Abstract
Protein tyrosine phosphatase 1B (PTP1B) appears to be an attractive target for the development of new drugs for type 2 diabetes and obesity. In our preliminary test, a MeOH extract of the stem barks of Sorbus commixta Hedl. (Rosaceae) showed strong PTP1B inhibitory activity. Bioassay−guided fractionation of the MeOH extract resulted in the isolation of two lupane−type triterpenes, lupenone (1) and lupeol (2). Compounds 1 and 2 inhibited PTP1B with IC50 values of 13.7 ± 2.1 and 5.6 ± 0.9 μM, respectively. Kinetic studies revealed that both the compounds 1 and 2 are non−competitive inhibitors of PTP1B that decrease Vmax values with no effect on Km values.
Introduction
Metabolic diseases such as type 2 diabetes and obesity are associated with insulin resistance [Citation1–3]. Recent studies suggest that protein tyrosine phosphatase 1B (PTP1B) plays a major role in the inhibition of insulin action [Citation1–3]. At the cellular level, the insulin signaling is initiated by means of phosphorylation of insulin receptor (IR) and insulin receptor substrates (IRS), which then activates several signaling cascades leading to biological responses such as glucose transport into the cells and glycogen synthesis [Citation1–3]. In this process, PTP1B blocks the signaling pathway by dephosphorylating the activated IR as well as IRS proteins. Accordingly, the enzyme is emerging as a potential therapeutic target for the treatment of type 2 diabetes and obesity [Citation1–4]. Although several types of synthetic PTP1B inhibitors have been developed and applied for clinical trials, due to the toxicity, side effects, and low bioavailability, new types of PTP1B inhibitors still need to be discovered [Citation1–4]. Because natural products are recognized as an attractive source for the development of new PTP1B inhibitors, we have screened for hundreds of plant extracts using in vitro enzyme assay [Citation5]. In the continuing study, we found that a MeOH extract of the stem barks of Sorbus commixta Hedl. (common names: mountain ash, scarlet rowan) in the family Rosaceae inhibited PTP1B activity (65% inhibition at 30 μg/mL). The stem bark of S. commixta has been used in traditional medicine as a tonic and to treat various respiratory diseases [Citation6]. The fruits have also been used as a laxative, gargle for sore throats, inflamed tonsils, and hoarseness [Citation6]. The biphenyls acuparin and its 29- and 49-oxygenated derivatives have been reported as phytoalexins of the genus Sorbus [Citation7]. Previous phytochemical investigations on this plant have resulted in the isolation of triterpenes, lignans and flavonoids [Citation8]. In our previous work, the flavanol glycosides, catechin-7-O-β-D-xylopyranoside and catechin-7-O-β-D-apiofuranoside, isolated from this species, were found to have antioxidant activity [Citation9]. Recently, its extract was demonstrated to have beneficial effects on atherosclerosis and protective effect on hepatic lipid peroxidation in acute-alcohol treated model [Citation10,Citation11]. However, there has been no study with regard to its inhibitory effect on PTP1B. Using a bioassay-guided fractionation, we finally purified two lupane type triterpenoids identified as lupenone (1) and lupeol (2) as active principles. In this paper, we describe the isolation of two active constituents, and the kinetic analyses of the compounds on the enzyme.
Materials and Methods
Plant material
The stem barks of S. commixta were collected in Mt. Sulak, Korea in June 1998, and identified by Professor. KiHwan Bae, College of Pharmacy, Chungnam National University. A voucher specimen (CNU 1081) has been deposited in the herbarium of the College of Pharmacy, Chungnam National University (Korea).
Extraction and isolation
The dried stem barks of S. commixta (500 g) were extracted with MeOH (3 L) by reflux. The MeOH extract (48 g) was suspended in H2O (1 L) and partitioned with EtOAc (900 mL × 3) and BuOH (900 mL × 3), sequentially. The EtOAc−soluble fraction showed PTP1B inhibitory activity (80% inhibition at 30 μg/mL). The EtOAc−soluble fraction (20 g) was chromatographed over silica gel (7 × 40 cm; 70 − 230 mesh) using a gradient of hexane−acetone (from 10:1 to 0:1) to yield seven fractions (Fr. 1 − Fr. 7). Of these, Fr. 2 and Fr. 3 showed the most potent PTP1B inhibitory activity (79 and 87% inhibition at 10 μg/mL). Fr. 2 (2.1 g) was chromatographed on silica gel (4.5 × 40 cm; 15 − 40 μm) with mixtures of hexane−acetone (15:1), to afford lupenone (1, 105 mg). The active fraction, Fr. 3 (3.2 g), was further separated by a silica gel column (4.5 × 40 cm; 15 − 40 μm), eluted with hexane−acetone (6:1), to give lupeol (2, 1100 mg).
