Abstract
Inhibitors of alpha glucosidase have potential use in the treatment of diabetes mellitus. The stem extract of Tinospora cordifolia was evaluated for inhibition of the enzyme. The extract was also found to inhibit the salivary and pancreatic amylase and therefore can effectively reduce an increase in postprandial glucose level. The crude ethyl acetate, dichloromethane (DCM), chloroform and hexane extracts of Tinospora cordifolia were studied. 15 mg of the DCM extract was most effective in that showed 100 % inhibition of the alpha glucosidase whereas salivary amylase was inhibited to the extent of 75 % and pancreatic amylase to 83 %. On giving a maltose load of 2mg / g along with 0.3 mg / g body weight of the DCM Tinospora stem extract a decrease was revealed in the hyperglycemic shoot up in normal and diabetic animals by 50 and 58 % respectively as compared to the controls. The extract was found to inhibit alpha glucosidase in a non-competitive manner.
Introduction
The prevalence and morbidity associated with type 2 diabetes mellitus continues to increase throughout the world [Citation1, Citation2]. The secondary complications in diabetes mostly stem from micro and macro vascular changes. Several studies on the treatment of Type 2 diabetes suggest that improved glycemic control reduces micro vascular risks [Citation3–7].
Glucosidase inhibitors are widely studied and isolated from different sources such as plants [Citation8] and microbes [Citation9]. These inhibitors have number of applications in therapeutics [Citation10]. In the 1970s, it was realized that inhibition of all or some of the intestinal disaccharidases and pancreatic alpha amylase by inhibitors could regulate the absorption of carbohydrate and these inhibitors could be used therapeutically in the oral treatment of the noninsulin-dependent diabetes mellitus (Type II diabetes). Bayer’s researchers found that the Actinoplanes strain SE 50 yields a potent sucrase inhibitor, acarbose, which inhibits pig intestinal sucrase with an IC50 value of 0.5 mM [Citation11]. However use of acarbose leads to intestinal disturbances in many patients and there exists a need for development of better and more tolerable alpha glucosidase inhibitors.
Tinospora cordifolia (TC) belonging to the Menispermaceae family is a plant indigenous to India. It is known as Giloe or Guduchi locally and has been documented in the ancient Ayurvedic writings to possess anti diabetic and immunomodulatory property.
Tinospora cordifolia has been reported to possess multiple activities such as antidiabetic [Citation12], immunomodulatury [Citation13–14], antioxidant [Citation15], and as a radioprotective agent [Citation16]. The plant extract has been reported to have hypolipedaemic action [Citation17–18]. The plant is used for hundreds of years in Ayurvedic medicine with no reported toxicity.
In the present study the glucosidase inhibitory activity of the Tinospora cordifolia (TC) has been evaluated in vitro and in vivo. TC has been demonstrated to possess inhibitory action against alpha glucosidase from rat intestine, pancreatic amylase and salivary amylase. It also has a good potential to control the increase in the post prandial glucose level as observed in the oral glucose tolerance test.
Methods and materials
Plant material
Tinospora cordifolia stem was collected fresh from Kolhapur and Sindhudurg Districts of Maharashtra, India and verified by the Botany Department of Shivaji University Kolhapur, India. It is deposited in the herbarium of the same department as specimen (voucher number ADC 2527).
Extraction
The shade dried stems were chopped into small pieces and pulverized into a fine powder. The plant material (20 g) was soaked in 100 mL hexane with occasional shaking, at room temperature. After 15 h, the hexane soluble material was filtered off. The filtrate was concentrated to dryness under vacuum at low temperature (408C ) using a rotary evaporator.
In the same way chloroform, ethyl acetate and dichloro methane extracts were obtained and used for the study.
Animals
Albino rats weighing between 150–200 g were used. The experimental protocol was submitted to and approved by the Animal Ethics Committee. The rats were fed with standard rat diet purchased from Amruth India. Animals were provided with food and water ad libitum and maintained between 25–288C.
Glucose tolerance test with maltose load
Glucose tolerance test was administered by feeding the rats with 2 mg maltose/g of body weight. Blood was withdrawn from tail vein at defined time intervals and blood glucose measured using Accu-check. All the results presented are average values of experiments conducted on a set of six rats.
Glucose measurement
Glucose was measured using glucose oxidase kits purchased from Qualigens, India. For Oral Glucose Tolerance Test, glucose was measured by a glucometer, using Accucheck purchased from Roche India, and results verified using appropriate controls. Diabetes was induced by injecting 160 mg of alloxan/Kg body weight intra-peritonealy to overnight fasted animals [Citation19]. The diabetic status of the animals was verified by checking their blood glucose.
