Abstract
A series of new 2-aryl-4-thiazolidinones (3 and 4) was synthesized from 2-hydroxy-2,2-diphenyl-N’-[(substituted phenyl)methylene]acetohydrazides (2) and mercaptoacetic acid or 2-mercaptopropionic acid. The antimycobacterial activity of these compounds was determined and several leads with 95–99% inhibition at 6.25 μg/mL test concentration were identified. In addition, antitumor activities were measured against several tumor cell lines, and significant growth inhibition was observed for compound 4p. Taken together, 2-aryl-4-thiazolidinones were shown to be promising scaffolds for both antimycobacterial and tumor-targeting compounds.
Introduction
Tuberculosis (TB) is a potentially lethal infectious disease of the lungs, which is mainly caused by Mycobacterium tuberculosis. This disease is one of the world’s greatest sources of mortality and morbidity. Approximately, 30% of the world’s population is believed to be infected with this pathogen [Citation1–3]. Every year, approximately 8 million of these infected people develop active TB and more than 2 million people die from pulmonary TB [Citation1–3]. Unfortunately, the incidence of TB infection has risen steadily in the past decades and an important reason for this is the development of drug resistance in M. tuberculosis. Multidrug-resistant tuberculosis (MDR-TB) is an emerging public health problem in many regions of the world, particularly in the developing countries. MDR-TB strains do not respond to most anti-TB drugs (including rifampin and isoniazide) that are still being used in clinics [Citation4]. Another cause is an increase in human immunodeficiency virus (HIV) infection. Even though the results are somewhat conflicting, it is to be expected that the increased vulnerability of immunocompromised patients for TB is life threatening [Citation5]. The association of TB and HIV infections is so dramatic that, in African countries, nearly two thirds of the patients diagnosed with TB are also HIV-1 seropositive [Citation6]. The lethal combination of drug-resistant TB and HIV infection is a growing problem that presents serious challenges for effective TB control. In view of this situation, in 1993, the World Health Organization (WHO) has declared TB as a global emergency.
No new drugs against tuberculosis has been developed in the past 30 years, while the development of drug resistance in M. tuberculosis is an ongoing process. Hence, there is an urgent need for new antituberculosis agents with new and unique mechanisms of action to avoid the resistance of M. tuberculosis.
Recently, there has been considerable interest in the chemistry and pharmacology of the thiazolidin-4-one containing scaffolds. These compounds display a broad spectrum of biological activities, such as antimycobacterial [Citation7–10], antifungal [Citation11–13], antiviral [Citation14–18], anticancer [Citation13], and anticonvulsant [Citation19] activities.
To further explore the pharmaceutical relevance of thiazolidinone containing compounds, we synthesized a wide variety of analogs and tested these compounds for antimycobacterial activities. As these compounds were also reported to have anticancer properties, we also tested our compounds for anticancer activity [Citation13]. In this study, we report several new 4-thiazolidinone derivatives with promising antimycobacterial and antitumor activities.
Experimental
Chemistry
All melting points were measured in open capillary tubes with Buchi 540 and are uncorrected. The compounds were checked for purity using thin layer chromatography on silica gel HF254 (E.Merck, Darmstadt, Germany). IR (KBr) spectra were recorded using a Perkin-Elmer 1600 FTIR spectrophotometer and all values are expressed as υmax cm−1. 1H-NMR, 13C-NMR(APT), and HSQC spectra were recorded on a VarianUNITYINOVA 500 MHz spectrometer using DMSO-d6 as solvent, and chemical shifts are given in ppm with TMS as a Standard. Elemental analysis of all new compounds was performed on a Thermo Finnigan Flash EA 1112 elemental analyzer. Mass spectral data (LC/MS-APCI) were recorded on a Finnigan™ LCQ™ Mass Spectrometer.
2-Hydroxy-2,2-diphenylacetohydrazide (1).
Methyl 2-hydroxy-2,2-diphenylacetate (0.05 mol), 12 mL hydrazine hydrate (98%) was heated under reflux for 12 h. The reaction mixture was transferred to a crystallizing dish and allowed to stand until crystallization occurred. The crude product thus obtained was then recrystallized from C2H5OH.
2-Hydroxy-2,2-diphenyl-N’-[(substituted phenyl)methylene]acetohydrazides (2).
A solution of 6 mmol 1 in 30 mL abs. C2H5OH and 6.6 mmol of an appropriate aromatic aldehyde was heated under reflux for 4 h. The precipitate obtained was purified either by recrystallization from C2H5OH or by washing with C2H5OH.
General method for the synthesis of 2-Hydroxy-N-(4-oxo-2-substitutedphenyl-1,3-thiazolidin-3-yl)-2,2-diphenylacetamide/2-Hydroxy-N-(5-methyl-4-oxo-2-substitutedphenyl-1,3-thiazolidin-3-yl)-2,2-diphenylacetamides (3,4).
A mixture of 5 mmol 2 and 20 mmol mercaptoacetic acid or α-mercaptoacetic acid was refluxed in 30 mL dry benzene (care-carcinogenic) for 6 h using a Dean-Stark water separator. Excess benzene was evaporated in vacuo. The resulting residue was triturated with saturated NaHCO3 solution until CO2 evolution ceased and refridgerated overnight. The crude product thus obtained was washed with H2O, dried, and recrystallized from C2H5OH/H2O.
N-[2-(2-fluorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3a).
White small needles. Yield 25%; m.p. 190–193°C; IR(KBr): υ 3291 (O-H/N-H), 1685,1722 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.69,3.83 (2H, 2d, J=15.61 Hz, thiazolidin C5-H2), 6.08 (1H, s, thiazolidin C2-H), 6.73 (1H, brs, COH), 7.12–7.16 (4H, m, Ar-H), 7.18–7.21 (3H, m, Ar-H), 7.23 (5H, s, Ar-H), 7.35–7.40 (1H, m, Ar-H), 7.60 (1H, t, J = 6.83 Hz, Ar-H), 10.33 (1H, brs, CONH). Anal. Calcd. for C23H19FN2O3S (422.47): C, 65.39; H, 4.53; N, 6.63. Found: C, 65.04; H, 4.22; N, 6.36%.
N-[2-(3-fluorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3b).
White needles. Yield 54%; m.p. 178–180°C; IR(KBr): υ 3276 (O-H/N-H), 1684,1719 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 3.60,3.77 (2H, 2d, J = 15.78 Hz, thiazolidin C5-H2), 5.76 (1H, s, thiazolidin C2-H), 6.66 (1H, brs, COH), 7.04–7.06 (3H, m, Ar-H), 7.09–7.15 (8H, m, Ar-H), 7.20–7.25 (3H, m, Ar-H), 10.24 (1H, brs, CONH). Anal. Calcd. for C23H19FN2O3S (422.47): C, 65.39; H, 4.53; N, 6.63. Found: C, 65.01; H, 4.22; N, 6.34%.
N-[2-(4-fluorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3c).
