Abstract
The in vitro effects of the analgesic drugs, lornoxicam, indomethacin, tenoxicam, diclofenac sodium, ketoprofen and lincomycine, on the activity of purified human serum paraoxonase (hPON1) (EC 3.1.8.1.) were evaluated. hPON1 was purified from human serum with a final specific activity of 3840 U mg−1 and a purity of 25.3 % using simple chromatographic methods, including DEAE-Sephadex anion exchange and Sepharose 4B-L-tyrozine-1-napthylamine hydrophobic interaction chromatography. SDS-polyacrylamide gel electrophoresis indicated a single protein band corresponding to hPON1. The six analgesics dose-dependently decreased in vitro hPON1 activity, with IC50 values for lornoxicam, indomethacin, tenoxicam, diclofenac sodium, ketoprofen and lincomycine of 0.136, 0.195, 0.340, 1.639, 6.23 and 9.638 mM, respectively. Ki constants were 0.009, 0.097, 0.306, 0.805, 13.010 and 11.116 mM, respectively. Analgesics showed different inhibition mechanisms: lornoxicam, diclofenac sodium and lincomycine were uncompetitive, indomethacin and tenoxicam were competitive, ketoprofen was noncompetitive. According to the results, inhibition potency was lornoxicam>indomethacin>tenoxicam> diclofenac sodium>ketoprofen> lincomycine.
| Abbreviations: | ||
| PON 1: | = | Paraoxonase 1 |
| OPs: | = | Organophosphates |
Introduction
Human paraoxonase 1 (arylesterase, EC 3.1.8.1, hPON1) is a calcium dependent enzyme which is exclusively associated with high-density lipoprotein (HDL). The enzyme is synthesized in the liver and then secreted into the blood, and exhibits broad-substrate specificity. The serum enzyme protects low-density lipoproteins (LDL) from oxidative modification in metabolism [Citation1–3]. Its gene family contains at least three members in mammals: PON1, PON2, and PON3, which are 65% and 70% similar at the nucleotide level [Citation4,Citation5]. PON1 and PON3 are expressed primarily in the liver while PON2 is widely expressed in various tissues, including brain, liver, and kidney [Citation6–8]. The enzyme was firstly characterized as an organophosphate hydrolyzer including paraoxon from which it takes its name [Citation9,Citation10]. PON1 hydrolyses several organophosphates, for instance the nerve agents: sarin, soman and the pesticides: chlorpyrifos oxon, diazoxon [Citation11–13]. It hydrolyses organophosphates (OPs), but physiological substrate and biological function of this enzyme is not clearly known yet. In addition to hydrolyzing OPs, PON1 also hydrolyses aromatic carboxyl esters, such as phenyl acetate, and is involved in drug and xenobiotic metabolism. Moreover, it hydrolyses various lactones, including naturally occurring lactone metabolites [Citation14–16]. Recently, a decrease in PON-I activity has been determined in some oxidative stress-associated diseases [Citation17]. PON1 acts as an antioxidant enzyme [Citation18] by protecting low-density lipoproteins (LDL) from oxidative damage, which is known to be associated with many vascular diseases, including atherosclerosis [Citation19]. PON1 activity reduces in cardiovascular diseases, such as diabetes mellitus, chronic renal failure, rheumatoid arthritis, hyperthyroidism and age-related macular degeneration [Citation20–28]. PON1 is thought to play its anti-atherogenic role by inhibiting the oxidation of LDL, and hydrolyzing lipid peroxides [Citation29]. HDL can influence different stages of the atherosclerotic process, and human paraoxonase-1 enzyme (PON1) contributes to the antiatherogenic effect of HDL.
PON was first purified 13-fold from rabbit kidney by Mazur in 1946 [Citation30]. Then, the enzyme has been purified using different methods from various sources. For instance, Mounter and colleagues purified the enzyme 65–100 fold in 1953 [Citation30]. In addition, PON3 from rat liver microsomes was purified using hydroxyapatite, DEAE-Sepharose, Cibacron Blue (M1), first DEAE (D1), second DEAE, and Con A methods [Citation1]. Sinan et al. purified hPON1 227-fold with ammonium sulfate fractionation and hydrophobic interaction chromatography [Citation9].