Preparation of rat intestinal alpha glucosidase
All procedures for preparation of rat intestinal alpha glucosidase were performed at 48C. The small intestinal mucosal tissue was collected by scraping the luminal surface firmly with a spatula. The mucosal scrapings were homogenized with 0.2 M sodium phosphate buffer pH 7.0 containing 1 % triton X 100, and then centrifuged at 12000 rpm for 15 min. The supernatant fraction contained rat small intestinal alpha glucosidase. Butanol was added to the supernatant fraction in 1: 1 proportion and centrifuged at 15000 rpm for 15 min. The aqueous layer was dialyzed overnight against the same buffer. After dialysis, the concentrated enzyme was stored at 48C. This crude enzyme was used in the study to observe inhibition by different extracts of Tinospora cordifolia.
Pancreatic amylase
Rat was sacrificed and quickly dissected and pancreas immediately washed with cold saline for 3 to 4 times. The pancreatic tissue was finely cut in to small pieces and homogenized in cold saline. The mixture was then centrifuged with protease inhibitor and supernatant used as crude enzyme. Procedure was carried out at 48C.
Salivary amylase
Salivary amylase was prepared by diluting the saliva with saline and stored in cold condition at 48C.
In vitro alpha glucosidase inhibition assay
The activity of the rat small intestinal alpha glucosidase was determined by measuring the formation of glucose. The standard incubation mixture contained 0.2 M sodium phosphate buffer (pH 6.6), 1 % starch and 0.2 mL enzyme. After incubation at 378C for 30 min, the liberated glucose was measured by GOD / POD kit. One unit of enzyme activity was defined as the quantity of enzyme producing 1mmol glucose per min at 378C at pH 6.6. In the same way pancreatic and salivary amylase assays were carried out. In all the inhibitor studies, enzyme was pre-incubated with the inhibitor for 3 min before addition of the substrate. The inhibition of alpha glucosidase was studied with increasing concentration of the plant extract. 1, 3 and 5 mg of the DCM extract (in 100 μL of DMSO) was used with increasing concentration of the substrate. Simultaneously a control test with only DMSO was carried out.
Statistical analysis
All data are expressed as mean ± S. D. for six experiments. The statistical significance was evaluated by ANOVA and individual comparisons were done by t test: (two samples assuming unequal variance).
Results and discussion
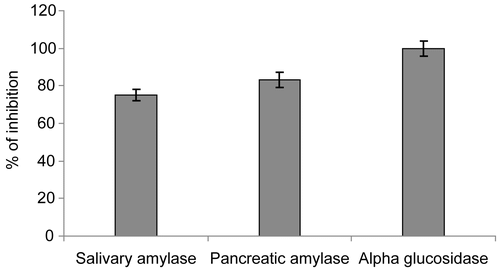
The various extracts of Tinospora cordifolia were studied for inhibitory activity of alpha glucosidase enzyme (). The dichloromethane (DCM) extract has shown highest inhibition as compared to the other extracts. The inhibition of the alpha glucosidase is greater than the inhibition of the salivary and pancreatic amylase at the same concentration of the DCM plant extract. The Tinospora extract therefore appears to inhibit all the amylases in a substantial manner with almost 100 percent inhibition of the intestinal alpha glucosidase ().
Figure 1. Inhibition of the alpha glucosidase by various solvent extracts of Tinospora cordifolia (15 mg/mL).
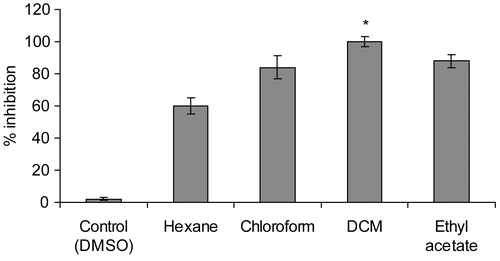
shows the effect of increasing concentration of the DCM extract. The extract has the potential to inhibit the enzyme almost linearly up to 15 mg. The IC50 value of this extract is 3 mg/mL.
Figure 3. Effect of various concentration of DCM extract (in 100 μL of DMSO) of Tinospora cordifolia (TC) on the activity of the alpha glucosidase.