Lupenone (1)
colorless needle (from CHCl3-MeOH); mp 169-170°C; [α]D +62.8° (c 1.0, CHCl3); IR νmax cm−1: 3080, 1700, 1648, 890; EIMS m/z: 424 [M]+, 409, 313, 218, 205, 189, 161; 1H-NMR (300 MHz, CDCl3) δ: 4.69 (1H, m, H-29β), 4.57 (1H, m, H-29α), 2.41 (1H, m, H-19), 1.68, 1.07, 1.07, 1.02, 0.96, 0.93, 0.80 (each 3H, s, 7×CH3); 13C-NMR (75 MHz, CDCl3) δ: 39.6 (C-1), 34.1 (C-2), 217.9 (C-3), 47.3 (C-4), 55.0 (C-5), 19.6 (C-6), 33.6 (C-7), 40.9 (C-8), 49.8 (C-9), 36.9 (C-10), 21.5 (C-11), 25.2 (C-12), 38.2 (C-13), 42.9 (C-14), 27.4 (C-15), 35.6 (C-16), 42.9 (C-17), 48.3 (C-18), 47.9 (C-19), 150.7 (C-20), 29.9 (C-21), 40.0 (C-22), 26.6 (C-23), 21.0 (C-24), 15.8 (C-25), 15.9 (C-26), 14.4 (C-27), 18.0 (C-28), 109.2 (C-29), 19.3 (C-30).
Lupeol (2)
white amorphous powder; mp 210°C; [α]D +26.0° (c 0.8, CHCl3); IR νmax cm−1: 3235, 1640, 1490, 1382, 1185, 1105, 1040, 984, 943; EIMS m/z: 426 [M]+, 218, 207, 189; 1H-NMR (300 MHz, CDCl3) δ: 4.69 (1H, m, H-29β), 4.57 (1H, m, H-29α), 3.18 (1H, dd, H-3), 2.29 (1H, m, H-19), 1.91 (1H, m, H-21), 1.68, 1.03, 0.97, 0.94, 0.83, 0.79, 0.76 (each 3H, s, 7×CH3); 13C-NMR (75 MHz, CDCl3) δ: 38.6 (C-1), 27.3 (C-2), 78.9 (C-3), 38.8 (C-4), 55.2 (C-5), 18.2 (C-6), 34.2 (C-7), 40.7 (C-8), 50.3 (C-9), 37.1 (C-10), 20.9 (C-11), 25.0 (C-12), 38.0 (C-13), 42.7 (C-14), 27.4 (C-15), 35.5 (C-16), 42.9 (C-17), 48.2 (C-18), 47.9 (C-19), 150.8 (C-20), 29.8 (C-21), 39.9 (C-22), 27.9 (C-23), 15.3 (C-24), 16.1 (C-25), 15.9 (C-26), 14.5 (C-27), 17.9 (C-28), 109.3 (C-29), 19.2 (C-30).
Assays for protein tyrosine phosphatases
PTP1B assay: PTP1B (human, recombinant) was purchased from BIOMOL® International LP (USA). The enzyme activity was measured using p−nitrophenyl phosphate (pNPP) as described previously [Citation12]. To each 96 well (final volume: 200 μL) was added 2 mM pNPP and PTP1B (0.05 − 0.1 μg) in a buffer containing 50 mM citrate (pH 6.0), 0.1 M NaCl, 1 mM EDTA and 1 mM dithiothreitol (DTT) with or without test compounds. Following incubation at 37°C for 30 min, the reaction was terminated with 1 M NaOH. The amount of produced p−nitrophenol was estimated by measuring the absorbance at 405 nm. The nonenzymatic hydrolysis of 2 mM pNPP was corrected by measuring the increase in absorbance at 405 nm obtained in the absence of PTP1B enzyme.