Many drugs are known to activate or inhibit several body enzymes in vivo [Citation31]. Should any drug inhibit human serum PON1, the increased oxidation of low-density lipoprotein (LDL) could cause many vascular diseases resulting in severe health problems, including atherosclerosis. Moreover, a decrease in the enzyme activity may cause vital effects in some oxidative stress-associated diseases. Many studies have been performed in our laboratory about interactions between drugs and different enzymes from a variety of sources [Citation31–33]. Indeed, due to the physiological role of PON, more studies about the inhibitory effects of medical drugs should be performed. However, there are few studies about the effects of medical drugs on PON1 activity. For example, in vitro and in vivo effects of some diuretic and hypocholesterolemic drugs on paraoxonase activity have been investigated, such as spironolactone, mevastatin, lovastatin, simvastatin, pravastatin and prulifloxacin [Citation34–36]. In addition, Sinan et al. reported the inhibition effects of cephalosporine and gentamycin sulfate on human serum PON1 activity. They found that gentamycin sulfate and cefazoline sodium were potential inhibitors [Citation9].
Here, we purified human serum PON1 using a simple modified method. The simple three-step procedure consists of ammonium sulfate precipitation, DEAE-Sephadex anion exchange chromatography, and Sepharose 4B-L-tyrozine-1-napthylamine hydrophobic interaction chromatography. We also examined the effects of commonly used analgesics on hPON1 activity.
Materials and methods
Materials
Materials, including DEAE-Sephadex A50, Sepharose 4B, 1-Naphthylamine, Paraoxon, protein assay reagents, and chemicals for electrophoresis, were obtained from Sigma Chem. Co (Sigma-Aldrich Chemie GmbH Export Department Eschenstrasse 5, 82024 Taufkirchen, Germany). All other chemicals used were of analytical grade and were obtained from either Sigma-Aldrich or Merck (Merck KGaA Frankfurter strasse 250, D-64293 Darmstadt, Germany). Lornoxicam, indomethacin, tenoxicam, diclofenac sodium, ketoprofen and lincomycine were provided from the University Hospital Pharmacy (Atatürk University, Erzurum, Turkey).
Paraoxonase activity assay
Paraoxonase activity was determined at 25°C with paraoxon (diethyl p-nitrophenyl phosphate) (1 mM) in 50 mM glycine/NaOH (pH 10.5) containing 1 mM CaCl2. The enzyme assay was based on the estimation of p-nitrophenol at 412 nm. The molar extinction coefficient of p-nitrophenol (ϵ = 18.290 M−1cm−1 at pH 10.5) was used to calculate enzyme activity [Citation37]. One enzyme unit was defined as the amount of enzyme that catalyzes the hydrolysis of 1 μmol of substrate at 25°C. Assays were performed using a spectrophotometer (CHEBIOS UV-VIS).
Ammonium sulfate precipitation
Twelve milliliters of Triton X-100-treated human serum was precipitated with ammonium sulphate. The precipitation fractions for paraoxonase were 60%-80% [Citation9]. The precipitate was collected by centrifugation at 15,000 rpm for 20 min and redissolved in 100 mM Na-phosphate buffer (pH 7.0).
DEAE-Sephadex A50 Anion Exchange Chromatography
The enzyme solution, which had been dialyzed in the presence of 1 mM Na-phosphate buffer (pH 7.0) at 4°C, was loaded onto the DEAE-Sephadex A50 anion exchange column (3 cm2 × 30 cm), which had been equilibrated with 100 mM Na-phosphate buffer (pH 7.0). The column was washed with 100 mM Na-phosphate buffer (pH 7.0), and then elution was performed with a linear gradient of 0-1.5 M NaCl. Eluted fractions were collected and enzyme activity was checked at 412 nm. Tubes with enzyme activity were combined. All purification procedures were performed at 4°C.
