Abstract
The potency of newly developed bispyridinium compounds (K250, K251) in reactivating tabun-inhibited acetylcholinesterase and reducing tabun-induced lethal toxic effects was compared with currently available oximes (obidoxime, trimedoxime, the oxime HI-6) using in vivo methods. Studies determined percentage of reactivation of tabun-inhibited blood and tissue AChE in poisoned rats and showed that the reactivating efficacy of both newly developed oximes is comparable with the oxime HI-6 but it is significantly lower than the reactivating effects of obidoxime and trimedoxime, especially in diaphragm and brain. Both newly developed oximes were also found to be able to slightly reduce lethal toxic effects in tabun-poisoned mice. Their therapeutic efficacy is higher than the potency of the oxime HI-6 but it is lower than the therapeutic effects of trimedoxime and obidoxime. Thus, the reactivating and therapeutic potency of both newly developed oximes (K250, K251) does not prevail over the effectiveness of currently available oximes and, therefore, they are not suitable for their replacement for the treatment of acute tabun poisoning.
Introduction
The current treatment of nerve agent poisoning usually includes an anticholinergic agent (preferably atropine) to block the overstimulation of cholinergic receptors, an oxime to reactivate nerve agent-inhibited acetylcholinesterase (AChE, EC 3.1.1.7) and an anticonvulsive drug to counteract centrally mediated seizures and subsequent tonic-clonic convulsions. Compounds with an oximate anion bound to the pyridinium ring are used to reactivate nerve agent-inhibited AChE by dephosphorylating the active site and restoring enzyme activity. Their reactivating potency is based on the nucleophilic properties of the oxime group [Citation1,Citation2].
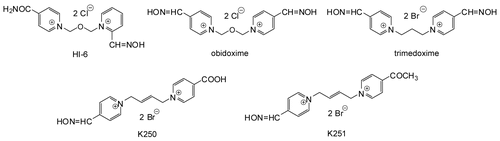
Tabun (O-ethyl-N,N-dimethyl phosphoramidocyanidate) is a highly toxic organophosphorus compound that presents a serious threat to both military and civilian populations. Its deleterious effects are extraordinarily difficult to antagonize because of the existence of a free electron pair located on the amidic nitrogen and conformational changes of AChE-tabun complex after an aging process in AChE active site that make the nucleophilic attack of oximes almost impossible [Citation3,Citation4,Citation5]. While anticholinergic drugs such as atropine are able to counteract the effects of tabun at peripheral cholinergic receptors [Citation6], commonly used reactivators of phosphorylated AChE based on monopyridinium (e.g. pralidoxime) and bispyridinium oximes (e.g. obidoxime, the oxime HI-6) are not able to sufficiently counteract the acute toxic effects of tabun because of their minimal reactivating efficacy [Citation7,Citation8,Citation9]. Therefore, the replacement of commonly used oximes (pralidoxime, obidoxime, HI-6) with a more effective oxime has been a long-standing goal for the treatment of tabun poisoning. New bispyridinium compounds, K250 (E)-1-(4-carboxypyridinium)-4-(4-hydroxyiminomethylpyridinium)-but-2-ene dibromide and K251 (E)-1-(4-hydroxyiminomethylpyridinium)-4-(4-methylcarbonylpyridinium)-but-2-ene dibromide () were synthesized at our Department of Toxicology [Citation10] to improve the efficacy of antidotal treatment in reactivating tabun-inhibited AChE and eliminating tabun-induced lethal toxicity. The evaluation of their potency to reactivate tabun-inhibited AChE using in vitro methods showed that the reactivating efficacy of both newly developed oximes is slightly lower than the effectiveness of obidoxime and better than the potency of HI-6 to reactivate tabun-inhibited AChE at both concentrations studied (10−3, 10−5 M) [Citation10]. In vitro assessment of reactivating efficacy of oximes is usually followed by the evaluation of their reactivating efficacy in vivo and their therapeutic efficacy against lethal nerve agent poisoning. The aim of this study was to compare the reactivating and therapeutic efficacy of newly developed oximes (K250, K251) with currently available oximes (obidoxime, trimedoxime, the oxime HI-6) against tabun using in vivo methods.