Dual-specificity protein tyrosine phosphatase (DS−PTP) assay
DS−PTP was assayed with the His tagged−VH1−related human protein (VHR) fusion enzyme. The full-length human VHR(residues 1-185) was expressed in Escherichia coli and the intact protein was purified [Citation12]. The reaction mixture containing VHR enzyme, 2 mM pNPP and assay buffer (50 mM succinate, 1 mM EDTA, 140 mM NaCl, 0.05% Tween 20, pH 6.0) was incubated at 37°C for 30 min. The reaction was terminated by the addition of 1 M NaOH, and the dephosphorylation activity measured at 405 nm [Citation13].
Protein serine/threonine phosphatase 1 (PP1) assay
The PP1 (Sigma Chemical Co., St. Louis, MO, USA) was measured at 37°C using pNPP as a substrate. Reactions were performed for 30 min in the assay buffer (50 mM Tris−HCl, 0.1% β-mercaptoethanol, 1 mM EDTA, 1 mM MnCl2, 20 mM MgCl2, pH 7.6). The reaction was stopped by the addition of 1 M NaOH, and the amount of p−nitrophenol was measured by absorbance at 405 nm [Citation12].
Inhibition kinetics
In the kinetic analysis, the reaction mixture consisting of six different concentrations of pNPP (0.5, 1.0, 2.0, 4.0, 8.0 and 16.0 mM) used as a PTP1B substrate in the absence or presence of compounds 1 and 2 [Citation12]. The Michaelis−Menten constant (Km) and maximum velocity (Vmax) of PTP1B were determined by Lineweaver−Burk plots using a GraphPad Prism® 4 program (GraphPad Software Inc., USA).
Results
Bioassay−guided fractionation on the EtOAc–soluble fraction led to the isolation of active compounds 1 and 2 (). The structures of compounds were identified as lupenone (1) and lupeol (2) by physicochemical (mp, [α]D) and spectroscopic data measurement (MS, 1H−NMR, 13C−NMR) and by comparison with published values [Citation8].
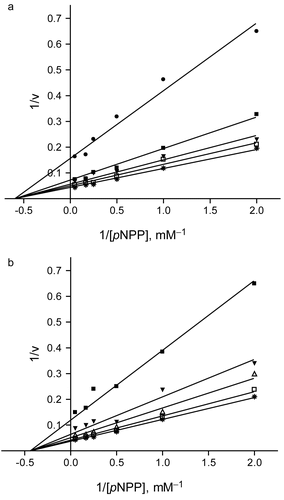
As shown in , lupenone (1) and lupeol (2) inhibited PTP1B activity in a dose−dependent manner with IC50 values of 13.7 ± 2.1 and 5.6 ± 0.9 μM, respectively. The known inhibitors RK-682 (IC50 = 4.5 ± 0.5 μM) and ursolic acid (IC50 = 3.5 ± 0.2 μM) were used as positive controls in this assay [Citation13,14]. In addition, both the compounds were tested for the inhibitory effects on other types of protein phosphatases, and it was revealed that the compounds 1 and 2 had no inhibitory effects toward dual−specificity protein tyrosine phosphatase (VHR) and protein serine/threonine phosphatase (PP1) at levels up to 100 μM. This suggests that 1 and 2 have specific inhibitory activity against PTP1B. To elucidate the inhibition mode of 1 and 2 on the activity of PTP1B, kinetic analyses were performed with different concentrations of substrate. As shown in , the mechanisms of inhibition by the two compounds were determined using a Lineweaver−Burk plot. When pNPP was used as substrate, both the 1 and 2 decreased the Vmax values, but did not alter the Km values of PTP1B (). Accordingly, both the 1 and 2 were determined as non−competitive inhibitors with Ki values of 11.8 and 3.4 μM, respectively.
Table 1. Comparison of the inhibitory activity of the compounds 1 and 2 isolated from S. commixta against PTP1B, VHR and PP1.
Figure 2. Inhibition kinetics of lupenone (1) and lupeol (2). Panel a shows a Lineweaver−Burk plot of the inhibitory effect of compound 1 on PTP1B−catalyzed hydrolysis of pNPP. Data are expressed as mean initial velocity for n = 3 replicates at each substrate concentration. Symbols: (*) 0 μM, (□) 1 μM, (▾) 5 μM, (▪) 10 μM, (•) 20 μM lupenone (1). Panel b shows a Lineweaver−Burk plot of the inhibitory effect of compound 2 on PTP1B. Data are expressed as mean initial velocity for n = 3 replicates at each substrate concentration. Symbols: (*) 0 μM, (□) 1 μM, (△) 3 μM, (▾) 5 μM, (▪) 10 μM lupeol (2).