Sepharose 4B-L-tyrozine-1-napthylamine hydrophobic interaction chromatography
Fractions from DEAE-Sephadex A50 column were mixed and prepared to apply to the Sepharose 4B-L-tyrozine-1-napthylamine hydrophobic interaction chromatography column which had been designed according to Sinan et al 2006 [Citation9]. After equilibring the column with 100 mM Na-phosphate buffer including NaCl (pH=7.0), elution was performed with a linear gradient of decreasing NaCl concentration 0–1,5 M NaCl. Fractions were analyzed for both protein amount (280 nm) and enzyme activity (412 nm). Tubes with enzyme activity were combined for other kinetic studies.
Protein Determination
During the purification steps, protein quantity was determined spectrophotometrically at 595 nm according to the Bradford method using bovine serum albumin as the standard [Citation38].
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed after purification of the enzyme with 10% and 3% acrylamide concentrations for the separating and stacking gels, respectively, and 0.1% SDS [Citation39]. Sample (20 μg) was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coomassie Brilliant Blue R-250, 50% methanol, and 10% acetic acid, then destained with several changes of the same solvent without dye.
In vitro studies for the drugs
We examined the inhibitory effects of six analgesic drugs: lornoxicam, indomethacin, tenoxicam, diclofenac sodium, ketoprofen and lincomycine. All compounds were tested in triplicate at each concentration used. PON activities were measured in the presence of different drug concentrations. Control activity was assumed to be 100% in the absence of inhibitor. For each drug, a percent activity versus drug concentration graph was drawn. For determination of Ki values, three different inhibitor concentrations were tested for each drug. In these experiments, paraoxone was used as substrate at five different concentrations (0.15, 0.3, 0.45, 0.6, and 0.75 mM). Lineweaver–Burk curves were used for determination of Ki and inhibitor type [Citation40].
Results and discussion
Human serum paraoxonase (PON 1), a calcium dependent HDL-associated enzyme, is known for its detoxification and antiatherogenic properties [Citation37]. The substrate specifity of PON is not well understood, so its physiologic function has not been clarified in vivo conditions. Yet, no natural substrates of this enzyme are known [Citation9,Citation30]. The enzyme catalyses the hydrolysis of organophosphates, aromatic carboxylic acid esters, lactones, and carbamates[Citation41]. Organophosphates (OP) are pesticides that inhibit cholinesterase. They cause poisonings and deaths [Citation42,Citation43]. Bioscavengers are enzymes or antibodies neutralising highly toxic OPs before they reach biological targets [Citation44]. Indeed, hPON1 also acts as an antioxidant enzyme, and is an in vivo bioscavencer [Citation18]. In the recent years, PON1, PON2 and PON3 have been shown to be members of a multigene family in mammals. PON1 and PON3 are expressed primarily in the liver while PON2 is widespread throughout almost all tissues, including brain, liver, kidney and testis, [Citation8].
Enzymes catalyze almost all chemical reactions in metabolism of the living organisms. Many chemicals influence metabolism at low concentrations by decreasing or increasing normal enzyme activity, especially by inhibiting specific enzymes [Citation45] with critical function [Citation46], and they are important drug targets [Citation47]. A number of studies about interactions between drugs and different enzymes have been carried out by our group. For instance, Carbonic anhydrase, glucose 6-phosphate dehydrogenase, and 6-phosphogluconate dehydrogenase were purified from different sources and investigated for inhibition and activation effects of commonly used drugs in medical applications [Citation31,Citation48,Citation49]. However, there are few studies about effects of medical drugs on PON1 activity in literature. For instance, Sinan et al. investigated the in vitro effects of gentamycin sulfate and cefazolin sodium on the purified human serum PON1 enzyme and human liver PON1 activity in human hepatoma HepG2 cells [Citation9]. In that study, they found that gentamycin sulfate and cefazolin sodium were potent inhibitors for human serum PON1, and IC50 values were 0.887 and 0.0084 mM, respectively. Metal ions, such as Co (II), Cu (II), Mn (II), Hg (II), and p-hydroxymercuribenzoate (pOHMB) change PON1 and PON3 activity in rat liver, indicating that their active sites may contain lysine, histidine, phenylalanine, cysteine, tryptophan, aspartic acid, glutamic acid, and asparagine residues, which can bind metals [Citation50]. The rank order of inhibitors were different: for PON1, Hg2+ > pOHMB > Co2+ > Mn2+ > Cu2+ and for PON3, Hg2+ > Cu2+ > pOHMB > Mn2+ > Co2+, suggesting that more work is necessary to determine the protective role of PONs against the toxic effects of xenobiotics, including environmental heavy metals and oxidative stress by-products.