White crystals. Yield 47%; m.p. 184–186°C; IR(KBr): υ 3278,3545 (O-H/N-H), 1670,1740 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.72,3.85 (2H, 2d, J = 15.62 Hz, thiazolidin C5-H2), 5.84 (1H, s, thiazolidin C2-H), 6.73 (1H, s, COH), 7.11–7.14 (4H, m, Ar-H), 7.21–7.25 (3H, m, Ar-H), 7.27 (5H, s, Ar-H), 7.46–7.49 (2H, m, Ar-H), 10.27 (1H, s, CONH); 13C-NMR (APT)(DMSO-d6/125 MHz) δ (ppm): 30.19 (C5), 61.85 (C2), 81.24 (C-OH), 127.98, 128.01, 128.04, 128.11, 128.19, 128.21, 115.66, 131.84, 131.27, 131.34 (ar. CH), 134.41, 144.10 (ar. C), 162.13 (ar. C-F), 169.10 (amide C=O), 172.14 (lactam C=O). LC/MS: m/z 421 (M-H)−. Anal. Calcd. for C23H19FN2O3S (422.47): C, 65.39; H, 4.53; N, 6.63. Found: C, 65.45; H, 4.31; N, 6.42%.
N-[2-(2-chlorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3d).
White small needles. Yield 72%; m.p. 185–187°C; IR(KBr): υ 3188,3307 (O-H/N-H), 1671,1714 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.71,3.85 (2H, 2d, J=15.62 Hz, thiazolidin C5-H2), 6.20 (1H, s, thiazolidin C2-H), 6.77 (1H, s, COH), 7.23 (10H, s, Ar-H), 7.33–7.35 (2H, m, Ar-H), 7.41–7.43 (1H, m, Ar-H), 7.76–7.78 (1H, m, Ar-H), 10.44 (1H, s, CONH). Anal. Calcd. for C23H19ClN2O3S (438.93): C, 62.94; H, 4.36; N, 6.38. Found: C, 62.70; H, 4.11; N, 6.06%.
N-[2-(3-chlorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3e).
White powder. Yield 42%; m.p. 162–164°C; IR(KBr): υ 3266 (O-H/N-H), 1681,1716 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.69,3.86 (2H, 2d, J=15.62 Hz, thiazolidin C5-H2), 5.82 (1H, s, thiazolidin C2-H), 6.73 (1H, brs, COH), 7.14–7.15 (2H, m, Ar-H), 7.21–7.23 (3H, m, Ar-H), 7.25 (5H, s, Ar-H), 7.29–7.35 (2H, m, Ar-H), 7.37–7.39 (1H, m, Ar-H), 7.57 (1H, brs, Ar-H), 10.34 (1H, brs, CONH). Anal. Calcd. for C23H19ClN2O3S (438.93): C, 62.94; H, 4.36; N, 6.38. Found: C, 62.83; H, 4.14; N, 6.21%.
N-[2-(4-chlorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3f).
White powder. Yield 69%; m.p. 183–185°C; IR(KBr): υ 3189,3459 (O-H/N-H), 1669,1707 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.69,3.83 (2H, 2d, J=15.74 Hz, thiazolidin C5-H2), 5.80 (1H, s, thiazolidin C2-H), 6.69 (1H, brs, COH), 7.07 (2H, d, J=7.32 Hz, Ar-H), 7.19–7.26 (8H, m, Ar-H), 7.33 (2H, d, J=8.53 Hz, Ar-H), 7.42 (2H, d, J=8.30 Hz, Ar-H), 10.25 (1H, brs, CONH). Anal. Calcd. for C23H19ClN2O3S (438.93): C, 62.94; H, 4.36; N, 6.38. Found: C, 62.69; H, 4.55; N, 6.05%.
N-[2-(2-bromophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3g).
White small needles. Yield 16%; m.p. 195–198°C; IR(KBr): υ 3175,3302 (O-H/N-H), 1674,1719 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.69,3.84 (2H, 2d, J=15.61 Hz, thiazolidin C5-H2), 6.16 (1H, s, thiazolidin C2-H), 6.78 (1H, s, COH), 7.22 (11H, d, J=6.83 Hz, Ar-H), 7.37 (1H, t, J=7.81 Hz, Ar-H), 7.58 (1H, d, J=7.81 Hz, Ar-H), 7.76 (1H, d, J=7.80 Hz, Ar-H), 10.47 (1H, s, CONH). Anal. Calcd. for C23H19BrN2O3S (483.38): C, 57.15; H, 3.96; N, 5.80. Found: C, 56.95; H, 3.91; N, 5.59%.
N-[2-(3-bromophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3h).
White powder. Yield 48%; m.p. 181–183°C; IR(KBr): υ 3292 (O-H/N-H), 1673,1704 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.70,3.87 (2H, 2d, J=16.10 Hz, thiazolidin C5-H2), 5.80 (1H, s, thiazolidin C2-H), 6.74 (1H, s, COH), 7.15 (2H, s, Ar-H), 7.21–7.23 (4H, m, Ar-H), 7.25–7.26 (5H, m, Ar-H), 7.39 (1H, d, J=7.80 Hz, Ar-H), 7.51–7.53 (1H, m, Ar-H), 7.68 (1H, d, J=1.96 Hz, Ar-H), 10.36 (1H, s, CONH). Anal. Calcd. for C23H19BrN2O3S (483.38): C, 57.15; H, 3.96; N, 5.80. Found: C, 57.45; H, 3.90; N, 5.94%.
N-[2-(4-bromophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3i).
White powder. Yield 72%; m.p. 170–172°C; IR(KBr): υ 3188,3307 (O-H/N-H), 1671,1714 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.68,3.83 (2H, 2d, J=16.10 Hz, thiazolidin C5-H2), 5.80 (1H, s, thiazolidin C2-H), 6.70 (1H, brs, COH), 7.07 (2H, d, J=6.83 Hz, Ar-H), 7.18–7.24 (8H, m, Ar-H), 7.35 (2H, d, J=8.30 Hz, Ar-H), 7.47 (2H, d, J=8.30 Hz, Ar-H), 10.25 (1H, brs, CONH). Anal. Calcd. for C23H19BrN2O3S (483.38): C, 57.15; H, 3.96; N, 5.80. Found: C, 56.97; H, 3.71; N, 5.50.
N-[2-(2,4-dichlorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3j).
White powder. Yield 53%; m.p. 204–206°C; IR(KBr): υ 3268 (O-H/N-H), 1681,1717 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.74,3.89 (2H, 2d, J=15.62 Hz, thiazolidin C5-H2), 6.17 (1H, s, thiazolidin C2-H), 6.80 (1H, s, COH), 7.21–7.26 (10H, m, Ar-H), 7.40 (1H, dd, J1=8.54, J2=2.20 Hz, Ar-H), 7.57 (1H, d, J=1.95 Hz, Ar-H), 7.77 (1H, d, J=8.30 Hz, Ar-H), 10.46 (1H, s, CONH). Anal. Calcd. for C23H18Cl2N2O3S (473.37): C, 58.36; H, 3.83; N, 5.92. Found: C, 58.22; H, 3.92; N, 5.75%.
N-[2-(3,4-dichlorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3k).
