Abstract
A series of aromatic disulfonamide (1-8) derivatives and 4-methylbenzenesulfonyl hydrazide (9) were synthesized and characterized. All compounds were evaluated in vitro for their antimicrobial activity against Staphylococcus aureus ATCC 25953, Bacillus cereus ATCC 6633, Bacillus magaterium RSKK 5117, Escherichia coli ATCC 11230, Salmonella enterititis ATCC 13076 by microdilution and disc diffusion methods. Antimicrobial activity of the aromatic disulfonamides decreased as the length of the carbon chain increased. An analysis of the structure- activity relationship (SAR) along with computational studies showed that the most active compound (9) possessed low lipophilicity (AlogP=0.59) and high solubility (logS = -1.33).
Introduction
Sulfa drugs are the oldest chemically synthesized antimicrobial agents and are still widely used today for the treatment of various bacterial, protozoal and fungal infections [Citation1,Citation2]. Sulfa drugs act by competitive inhibition of the enzyme dihydropteroate synthase, which is a key enzyme involved in folate synthesis [Citation3]. Although a number of antimicrobial drugs are available commercially, the need for more effective ones continues to exist, because the most common bacteria are resistant to available drugs. In fact, multidrug resistance (MDR) remains a significant problem for the treatment of microbial infections [Citation4–7]. Furthermore, the threat of bioterrorism using agents such as weaponized Bacillus anthracis and Yersinia pestis highlight the need for continuing research on infectious diseases and the search for new therapeutic agents [Citation8]. Recently, a few substituted derivatives of aromatic/heterocyclic sulfonamides and their complexes have been synthesized and tested for their antimicrobial activity [Citation9–13].
In our previous studies, aliphatic and aromatic disulfonamid es were synthesized and evaluated for antimicrobial activity [Citation14–16]. In addition, methanesulfonic acid hydrazides and their hydrazones were obtained and screened for their antimicrobial and cytotoxic activity [Citation17–19]. Metal carbonyl complexes of these sulfonyl hydrazones were also reported [Citation20–26]. In this paper, continuing our studies, a series of novel aromatic sulfonamide derivatives (1–8) and sulfonyl hydrazide (9) were synthesized and characterized. Their antibacterial activities were evaluated against Gram-positive bacteria (Staphylococcus aureus ATCC 25953, Bacillus cereus ATCC 6633, Bacillus magaterium RSKK 5117) and Gram-negative bacteria (Escherichia coli ATCC 11230, Salmonella enterititis ATCC 13076) by disc diffusion and microdilution methods. Structure–activity relationships (SAR) between the antimicrobial activities and the physicochemical/ structural properties were also evaluated.
Materials and methods
The elemental analyses (C, H, N and S) were performed on a LECO–CHSNO - 9320 type elemental analyzer. The IR spectra (4000-400 cm−1) were recorded on a Mattson-1000 FT-IR spectrophotometer with samples prepared as KBr pellets. NMR spectra were recorded on a Bruker-Spectrospin Avance DPX - 400 Ultra – Shield (400 MHz) using DMSO-d6 and CDCl3 as a solvent and TMS as an internal standard. LC/MS-APCl were recorded on AGILENT 1100. The melting point was recorded on a OptiMelt apparatus. TLC was conducted on 0.25 mm silica gel plates (60F254, Merck). Visualization was made with ultraviolet light. All extracted solvents (all from Merck) were dried over anhydrous Na2SO4 and evaporated with a BUCHI rotary evaporator. Reagents were obtained commercially from Aldrich (ACS grade) and used as received.
Dipole moment, HOMO and LUMO energies and local charges were calculated using AM1 semi-empirical method with HyperChem software (version 7, Hypercube Inc.). Total topological parameter was generated from DRAGON software [Citation40].