In the present study, human serum PON1 (hPON1) was purified using only three procedures. The procedure consists of ammonium sulfate fractionation (60-80%), DEAE-Sephadex anion exchange and Sepharose 4B-L-tyrosine-1-napthylamine hydrophobic interaction chromatography. The purification procedure is relatively fast and takes only 7 to 8 h. The enzyme, with a specific activity of 3840 U/mg protein, was purified 314.9-fold with a yield of 25.3% (). PON has been purified using different purification procedures from a wide range of sources. In a study, human serum PON enzyme was purified approx. 62.1-fold using Agarose blue, Sephadex G 200, DEAE-Trisacryl M, Sepladex G 75 chromatography techniques. Pla and colleagues purified PON3 from rat liver with a final specific activity of 461 μmol min−1 mg−1 and a yield of 0.4% [Citation50]. They obtained an overall purification factor of 177-fold using six steps, including hydroxyapatite adsorption, DEAE-Sepharose CL-6B chromatography, Cibacron Blue 3GA non-specific affinity chromatography, anion exchange on Mono Q HR 5/5, DEAE-cellulose, and a final affinity chromatography on Concovalin A-Sepharose. Thus, the present purification procedure both takes less time and has higher specific activity, yield, and purification. The final purified hPON1 had only one protein band on SDS-PAGE, with a molecular weight of 43 kDa, which is in agreement with other studies [Citation9,Citation11], although others show different migration patterns [Citation51]. We also examined the in vitro effects of some analgesic drugs, such as lornoxicam, indomethacin, tenoxicam, diclofenac sodium, ketoprofen and lincomycine, on human serum PON1 activity. Both the IC50 and Ki parameters were determined. Inhibitory effects of the drugs on enzyme activities were tested under in vitro conditions. Both the IC50 and Ki parameters were determined and are given in and the representative graphs for lead are shown in and . It is important that the drugs inhibited the enzyme activity at low concentrations (), and that they are potent inhibitors for human serum PON1. Especially, lornoxicam, indomethacin and tenoxicam have inhibitory effects at lower concentrations according to IC50 values, 0.136, 0.195 and 0.34 mM, respectively ( and ). According to these results, inhibition range can be given as; lornoxicam > indomethacin > tenoxicam > diclofenac sodium > ketoprofen > lincomycine. Ki graphs show that lornoxicam, diclofenac sodium and lincomycine inhibit the enzyme in an uncompetitive manner ( and ), ketoprofen inhibits in noncompetitive manner (), indomethacin and tenoxicam inhibit in competitive manner ( and ). Since lower Ki indicates greater inhibition, lornoxicam has a high inhibition rate for the enzyme in comparison with that of other drugs. When the chemical structures of the drugs are investigated, it can be clearly seen that the enzyme is susceptible to the molecules having electronegative atoms like chlor, sulphur, nitrogen and oxygen (). It is so interesting that IC50 and Ki values of lornoxicam and tenoxicam are so different even though their chemical structures are the same except for an additional chlorine atom in lornoxicam. This may indicate that molecules containing chlor atoms are strong inhibitors for PON-I, but this is a little contradictory because PON activity is known to be stimulated in the presence of NaCl and CaCl2 salts. So, these results should be supplemented with additional studies such as X-ray analysis and, etc.
Figure 1. In vitro effect of the drugs – (a) lornoxicam, (b) tenoxicam at five different concentrations on paraoxonase-1 activity.