Material and Methods
Animals
Male albino Wistar rats weighing 200-230 g and NMRI male mice weighing between 20 and 24 g were purchased from Konarovice, Czech Republic. They were kept in an air-conditioned room with the light from 07:00 to 19:00 hr and were allowed access to standard food and tap water ad libitum. The rats were divided into groups of 8 animals. Handling of the experimental animals was done under the supervision of the Ethics Committee of the Faculty of Military Health Sciences, Czech Republic.
Chemicals
Tabun was obtained from the Technical Institute in Brno (Czech Republic) and was 95% pure. All oximes (obidoxime, trimedoxime, the oxime HI-6, K250, K251) were synthesized at the Department of Toxicology of the Faculty of Military Health Sciences (Czech Republic). Their purities were analyzed using a HPLC technique. All other drugs and chemicals of analytical grade were obtained commercially and used without further purification. All substances were administered intramuscularly (i.m.) at a volume of 1 ML/kg body weight (b.w.).
Evaluation of acute toxicity of oximes
Before starting the evaluation of reactivating and therapeutic efficacy of oximes, the acute toxicity of tested oximes was evaluated in rats and mice by the assessment of their LD50 values and their 95% confidence limits using probit-logarithmical analysis of death occuring within 24 h after i.m. administration of each oxime at five different doses with eight animals per dose [Citation11].
Evaluation of reactivating efficacy of oximes
To evaluate the reactivating efficacy of the oximes, the rats were injected i.m. with either atropine (21 mg/kg) alone (atropine control) or atropine (21 mg/kg) in combination with one of the oximes studied in equimolar dose (50 μmol/kg) 5 min before the rats received tabun i.m. at a dose of 220 μg/kg (LD50). The prophylactic administration of antidotes was used because this procedure is suitable for a mechanistic study that compares the reactivating efficacy of various oximes. The technique should give better results than the treatment of animals after poisoning and reduce the influence of aging of nerve agent-AChE complex [Citation12]. Moreover, some oximes are planned to be used prophylactically in certain chemical warfare scenarios [Citation6]. The rats were decapitated and exsanguinated to obtain the blood 30 min following tabun poisoning. The blood was hemolyzed in Tris-HCl buffer (0.02 mol/L, pH 7.6, 1:20). The tissues, diaphragm and brain were removed and homogenized in Tris-HCl buffer (0.02 mol/L, pH 7.6, 1:10) to determine AChE activity by the standard spectrophotometric method of Ellman et al. [Citation13]. Acetylthiocholine was used as substrate (Tris-HCl buffer, 0.1 mol/L, pH 7.6). Helios Alpha was the spectrophotometer was used for determination of absorbance at 436 nm. The AChE activity was expressed as μkat/kg or L (μmol substrate hydrolyzed/kg wet tissue or L blood within 1 s). The untreated control values for blood, diaphragm and brain AChE activity were obtain from rats administered with saline instead of tabun and antidotes (saline control). The reactivation % extent was calculated using the AChE activity values: {1- [((saline control) – (oxime + atropine))/((saline control) – (atropine control))]} × 100 [Citation12].
Evaluation of therapeutic efficacy of oximes
The potency of oximes in combination with atropine to eliminate tabun-induced lethal effects in mice was determined as follows. The LD50 value of tabun and its 95% confidence limit in tabun-poisoned mice was assessed using probit-logarithmical analysis of death occuring within 24 h after i.m. administration of tabun at five different doses with eight mice per dose [Citation11]. Then, tabun-poisoned mice were treated i.m. with one of tested oximes at equitoxic doses (5% LD50) in combination with atropine (21 mg/kg) at 1 min after i. m. challenge of tabun. The LD50 values of tabun and their 95% confidence limit in treated, tabun-poisoned mice were assessed by the same method. The efficacy of tested antidotal mixtures was expressed as protective ratio (LD50 value of tabun in protected mice/ LD50 value of tabun in unprotected mice). Statistical significance was determined by the use of Student’s t-test and differences were considered significant when P < 0.05. Statistical evaluation was determined with the relevant computer programs [Citation11].
Results
The acute i.m. toxicity of tested oximes is summarized in . The results show that the acute toxicity of newly developed oxime K250 corresponds to the acute toxicity of HI-6 that is considered to be the least toxic oxime for both animal species and it is much lower toxic than obidoxime and trimedoxime in rats as well as mice. Unfortunately, we were not able to calculate the LD50 value for K250 in rats due to the limitation of its solubility. The acute toxicity of another newly developed oxime K251 corresponds to the acute toxicity of obidoxime and trimedoxime in mice but it is less toxic than obidoxime and trimedoxime in rats.