Discussion
PTP1B appears to be a promising therapeutic target because the level of PTP1B expression in muscles and adipose tissues is associated with the degree of insulin resistance in subjects with diabetes and obesity [Citation1–4].
During the screening effort we found that a MeOH extract of the stem barks of S. commixta inhibited around 65% PTP1B activity at a level of 30 μg/ml. After solvent fractionation, the activity was concentrated in EtOAc–soluble fraction (80% inhibition at 30 μg/ml). Further bioassay−guided fractionation of the EtOAc–soluble fraction resulted in the isolation of two lupane−type triterpenes, lupenone (1) and lupeol (2) as the active principles. Both the compounds isolated showed selective inhibitory activity against PTP1B. To examine whether 1 and 2 inhibit PTP1B by interacting with the enzyme’s active site, we tested the inhibition kinetics of the compounds with pNPP as the substrate. From the kinetic studies, we found that both 1 and 2 inhibited PTP1B in a non−competitive manner, indicating that they may bind to the enzyme-substrate complex or interact with a specific binding site distinct from the active site of the enzyme [Citation15]. Lupane−type triterpenes including lupenone and lupeol have been reported to possess a wide range of bioactivities that include anti-inflammatory, antiviral, antimicrobial, antioxidant, antitumor, antiangiogenic and antimalarial effects [Citation16–21]. Recent studies suggest that various bioactivities of lupeol and lupenone are associated with the inhibition of key enzymes and/or transcription factors such as NF-κB and PI3K/Akt [Citation19,Citation22,Citation23]. Those findings indicate that lupeol and lupenone are capable of modulating signaling cascades in cells, which may be useful for developing a new PTP1B inhibitor because PTP1B is an intracellular enzyme. Interestingly, some plants containing lupeol and lupeol itself were demonstrated to have hypoglycemic activity as well as α-amylase inhibitory activity useful for treating diabetes [Citation24,Citation25]. These evidences strongly support that lupane−type triterpenoids can be a lead moiety for the development of new PTP1B inhibitors. Among hundreds of PTP1B inhibitors developed so far, only a few types of molecules such as ursane and oleane type triterpenoids, and kaurane diterpenoids are known as non azole−type inhibitors of PTP1B with micromolar level IC50 values [Citation12,Citation26,Citation27]. A lupane structure, as a non azole−type inhibitor, may provide us with better understanding of inhibition mechanism, and be useful in drug design.
In conclusion, bioassay−guided investigation of the MeOH extract of the stem barks of S. commixta afforded two lupane−type triterpenes, lupenone (1) and lupeol (2) as active constituents. The present study indicates that these compounds are selective and non−competitive inhibitors of PTP1B. To our knowledge, this is the first time that lupane−type triterpenes have been described as PTP1B inhibitors. Because PTP1B is an intracellular enzyme, further studies to confirm their cellular effects is in progress.
Declaration of interest: This work was supported partly by the Global Partnership Program (No. M60602000001-06E0200-00100) of Korea Foundation for International Cooperation of Science & Technology (KICOS) and KRIBB Research Initiative Program. The authors alone are responsible for the content and writing of the paper.
References
- Johnson TO, Ermolieff J, Jirousek MR. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat Rev Drug Discov 2002;1:696–709.
- Asante-Appiah E, Kennedy BP. Protein tyrosine phosphatases: the quest for negative regulators of insulin action. Am J Physiol Endocrinol Metab 2003;284:E663–70.
- Taylor SD, Hill B. Recent advances in protein tyrosine phosphatase 1B inhibitors. Expert Opin Investig Drugs 2004;13:199–214.
- Bialy L, Waldmann H. Inhibitors of protein tyrosine phosphatases: Next-generation drugs? Angew Chem Int Ed 2005;44:3814–3839.
- Hong JH, Lee MS, Bae EY, Kim YH, Oh H, Oh WK, Kim BY, Ahn JS. Screening of the inhibitory activity of medicinal plants against protein tyrosine phosphatase 1B. Kor J Pharmacogn 2004;35:16–21.
- Bae K. The medicinal plants of Korea. Seoul: Kyo-Hak Publishing Co.;2000. p. 236.
- Kokubun T, Harborne JB, Eagles J, Waterman PG. Antifungal biphenyl compounds are the phytoalexins of the sapwood of Sorbus acuparia. Phytochemistry 1995;40:57–59.