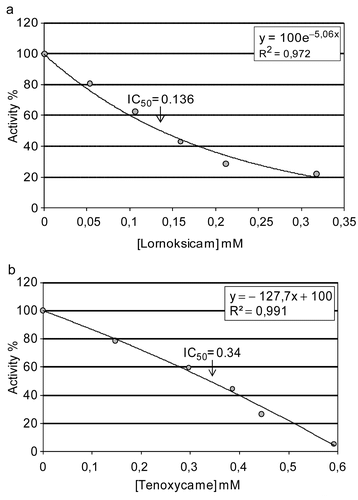
Figure 2. Ki graphs for paraoxonase from human serum. (a) and (b), Lineweaver-Burk graphs in 5 different substrate (paraoxon) concentrations and 3 different (a) lornoxicam, or (b) tenoxicam, concentrations for determination of Ki.
Figure 3. Structures of lornoxicam, indomethacin, tenoxicam, diclofenac sodium, ketoprofen and lincomycine.
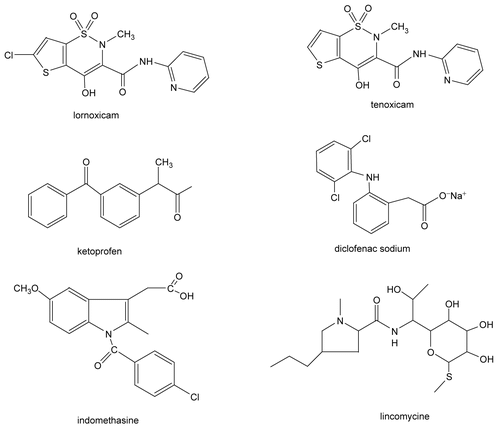
Table 1. Summary of purification procedure of PON 1.
Table 2. Ki values and inhibition types of 6 inhibitors for PON1.
In conclusion, it was aimed in this study to purify PON1 from human serum, and understand the inhibition effects of some drugs on this crucial enzyme.
Acknowledgement
The authors thank to Baris and Elif Cinel for their kind help in providing fresh blood and serum samples.
Declaration of interest: The authors report no conflicts of interest.
References
- Rodrigo L, Gil F, Hernandez AF, Lopez O, Pla A. Identification of paraoxonase 3 in rat liver microsomes: purification and biochemical properties. Biochem J 2003;376:261–268.
- La Du BN. Human serum paraoxonase/arylesterase, In Pharmacogenetics of Drug Metabolism (Kalow, W., ed.). Pergamon, Elmford, NY; 1992. p 51–91.
- Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet 1996;14:334–336.
- Billecke S, Draganov D, Councell R, Stetson P, Watson C, Hsu C, La Du BN. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab Dispos 2000;28:1335–1342.
- Lu H, Zhu J, Zang Y, Ze Y, Qın J. Cloning, purifcation, and refolding of human paraoxonase-3 expressed in Escherichia coli and its characterization. Protein Expres Purif 2006;46:92–99.
- Durringhton PN, Mackness B, Mackness MJ. Paraoxonase and atherosclerosis. Arterioscl Throm and Vas 2001;21:473–480.
- Primo-Parmo SL, Sorenson RS, Teiber J, La Du BN. The human serum paraoxonase / arylesterase gen (PON1) is one member of a multigene family. Genomics 1996;33:498–507.
- La Du N, Aviram S, Billecke M, Navabc M, Primo-Parmoa S, Sorensona RC, Standifordd TJ. On the physiological role(s) of the paraoxonases. Chem-Biol Interac 1999;119–120:379–388.
- Sinan S, Kockar F, Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie 2006;88:565–574.
- Costa LG, Li WF, Richter RJ, Shihc DM, Lusisc A, Furlongb CE. The role of paraoxonase (PON1) in the detoxification of organophosphates and its human polymorphism. Chem-Biol Interac 1999;119–120:439–444.
- Golmanesh L, Mehrani H, Tabei M. Simple procedures for purification and stabilization of human serum paraoxonase-1. J Biochem and Bioph Meth 2008;70:1037–42.