Chemistry
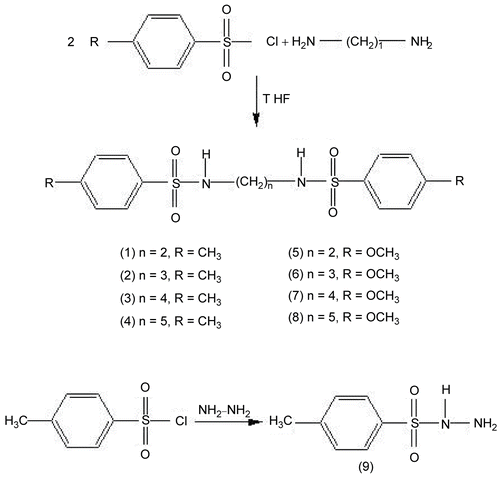
According to the general procedure shown in , the synthesis of a series of aryldisulfonamides compounds (1-8) and 4-methylbenzenesulfonyl hydrazide analogues (9) could be carried out easily in THF, which gives the best result among the other solvents such as, acetonitrile, N,N-dimethylformamide dichloromethane, ethylacetate, just by the nucleophilic substitution reaction of aromatic diamines/ hydrazine hydrate with substitued aromatic sulfonyl chloride. The crystal lattice structure [Citation28] and synthesis [Citation27] of compound (1) and the synthesis and antitumor activity of compound (5) [Citation29] as well as the synthesis, 1H-NMR, 13C-NMR spectroscopy [Citation30] and x-ray powder diffraction data [Citation31] of compound (9) have been published.
General synthesis procedure
The nucleophilic substitution reaction of the aromatic diamines with aryl sulfonyl chlorides were carried out as follows: THF solution of aromatic diamines was added slowly to the THF solution of sulfonyl chlorides, maintaining the temperature at -5 °C. Then, the reaction mixture was stirred for 24 h at room temperature (completion of the reaction was monitored by TLC) then solvent was evaporated in vacuum and the solid residue purified by column chromatography.
p-toluenesulfonamide N,N′-1,2-ethanediylbis (1) The title compound was obtained from ethylenediamine (2.71 mL, 0.08 mol) and p-toluenesulfonyl chloride (3.85 g, 0.04 mol) as described above. The white crystalline solid was recrystallized from tetrahydrofurane/n-hekzan mixture. Product was dried in vacuo and stored at THF vapour. Yield 72%; mp 158-161 °C; MS (70 eV, APCl): 368.8 (M+), 369.8 (M++1), 370.8 (M++2); Anal. Calcd for C16H20N2O4S2: C, 40.0; H, 8.0; N, 9.33; S, 21.3. Found: C, 40.23; H, 7.94; N, 9.10; S, 21.51%.
p-toluenesulfonamide N,N′-1,3-propanediylbis (2) The title compound was obtained from 1,3-diamino propane (3.36 mL, 0.08 mol) and p-toluenesulfonyl chloride (3.85 g, 0.04 mol). The white crystalline solid was filtered and recrystallized from ethanol/n-hekzan mixture. Product was dried in vacuo and stored at ethanol vapour. Yield 72%; mp 173-174 °C; MS (70 eV, APCl): 382.8 (M+), 383.9 (M+1), 384.8 (M+2). Anal. Calcd for: C17H22N2 O4S2 C, 53.38; H, 5.80; N, 7.32; S, 16.77. Found: C, 53.94; H, 5.21; N, 7.04; S, 15.82%.
p-toluenesulfonamide N,N′-1,4-buthanediylbis (3) The title compound was obtained from 1,4-diamino buthane (4.06 mL, 0.08 mol) and p-toluenesulfonyl chloride (3.85 g, 0.04 mol). The white crystalline solid was filtered and recrystallized from ethanol/n-hekzan mixture. Product was dried in vacuo and stored at ethanol vapour. Yield 72%; mp 183-184 °C; MS (70 eV, APCl): 396.9 (M+), 397.9 (M+1), 398.9 (M+2). Anal. Calcd for: C18H24N2O4S2, C, 54.52; H, 6.10; N, 7.06; S, 16.01. Found: C, 54.72; H, 5.14; N, 7.35; S, 14.50%.
p-toluenesulfonamide N,N′-1,5-pentanediylbis (4) The title compound was obtained from 1,5-diamino pentane (4.93 mL, 0.08 mol) and p-toluenesulfonyl chloride (3.85 g, 0.04 mol) as described above. The white crystalline solid was filtered and recrystallized from ethanol/n-hexane mixture. Product was dried in vacuo and stored at ethanol vapour. Yield 72%; mp 192-194 °C; MS (70 eV, APCl): 410.9 (M+), 411.9 (M+1) 412.9 (M+2). Anal. Calcd for: C19H26N2O4S2, C, 55.58; H, 6.38; N, 6.82; S, 15.62. Found: C, 55.82; H, 5.19; N, 7.20; S, 13.35%.