Table 1. LD50 values of oximes following i.m. administration in rats and mice.
The ability of oximes to reactivate tabun-inhibited AChE in rat blood, diaphragm and brain in vivo is shown in . Both newly developed oximes seem to be relatively poor reactivators of tabun-inhibited AChE in blood, diaphragm as well as brain. Their reactivating efficacy is significantly lower in comparison with the potency of obidoxime and trimedoxime to reactivate tabun-inhibited AChE, especially in diaphragm and brain. Additionally, their reactivating efficacy corresponds to the reactivating potency of the oxime HI-6 that is considered to be the worst reactivator of tabun-inhibited AChE among currently available oximes.
Table 2. Percent reactivation of tabun-inhibited AChE by oximes in rat blood, diaphragm and brain in vivo.
These results correlate with the therapeutical potency of the oximes tested against lethal tabun poisoning in mice (). Tabun – poisoned mice showed wide spectrum of clinical signs of poisoning including muscarinic (salivation) and niconitic (tonic-clonic convulsions) signs within a few minutes regardless of the type of antidotes. They died within 25-35 minutes after poisoning with tabun. Both newly developed oximes (K250, K251) were able to decrease the acute toxicity of tabun approximately 1.3-fold. Their therapeutic efficacy is slightly higher than the potency of the oximes HI-6 in decreasing acute toxicity of tabun. On the other hand, obidoxime and trimedoxime showed higher potency to reduce acute lethal toxic effects of tabun in mice when compared with newly synthesized oximes K250 and K251.
Table 3. The influence of the type of oxime on the potency of antidotal treatment to eliminate acute lethal effects of tabun in mice.
Discussion
It has been described many times that commonly used monopyridinium and bispyridinium oximes seem to be relatively poor reactivators of tabun-inhibited AChE. The evaluation of their kinetic parameters characterizing in vitro reactivation of tabun-inhibited AChE showed that dissociation constants and rate constants are lower compared to kinetic parameters describing the reactivation of sarin, soman or cyclosarin-inhibited AChE by these oximes [Citation14,Citation15,Citation16]. Therefore, a lot of new structural analogues of currently available oximes have been developed to increase the potency of oximes to reactivate tabun-inhibited AChE [Citation17,Citation18, Citation19, Citation20].
The reactivating efficacy of oximes depends upon their chemical structure. The main structural features which influence their reactivation potency are the oxime functional group (position and number of groups), the connecting linker for bisquaternary reactivators and other substituent(s) on the second heteroaromatic ring [Citation21,Citation22,Citation23]. For tabun-inhibited AChE at least one oxime in position four on the heteroaromatic ring is necessary for substantial reactivation whilst an oxime in position two has a low or no reactivation capability [Citation22]. Additionally, the optimal linker length suitable for reactivation of tabun-inhibited AChE varies from three to four carbon-carbon bonds [Citation21]. The (E)-but-2-ene linker involved into the structure of both newly developed oximes (K250, K251) is slightly longer than three but slightly shorter than four carbon-carbon bonds due to the presence of double bond, which also restricts the conformational flexibility of (E)-but-2-ene linker [Citation24]. Moreover, the necessity of (E)- instead of (Z)-but-2-ene linker has also been established [Citation25]. These data can explain relatively low efficacy of the oxime HI-6, which is effective against fluorophosphonates [Citation14,Citation15,Citation16], because the oxime HI-6 contains a dimethylether bridge and the oxime group at position 2. The chemical structure of the oxime HI-6 compared to other oximes studied is disadvantageous for the reactivation of tabun-inhibited AChE [Citation21].
Previously published in vitro results correlate with our results obtained in vivo. Both newly developed oximes (K250, K251), that were characterized by moderate percentage of reactivation of tabun-inhibited AChE in vitro [Citation10], were found to be relatively week reactivators of tabun-inhibited AChE in blood, diaphragm and brain of tabun-poisoned rats and slightly efficacious to protect mice poisoned with lethal doses of tabun. In addition, K250 or K251-induced % reactivation of tabun-inhibited AChE in blood, diaphragm and brain did not reach 10% that is considered to be necessary for survival of nerve agent - poisoned animals [Citation26].