- Furlong CE, Li WF, Brophy VH, Jarvik GP, Richter RJ, Shih DM, Lusis AJ, Costa LG. The PON1 gene and detoxication. Neurotoxicology 2000;21:581–588.
- Josse D, Masson P. Human plasma paraoxonase (HUPON1): an atherogenic enzyme with organophosphate hydrolase activity. Ann Pharmaceut Fran 2001;59:108–118.
- Aharoni A, Gaidukov L, Khersonsky O, Gould SM, Roodveldt C, Tawfik DS. The ‘evolvability’ of promiscuous protein functions. Nat Genet 2005;37:73–76.
- Khersonsky O, Tawfik DS. Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry-US 2005;44: 6371–6382.
- Rochua D, Chabri`ere E, Massona P. Human paraoxonase: A promising approach for pre-treatment and therapy of organophosphorus poisoning. Toxicology 2007;233:47–59.
- Nguyen SD, Sok DE. Preferable stimulation of PON1 arylesterase activity by phosphatidylcholines with unsaturated acyl chains or oxidized acyl chains at sn-2 position. BBA Biomembranes 2006;1758:499–508.
- Akgur SA, Ozturk P, Solak I, Moralb AR, Ege B. Human serum paraoxonase (PON1) activity in acute organophosphorus insecticide poisoning. Forensic Sci Int 2003;133:136–140.
- Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL, Newton RS, La Du B. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radical Bio Med 1999;26:892–904.
- Navab M, Hama-Levy S, Van Lenten BJ, Fonarow GC, Cardinez CJ, Castellani LW, Brennan ML, Lusis AJ, Fogelman AM, La Du BN. Mildly oxidized LDL induces an increased apolipoprotein J/ paraoxonase ratio. J Clin Invest 1997;99:2005–19.
- McElveen J, Mackness MI, Colley CM, Peard T, Warner S, Walker CH. Distribution of paraoxon hydrolytic activity in the serum of patients after myocardial infarction. Clin Chem 1986;32:671–3.
- Abbott CA, Mackness MI, Kumar S, Boulton AJ, Durrington PN. Serum paraoxonase activity, concentration and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscl Throm and Vas 1995;15:1812–18.
- Mackness B, Mackness MI, Arrol S, Turkie W, Julier K, Abuasha B, Miller JE, Boulton AJM, Durrington PN. Serum paraoxonase (PON1) 55 and 192 polymorphism and paraoxonase activity and concentration in non-insulin dependent diabetes mellitus. Atherosclerosis 1998;139:341–9.
- Sakai T, Matsuura B, Onji M. Serum paraoxonase activity and genotype distribution in Japanese patients with diabetes mellitus. Internal Med 1998;37:581–4.
- Dantoine TF, Debord J, Charmes JP, Merle L, Marquet P, Lachatre G, Leroux-Robert C. Decrease of serum paraoxonase activity in chronic renal failure. J Am Soc Nephrol 1998;9:2082–2088.
- Baskol G, Demir H, Baskol M, Kilic E, Ates F, Kocer D, Muhtaroglu S. Assessment of Paraoxonase 1 activity and malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem 2005;38:951–955.
- Raiszadeh F, Solati M, Etemadi A, Azizi F. Serum paraoxonase activity before and after treatment of thyrotoxicosis.Serum paraoxonase activity before and after treatment of thyrotoxicosis. Clin Endocrinol 2004;60:75–80.
- Baskol G, Karakucuk S, Oner AO, Baskol M, Kocer D, Mirza E, Saraymen R, Ustdal M, Serum paraoxonase 1 activity and lipid peroxidation levels in patients with age-related macular degeneration. Ophthalmologica 2006;220:12–16.
- Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Navab M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 1995;96:2882–2891.
- Rodrigo L, Gil F, Hernandez AF, Marina A, Vazquez J, Pla A, Purification and characterization of paraoxon hydrolase from rat liver. Biochem J 1997;321:595–601.