- Na M, An RB, Min BS, Lee SM, Kim YH, Bae K. Chemical constituents from Sorbus commixta. Nat Prod Sci 2002;8:62–65.
- Na M, An RB, Lee SM, Min BS, Kim YH, Bae K, Kang SS. Antioxidant compounds from the stem bark of Sorbus commixta. Nat Prod Sci 2002;8:26–29.
- Sohn EJ, Kang DG, Mun YJ, Woo WH, Lee HS. Anti-atherogenic effects of the methanol extract of Sorbus cortex in atherogenic-diet rats. Biol Pharm Bull 2005;28:1444–1449.
- Lee SO, Lee HW, Lee IS, Im HG. The pharmacological potential of Sorbus commixta cortex on blood alcohol concentration and hepatic lipid peroxidation in acute alcohol-treated rats. J Pharm Pharmacol 2006;58:685–693.
- Kim S, Na M, Oh H, Jang J, Sohn CB, Kim BY, Oh WK, Ahn JS. PTP1B inhibitory activity of kaurane diterpenes isolated from Siegesbeckia glabrescens. J Enz Inhib Med Chem 2006;21:379–383.
- Hamaguchi T, Sudo T, Osada H. RK-682, a potent inhibitor of tyrosine phosphatase VHR, arrested the mammalian cell cycle progression at G1 phase. Chem Biol 2001;8:1209–1220.
- Na M, Jang J, Njamen D, Mbafor JT, Fomum ZT, Kim BY, Oh WK, Ahn JS. Protein tyrosine phosphatase-1B inhibitory activity of isoprenylated flavonoids isolated from Erythrina mildbraedii. J Nat Prod 2006;69:1572–1576.
- Wiesmann C, Barr KJ, Kung J, Zhu J, Erlanson DA, Shen W, Fahr BJ, Zhong M, Taylor L, Randal M, McDowell RS, Hansen SK. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat Struc Mol Biol 2004;11:730–737.
- Geetha T, Varalakshmi P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J Ethnopharmacol 2001;76:77–80.
- Madureira AM, Ascenso JR, Valdeira L, Duarte A, Frade JP, Freitas G, Ferreira MJ. Evaluation of the antiviral and antimicrobial activities of triterpenes isolated from Euphorbia segetalis. Nat Prod Res 2003;17:375–380.
- Nagaraj M, Sunitha S, Varalakshmi P. Effect of lupeol, a pentacyclic triterpene, on the lipid peroxidation and antioxidant status in rat kidney after chronic cadmium exposure. J Appl Toxicol 2000;20:413–417.
- Saleem M, Afaq F, Adhami VM, Mukhtar H. Lupeol modulates NF-κB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene 2004;23:5203–5214.
- You YJ, Nam NH, Kim Y, Bae K, Ahn BZ. Antiangiogenic activity of lupeol from Bombax ceiba. Phytother Res 2003;17:341–344.
- Ziegler HL, Staerk D, Christensen J, Hviid L, Hägerstrand H, Jaroszewski JW. In vitro Plasmodium falciparum drug sensitivity assay: inhibition of parasite growth by incorporation of stomatocytogenic amphiphiles into the erythrocyte membrane. Antimicrob Agents Chemother 2002;46:1441–1446.
- Rajic A, Kweifio-Okai G, Macrides T, Sandeman RM, Chandler DS, Polya GM. Inhibition of serine proteases by anti-inflammatory triterpenoids. Planta Med 2000;66:206–210.
- Wada S, Iida A, Tanaka R. Screening of triterpenoids isolated from Phyllanthus flexuosus for DNA topoisomerase inhibitory activity. J Nat Prod 2001;64:1545–1547.
- Ortiz-Andrade RR, García-Jiménez S, Castillo-España P, Ramírez-Avila G, Villalobos-Molina R, Estrada-Soto S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: an anti-hyperglycemic agent. J Ethnopharmacol 2007;109:48–53.
- Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol 2006;107:449–455.
- Na M, Yang S, He L, Oh H, Kim BS, Oh WK, Kim BY, Ahn JS. Inhibition of protein tyrosine phosphatase 1B by ursane-type triterpenes isolated from Symplocos paniculata. Plant Med 2006;72:261–263.
- Zhang YN, Zhang W, Hong D, Shi L, Shen Q, Li JY, Li J, Hu LH. Oleanolic acid and its derivatives: new inhibitor of protein tyrosine phosphatase 1B with cellular activities. Bioorg Med Chem 2008;16:8697–8705.