Our results confirm that there is no single, broad-spectrum oxime suitable for the antidotal treatment of poisonings with all organophosphorus agents [Citation1,Citation27]. While trimedoxime and obidoxime are preferred for the treatment of acute poisoning with organophosphorus insecticides (OPI) because they are considered to be sufficiently effective reactivators of OPI-inhibited AChE [Citation28,Citation29,Citation30], the oxime HI-6 appears to be a promising antidote against highly toxic fluorophosphonates, especially soman and cyclosarin, because it is able to protect experimental animals from adverse effects and improve survival of poisoned animals [Citation14,Citation15]. Nevertheless, our results clearly demonstrate its low potency to reactivate tabun-inhibited AChE in rats and protect tabun-poisoned mice from its lethal toxic effects [Citation31,Citation32]. Trimedoxime as well as obidoxime seem to be more effective oximes for the treatment of acute tabun poisonings than the oxime HI-6 but their potency to eliminate tabun-induced lethal effects is limited, when they are administered at low, human-relevant doses [Citation32]. They are not able to reach 70% reactivation of tabun-inhibited AChE that is necessary for non-toxic equilibrium state [Citation26]. The potency of both newly developed oximes (K250, K251) to reactivate tabun-inhibited AChE in rats and to reduce lethal toxic effects of tabun in mice corresponds to the potency of the oxime HI-6 and it is lower compared to obidoxime and trimedoxime. Therefore, they are not suitable for the replacement of commonly used oximes for the treatment of acute tabun poisoning.
Acknowledgements
The authors wish to thank Mrs Martina Hrabinova and Mrs Jana Uhlirova for their skilful assistance.
Declaration of interest: The study was supported by the grant of Ministry of Defence, No OPUOFVZ200603.
References
- Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol 2002;40:803–816.
- Marrs TC. Organophosphate poisoning. Pharmacol Ther 1993;58:51–66.
- Cabal J, Bajgar J. Tabun – reappearance 50 years later (in Czech). Chem Listy 1999;93:27–31.
- Ekström F, Akfur C, Tunemalm AK, Lundberg S. Structural changes of phenylalanine 338 and histidine 447 revealed by the crystal structures of tabun-inhibited murine acetylcholinesterase. Biochemistry 2006;45:74–81.
- Jokanovic M, Maksimovic M, Kilibarda V, Jovanovic D, Savic D. Oxime-induced reactivation of acetylcholinesterase inhibited by phosphoramidates. Toxicol Lett 1996;85:35–39.
- Bajgar J. Organophosphate/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis and treatment. Adv Clin Chem 2004;38:151–216.
- Koplovitz I, Stewart JR. A comparison of the efficacy of HI-6 and 2-PAM against soman, tabun, sarin and VX in the rabbit. Toxicol Lett 1994;70:169–179.
- Puu G, Artursson E, Bucht G. Reactivation of nerve agent inhibited acetylcholinesterases by HI-6 and obidoxime. Biochem Pharmacol 1986;35:1505–1510.
- Worek F, Widmann R, Knopff O, Szinicz L. Reactivating potency of obidoxime, pralidoxime, HI-6 and HLö-7 in human erythrocyte acetylcholinesterase inhibited by highly toxic organophosphorus compounds. Arch Toxicol 1998;72:237–243.
- Musilek K, Holas O, Jun D, Dohnal V, Gunn-Moore F, Opletalova V, Dolezal M, Kuca K. Monooxime reactivators of acetylcholinesterase with (E)-but-2-ene linker – Preparation and reactivation of tabun and paraoxon-inhibited acetylcholinesterase. Bioorg Med Chem,2007;15: 6733–6741.
- Tallarida R. Murray R. Manual of Pharmacological Calculation with Computer Programs. New York:Springer-Verlag;1987.
- Clement JG, Hansen AS, Boulet CA. Efficacy of HLö-7 and pyrimidoxime as antidotes of nerve agent poisoning in mice. Arch Toxicol 1992;66:216–219.
- Ellman GL, Courtney DK, Andres VJr, Feartherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–93.
- Kassa J, Cabal J. A comparison of the efficacy of a new asymmetric bispyridinium oxime BI-6 with currently available oximes and H oximes against soman by in vitro and in vivo methods. Toxicology 1999;132:111–118.
- Kassa J, Cabal J. A comparison of the efficacy of a new asymmetric bispyridinium oxime BI-6 with presently used oximes and H oximes against sarin by in vitro and in vivo methods. Hum Exp Toxicol 1999;18:560–565.