White small needles. Yield 74%; m.p. 199–203°C; IR(KBr): υ 3268 (O-H/N-H), 1681,1717 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.69,3.87 (2H, 2d, J=16.10 Hz, thiazolidin C5-H2), 5.82 (1H, s, thiazolidin C2-H), 6.73 (1H, brs, COH), 7.11 (2H, d, J=6.83 Hz, Ar-H), 7.17–7.22 (3H, m, Ar-H), 7.24 (5H, s, Ar-H), 7.35 (1H, d, J=6.78 Hz, Ar-H), 7.49 (1H, dd, J1=8.30, J2=2.44 Hz, Ar-H), 7.67 (1H, s, Ar-H), 10.35 (1H, brs, CONH). Anal. Calcd. for C23H18Cl2N2O3S (473.37): C, 58.36; H, 3.83; N, 5.92. Found: C, 58.18; H, 3.66; N, 5.69%.
N-[2-(2,6-dichlorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3l).
White crystals. Yield 57%; m.p. 191–193°C; IR(KBr): υ 3252 (O-H/N-H), 1682,1716 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.82,3.90 (2H, 2d, J=15.37 Hz, thiazolidin C5-H2), 6.63 (1H, d, J=2.44 Hz, thiazolidin C2-H), 6.73 (1H, s, COH), 7.23–7.29 (10H, m, Ar-H), 7.37–7.44 (2H, m, Ar-H), 7.50 (1H, dd, J1=7.81, J2=1.46 Hz, Ar-H), 10.23 (1H, s, CONH); 13C-NMR (APT)(DMSO-d6/125 MHz) δ (ppm): 31.26 (C5), 56.94 (C2), 81.34 (C-OH), 127.94, 128.21, 128.24, 129.23, 131.84 (ar. CH), 136.13, 136.52 (ar. C-Cl), 143.90, 144.04 (ar. C), 169.01 (amide C=O), 172.74 (lactam C=O). Anal. Calcd. for C23H18Cl2N2O3S (473.37): C, 58.36; H, 3.83; N, 5.92. Found: C, 58.12; H, 3.62; N, 5.88%.
N-[2-(2-chloro-6-fluorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3m).
White powder. Yield 65%; m.p.171–173°C; IR(KBr): υ 3285 (O-H/N-H), 1688,1732 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.73,3.84 (2H, 2d, J=15.62 Hz, thiazolidin C5-H2), 6.27 (1H, s, thiazolidin C2-H), 6.73 (1H, s, COH), 7.15–7.19 (2H, m, Ar-H), 7.23–7.26 (10H, m, Ar-H), 7.37–7.42 (1H, m, Ar-H), 10.44 (1H, s, CONH). LC/MS: m/z 471 (M-H)−. Anal. Calcd. for C23H18ClFN2O3S (456.918): C, 60.46; H, 3.97; N, 6.13. Found: C, 60.26; H, 3.62; N, 5.98%.
N-[2-(4-trifluoromethylphenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3n).
White powder. Yield 82%; m.p. 198–199°C; IR(KBr): υ 3252 (O-H/N-H), 1682,1716 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.72,3.88 (2H, 2d, J=16.10 Hz, thiazolidin C5-H2), 5.88 (1H, s, thiazolidin C2-H), 6.72 (1H, s, COH), 7.08 (2H, d, J=7.80 Hz, Ar-H), 7.16–7.24 (8H, m, Ar-H), 7.63 (4H, s, Ar-H), 10.33 (1H, s, CONH). Anal. Calcd. for C24H19F3N2O3S (472.47): C, 61.01; H, 4.05; N, 5.93. Found: C, 60.98; H, 4.19; N, 5.58%.
N-[2-(4-cyanophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3o).
Yellow powder. Yield 55%; m.p. 212–214°C; IR(KBr): υ 3379 (O-H/N-H), 1681,1725 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.74,3.91 (2H, d, J=15.62 Hz, thiazolidin C5-H2), 5.89 (1H, s, thiazolidin C2-H), 6.75 (1H, s, COH), 7.12 (2H, d, J=7.33 Hz, Ar-H), 7.22–7.28 (8H, m, Ar-H), 7.62–7.74 (4H, 2d, J=8.3 Hz, Ar-H), 10.37 (1H, s, CONH); 13C-NMR (APT)(DMSO-d6/125 MHz) δ (ppm): 30.07 (C5), 61.75 (C2), 81.23 (C-OH), 119.28 (CN), 127.99, 128.08, 128.10, 128.22, 129.83, 132.83 (ar. CH), 143.97, 144.08, 144.09 (ar. C), 169.04 (amide C=O), 172.19 (lactam C=O). Anal. Calcd. for C24H19N3O3S (429.49): C, 67.12; H, 4.46; N, 9.78. Found: C, 67.11; H, 4.41; N, 9.52%.
N-[2-(4-benzyloxyphenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3p).
White powder. Yield 68%; m.p. 210–213°C; IR(KBr): υ 3346 (O-H/N-H), 1670,1725 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 3.60 (2H, s, thiazolidin C5-H2), 5.11–5.14 (2H, m, –OCH2C6H5), 5.73 (1H, s, thiazolidin C2-H), 6.88 (1H, brs, COH), 7.19 (4H, d, J=7.32 Hz, Ar-H), 7.27–7.33 (5H, m, Ar-H), 7.36 (5H, t, J=7.16 Hz, Ar-H), 7.50 (5H, d, J=7.16 Hz, Ar-H), 10.28 (1H, s, CONH). Anal. Calcd. for C30H26N2O4S (510.61): C, 70.57; H, 5.13; N, 5.49. Found: C, 70.38; H, 5.45; N, 5.67%.
N-[2-(4-methylthiophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (3r).
White powder. Yield 85%; m.p. 122–124°C; IR(KBr): υ 3253 (O-H/N-H), 1674,1715 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 3.32 (3H, brs, SCH3), 3.60,3.73 (2H, 2d, J=15.79 Hz, thiazolidin C5-H2), 5.68 (1H, s, thiazolidin C2-H), 6.57 (1H, s, COH), 6.96 (2H, d, J=5.39 Hz, Ar-H), 7.07–7.18 (10H, m, Ar-H), 7.26 (2H, d, J=8.38 Hz, Ar-H), 10.16 (1H, s, CONH). Anal. Calcd. for C24H22N2O3S2 (450.575): C, 63.98; H, 4.92; N, 6.22. Found: C, 63.85; H, 4.65; N, 5.92%.
N-[2-(2-fluorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4a).
White powder. Yield 68%; m.p. 163–165°C; IR(KBr): υ 3375 (O-H/N-H), 1682,1715 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.50 (3H, d, J=6.83 Hz, 5-CH3), 4.02,4.11 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 6.09 (1H, s, thiazolidin C2-H), 6.73 (1H, s, COH), 7.13–7.17 (3H, m, Ar-H), 7.19–7.25 (9H, m, Ar-H), 7.37–7.41 (1H, m, Ar-H), 7.68 (1H, t, J=7.81 Hz, Ar-H), 10.36 (1H, s, CONH). Anal. Calcd. for C24H21FN2O3S (436.50): C, 66.04; H, 4.85; N, 6.42. Found: C, 65.82; H, 4.87; N, 6.34%.
N-[2-(3-fluorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4b).