- Çiftçi M, Beydemir ş, Yılmaz H, Bakan E. Effects of some drugs on rat erythrocyte 6-phosphogluconate dehydrogenase: an in vitro and in vivo study. Pol J Pharmacol 2002;54:275–280.
- Beydemir Ş, Gülçin İ, Küfrevioğlu Öİ, çiftci M. Glucose 6-phosphate dehydrogenase: In vitro and in vivo effects of dantrolene sodium. Pol J Pharmacol 2003;55:787–792.
- Ekinci D, Beydemir ş, Alım Z. Some drugs inhibit in vitro hydratase and esterase activities of human carbonic anhydrase-I and II. Pharmacol Rep 2007;59:580–587.
- Malin R, Laaksonen R, Knuuti J, Janatuinen T, Vesalainen R, Nuutila P, Lehtimäki T. Paraoxonase genotype modifies the effect of pravastatin on high-density lipoprotein cholesterol. Pharmacogenetics 2001;11:625–633.
- Tomas M, Senti M, Garcia-Faria F, Vila J, Torrents A, Covas M, MarrugatJ. Effect of simvastatin therapy on paraoxonase activity and related lipoproteins in familial hypercholesterolemic patients. Arterioscl Throm and Vas 2000;20:2113–2119.
- Leviev I, James R. Simvastatin increases plasma levels of the antioxidant enzyme paraoxonase by PON1 gene activation, Atherosclerosis 2000;151:41.
- Renault F, Chabrière E, Andrieu JP, Dublet B, Massona P, Rochua D, Tandem purification of two HDL-associated partner proteins in human plasma, paraoxonase (PON1) and phosphate binding protein (HPBP) using hydroxyapatite chromatography. J Chromatogr B 2006;836:15–21.
- Bradford MM. A rapid and sensitive method for the quantition of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72: 48–251.
- Laemli DK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970;227:680–683.
- Lineweaver H, Burk D, The determination of enzyme dissocation constants. J Am Chem Soc 1934;57:685.
- La Du BN. Structural and functional diversity of paraoxonases. Nat Med 1996;2: 1186–1187.
- Alici HA, Ekinci D, Beydemir ş. Intravenous anesthetics inhibit human paraoxonase-1 (PON1) activity in vitro and in vivo. Clin Bioch 2008 ;(in press).
- Shadnia S, Azizi E, Hosseini R, Khoei S, Fouladdel S, Pajoumand A, Jalali N, Abdollahi M. Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Hum Exp Toxicol 2005;9:439–445.
- Ashani Y, Pistinner S. Estimation of the upper limit of human butyrylcholinesterase dose required for protection against organophosphates toxicity: a mathematically based toxicokinetic model. Toxicol Sci 2004;77:358–367.
- Hochster RM, Kates M, Quastel JH. Metabolic Inhibitors, New York: Academic Press, 1973.
- Christensen GM, Olson D, Riedel B. Chemical effects on the activity of eight enzymes: a review and a discussion relevant to environmental monitoring. Environ Res 1982;29:247–255.
- Robertson JG, Enzymes as a special class of therapeutic target: clinical drugs and modes of action. Curr Opin Struc Biol 2007;17:674–679.
- Yılmaz H, Ciftci M, Beydemir S, Bakan E. Purification of glucose 6-phosphate dehydrogenase from chicken erythrocytes. investigation of some kinetic properties. Prep Bıochem Bıotech 2002;32:287–301.
- Hisar O, Beydemir S, Gülçin I, Küfrevioğlu OI, Supuran CT. Effects of low molecular weight plasma inhibitors rainbow trout (Oncorhynchus mykiss) on human erythrocyte carbonic anhydrase-II isozyme activity in vitro and rat erythrocytes in vivo”. J Enz Inhib Med Ch 2005;20:35–39.
- Pla A, Rodrigo L, Hernandez AF, Gil Lopez FO. Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chem-Biol Interact 2007;167:63–70.
- Furlong CE, Richter RJ, Chapline C, Crabb JW. Purification of Rabbit and Human Serum Paraoxonase. Biochemistry 1991;30:10133–10140.