 (s), 2981 (s), 1657 (w), 2971 (s), 1450 (m), 1232 (s), 1537(s), 3259 (s), 2862 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.82 (s, 3H, -OCH3), 3.91 (s, 3H, -OCH3), 3.29 (t, 2H, -CH2), 3.19 (t, 2H, -CH2), 9.88 (s, 1H, -NH), 7.15-8.27(m 7H, Ar-H), 8.78 (s, 1H, -NH), 4.48 (s, 1H, -NH), 4.55 (s, 1H, -NH), 2.51 (m, 1H, -CH), 0.84-1.95 (m, 10H, -CH2). Anal. Found: C 61.25, H 6.30, N 19.20%. Calcd for C26H32FN7O3: C 61.28, H 6.33, N 19.24%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(2-methoxy phenyl ureido) s-triazine (5j)
m. p. 172-174 °C and yield 65%. IR (KBr): 1560 (s), 2949 (s), 1639 (w), 2989 (s), 1465 (m), 1234 (s), 1512(s), 3276 (s), 2841 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.82 (s, 3H, -OCH3), 3.94 (s, 3H, -OCH3), 3.21 (t, 2H, -CH2), 3.39 (t, 2H, -CH2), 10.04 (s, 1H, -NH), 7.23-8.25(m 7H, Ar-H), 9.02 (s, 1H, -NH), 4.61 (s, 1H, -NH), 4.51 (s, 1H, -NH), 2.83 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2). Anal. Found: C 62.15, H 6.73, N 18.78%. Calcd for C27H35N7O4: C 62.17, H 6.76, N 18.80%.
3,4-dimethoxy phenyl ethyl thiourea (2)
To a stirred solution of 3,4-dimethoxy phenyl ethyl amine (0.1 mol, 17.93 ML) in T.H.F. (92 ML) was added potassium thiocynate (0.1 mol, 9.7 g). The mixture was heated at reflux for 24h. The mixture was diluted with water (100 ML) and extracted with ethylacetate (200 ML). The interfacial solids and ethyl acetate extracts were combined and washed with 1N HCl (100 ML) and brine. The organic layer was dried (over sodium sulphate) and concentrated under reduced pressure to give solid product. The crude product was purified by crystallization from absolute alcohol. m.p. 130-136 °C, yield 67 % IR (KBr): 3049 (s), 1504(s), 1529 (s), 1446 (s), 1331 (s), 3302 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.84(s, 3H), 4.0(s, 3H), 3.45 (t, 2H), 3.34 (t, 2H), 6.12 ( s, 2H), 7.17-7.42(m 4H).
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(chloro) s-triazine (6)
To a stirred solution of (3) (0.1 mol, 24.7 g) in D.M.F. (92 ML) at 0-5°C, the solution of 3,4-dimethoxy phenyl ethyl thiourea (2) (0.1 mol, 24.0 g) in D.M.F. (25 ML) was added drop wise keeping the temperature 35°C. The pH was adjusted neutral by the addition of 10% sodium carbonate solution. The temperature was gradually raised to 45°C during 2h. After the completion of reaction, the content was poured into ice cold water. The solid product obtained was filtered and dried. The crude product was purified by crystallization from absolute alcohol. m. p. 233°C, yield 77%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(phenyl ureido) s-triazine (7a)