White crystals. Yield 55%; m.p. 183–185°C; IR(KBr): υ 3379 (O-H/N-H), 1685,1717 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.50 (3H, d, J=6.83 Hz, 5-CH3), 3.99,4.11 (1H, 2q, J=7.32 Hz, thiazolidin C5-H), 5.82 (1H, s, thiazolidin C2-H), 6.72 (1H, s, COH), 7.14–7.19 (3H, m, Ar-H), 7.20–7.25 (9H, m, Ar-H), 7.28–7.36 (2H, m, Ar-H), 10.35 (1H, s, CONH). Anal. Calcd. for C24H21FN2O3S (436.50): C, 66.04; H, 4.85; N, 6.42. Found: C, 65.91; H, 4.72; N, 6.34%.
N-[2-(4-fluorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4c).
White powder. Yield 47%; m.p. 107–110°C; IR(KBr): υ 3278,3545 (O-H/N-H), 1670,1710 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.50 (3H, d, J=6.83 Hz, 5-CH3), 3.98,4.09 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 5.78 (1H, s, thiazolidin C2-H), 6.69 (1H, s, COH), 7.09–7.12 (4H, m, Ar-H), 7.17–7.25 (8H, m, Ar-H), 7.44–7.46 (2H, m, Ar-H), 10.26 (1H, s, CONH). Anal. Calcd. for C24H21FN2O3S (436.50): C, 66.04; H, 4.85; N, 6.42. Found: C, 65.95; H, 4.92; N, 6.22%.
N-[2-(2-chlorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4d).
White powder. Yield 35%; m.p. 135–137°C; IR(KBr): υ 3326,3369 (O-H/N-H), 1676,1718 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.49 (3H, d, J=6.83 Hz, 5-CH3), 4.03,4.11 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 6.17,6.23 (1H, 2s, thiazolidin C2-H), 6.79 (1H, brs, COH), 7.25 (10H, d, J=5.86 Hz, Ar-H), 7.34–7.37 (2H, m, Ar-H), 7.42–7.44 (1H, m, Ar-H), 7.78–7.81 (1H, m, Ar-H), 10.51 (1H, s, CONH); 13C-NMR (APT)(DMSO-d6/125 MHz) δ (ppm): 19.95 (5-CH3), 38.24 (C5), 57.48 (C2), 81.26 (C-OH), 127.99, 128.02, 128.05, 128.16, 128.23, 128.46, 129.38, 130.87, 130.74, 134.04 (ar. CH), 136.50, 144.09, 144.11, 144.16 (ar. C), 172.28 (amide C=O), 172.75 (lactam C=O). Anal. Calcd. for C24H21ClN2O3S (452.96): C, 63.64; H, 4.67; N, 6.18. Found: C, 63.50; H, 4.96; N, 5.31%.
N-[2-(3-chlorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4e).
White small needles. Yield 38%; m.p. 200–202°C; IR(KBr): υ 3292,3386 (O-H/N-H), 1682,1716 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.50 (3H, q, J=6.83 Hz, 5-CH3), 3.99,4.12 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 5.83 (1H, s, thiazolidin C2-H), 6.73 (1H, brs, COH), 7.14–7.23 (5H, m, Ar-H), 7.26–7.27 (5H, m, Ar-H), 7.32–7.36 (2H, m, Ar-H), 7.38–7.41 (1H, m, Ar-H), 7.53 (1H, m, Ar-H), 10.36 (1H, brs, CONH). Anal. Calcd. for C24H21ClN2O3S (452.96): C, 63.64; H, 4.67; N, 6.18. Found: C, 63.42; H, 4.48; N, 5.89%.
N-[2-(4-chlorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4f).
White powder. Yield 62%; m.p. 122–125°C; IR(KBr): υ 3393 (O-H/N-H), 1682,1715 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.51 (3H, t, J=7.32 Hz, 5-CH3), 4.00,4.11 (1H, 2q, J=6.86 Hz, thiazolidin C5-H), 5.80 (1H, s, thiazolidin C2-H), 6.72 (1H, brs, COH), 7.13–7.19 (2H, m, Ar-H), 7.22–7.24 (2H, m, Ar-H), 7.27 (6H, s, Ar-H), 7.34 (2H, dd, J1=8.69, J2=2.97 Hz, Ar-H), 7.44 (2H, dd, J1=8.69, J2=2.29 Hz, Ar-H), 10.29 (1H, brs, CONH); 13C-NMR (HSQC)(DMSO-d6/125 MHz) δ (ppm): 20.13 (5-CH3), 39.41 (C5), 60.81 (C2), 81.24 (C-OH), 127.94, 128.00, 128.06, 128.10, 128.12, 128.15, 128.17, 128.22, 128.91, 128.94, 129.12, 130.83, 131.15, 134.13 (ar. CH), 134.33, 136.67, 144.05, 144.08 (ar. C), 171.98 (amide C=O), 172.15 (lactam C=O); LC/MS: m/z 451 (M-H)−. Anal. Calcd. for C24H21ClN2O3S (452.96): C, 63.64; H, 4.67; N, 6.18. Found: C, 63.42; H, 4.76; N, 5.88%.
N-[2-(2-bromophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4g).
White powder. Yield 27%; m.p. 170–174°C; IR(KBr): υ 3283,3379 (O-H/N-H), 1682,1717 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.46–1.48 (3H, m, 5-CH3), 4.00,4.08 (1H, 2q, J=6.84 Hz, thiazolidin C5-H), 6.12 (1H, s, thiazolidin C2-H), 6.79 (1H, s, COH), 7.23–7.27 (11H, m, Ar-H), 7.36–7.40 (1H, m, Ar-H), 7.57–7.59 (1H, m, Ar-H), 7.76–7.80 (1H, m, Ar-H), 10.53 (1H, s, CONH). Anal. Calcd. for C24H21BrN2O3S (497.41): C, 57.95; H, 4.26; N, 5.63. Found: C, 57.65; H, 4.53; N, 5.42%.
N-[2-(3-bromophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4h).
White powder. Yield 25%; m.p. 198–200°C; IR(KBr): υ 3318 (O-H/N-H), 1673,1714 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.49 (3H, m, 5-CH3), 3.99,4.12 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 5.78 (1H, s, thiazolidin C2-H), 6.73 (1H, s, COH), 7.14–7.16 (2H, m, Ar-H), 7.22–7.26 (9H, m, Ar-H), 7.40 (1H, t, J=7.32 Hz, Ar-H), 7.52 (1H, t, J=7.81 Hz, Ar-H), 7.67 (1H, s, Ar-H), 10.38 (1H, s, CONH). Anal. Calcd. for C24H21BrN2O3S (497.41): C, 57.95; H, 4.26; N, 5.63. Found: C, 57.68; H, 4.39; N, 5.78%.
N-[2-(4-bromophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4i).
White powder. Yield 77%; m.p. 165–168°C; IR(KBr): υ 3390,3564 (O-H/N-H), 1682,1714 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.51 (3H, t, J=7.08 Hz, 5-CH3), 4.01,4.12 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 5.79 (1H, s, thiazolidin C2-H), 6.72 (1H, s, COH), 7.09–7.12 (2H, m, Ar-H), 7.21–7.28 (8H, m, Ar-H), 7.38 (2H, dd, J1=8.53, J2=2.19 Hz, Ar-H), 7.49–7.51 (2H, m, Ar-H), 10.30 (1H, s, CONH). Anal. Calcd. for C24H21BrN2O3S (497.41): C, 57.95; H, 4.26; N, 5.63. Found: C, 57.61; H, 4.53; N, 5.92%.
N-[2-(2,4-dichlorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4j).