p-methoxybenzenesulfonamide N,N′-1,2-ethanediylbis (5) The title compound was obtained from ethylendiamine (2.71 mL, 0.08 mol) and p-methoxybenzenesulfonyl chloride (3.85 g, 0.04 mol). The white crystalline solid was recrystallized from tetrahydrofurane/n-hekzan mixture. Product was dried in vacuo and stored at tetrahydrofurane vapour. Yield 72%; mp 166-168 °C; MS (70 eV, APCl): 401.1 (M+1) 402.1 (M+2+), 213.1 [CH3OC6H4SO2+]. Anal. Calcd for: C16H20N2O6S2, C, 47.99; H, 5.03; N, 7.00; S, 16.01. Found: C, 48.30; H, 5.79; N, 6.99; S, 15.79%.
p-methoxybenzenesulfonamide N,N′-1,3-propanediylbis (6) The title compound was obtained from 1,3-diamino propane (2.71 mL, 0.08 mol) and p-methoxybenzenesulfonyl chloride (3.85 g, 0.04 mol). The white crystalline solid was recrystallized from tetrahydrofurane/n-hekzan mixture. Product was dried in vacuo and stored at tetrahydrofurane vapour. Yield 72%; mp 177-178 °C; MS (70 eV, APCl): 414.8 (M+), 415.8 (M+1). Anal. Calcd for: C17H22N2O6S2, C, 49.26; H, 5.35; N, 6.76; S, 15.47 Found: C, 49.00; H, 5.50; N, 6.79; S, 15.76%.
p-methoxybenzenesulfonamide N,N′-1,4-buthanediylbis (7) The title compound was obtained from 1,4-diamino buthane (2.71 mL, 0.08 mol) and p-methoxybenzenesulfonyl chloride (3.85 g, 0.04 mol). The white crystalline solid was recrystallized from tetrahydrofurane/n-hekzan mixture. Product was dried in vacuo and stored at tetrahydrofurane vapour. Yield 72%; mp 177-178 °C; MS (70 eV, APCl): 428.11 (M+), 429.11 (M+1). Anal. Calcd for: C18H24N2O6S2, C, 50.45; H, 5.65; N, 6.54; S, 14.97 Found: C, 50.30; H, 5.70; N, 6.75; S, 14.76%
p-methoxybenzenesulfonamide N,N′-1,5-pentanediylbis (8) The title compound was obtained from 1,5-diamino pentane (2.71 mL, 0.08 mol) and p-methoxybenzenesulfonyl chloride (3.85 g, 0.04 mol). The white crystalline solid was recrystallized from tetrahydrofurane/n-hekzan mixture. Product was dried in vacuo and stored at tetrahydrofurane vapour. Yield 72%; mp 185-186 °C; MS (70 eV, APCl): 442.12 (M+) 443.13 (M+1). Anal. Calcd for: C19H26N2O6S2, C, 51.57; H, 5.92; N, 6.33; S, 14.49 Found: C, 51.30; H, 5.88; N, 6.36; S, 14.70%.
Microbiology
S. aureus ATCC 25953, B. cereus ATCC 6633, B. magaterium RSKK 5117, E. coli ATCC 11230 and S. enterititis ATCC 13076 cultures were obtained from Gazi University, Biology Department. Bacterial strains were cultured overnight at 37 °C in Nutrient Broth. These stock cultures were stored in the dark at 4 °C during the survey.
Antibacterial activity was evaluated by Broth dilution technique following the procedures recommended by the National Commite for Clinical Laboratory Standards [Citation42]. MICs were determined twice in duplicate experiments. The Nutrient Broth, which contained logarithmic serially two fold diluted amount of test compound and controls, was inoculated with approximately 5×105 c.f.u. Aqueous DMSO (20%) was used as negative control (containing compounds but no inoculum).
Inhibition zones of compounds were determined by the disc diffusion method [Citation43]. The sterilized (autoclaved at 120 °C for 30 min), liquified Mueller Hinton agar (40–50 °C) was inoculated with the suspension of the microorganism (matched to 0.3 Mc Farland) and poured into a Petri dish to give a depth of 3–4 mm. The paper discs impregnated with the test compounds (60μg) were placed on the solidified medium. Discs were placed on agar plates and the cultures were incubated at 37 °C for 24 h for bacteria. Inhibition zones formed on the medium were evaluated in mm. Ciprofloxacin was chosen as a standard in antibacterial activity measurements (positive control).