A mixture of (6) (0.01 mol, 4.5 g) and phenyl urea (0.01 mol, 1.36 g) in acetone (15 ML) was refluxed on a water bath. The temperature was gradually raised to 80-90°C during 3h. The pH was adjusted neutral by the addition of 10% sodium carbonate solution. After the completion of reaction, the refluxed content was added to cold water. The solid product obtained was filtered and dried. The crude product was purified by crystallization from absolute alcohol. m. p. 252°C and yield 63%. IR (KBr):784 (s), 3442 (s), 1650(s),1529 (s), 3302 (s), 1608 (s), 3049 (s), 1504 (w), 3203 (s) cm−1. 1H NMR (DMSO –d6, δ): 4.0 (s, 6H, -OCH3), 3.45 (t, 2H, -CH2), 3.34 (t, 2H, -CH2), 9.73 (s, 1H, -NH), 7.20-8.25(m 8H, Ar-H), 8.82 (s, 1H, -NH), 4.61 (s, 1H, -NH), 9.62 (s, 1H, -NH), 3.73 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2), 8.94 (s, 1H, -NH). Anal. Found: C 58.87, H 6.20, N 20.32%. Calcd for C27H34N8O3S: C 58.89, H 6.22, N 20.35%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(2-methyl phenyl ureido) s-triazine (7b)
m. p. 160°C and yield 56%. IR (KBr): 784 (s), 3434 (s), 1644(s), 1529 (s), 3296 (s), 1620 (s), 3055 (s), 1512 (w), 3192 (s) cm−1. 1H NMR (DMSO –d6, δ): 4.10 (s, 6H, -OCH3), 3.41 (t, 2H, -CH2), 2.81 (s, 3H, -CH3), 3.32 (t, 2H, -CH2), 9.71 (s, 1H, -NH), 7.20-8.25(m 7H, Ar-H), 8.79 (s, 1H, -NH), 4.57 (s, 1H, -NH), 9.55 (s, 1H, -NH), 3.68 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2), 8.84 (s, 1H, -NH). Anal. Found: C 59.52, H 6.41, N 19.81%. Calcd for C28H36N8O3S: C 59.55, H 6.43, N 19.84%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(3-methyl phenyl ureido) s-triazine (7c)
m. p. 160°C and yield 56%. IR (KBr): 780 (s), 3444 (s), 1654(s), 1534 (s), 3310 (s), 1609 (s), 3045 (s), 1505 (w), 3191 (s) cm−1. 1H NMR (DMSO –d6, δ): 4.10 (s, 6H, -OCH3), 3.41 (t, 2H, -CH2), 3.32 (t, 2H, -CH2), 9.71 (s, 1H, -NH), 7.20-8.25(m 7H, Ar-H), 8.79 (s, 1H, -NH), 2.41 (s, 3H, -CH3), 4.57 (s, 1H, -NH), 9.55 (s, 1H, -NH), 3.68 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2), 8.84 (s, 1H, -NH). Anal. Found: C 59.53, H 6.40, N 19.82%. Calcd for C28H36N8O3S: C 59.55, H 6.43, N 19.84%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(4-methyl phenyl ureido) s-triazine (7d)
m. p. 157-159°C and yield 67%. IR (KBr): 788 (s), 3439 (s), 1651(s), 1531 (s), 3289 (s), 1615 (s), 3040 (s), 857(s), 1502 (w), 3210 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.93 (s, 6H, -OCH3), 3.31 (t, 2H, -CH2), 2.61 (s, 3H, -CH3), 3.52 (t, 2H, -CH2), 9.61 (s, 1H, -NH), 7.20-8.25(m 7H, Ar-H), 8.71 (s, 1H, -NH), 4.52 (s, 1H, -NH), 9.45 (s, 1H, -NH), 3.68 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2), 8.74 (s, 1H, -NH). Anal. Found: C 59.51, H 6.42, N 19.81%. Calcd for C28H36N8O3S: C 59.55, H 6.43, N 19.84%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(2-chloro phenyl ureido) s-triazine (7e)
m. p. 174°C and yield 70%. IR (KBr): 779 (s), 3445 (s), 1639(s), 1543 (s), 3321 (s), 1613 (s), 3044 (s), 1502 (w), 3199 (s) cm−1. 1H NMR (DMSO –d6, δ): 4.02 (s, 6H, -OCH3), 3.45 (t, 2H, -CH2), 3.39 (t, 2H, -CH2), 9.72 (s, 1H, -NH), 7.20-8.25(m 7H, Ar-H), 8.81 (s, 1H, -NH), 4.51 (s, 1H, -NH), 9.52 (s, 1H, -NH), 3.63 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2), 8.88 (s, 1H, -NH). Anal. Found: C 55.39, H 5.66, N 19.13%. Calcd for C27H33ClN8O3S: C 55.42, H 5.68, N 19.15%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(3-chloro phenyl ureido) s-triazine (7f)