White powder. Yield 60%; m.p. 183–186°C.; IR(KBr): υ 3339 (O-H/N-H), 1676,1726 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.48 (3H, d, J=5.86 Hz, 5-CH3), 4.03,4.12 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 6.16 (1H, s, thiazolidin C2-H), 6.78 (1H, s, COH), 7.21–7.25 (10H, m, Ar-H), 7.39 (1H, d, J=8.30 Hz, Ar-H), 7.55–7.56 (1H, m, Ar-H), 7.76 (1H, t, J=5.85 Hz, Ar-H), 10.48 (1H, s, CONH); 13C-NMR (APT)(DMSO-d6/125 MHz) δ (ppm): 20.64 (5-CH3), 39.94 (C5), 57.42 (C2), 81.21 (C-OH), 128.03, 128.13, 128.20, 129.36, 129.42, 131.45 (ar. CH), 134.01, 134.67 (ar. C-Cl), 135.51, 143.94, 144.02 (ar. C), 172.34 (amide C=O), 172.51 (lactam C=O). Anal. Calcd. for C24H20Cl2N2O3S (487.399): C, 59.14; H, 4.14; N, 5.75. Found: C, 59.17; H, 3.93; N, 5.83%.
N-[2-(3,4-dichlorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4k).
White powder. Yield 77%; m.p. 175–178°C; IR(KBr): υ 3286,3374 (O-H/N-H), 1674,1724 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.47–1.51 (3H, m, 5-CH3), 4.01,4.15 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 5.79 (1H, s, thiazolidin C2-H), 6.75 (1H, s, COH), 7.10–7.14 (2H, m, Ar-H), 7.18–7.23 (3H, m, Ar-H), 7.26 (5H, d, J=5.36 Hz, Ar-H), 7.37–7.40 (1H, m, Ar-H), 7.51 (1H, dd, J1=8.30, J2=2.44 Hz, Ar-H), 7.68 (1H, dd, J1=5.86, J2=1.95 Hz, Ar-H), 10.39 (1H, s, CONH). Anal. Calcd. for C24H20Cl2N2O3S (487.399): C, 59.14; H, 4.14; N, 5.75. Found: C, 59.43; H, 3.86; N, 5.70%.
N-[2-(2,6-dichlorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4l).
White crystals. Yield 12%; m.p. 229–232°C. IR(KBr) (υ, cm−1), 3277 (O-H/N-H), 1681,1713 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.50 (3H, t, J=6.35 Hz, 5-CH3), 4.05,4.14 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 6.12,6.18 (1H, 2s, thiazolidin C2-H), 6.80 (1H, brs, COH), 7.23–7.27 (10H, m, Ar-H), 7.41 (1H, d, J=8.54 Hz, Ar-H), 7.56–7.58 (1H, m, Ar-H), 7.78–7.80 (1H, m, Ar-H), 10.48,10.52 (1H, 2s, CONH). Anal. Calcd. for C24H20Cl2N2O3S (487.399): C, 59.14; H, 4.14; N, 5.75. Found: C, 58.97; H, 4.05; N, 5.75%.
N-[2-(2-chloro-6-fluorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4m).
White small needles. Yield 43%; m.p. 195–198°C; IR(KBr): υ 3265 (O-H/N-H), 1686,1718 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 1.38,1.44 (3H, 2d, J=6.98 Hz, 5-CH3), 4.00,4.04 (1H, m, thiazolidin C5-H), 6.12,6.20 (1H, 2s, thiazolidin C2-H), 6.66 (1H, brs, COH), 7.05–7.18 (11H, m, Ar-H), 7.28–7.35 (2H, m, Ar-H), 10.38,10.44 (1H, 2s, CONH). Anal. Calcd. for C24H20ClFN2O3S (470.944): C, 61.21; H, 4.28; N, 5.95. Found: C, 60.94; H, 4.42; N, 6.04%.
N-[2-(4-(trifluoromethyl)phenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4n).
White powder. Yield 84%; m.p. 157–158°C; IR(KBr): υ 3389 (O-H/N-H), 1687,1718 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.53 (3H, t, J=8.54 Hz, 5-CH3), 4.04,4.16 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 5.91 (1H, s, thiazolidin C2-H), 6.75 (1H, s, COH), 7.11–7.17 (2H, m, Ar-H), 7.18–7.23 (3H, m, Ar-H), 7.26 (5H, s, Ar-H), 7.66 (4H, s, Ar-H), 10.38 (1H, 2s, CONH); 13C-NMR (APT)(DMSO-d6/125 MHz) δ (ppm): 20.31 (5-CH3), 38.97 (C5), 60.78 (C2), 81.19 (C-OH), 125.80 (CF3), 125.84, 125.89, 128.11, 129.97, 129.91, 129.65 (ar. CH), 142.69, 144.13 (ar. C), 172.13 (amide C=O), 172.27 (lactam C=O). Anal. Calcd. for C25H21F3N2O3S (486.51): C, 61.72; H, 4.35; N, 5.76. Found: C, 62.02; H, 4.09; N, 5.77%.
N-[2-(4-cyanophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4o).
White powder. Yield 63%; m.p. 195–197°C; IR(KBr): υ 3269,3444 (O-H/N-H), 1676,1721 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.51 (3H, t, J=6.35 Hz, 5-CH3), 4.02,4.11 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 5.85 (1H, s, thiazolidin C2-H), 6.72 (1H, s, COH), 7.08–7.12 (1H, m, Ar-H), 7.18–7.27 (9H, m, Ar-H), 7.60 (2H, d, J=8.30 Hz, Ar-H), 7.72 (2H, d, J=7.81 Hz, Ar-H), 10.36 (1H, 2s, CONH). Anal. Calcd. for C25H21N3O3S (443.52): C, 67.70; H, 4.77; N, 9.47. Found: C, 67.41; H, 4.95; N, 9.22%.
N-[2-(4-benzyloxyphenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4p).
White powder. Yield 51%; m.p. 203–204°C; IR(KBr): υ 3395 (O-H/N-H), 1682,1721 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.42 (3H, d, J=6.83 Hz, 5-CH3), 4.00,4.11 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 5.12–5.14 (2H, m, –OCH2C6H5), 6.12 (1H, s, thiazolidin C2-H), 6.82 (1H, s, COH), 7.17 (4H, d, J=7.32 Hz, Ar-H), 7.25–7.29 (5H, m, Ar-H), 7.32–7.35 (5H, m, Ar-H), 7.47–7.49 (5H, m, Ar-H), 10.31 (1H, s, CONH). 13C-NMR (APT)(DMSO-d6/125 MHz) δ (ppm): 20.42 (5-CH3), 37.59 (C5), 58.95 (C2), 71.45 (OCH2), 81.59 (C-OH), 112.95, 126.83, 127.24, 128.27, 128.35, 129.08 (ar. CH), 144.63, 146.50, 149.25 (ar. C), 166.70 (ar. C-OR), 170.57 (amide C=O), 173.45 (lactam C=O). Anal. Calcd. for C31H28N2O4S (524.63): C, 70.97; H, 5.38; N, 5.34. Found: C, 71.02; H, 5.56; N, 5.13%.
N-[2-(4-methylthiophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-hydroxy-2,2-diphenylacetamide (4r).