Result and discussion
Chemistry
NMR spectra of compounds were recorded in CDCl3 taking using TMS as an internal standard. 1H NMR and 13C NMR data for the compounds are given in and , respectively. It is known that symmetric C and H atoms in the compounds show the same chemical shift because of having same chemical environment. Shifting values of compounds (1–8) is in reasonable agreement with literature data for the similar compounds [Citation14, Citation30]. In NMR spectra, aromatic proton peaks are observed at about 7. 5 ppm, and aromatic carbon peaks are shown at ~ 126 ppm. The chemical shift of the NH group of the compounds in CDCl3 is in the range of 4.62-5.02 ppm, however, in d6-DMSO at ~ 6.9 ppm. Difference of chemical shift may be attributed to the formation of intermolecular interactions with polar solvent DMSO.
Wave numbers of the selected vibration of IR spectra of the compounds are listed in . The IR spectra of the compounds were found to be very similar to each other. More characteristic vibrations of the compounds are as follows: asymmetric and symmetric stretching vibrations of SO2 groups are observed at ~1115 and ~1135 cm−1, respectively. Terminal methyl stretching vibrations are found in the range of 2970-1875 cm−1. The absence of NH2 vibrations which are observed between 3300 and 3350 cm−1 as double bonds and presence of single and strong NH band at~3280 cm−1 confirms the presence of secondary amine group in compounds (1–8).
Table 1. The 1H NMR data of the compounds (for half of molecules).
Table 2. The 13C NMR data of the compounds.
Table 3. Wavenumber (cm−1) of selected vibration of the compounds.
Antimicrobial activity
Antimicrobial activity of compounds tested is presented in . Microbiological results indicated that the synthesized compounds possessed a broad spectrum of activity against the tested microorganisms and showed relatively better activity against Gram-negative than Gram- positive bacteria. Sulfonyl hydrazide (9) showed the best activity with lowest MIC (42 μg/mL) against E.coli and S.enteritidis bacteria. Among the disulfonamides, compound (5) possessed great activity with MIC of 100 μg/mL against Gram-negative E.coli and S.enteritidis bacteria. All compound showed poor activity against B. Magaterium, and compound (4) exhibited the lowest inhibitory activity with MIC of 440 μg/mL.
Table 4. Antimicrobial activity of compounds with microdilution method.
It is known that, in general, para-aminobenzenesulfonamide derivatives possessing one seconderary amine group show good antimicrobial activity as competitive dihydropteroate synthase inhibitors. In this study, sulfonyl hydrazide having one amino and one seconderary amine groups showed the best activity (9), however, sulfonamides having two seconderary amine groups showed a profound drop in activity (1-8). Furthermore, replacing the methyl group at the 4-position of the aryl ring with electron-donating methoxy group resulted in modera increase in activity (5-8). In addition, antibacterial activity increased proportionately as the length of the carbon chain between NH groups decreased. This could be due to bulkiness of the carbon chain, which render the molecule unable to penetrate through the cell wall of the bacteria. As shown in , all tested compounds, although applied in 12-fold higher concentrations (60 μg) than the reference ciprofloxacin (5 μg), displayed weak activity against bacteria.
Table 5. Result of the antimicrobial test of compounds (disc potency 60 µg).
Structure–activity relationship: log P (octanol–water partition coefficient) is an important physicochemical parameter in the development of lipophilicity index [Citation32–35]. It is generally accepted that more lipophilic molecule will interact more easily with the fatty acid tails of the lipid bilayer, thus allowing the molecule to cross cell membranes. Lipophilicity values () of our compounds have been computed using AlogPs, AC logP and AB/logP methods [Citation36]. Calculated values spans the 0.26-1.61 region with AlogPs, the minus 0.42-3.24 region with AC logP and the 0.53-4.42 region with AB/logP methods. The most active compound (9) has the least lipophilicity (AlogP = 0.26). Calculated AlogP values of dichlorophenamide (0.95) and sulfanilamide( -0.16) taken from Remko and Lieth are included for comparison [Citation37]. As shown in , although different values were calculated using different methods, same order were obtained that increasing antibacterial activity followed decreasing lipophilicity. After plotting the calculated values versus the experimental antimicrobial activity data (expessed as pMIC, in which MIC values converted to mM) regression coefficients(r) were found between 0.65 and 0.97 (). This result shows that there are strong dependency between activities and lipophilicity for all bacteria.