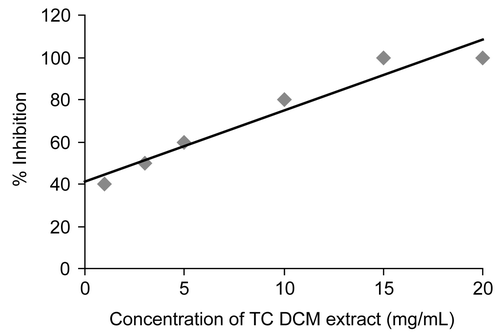
The double reciprocal Lineweaver-Burk plot shows that the DCM extract is a non competitive inhibitor of alpha glucosidase (). The Ki value for DCM extract of the TC is 9.86 mg/mL. The commercially used alpha glucosidase inhibitor, acarbose is a competitive inhibitor of the enzyme [Citation20] and the inhibition would be overcome by higher substrate concentration. Hence, a heavy meal would need a higher concentration of acarbose to reduce postprandial glucose level. On the other hand a non-competitive inhibitor would bind to a site other than the active site and would be able to inhibit better even after a heavy meal.
Figure 4. Lineweaver-Burk plot of alpha glucosidase inhibition by different concentration of the DCM extract (in 100 μL of DMSO).
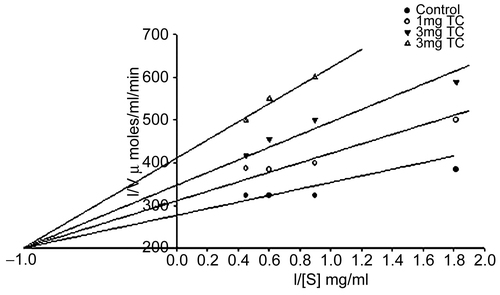
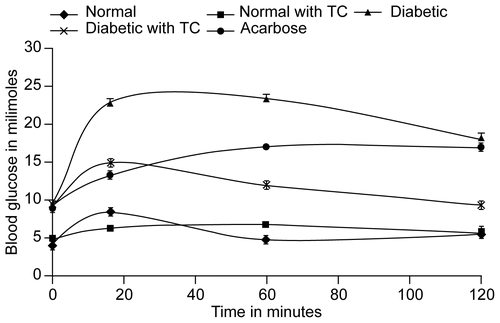
The inhibitors of the alpha glucosidase, salivary and pancreatic amylases are useful in the diabetes treatment [Citation10]. As the inhibitors cause delay in the digestion of complex carbohydrate, they prevent the rise in the postprandial glucose level. depicts the variation in the blood glucose values of diabetic and normal rats after administration of the maltose with or without the DCM extract (0.3 mg/g). It can be observed that while in case of the diabetic controls, the blood glucose level increased to 22.83 ± 0.7 mM by 16 minutes, it continues to rise to 23.38 ± 0.6 mM in one hour. As against this the diabetic rats with the plant extract show a decrease by 7.77 to 11.44 mM in the same time period. Acarbose reveals a decrease by about 9.44 ± 0.7 mM by the 16th minute it shows lesser difference by one hour (6.22 mM). It can be observed from the that by 2 hours while blood glucose level for diabetic control and acarbose are on par, the plant-treated animals show a drastic decrease by almost 8.88 mM. This implies that the Tinospora extract may be having other antidiabetic activity apart from alpha glucosidase activity.
Figure 5. Variations in levels of blood glucose in diabetic and normal rats after administration of maltose (2 mg/g) in presence of Acarbose 60 mg/Kg, or TC 300 mg/Kg.
Several reports on the phytochemical analysis of Tinospora cordifolia have been recently published. The major isolated compounds include the norditerpene furan glycosides cordiofolisides A, B, and C [Citation21] the daucane-type sesquiterpenes tinocordifolin and tinocordifolioside [Citation22, Citation23]. In addition, syringin, cordiol, cordioside, and the phenylpropene disaccharides cordifoliosides A and B were identified as the active principles with anticomplement and immunomodulatory activities. [Citation24–26]. But not much information is available on their biological activity regarding the active amylase inhibitor compound.
In conclusion, Tinospora cordifolia stem extract demonstrates good alpha glucosidase inhibitory activity. It exhibits a predominant non-competitive type of inhibition, which is not commonly observed. This would apparently be more advantageous than competitive inhibitors, which would be needed in larger quantity. Further attempts at isolation and purification of the active constituent from the extract are ongoing. In general, a good glycemic regime is maintained by administering a secretgouge or insulin or insulin sensitizer along with alpha glucosidase inhibitors. Tinospora cordifolia stem extract has the dual advantage of having alpha glucosidase inhibitory action as well as good antidiabetic action and hence could prove to be an effective treatment for diabetes mellitus.
Declaration of interest: The authors report no financial conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004; 140: 945–950.