m. p. 155-156 °C and yield 50%. IR (KBr): 788 (s), 3439 (s), 1639 (s), 1541 (s), 3301 (s), 1619 (s), 3030 (s), 1512 (w), 3217 (s) cm−1. 1H NMR (DMSO –d6, δ): 4.02 (s, 6H, -OCH3), 3.49 (t, 2H, -CH2), 3.32 (t, 2H, -CH2), 9.85 (s, 1H, -NH), 7.20-8.25(m 7H, Ar-H), 8.89 (s, 1H, -NH), 4.59 (s, 1H, -NH), 9.42 (s, 1H, -NH), 3.69 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2), 8.76 (s, 1H, -NH). Anal. Found: C 55.41, H 5.67, N 19.12%. Calcd for C27H33ClN8O3S: C 55.42, H 5.68, N 19.15%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(4-chloro phenyl ureido) s-triazine (7g)
m. p. 169 °C and yield 48%. IR (KBr): 851(s), 778 (s), 3431 (s), 1631 (s), 1545 (s), 3322 (s), 1631 (s), 3032 (s), 1533 (w), 3254 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.87 (s, 6H, -OCH3), 3.39 (t, 2H, -CH2), 3.29 (t, 2H, -CH2), 9.81 (s, 1H, -NH), 7.20-8.25(m 7H, Ar-H), 8.81 (s, 1H, -NH), 4.49 (s, 1H, -NH), 9.39 (s, 1H, -NH), 3.61 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2), 8.71 (s, 1H, -NH). Anal. Found: C 55.38, H 5.65, N 19.14%. Calcd for C27H33ClN8O3S: C 55.42, H 5.68, N 19.15%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(3-nitro phenyl ureido) s-triazine (7h)
m. p. 157-159°C and yield 67%. IR (KBr): 788 (s), 3439 (s), 1651(s), 1531 (s), 3289 (s), 1615 (s), 3040 (s), 857(s), 1502 (w), 3210 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.93 (s, 6H, -OCH3), 3.31 (t, 2H, -CH2), 3.42 (t, 2H, -CH2), 9.66 (s, 1H, -NH), 7.20-8.25(m 7H, Ar-H), 8.74 (s, 1H, -NH), 4.58 (s, 1H, -NH), 9.42 (s, 1H, -NH), 3.61 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2), 8.70 (s, 1H, -NH). Anal. Found: C 54.41, H 5.56, N 21.14%. Calcd for C27H33N9O5S: C 54.44, H 5.58, N 21.16%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(4-fluoro phenyl ureido) s-triazine (7i)
m. p. 120 °C and yield 63%. IR (KBr): 858(s), 786 (s), 3431 (s), 1641 (s), 1542 (s), 3314 (s), 1601 (s), 3012 (s), 1523 (w), 3211 (s) cm−1. 1H NMR (DMSO –d6, δ): 4.02 (s, 6H, -OCH3), 3.49 (t, 2H, -CH2), 3.32 (t, 2H, -CH2), 9.85 (s, 1H, -NH), 7.20-8.25(m 7H, Ar-H), 8.89 (s, 1H, -NH), 4.59 (s, 1H, -NH), 9.42 (s, 1H, -NH), 3.69 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2), 8.76 (s, 1H, -NH). Anal. Found: C 57.01, H 5.82, N 19.67%. Calcd for C27H33FN8O3S: C 57.03, H 5.85, N 19.70%.
2-(Cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(2-methoxy phenyl ureido) s-triazine (7j)
m. p. 165-167 °C and yield 75%. IR (KBr): 859(s), 768 (s), 3434 (s), 1639 (s), 1541 (s), 3324 (s), 1635 (s), 3037 (s), 1538 (w), 3259 (s) cm−1. 1H NMR (DMSO –d6, δ): 3.98 (s, 6H, -OCH3), 3.44 (t, 2H, -CH2), 3.32 (t, 2H, -CH2), 9.83 (s, 1H, -NH), 7.20-8.25(m 7H, Ar-H), 8.87 (s, 1H, -NH), 4.42 (s, 1H, -NH), 9.32 (s, 1H, -NH), 3.63 (m, 1H, -CH), 0.81-1.91 (m, 10H, -CH2), 8.66 (s, 1H, -NH). Anal. Found: C 57.88, H 6.22, N 19.27%. Calcd for C28H36N8O4S: C 57.91, H 6.25, N 19.30%.
Biological assays
Compounds
Test compounds were dissolved in DMSO (12.5%) at an initial concentration of 20 mg ML −1 and then were serially diluted in culture medium.
Cells
Bacterial strains Staphylococcus aureus, Bacillus subtillis, Escherichia coli, Salmonella typhosa and yeast-like fungi stain Candida albicans.