Table 6. Calculated partition coefficients and aqueous solubility of the compound studied.
Table 7. Antimicrobial activity of compounds and regression coefficient.
LogS is indicative of the intrinsic solubility of compounds in neutral state. The calculated aqueous solubility values with AlogPS, AClogS, AB/logS methods are given in . The highly active compound (9) has the most solubility (AlogPS= -1.21 or 10.69 g/L). As seen in , positive correlation between calculated solubility values and antibacterial activity of compounds was found. As seen in , there are also significant correlation between solubility and the antimicrobial activity (regression coefficient = 0.69 - 0.84).
Dissociation constant (pKa) which is a measure of the strength of an acid or a base is very useful in understanding the behavior of drug molecules at the site of action. Calculated dissociation constant (pKa) of compounds (5-9) shows that sulfonyl hydrazide is more acidic (6.20) than the disulfonamides (9.70). In the literature, Bell and Roblin previously reported that there were relationship between the pKa and antibacterial activity of an extensive series of sulfonamides, and maximum pKa values lay between 6.0 and 7.4 [Citation38].
In this research, numerous studies have been made to find a correlation between the physicochemical properties of the compounds and their antibacterial activity, some physicochemical properties (molecular volume, molecular refractivity, HOMO, LUMO, dipole moment) were calculated using HyperChem [Citation39] and total topological polar surface area was calculated using DRAGON software [Citation40]. Surface area, molecular volume, and topological indices have been used as an expression of the steric effects. As seen in , the most active compound (9) has the lowest value of molecular weight, molecular volume, total topological polar surface area and molecular refractivity. Generally speaking, as steric effects is lowered, the activity of the compounds is increased. The attempt to correlate antibacterial activities with electronic parameter such as HOMO, LUMO, dipole moment(Dμ) and local charges was unsuccessful (r = 0.055-0.197).
Table 8. Some physicochemical properties of compounds.
Bioavailability of compounds have been connected to some parameters (logP, molecular weight, counts of hydrogen bond acceptors and donors), which is expressed by Rule of 5 or the Lipinski rule [Citation41]. Rule of 5 have also been calculated for compounds (1-9) and they obey the Lipinski rule. This indicates favorable properties for drug absorption and less probability of problems in permeation.
Declaration of interest: The authors thank the TUBITAK Foundation (No: TBAG 104 T 390) and Gazi University Scientific Research Projects (No : 05/2008-05) for financial supports.
References
- Anand, N. In Burger’s Medicinal Chemistry and Drug Discovery; Wolff, M E., Ed., 5th ed.; Therapeutic Agents; J. Wiley & Sons: New York, 1996; Vol. 2, pp 527–544.
- Mastrolorenzo A, Scozzafava A, and Supuran C T. Antifungal activity of silver and zinc complexes of sulfadrug derivatives incorporating arylsulfonylureido moieties. Eur. J Pharm Sci 2000; 11(2): 99–107.
- Onisha G P, Eddy K M, Alexis M N, and Ian G M. Sulfa drugs strike more than once. Trends Parasitol 2004; 20(1):1–3.
- Kamel A M, Lobna M A-A, El-Sayed M L, Mohamed I H, and Rania H B. Hydrazones of 2-aryl-quinoline-4-carboxylic acid hydrazides: Synthesis and preliminary evaluation as antimicrobial agents. Bioorg Med Chem. 2006; 14:8675–8682.
- Hadj-esfandiari N, Navidpour L, Shadnia H, Amini M, Samadi N, Faramarzi M A, and Shafiee A. Synthesis, antibacterial activity, and quantitative structure-activity relationships of new (Z)-2-(nitroimidazolylmethylene)-3(2H)-benzofuranone derivatives. Bioorg Med Chem Lett. 2007; 17:6354–6363.
- Khan M W, Alam M J, Rashid M A, Chowdhury R. A new structural alternative in benzo[b]furans for antimicrobial activity. Bioorg Med Chem. 2005; 13(16): 4796–4805.