- Kassa J, Cabal J. A comparison of the efficacy of acetylcholinesterase reactivators against cyclohexylmethylphosphonofluoridate (GF agent) by in vitro and in vivo methods. Pharmacol Toxicol 1999;84:41–45.
- Kuca K, Kassa J. A comparison of the ability of a new bispyridinium oxime – 1-(4- hydroxyiminomethylpyridinium-4-(4-carbamoylpy ridinium)butane dibromide and currently used oximes to reactivate nerve agent-inhibited rat brain acetylcholinesterase by in vitro methods. J Enz Inhib Med Chem 2003;18:529–535.
- Picha J, Kuca K, Kivala M, Kohout M, Cabal J, Liska F. New group of monoquaternary reactivators of the acetylcholinesterase inhibited by nerve agents. J Enz Inhib Med Chem 2005;20:233–237.
- Musilek K, Holas O, Kuca K, Jun D, Dohnal V, Dolezal M. Synthesis of a novel non-symmetrical bispyridinium compounds bearing a xylene linker and evaluation of their reactivation activity using tabun and paraoxon-inhibited acetylcholinesterase. J Enz Inhib Med Chem 2007;22:425–432.
- Musilek K, Holas O, Kuca K, Jun D, Dohnal V, Opletalova V, Dolezal M. Synthesis of monooxime-monocarbamoyl bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase. J Enz Inhib Med Chem 2008;23:70–76.
- Cabal J, Kuca K, Kassa J. Specification of the structure of oximes able to reactivate tabun-inhibited acetylcholinesterase. Pharmacol Toxicol 2004;95:81–86.
- Kuca K, Jun D, Musilek K. Structural requirements of acetylcholinesterase reactivators. Mini Rev Med Chem 2006;6:269–277.
- Musilek K, Kuca K, Jun D, Dolezal M. Progress in synthesis of new acetylcholinesterase reactivators during the period 1990-2004. Curr Org Chem 2007; 11:229–238.
- Carey FA, Sundberg RJ. In: The Structure and Mechanisms, 4th ed.; Plenum Publishers, New York, 2000, p. 13.
- Musilek K, Holas O, Kuca K, Jun D, Dohnal V, Opletalova V, Dolezal M. Novel series of bispyridinium compounds bearing a (Z)-but-2-ene linker – synthesis and evaluation of their reactivation activity against tabun and paraoxon-inhibited acetylcholinesterase. Bioorg Med Chem Lett 2007;17:3172–3176.
- Bajgar J, Fusek J, Kuca K, Bartosova L, Jun D. Treatment of organophosphate intoxication using cholinesterase reactivators: facts and fiction. Mini-Rev Med Chem 2007;7:461–466.
- Kassa J, Kuca K, Bartosova L, Kunesova G. The development of new structural analogues of oximes for the antidotal treatment of poisoning by nerve agents and the comparison of their reactivating and therapeutic efficacy with currently available oximes. Curr Org Chem 2007;11:267–283.
- Jokanovic M, Maksimovic M. A comparison of trimedoxime, obidoxime, pralidoxime and HI-6 in the treatment of oral organophosphorus insecticide poisoning in the rat. Arch Toxicol 1995;70:119–123.
- Petroianu GA, Nurulain SM, Nagelkerke N, Shafiullah M, Kassa J, Kuca K. Five oximes (K-27, K-48, obidoxime, HI-6 and trimedoxime) in comparison with pralidoxime: survival in rats exposed to methyl-paraoxon. J Appl Toxicol 2007;27: 453–457.
- Worek F, Kirchner T, Bäker M, Szinicz L. Reactivation by various oximes of human erythrocyte acetylcholinesterase inhibited by different organophosphorus compounds. Arch Toxicol 1996;70:497–503.
- Kassa J, Jun D, Kuca K. A comparison of reactivating efficacy of newly developed oximes (K074, K075) and currently available oximes (obidoxime, HI-6) in cyclosarin and tabun-poisoned rats. J Enz Inhib Med Chem. 2007; 22:297–300
- Kassa J, Karasova J, Bajgar Kuca, K, Musilek KA comparison of the therapeutic and reactivating efficacy of newly developed bispyridinium compounds (K206, K269) with currently available oximes against tabun in rats and mice. J Enz Inhib Med Chem 2008; 23: 776–780