Antimicrobial assays
The MICs of the chemical compounds assays were carried out as described by Clause [Citation17] with minor modifications. Tetracycline was used as reference antibacterial agent. Solutions of the test compounds and reference drug were dissolved in DMSO at a concentration of 20 mg ML−1. The twofold dilution of the compounds and reference drug were prepared (20, 10, 5.0, 2.5, 1.25, 0.625, 0.31, 0.15, 0.07, 0.03, 0.019, 0.01, 0.005 >) mg ML−1. Antibacterial activities of the bacterial strains were carried out in Muller– Hinton broth (Difco) medium, at pH 6.9, with an inoculum of (1– 2) × 103 cells ML−1 by the spectrophotometric method and an aliquot of 100 μL was added to each tube of the serial dilution. The chemical compounds-broth medium serial tube dilutions inoculated with each bacterium were incubated on a rotary shaker at 37 °C for 24 h at 150 rpm. The minimum inhibitory concentrations of the chemical compounds were recorded as the lowest concentration of each chemical compounds in the tubes with no growth (i.e. no turbidity) of inoculated bacteria. A fungi was cultivated in Sabouraud Dextrose Agar (Merck). The fungi inoculums was prepared in Sabouraud liquid medium (Oxoid) which had been kept at 36°C overnight and was diluted with RPMI-1640 medium with L-glutamine buffered with 3-[N-morpholino]-propansulfonic acid (MOPS) at pH 7 to give a final concentration of 2.5 × 103 cfu/ML. The twofold dilution of the compounds and reference drug were prepared (20, 10, 5.0, 2.5, 1.25, 0.625, 0.31, 0.15, 0.07, 0.03, 0.019, 0.01, 0.005 > mg ML−1). The micro tubes were incubated at 36°C and read visually after 24 h. The incubation chamber was kept humid. At the end of the incubation period, MIC values were recorded as the lowest concentrations of the substances that gave no visible turbidity. Fluconazole was used as reference antifungal agent. Solutions of the test compounds and reference drug were dissolved in DMSO at a maximum final concentration of 12.5% which had no effect on the microorganism’s growth.
Result and discussion
Chemistry
Commercially available 3,4-dimethoxy phenyl ethyl amine (1) was converted into 3,4-dimethoxy phenyl ethyl thiourea (2) in acceptable yield according to literature [Citation18] (). The identity of the product was determined by IR and 1H NMR spectral studies. The IR spectrum of compound (2) revealed absorption band at 1506 cm−1 (-C=S- stretching in thiourea), 3302 cm−1 and 3294 cm−1 (-NH- stretching in thiourea) indicating the formation of thiourea. NMR spectra shown 6.50 (s, 2H, -NH2), indicating the formation of thiourea in the heterocyclic frame work. Commercially available s-triazine are converted in to 2-(cyclohexyl amine)-4,6-(dichloro)-s-triazine (3) by using cyclohexyl amine in presence of sodium carbonate in DMF at 0-5 °C. The compound 2-(cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(chloro)-s-triazine (4) were synthesized by condensation of (3) and 3,4-dimethoxy phenyl ethyl amine (1) in presence of DMF at 30-40 °C. The reaction of compound (4) with aryl urea yielded 2-(cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl amino)-6-(aryl ureido) s-triazine (5a-j).
The compound 2-(cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(chloro) s-triazine (6) was prepared by reaction of 3,4-dimethoxy phenyl ethyl thiourea (2) and compound (3) in presence of DMF at 30-40 °C. Compound (6) reacted with aryl urea yielded 2-(cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(aryl ureido) s-triazine (7a-j). All the synthesized compounds were well characterized by spectroscopic data as IR, NMR and elemental analysis. The IR spectra of the synthesized compounds generally show the characteristic bands corresponding to the carbonyl and the thiocarbonyl functions, in addition to the NH moieties. The 1H-NMR spectra of the compounds showed the broad exchangeable singlets due to NH urea and thiourea protons. All other aromatic and aliphatic protons were observed in the expected regions.
Antibacterial activity
The present paper is focused on the synthesis of novel heterocyclic compounds as possible antibacterial agents. The minimal inhibitory concentration (MICs, mg ML−1) of tested compounds against bacteria are shown in . A series of novel compound (5a-j) and (7a-j) were prepared and tested for their in vitro antibacterial activity against the four strains of bacteria (two gram +ve, two gram –ve) and yeast-like fungi. Two compounds of the obtained series showed high in vitro antimicrobial activity. Compounds 2-(cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(4-chloro phenyl ureido) s-triazine (7g) showed excellent activity against E. coli (0.019 mg ML−1) and 2-(cyclohexyl amino)-4-(3,4-dimethoxy phenyl ethyl thioureido)-6-(2-chloro phenyl ureido) s-triazine (7e) showed excellent activity against S. typhi (0.019 mg ML−1) comparable to or slightly lower than analogous to the standard drug.. The presence of electron-withdrawing group on the aromatic ring in general increase the antimicrobial activities of the tested compounds compared to compounds having electron donating groups. Based upon the results it will also be necessary to optimize the lead compound by substitution in the C2 and C4 position in of phenyl ring by chloro seem to be very important for antibacterial effect, as well as the presence and the position of –NHCSNH- group in the connecting linker between the aromatic ring seems to be very important for antibacterial effect. Most of the synthesized compounds were showed moderate activity against C. albicans whereas others were inactive compared with flucocazole”.