- Zanatta N A, Coelho S H, Borchhardt H S, Machado D M, Flores P, Silva K M, Spader F M, Santurio T B, Bonacorso J M, Helio G, Martins M AP. Synthesis, antimicrobial activity, and QSAR studies of furan-3-carboxamides. Bioorg Med Chem. 2007;15(5):1947–1958.
- Greenfield R A, and Bronze M S. Prevention and treatment of bacterial diseases caused by bacterial bioterrorism threat agents. Drug Discov. 2003; 8(19):881–888.
- Ezabadi I R, Camoutsis C, Zoumpoulakis P, Geronikaki A, Sokovic M, Glamocilija J, and Ciric A. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg Med Chem. 2008; 16(3): 1150–1161.
- Patel P R, Ramalingan C, and Park Y-T. Synthesis and antimicrobial evaluation of guanylsulfonamides. Bioorg Med Chem Lett. 2007;1 7(23): 6610–6614.
- Isik, K.; Kocak F. O., Microbiol. Res., Available online 27 February 2007.
- Fathalla O A, Zaghary W A, Radwan H H, Awad S M, and Mohamed M S. Synthesis of new 2-thiouracil-5-sulfonamide derivatives with biological activity. Arch Pharm Res. 2002; 3(25): 258–269.
- Mastrolorenzo A, Scozzafava A, Supuran C T. Antifungal activity of silver and zinc complexes of sulfadrug derivatives incorporating arylsulfonylureido moieties. Eur J Pharm Sci. 2000; 11(2): 99–107.
- Ozbek N, Katırcıoglu H, Karacan N, and Baykal T. Synthesis, characterization and antimicrobial activity of new aliphatic sulfonamide. Bioorg Med Chem. 2007;15(15): 5105–5109.
- Alyar S, Ozbek N, Karacan N. Synthesis and antimicrobial activity of new substituted arylsulfonamides. Drug Future. 2007; 32: 126–127 Suppl. A
- Ozbek N, Alyar S, Karacan N. Synthesis, characterization and antimicrobial activity of new butanesulfonamides. Drug Future 2007; 32: 128 Suppl. A
- Ienco, A, Mealli C, Paoli P. Dodoff N I, Kantarci Z, Karacan N. Structure and vibrational spectrpscopy of methanesulfonic acid hydrazide: an experimental and theoretical study New J Chem. 1999; 23:1253–1260.
- Dodoff N I, Ozdemir U, Karacan N, Georgieva M Ch,Konstantinov S M, and Stefanova M E. Schiff Bases of Methanesulfonylhydrazine. Synthesis, Spectroscopic Characterization, Conformational Analysis and Biological Activity. Z Naturforsch. 1999;54b:1553–1562.
- Alyar S, Ozmen U O, Karacan N, Sentürk O S, and Udachin K.A. Tautomeric properties, conformations and structure of 2-hydroxyacetophenone methanesulfonylhydrazone. J Mol Struct., Available online 12 February 2008
- Ozdemir U, Sentürk O S, Sert S, Karacan N, Ugur F. Photochemical reactions of metal carbonyls [M(CO)(6) (M=Cr, Mo and W), Re(CO)(5)Br, Mn(CO)(3)Cp] with 2-hydroxyacetophenone methanesulfonylhydrazone (apmsh) Trans Met Chem. 2003; 28(4): 443–446.
- Sert S, Sentürk O S, Ozdemir U, Karacan N, U_ur F. Synthesis and characterization of the products from reaction of metal carbonyls [M(CO)(6) (M = Cr, Mo, W), Re(CO)(5)Br, Mn(CO)(3)Cp] with salicylaldehyde methanesulfonylhydrazone J Coord Chem. 2004; 57(3): 183–188.
- Ozdemir U, Karacan N, Sentürk O S, Sert S, U_ur F. Synthesis and characterization of metal carbonyl complexes of M(CO)(6)(M = Cr, Mo, and W), Re(CO)(5)Br, and Mn(CO)(3)CP with - Acetonemethanesulfonylhydrazone (amsh) and methanesulfonylhydrazone (msh). Synth React Inorg Met Org Chem. 2004; 34(6): 1057–1067.