- World Health Organization. Diabetes Programme. Facts and Figures. Accessed on 11 June 2007 at http://www.who.int/diabetes/facts/en/.
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352: 854–865.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352: 837–853.
- Gaster B, Hirsch IB. The effects of improved glycemic control on complications in type 2 diabetes. Arch Intern Med. 1998; 158: 134–140.
- Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995; 28: 103–17.
- Vijan S, Hofer TP, Hayward RA. Estimated benefits of glycemic control in microvascular complications in type 2 diabetes. Ann Intern Med. 1997; 127: 788–95.
- Yoshikawa M, Murakami T, Yashiro K, Matsuda H. Kotalanol a potent alpha glucosidase inhibitor with thiosugar sulfonium sulphate structure, from antidiabetic ayurvedic medicine Salacia reticulata. Chem. Pharma. Bull. 1998; 46: 1339–1340.
- Kameda Y, Asano N, Yoshikawa M, Takeuchi M, Yamaguchi T, Matsui K, Horri S, Fukase H, Valiolamine, a new alpha glucosidase inhibiting aminocyclitol produced by Streptomyces hygroscopicus J. Antibiotic 1984; 37: 1301–1307.
- Asano N,Glycosidase inhibitor: update and prospectives on practical use. Glycobiology 2003; 13(10): 93R–104R.
- Schmidt DD., Frommer, W., Mouller, L.,Truscheit, E. Glucosidase inhibitors from bacilli. Naturwissenshaften, 1979; 66: 584–585.
- Grover JK, Vats V, Rathi SS. Anti-hyperglycemic effect of Eugenia jambolana and Tinospora cordifolia in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. J Ethnopharmacol 2000; 73(3): 461–470.
- Kapil A, Sharma S. Immunopotentiating compounds from Tinospora cordifolia. J Ethnopharmacol 1997; 58: 89–95.
- Manjrekar PN, Jolly CI,Narayanan S. Comparative studies of the immunomodulatory activity Tinospora cordifolia and Tinospora sinensis. Fitoterapia 2000; 71(3): 254–257.
- Prince SM, Menon VPAntioxidant activity of Tinospora cordifolia roots extract in alloxan diabetes rats. Phytotherapy Res 2001; 15(3): 213–221.
- Goel HA, Prasad J, Singh S, Sagar RK, Agrawala PK, Bala M, Sinha K,Dorga R. Radioprotective potential of Herbal Extract of Tinospora cordifolia. J Radiat Res 2004; 45: 61–68.
- Prince SM, Padmanabhan M, Menon VP. Restoration of antioxidant defence by ethanolic of Tinospora cordifolia root extract in alloxan induced diabetic liver and kidney. Phytotherapy Res 2004; 18(9): 785–787.
- Prince SM, Menon VP. Hypogycemic and hypolipidemic action of action of alcohol extract of Tinospora cordifolia roots in chemical induced diabetic rats. Phytotherapy Res 2003; 17(4): 410–428.
- Chougale AD, Panskar SN, Gurao PG, and Arvindekar AU. Optimisation of Alloxan dose for induction of stable diabetes for prolonged period. Asian J Biochem 2007; 2: 402–408.
- Madar Z. The effect of Acarbose and miglitol (BAY- M-1099) on post prandial glucose levels following ingestion of various sources of starch by non diabetic and streptozotocin – induced diabetic rats. Nut Phara Toxico 1989; 119: 2023–2029.
- Gangan VD, Pradhan P, Sipahimalani AT, Banerji A., Cordifolisides, A, B, C: norditerpene furan glycosides from Tinospora cordifolia. Phytochemistry. 1994; 37: 781–786
- Maurya R., Dhar KL, Handa SS. A sesquiterpene glucoside from Tinospora cordifolia. Phytochemiatry 1997; 44: 749–750.
- Maurya R., Handa SS. Tinocordifolin, a sesquiterpene from Tinospora cordifolia. Phytochemistry 1998; 49: 1343–1345.
- Kapil A, Sharma S. Immunopotentiating compounds from Tinospora cordifolia. J Ethnopharmacol 1997; 58: 89–95.
- Maurya R Dhar KL, Handa SSCordifolisides A and B, two new phenylpropene disaccharides from Tinospora cordifolia possessing immunostimulant activity. Nat Prod Lett 1996; 8: 7–10.
- Chintalwar G, Jain A, Sipahimalani A, Banerji A, Sumariwalla P, Ramakrishnan R, Sainis K. An immunologically active arabinoga-lactan from Tinospora cordifolia. Phytochemistry 1999; 52: 1089–1093.