Table 1. Antimicrobial activity of compounds (5a-j) and (7a-j).
Acknowledgement
The authors are thankful to the Dr. Prof K. R. Desai, Head of the chemistry and Bioscience Department of Veer Narmad South Gujarat University, Surat. The authors also express their sincere thanks to the SAIF, Punjab University, Chandigarh for spectral analysis.
Declaration of interest: The authors report no conflicts of interest.
References
- Harbarth S, Albrich W, Goldmann DA, Huebner J. Control of multiply resistant cocci: do international comparisons help? Lancet Infect Dis 2001;1:251–261
- Mitscher LA, Pillai SP, Gentry EJ, Shankel DM. Multiple drug resistance. Med Res Rev 1999;19(6):477–496
- Berber I, Cokmus C, Atalan E. Characterization of Staphylococcus species by SDS-PAGE of whole-cell and extracellular proteins. Microbiology 2003;72:54–59
- Jeewoo L, Jiyoun L, Jiyoung K, Soo Y K. N-(3-acyloxy-2-benzylpropyl)-N9-(4-hydroxy-3-methoxybenzyl)thiourea derivatives as potent vanilloid receptor agonists and analgesics. Bioorgan Med Chem Lett 2001; 9(1):19–32
- Schoeder MC, Showalter HD. Soluble 2-Substituted Aminopyrido[2,3-d]pyrimidin-7-yl Ureas. Structure-Activity Relationships against Selected Tyrosine Kinases and Exploration of in Vitro and in Vivo Anticancer Activity. J Med Chem 2001; 44:1915–1926
- Venkatachalam TK, Qazi S, Samuel P, Uckun FM. Substituted heterocyclic thiourea compounds as a new class of anti-allergic agents inhibiting IgE/Fci RI receptor mediated mast cell leukotriene release. Bioorgan Med Chem 2003;11(6):1095–1105
- Tilley JW. Levitan P, Kramer MJ. Adamantylthiourea derivatives as antiviral agents. J Med Chem 1979;22(8):1009–1010
- Siddique B, Pandeya SN, Khan SA, Stables JS. Synthesis and anticonvulsant activity of sulfonamide derivatives-hydrophobic domain. Bioorgan Med Chem Lett. 2007;17:255–259
- Bell FW, Cantrell AS, Hogberg M. Phenethylthiazolethiourea (PEW) Compounds, a New Class of HIV- 1 Reverse Transcriptase Inhibitors. 1. Synthesis and Basic Structure-Activity Relationship Studies of PEW Analogues. J Med Chem 1995;38:4929–4936
- Young GS, Kyung HM, Jin KK. Novel Potent Antagonists of Transient Receptor Potential Channel Vanilloid Subfamily Member 1: Structure- Activity Relationship of 1,3-Diarylalkyl Thiourea Possessing New Vanilloid Equivalents. J Med Chem., 2005;48:5823–5836
- Cappola GM, Damon RE, Paterniti JR. Biological evaluation of 1-alkyl-3-phenylthioureas as orally active HDL-elevating agents. Bioorgan Med Chem Lett. 2006:16:113–117
- Cappola GM, Damon RE, Paterniti JR. Thiourea-Based Gemfibrozil Analogues as HDL-Elevating Agents. Bioorgan Med Chem Lett. 2002;12:2439–2442
- Esteves-Souza A, Pissinate K, Nascimento Md-G, Grynberg NF. Synthesis, cytotoxicity, and DNA-topoisomerase inhibitory activity of new asymmetric ureas and thioureas. Bioorgan Med Chem. 2006;14(2):492–499
- loev B, Bender PE, Bowman H. Amidines. 31.Thiourea possessing antihypertensive activity. J Med Chem 1972;15:1024–1025
- Phetsuksiri B, Baulard AR, Cooper AM, Minnikin DE, Douglas JD, Besra GS. J. Antimycobacterial Activities of Isoxyl and New Derivatives through the Inhibition of Mycolic Acid Synthesis. Antimicrob. Agents Chemother 1999;43:1042–1051
- Seth P, Ranken R, Robinson DE, Osgood SA, Risen LM. Aryl urea analogs with broad–spectrum antibacterial activity. Bioorgan Med Chem 2004;14(22):5569–5572
- Clause GW, Understanding Microbes: A Laboratory Textbook for Microbiology, W.H. Freeman and Company, New York, USA, 1989
- Herr RJ, Kuhler JL, Meckler H, Opalka CJ. A Convenient Method for the Preparation of Primary and Symmetrical N,N’–Disubstituted Thiourea. Synthesis 2000;11:1569–1574