- Ozdemir U, Sentürk O S, Sert S, Karacan N, U_ur F. Reaction of metal carbonyls with 2-hydroxy-1-naphthaldeyde methanesulfonylhydrazone and characterization of the substitution products. J Coord Chem. 2006; 59(17): 1905–1911.
- Ozmen U O, Olgun G. Synthesis, characterization and antibacterial activity of new sulfonyl hydrazone derivatives and their nickel(II) complexes Spectrochim Acta Part A 2008; 70(3):641–645.
- Senturk O S, Ozdemir U, Sert S, Karacan N, U_ur F. Photochemical reactions of metal carbonyls [M(CO)(6) (M = Cr, Mo, W), Re(CO)(5)Br, Mn(CO)(3)Cp] with salicylaldehyde ethanesulfonylhydrazone (Hsalesh). J Coord Chem. 2007; 60(2):229–232.
- Senturk O S, Ozdemir U, Sert S, Karacan N, U_ur F. Synthesis and characterization of some metal carbonyls with 2-hydroxyacetophenone ethanesulfonylhydrazone. Inorg Chem Commun. 2003; 6(7):926–929.
- Rastenyte, L.; Jokubaityte, S.; Mozolis, V., Lietuvos TSR Mokslu Akademijos Darbai, Serija B: Chemija, Technika, Fizine Geografija, 1974, 1, 69.
- Gajadhar-Plummer A S, Kahwa A, Mague J T. N,N0-Ethylenebis(p-toluenesulfonamide). Acta Crystallogr. Section E: 2001; E57(1): 68–69.
- Grigoryan L A, Kaldrikyan M R, Arsenyan E G, Stepanyan G M, and Garibdzhanyan B T. Arylsulfonic acid derivatives. Synthesis and antitumor activity of di- and tetra(benzylsulfonyl)diamines. Pharm Chem J. 2000; 4(34):186–188.
- Friedman L, Litle R L, and Reichle W R.,p-toluensulfonylhydrazide. Org Syntheses. 1960;40:93.
- Tremayne M, Lightfoot P, Glidewell C, Harris K D M, Shankland K Gilmore C J Bricogne G, Bruce P G. Materials chemistry communications. Application of the combined maximum entropy and likelihood method to the ab initio determination of an organic crystal structure from X-ray powder diffraction data. J Mater Chem. 1992;2(12):1301–1302.
- Grande F, Aiello F, Grazia O D, Brizzi A, Garofalo A, Neamati N. Synthesis and antitumor activities of a series of novel quinoxalinhydrazides. Bioorg Med Chem 2007;15(1):288–294.
- Yakaiah T, Lingaiah B P V, Narsaiah B, Shireesha B,Ashok Kumar B, Gururaj S, Parthasarathy T, Sridhar B. Synthesis and structure-activity relationships of novel pyrimido[1,2-b]indazoles as potential anticancer agents against A-549 cell lines. Bioorg Med Chem Lett. 2007;17(12):3445–3453.
- Kamal A, Khan M N A, Reddy K S, Rohini K. Synthesis of a new class of 2-anilino substituted nicotinyl arylsulfonylhydrazides as potential anticancer and antibacterial agents. Bioorg Med Chem. 2007; 15(2), 1004–1013.
- Leo A J. Calculating log Poct from structures. Chem Rev. 1993; 93:1281–1306.
- ALOGPS 2.1 software available at http://www.vcclab.org/lab/alogps.
- Remko M, and Lieth C W. Theoretical study of gas-phase acidity, pKa, lipophilicity, and solubility of some biologically active sulfonamides. Bioorg Med Chem. 2004; 12(20): 5395–5403.
- Bell P H, and Roblin R O. Studies in Chemotherapy. VII. A Theory of the Relation of Structure to Activity of Sulfonilamide Type compounds. J Am Chem Soc. 1942; 64:2905–2017.
- HyperChem 7.5 program, Hypercube Inc Toronto, Canada, 2002.
- http://www.talete.mi.it/ dragon_exp.htm, Italia
- Lipinski C A, Lombardo F, Dominy B W, and Feeney P J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliver Rev. 1997; 23(1-3): 3–25.
- Wayne P A. National Committee for Clinical Laboratory Standards. Approved Standard M7-A4. 1997 and M27. 1997.
- Bauer A W, Kirby W M, Sherries J C, Truck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966; 45(4): 493–496.