White powder. Yield 80%; m.p. 173–174°C; IR(KBr): υ 3397 (O-H/N-H), 1689,1714 (C=O); 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 1.49 (3H, d, J=6.83 Hz, 5-CH3), 2.48 (3H, s, SCH3), 3.96,4.07 (1H, 2q, J=6.83 Hz, thiazolidin C5-H), 5.74 (1H, s, thiazolidin C2-H), 6.67 (1H, s, COH), 7.16–7.25 (10H, m, Ar-H), 7.34 (2H, d, J=7.81 Hz, Ar-H), 10.22 (1H, s, CONH). Anal. Calcd. for C25H24N2O3S2 (464.602): C, 64.63; H, 5.21; N, 6.03. Found: C, 64.42; H, 5.00; N, 6.11%.
Biology
In vitro evaluation of antituberculosis activity
Primary screening was conducted at 6.25 μg/mL against M. tuberculosis H37Rv in BACTEC 12B medium using the Microplate Alamar Blue Assay (MABA). Compounds exhibiting fluorescence were tested in the BACTEC 460 radiometric system. Compounds showing ≥90 inhibition in the primary screen were considered active and subsequently retested at lower concentrations against M. tuberculosis H37Rv in order to determine the actual minimum inhibitory concentration (MIC). The MIC is defined as the lowest concentration effecting a reduction in fluorescence of 90% with respect to the controls.
Microplate alamar blue susceptibility assay
Antimicrobial susceptibility testing was performed in black, clear-bottomed, 96-well microplates (black view plates; Packard Instrument, Meriden, CN) to minimize background fluorescence. Outer perimeter wells were filled with sterile water to prevent dehydration in experimental wells. Initial drug dilutions were prepared in either dimethyl sulfoxide or distilled deionized water, and subsequent twofold dilutions were performed in 0.1 mL 7H9GC (no Tween 80) in the microplates. BACTEC 12B-passaged inocula were initially diluted 1:2 in 7H9GC, and 0.1 mL was added to wells. Subsequent determination of bacterial titer yielded 1 × 106 CFU/mL in plate wells for H37Rv. Frozen inocula were initially diluted 1:20 in BACTEC 12B medium followed by a 1:50 dilution in 7H9GC. The addition of 1/10 mL to wells resulted in a final bacterial titer of 2.0 × 105 CFU/mL for H37Rv. Wells containing drug only were used to detect autofluorescence of compounds. Addition control wells consisted of bacteria only (B) and medium only (M). Plates were incubated at 37°C. Starting at day 4 of incubation, 20 μL of 10× Alamar Blue solution (Alamar Biosciences/Accumed, Westlake, OH) and 12.5 μL of 20% Tween 80 were added to one B well and one M well, and plates were reincubated at 37°C. Wells were observed at 12 and 24 h for a color change from blue to pink and for a reading of ≥50,000 fluorescence units (FU). Fluorescence was measured in a Cytofluor II microplate fluorometer (PerSeptive Biosystems, Framingham, MA) in bottom-reading mode with excitation at 530 nm and emission at 590 nm. If the B wells became pink by 24 h, reagent was added to the entire plate. If the well remained blue or ≤50,000 FU was measured, additional M and B wells were tested daily until a color change occurred, at which time reagents were added to all remaining wells. Plates were then incubated at 37°C, and results were recorded at 24 h post-reagent addition. Visual MICs were defined as the lowest concentration of drug that had prevented a color change. For fluorometric MICs, a background subtraction was performed on all wells with a mean of triplicate M wells. Percent inhibition was defined as 1 − (test well FU/mean FU of triplicate B wells) × 100. The lowest drug concentration effecting an inhibition of ≥90% was considered the MIC.
Methodology of the in vitro cancer screen
The human tumor cell lines of the cancer screening panel were grown in RPMI 1640 medium containing 5% fetal bovine serum and 2 mM l-glutamine. For a typical screening experiment, cells were inoculated into 96-well microtiter plates in 100 μL at plating densities ranging from 5000 to 40000 cells/well depending on the doubling time of individual cell lines. After cell inoculation, the microtiter plates were incubated at 37°C, 5% CO2, 95% air, and 100% relative humidity for 24 h before adding experimental drugs. After 24 h, two plates of each cell line were fixed in situ with TCA to represent a measurement of the cell population for each cell line at the time of drug addition (Tz). Experimental drugs were solubilized in dimethyl sulfoxide at 400-fold the desired final maximum test concentration and stored frozen before use. At the time of drug addition, an aliquot of frozen concentrate was thawed and diluted to twice the desired final maximum test concentration with complete medium containing 50 μg/mL gentamicin. Additional fourfold, 10-fold, or ½ log serial dilutions were made to provide a total of five drug concentrations plus control. Aliquots of 100 μL of these different drug dilutions were added to the appropriate microtiter wells already containing 100 μL of the medium, resulting in the required final drug concentrations. Following drug addition, the plates were incubated for an additional 48 h at 37°C, 5% CO2, 95% air, and 100% relative humidity. For adherent cells, the assay was terminated by the addition of ice-cold TCA. Cells were fixed in situ by the gentle addition of 50 μL of ice-cold 50% (w/v) TCA (final concentration, 10% TCA) and incubated for 60 min at 4°C. The supernatant was discarded, and the plates were washed five times with tap water and air dried. Sulforhodamine B (SRB) solution (100 μL) at 0.4% (w/v) in 1% acetic acid was added to each well, and plates were incubated for 10 min at room temperature. After staining, unbound dye was removed by washing five times with 1% acetic acid and the plates were air dried. Bound stain was subsequently solubilized with 10 mM trizma base, and the absorbance was read on an automated plate reader at a wavelength of 515 nm. For suspension cells, the methodology was the same except that the assay was terminated by fixing settled cells at the bottom of the wells by gently adding 50 μL of 80% TCA (final concentration, 16% TCA). Using the seven absorbance measurements [time zero, (Tz), control growth, (C), and test growth in the presence of drug at the five concentration levels (Ti)], the percentage growth was calculated at each of the drug concentrations levels. Percentage growth inhibition was calculated as follows:
[(Ti−Tz)/(C−Tz)] × 100 for concentrations for which Ti ≥ Tz.
[(Ti−Tz)/Tz] × 100 for concentrations for which Ti<Tz.
Three dose response parameters were calculated for each experimental agent. Growth inhibition of 50% (GI50) was calculated from [(Ti−Tz)/(C−Tz)] × 100 = 50, which was the drug concentration resulting in a 50% reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation. The drug concentration resulting in total growth inhibition (TGI) was calculated from Ti = Tz. The LC50 (concentration of drug resulting in a 50% reduction in the measured protein at the end of the drug treatment as compared to that at the beginning), indicating a net loss of cells following treatment was calculated from [(Ti−Tz)/Tz] × 100 = −50. Values were calculated for each of these three parameters if the level of activity was reached; however, if the effect was not reached or was exceeded, the value for that parameter was expressed as greater or less than the maximum or minimum concentration tested.
Results and discussion
Chemistry
Novel 2-aryl-4-thiazolidinones were obtained by using a three-step synthesis pathway (). Methyl-2-hydroxy-2,2-diphenylacetate was converted to 2-hydroxy-2,2-diphenylacetohydrazide (1) by refluxing in ethanol in the presence of hydrazine hydrate for 6 h. Compound 1 reacted with substituted aromatic aldehydes to give intermediate Schiff bases (2). Finally, 2-hydroxy-N-(4-oxo-2-substituted phenyl-1,3-thiazolidin-3-yl)-2,2-diphenylacetamides (3) and 2-hydroxy-N-(5-methyl-4-oxo-2-substituted phenyl-1,3-thiazolidin-3-yl)-2,2-diphenylacetamides (4) were obtained after cyclodehyration with mercaptoacetic acid or 2-mercaptopropionic acid, respectively.
Scheme 1. Reagents: (i) hydrazine hydrate, EtOH, reflux, 6 h; (ii) EtOH, reflux, 4 h; (iii) mercaptoacetic acid/2-mercaptopropionic acid, dry benzene, reflux, 6 h.
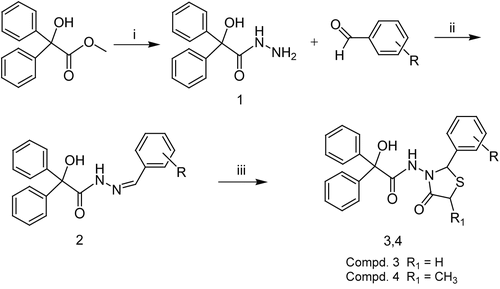
The structures of the new compounds were confirmed using elemental analysis, IR, 1H-NMR, HSQC, 13C-NMR(APT), and atmospheric pressure chemical ionization [APCI(−)] mass spectrometry.
The IR spectra exhibited O-H/N-H and C=O bands in the 3275–3565 cm−1 and 1669–1689 cm−1 regions attributed to the common CONH functions of 3 and 4 [Citation20]. Observation of new endocyclic C=O bands (1704–1740 cm−1) characteristic for such structures besides C=O amide bands (1669–1689 cm−1) in the IR spectra of 3 and 4 supported the aimed cyclization [Citation7,Citation11].
The 1H-NMR spectra of 3 and 4 displayed two singlets and two quartets attributed to the methylene (SCH2) and methine (SCHCH3) ring protons at 2-position of the 4-thiazolidinone system at about δ 3.60–3.91 and 3.96–4.15 ppm, respectively.Citation21 The C2-H protons were observed at about δ 5.68–6.63 ppm. The C-OH and CONH protons were observed at about δ 6.57–6.88 and δ 10.16–10.53 ppm, respectively [Citation22].
13C-APT run on 3c, 3l, 3n, 4d, 4j, 4o, and 2D NMR experiment HSQC run on 4f allowed explicit assignments for the proton and carbon chemical shifts. The spectra substantiated the expected conversion and revealed the typical 4-thiazolidinone C2, C4 (lactam C=O), C5 (compd. 3), and C5 (compd. 4) resonances at δ 56.94–61.85, 172.14–173.45, 30.07–31.26, and 37.59–39.94 ppm, respectively [Citation7,Citation22,Citation23]. Existence of cross peaks connecting C5-CH3 (δ 20.13 ppm) with the triplet at δ 1.51 ppm; C5 (δ 39.41 ppm) with the two quartets at δ 4.00, 4.11 ppm; and C2 (δ 60.81 ppm) with the singlet at δ 5.81 ppm was decisive evidence for unambiguous assignment. Carbon resonances at δ 81.24, 171.98, and 172.15 ppm showed no cross peaks and were thus assigned to C-OH, CONH, and C4 (lactam C=O) carbons, respectively.
(M-H)− ions with 100% relative abundance observed in the APCI(−) mass spectra of 3c, 3m, and 4f provided further confirmation that the expected structures had formed [Citation24]. MS/MS clearly showed the [(M-H)-SCH2CO]− fragments of the 4-thiazolidinone system cited in the literature [Citation25].
Further spectral details have already been presented in the Materials and methods section.
Biological activity
Antimycobacterial activity
The in vitro antimycobacterial activity of the 34 novel 2-aryl-4-thiazolidinones (compounds 3 and 4) against M. tuberculosis H37Rv (ATCC 27294) was determined using the Microplate Alamar Blue Assay (MABA) at a test concentration of 6.25 μg/mL (). Compounds exhibiting fluorescence were further characterized in the BACTEC 460 radiometric system [Citation26].
Table 1. The primary in vitro antimycobacterial activity of compounds 3a-r and 4a-r were determined against M. tuberculosis H37Rv using Microplate Alamar Blue Assay (MABA). The compounds were tested at a concentration of 6.25 µg/mL. The inhibition values of the compounds with more than 90% growth inhibition are indicated in boldface.
Several compounds have been identified with more than 90% growth inhibition of M. tuberculosis H37Rv at 6.25 μg/mL test concentration, i.e., compounds 3f (99%), 3o (98%), 3p (98%), 4d (95%), 4f (99%), 4o (99%), and 4p (98%) (). Interestingly, all these compounds are substituted on the para position of the phenyl moiety (position R; ) with chloro (3f and 4f), cyano (3o and 4o), or benzoxy (3p and 4p) functions. No influence of the substituent on R1 is observed for the activity of these compounds. In fact, this is observed for all synthesized compounds, except for 3c/4c, 3d/4d, 3j/4j, and 3r/4r. A methyl substituent on R1 increases the growth inhibition of these latter compounds compared with a hydrogen substituent. This effect is very strong for compounds 3d/4d. A chloro substituent on the ortho position of the phenyl moiety and a hydrogen on R1 (3d, 0% inhibition) shows no growth inhibition at all. However, a methyl group on R1 increases the activity enormously (4d, 95% inhibition).
Antitumor activity
Compounds 3c, 3m, 3n, 3p, 4a, 4i, and 4o were chosen by the National Cancer Institute (NCI) and screened for antitumor activity. Primary anticancer assay was performed in accordance with the protocol of the Drug Evaluation Branch of the National Cancer Institute (Bethesda)[Citation27–29] and compound 4p was chosen for a second anticancer test. The 50% growth inhibition (GI50) and total growth inhibition (TGI) were obtained for the selected compound (4p) for each cell line (). The log10GI50 and log10TGI defined as the mean of the log10’s of the individual GI50 and TGI values were then determined. Negative values indicated the most sensitive cell lines. Compounds having values ≤−4 are declared to be active. All values for log10GI50 are found to be <−5.00 for compound 4p on all cell lines.
Table 2. In vitro tumor cell growth inhibition of 4p.
In conclusion, a series of 34 new 2-aryl-4-thiazolidinones were synthesized and tested for biological activity. The antimycobacterial activity of these compounds was determined and seven compounds showed inhibition values between 95% and 99% at a test concentration of 6.25 μg/mL. In addition, antitumor activities of several compounds was tested by the NCI and compound 4p was found to be active on a wide variety of tumor cell lines. Taken together, 2-aryl-4-thiazolidinones showed to be promising scaffolds for both antimycobacterial and tumor targeting compounds.
Acknowledgments
Antimycobacterial data were provided by the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) through a research and development contract with the U.S. National Institute of Allergy and Infectious Diseases. Dr. Joseph A. Maddry (TAACF) and his team are recognized for their collaboration. We thank the Division of Cancer Research, National Cancer Institute, Bethesda, MD, for the anticancer activity screening.
Declaration of interest: This work was supported by The Research Fund of Istanbul University (Project Numbers 290/05012005 and UDP-731/05052006).
References
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. (1999). Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. J Am Med Assoc, 282, 677–686.
- Bass JB, Farer LS, Hopewell PC, O’Brein R, Jacob RF, Ruben F, Snider DE Jr, Thornton G. (1994). Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and the Centers for Disease Control and Prevention. Am J Respir Crit Care Med, 149, 1359–1374.
- Farmer P, Bayona J, Becerra M, Furin J, Henry C, Hiatt H, Kim JY, Mitnick C, Nardell E, Shin S. (1998). The dilemma of MDR-TB in the global era. Int J Tuberc Lung Dis, 2, 869–876.
- World Health Organization. (2000). Anti-Tuberculosis Drug Resistance in the World-Report No. 2. Prevalence and Trends. Geneva, Switzerland: WHO.
- Ginsburg AS, Grosset JH, Bishai WR. (2003). Fluoroquinolones, tuberculosis, and resistance. Lancet Infect Dis, 3, 432–442.
- Alland D, Kalkut GE, Moss AR, McAdam RA, Hahn JA, Bosworth W, Drucker E, Bloom BR. (1994). Transmission of tuberculosis in New York city—an analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med, 330, 1710–1716.
- Ulusoy N. (2002). Synthesis and antituberculosis activity of cycloalkylidene-hydrazide and 4-aza-1-thiaspiro[4.5]decan-3-one derivatives of imidazo[2,1-b]thiazole. Arzneim-Forsch/Drug Res, 52, 565–571.
- Srivastava T, Gaikwad AK, Haq W, Sinha S, Kati SB. (2005). Synthesis and biological evaluation of 4-thiazolidinone derivatives as potential antimycobacterial agents. Arkivoc, ii, 120–130.
- Bukowski L, Janowiec M, Zwolska-Kwiek Z, Andrezejczyk Z. (1998). Some reactions of 2-cyanomethylimidazo[4,5-b]pyridine with isothiocyanates. Antituberculotic activity of the obtained compounds. Pharmazie, 53, 373–376.
- Babaoglu K, Page MA, Jones VC, McNeil MR, Dong C, Naismith JH, Lee RE. (2003). Novel inhibitors of an emerging target in Mycobacterium tuberculosis; substituted thiazolidinones as inhibitors of dTDP-rhamnose synthesis. Bioorg Med Chem Lett, 13, 3227–3230.
- Çapan G, Ulusoy N, Ergenç N, Kiraz M. (1999). New 6-phenylimidazo[2,1-b]thiazole derivatives: synthesis and antifungal activity. Monatsh Chem, 130, 1399–1407.
- Karali N, İlhan E, Gürsoy A, Kiraz M. (1998). New cyclohexlidenehydrazide and 4-aza-1-thiaspiro[4,5]decan-3-one derivatives of 3-phenyl-4(3H)-quinazolinones. Farmaco, 53, 346–349.
- Fahmy HTY. (2001). Synthesis of some new triazoles as potential antifungal agents. Boll Chim Farmaco, 140, 422–427.
- Barreca ML, Chimirri A, Luca LD, Monforte AM, Monforte P, Rao M, Zappalá M, Balzarini J, De Clercq E, Pannecouque C, Witvrouw P. (2001). Discovery of 2,3-diaryl-1,3-thiazolidin-4-ones as potent anti-HIV agents. Bioorg Med Chem Lett, 11, 1793–1796.
- Rao A, Carbone A, Chimirri A, De Clercq E, Monforte AM, Monforte P, Pannecouque C, Zappalá M. (2002). Synthesis and anti-HIV activity of 2,3-diaryl-1,3-thiazolidin-4-(thi)one derivatives. Farmaco, 57, 747–751.
- Rao A, Carbone A, Chimirri A, De Clercq E, Monforte AM, Monforte P, Pannecouque C, Zappalá M. (2003). Synthesis and anti-HIV activity of 2,3-diaryl-1,3-thiazolidin-4-ones. Farmaco, 58, 115–120.
- Barreca ML, Chimirri A, De Clercq E, Luca LD, Monforte AM, Monforte P, Rao A, Zappalá M. (2003). Design and discovery of new potent RT inhibitors. Farmaco, 58, 259–263.
- Rao A, Balzarini J, Carbone A, Chimirri A, De Clercq E, Luca LD, Monforte AM, Monforte P, Pannecouque C, Zappalá M. (2004). Synthesis of new 2,3-diaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Farmaco, 59, 33–39.
- Ragab FA, Eid NM, El-Tawab HA. (1997). Synthesis and anticonvulsant activity of new thiazolidinone and thioxoimidazolidinone derivatives derived from furochromones. Pharmazie, 52, 926–929.
- Kaynak FB, Öztürk D, Özbey S, Çapan G. (2005). New N’-alkylidene/cycloalkylidene derivatives of 5-methyl-3-phenyl-1H-indole-2-carbohydrazide: synthesis, crystal structure, and quantum mechanical calculations. J Mol Struct, 740, 213–221.
- Bicking JB, Bock MG, Cragoe EJ, Dipardo RM, Gould NP, Holltz WJ, Lee TJ, Robb CM, Smith RL, Springer JP, Blaine EH. (1983). Prostaglandin isosteres. 2. Chain-modified thiazolidinone prostaglandin analogs as renal vasodilators. J Med Chem, 26, 342–348.
- Güzel Ö, İlhan E, Salman A. (2006). Synthesis and antimycobacterial activity of new 2-hydroxy-N-(3-oxo-1-thia-4-azaspiro[4.4]non-4-yl)/(3-oxo-1-thia-4-azaspiro-[4.5]dec-4-yl)-2,2-diphenylacetamide derivatives. Monatsh Chem, 137, 795–801.
- Karalı N, İlhan E, Gürsoy A, Kiraz M. (1998). New cyclohexylidenhydrazide and 4-aza-1-thiaspiro[4,5]decan-3-one derivatives of 3-phenyl-4-(3H)-quinazolinones. Farmaco, 52, 1–5.
- Peter R, Hellenbrand J, Mengerink Y, Van der Wal Sj J. (2004). On-line determination of carboxylic acids, aldehydes and ketones by high-performance liquid chromatography-diode array detection-atmospheric pressure chemical ionisation mass spectrometry after derivatization with 2-nitrophenylhydrazine. Chromatogr A, 1031, 35–50.
- Çapan G, Ulusoy N, Ergenç N, Ekinci AC, Vidin A. (1996). Synthesis and anticonvulsant activity of new 3-[(2-furyl)carbonyl]amino-4-thiazolidinone and 2-[(furyl)carbonyl]hydrazono-4-thiazoline derivatives. Farmaco, 51, 729–732.
- Collins LA, Franzblau SG. (1997). Microplate Alamar Blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother, 41, 1004–1009.
- Alley MC, Scudiero DA, Monks PA, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. (1988). Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res, 48, 589–601.
- Grever MR, Schepartz SA, Chabner BA. (1992). The National Cancer Institute: Cancer Drug Discovery and Development Program. Semin Oncol, 19(6), 622.
- Boyd MR, Paull KD. (1995). Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Devel Res, 34, 91–109.