Abstract
A new group of 1-phenylpyrazolo[3,4-d]pyrimidine derivatives 14a–d–21 were synthesized from 2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetohydrazide (12). All the synthesized compounds were evaluated for their cyclooxygenase (COX) inhibition, anti-inflammatory activity and ulcerogenic liability. All the target compounds were more potential in inhibiting COX-2 than COX-1. Compounds having pyrazolyl moiety in a hybrid structure with pyrazolo[3,4-d]pyrimidine scaffold (14a–d, 16 and 17) showed higher edema inhibition percentage activities (34–68%) and the 5-aminopyrazole derivative (14c, ED50 = 87.9 μmol/kg) was the most potent one > celecoxib (ED50 = 91.9 μmol/kg). While, the in vivo potent compounds (14a–d, 16, 17 and 21) caused variable ulceration effect (ulcer index = 0.33–4.0) comparable to that of celecoxib (ulcer index = 0.33), the pyrazol-3-one derivative (16) and the acetohydrazide (21) were the least ulcerogenic derivatives showing the same ulcerogenic potential of celecoxib.
Introduction
Inflammation is an essential physiological response to a wide variety of stimuli (infection, trauma, burns, surgery and injury), and aimed at limiting damage and promoting tissue healingCitation1. Non-steroidal anti-inflammatory drugs (NSAIDs) treat pain and inflammation via inhibition of cyclooxygenase enzyme (COX), a protein responsible for prostaglandins (PGs) biosynthesis from arachidonic acidCitation2,Citation3. COX exists in at least two distinct isoforms: a constitutive form (COX-1) and an inducible form (COX-2)Citation4. While, the constitutive isoform (COX-1) is essential for the synthesis of cytoprotective PGs, biosynthesis of pro-aggregatory thromboxaneA2 (TXA2) and maintenance of renal function, the inducible one (COX-2) is induced in response to pro-inflammatory stimuli and is responsible for the progression of inflammationCitation5–7. Traditional NSAIDs such as aspirin (1), indomethacin (2) and ibuprofen (3) inhibit both the isoforms leading to side effects ranging from ulcers to perforation and bleedingCitation8. In an attempt to circumvent these side effects, selective COX-2 inhibitor drugs, such as celecoxib (4), rofecoxib (5) and valdecoxib (6), have been developed where they exhibited equivalent anti-inflammatory/analgesic activities to nonselective COX inhibitors, but with less GI toxicityCitation9 (). Unfortunately, some cardiovascular side effects such as myocardial infarction and increased incidences of high blood pressure caused by the highly selective COX-2 inhibitors led to the withdrawal of rofecoxib and valdecoxib from the marketCitation10,Citation11.
Figure 1. Chemical structures of traditional NSAIDs; aspirin (1), indomethacin (2) and ibuprofen (3), selective COX-2 inhibitors; celecoxib (4), rofecoxib (5), and valdecoxib (6) and reported pyrazolo[3,4-d]pyrimidine derivatives (7–9) with anti-inflammatory activity.
![Figure 1. Chemical structures of traditional NSAIDs; aspirin (1), indomethacin (2) and ibuprofen (3), selective COX-2 inhibitors; celecoxib (4), rofecoxib (5), and valdecoxib (6) and reported pyrazolo[3,4-d]pyrimidine derivatives (7–9) with anti-inflammatory activity.](/cms/asset/741de061-1a8b-4eb5-96ac-b67d73540ca8/ienz_a_1186018_f0001_b.jpg)
Pyrazolo[3,4-d]pyrimidine represents one of the most frequently found scaffold in a wide variety of anti-inflammatory agentsCitation12,Citation13. The 5-benzamido-1H-pyrazolo[3,4-d]pyrimidin-4-one derivative (7) showed superior inhibitory profile against COX-2 when compared to that of reference standards N-[2-(cyclohexyloxy)4-nitrophenyl]methane sulfonamide (NS398) and indomethacinCitation14. Also, the sulfamoylphenylpyrazolopyrimidine derivative (8) was reported to exhibit comparable anti-inflammatory activity with celecoxib (4) at a dose of 25 mg/kgCitation15. Furthermore, the 4-substituted-1-phenylpyrazolo[3,4-d]pyrimidine derivative (9) showed considerable anti-inflammatory activityCitation16. Guided by the previously mentioned studies and as a continuation of our previous workCitation17–21 for the development of safe anti-inflammatory derivatives,here wedescribe the synthesis, in vitro evaluation as COX-1/COX-2 inhibitors, in vivo anti-inflammatory (AI) activity and ulcerogenic liability for some new 1-phenylpyrazolo[3,4-d]pyrimidine derivatives (14a–d–21) with the hope of realizing compounds with improved anti-inflammatory activity and diminished side effects.
Experimental
Chemistry
Melting points were determined using a Griffin apparatus and are uncorrected. Infrared (IR) spectra were recorded on a Shimadzu 435 spectrometer (Palo Alto, CA) using KBr discs. 1H NMR and 13C NMR spectra were measured on a Bruker 400 MHz spectrometer (Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt) in D2O, DMSO-d6 with TMS as the internal standard, where J (coupling constant) values were estimated in Hertz (Hz). Mass spectra were run on Hewlett Packard 5988 spectrometer. Microanalysis was performed for C, H, N at the Micro Analytical Center, Cairo University, Egypt, and was within ± 0.4% of theoretical values. All other reagents, purchased from the Acros Chemical Company (Milwaukee, WI), were used without further purification. 6-Methyl-1-phenyl-1,5-dihydropyrazolo[3,4-d]pyrimidin-4-one (10)Citation23, 2-ethoxymethylenemalononitrile (13a)Citation24, 2–(1-ethoxy-ethylidene)malononitrile (13b)Citation25, 2-cyano-3-ethoxy-acrylic acid ethyl ester (13c)Citation25, 2-cyano-3-ethoxybut-2-enoic acid ethyl ester (13d)Citation25 and 4–(2-chloroacetylamino)-benzoic acid ethyl ester (18)Citation26 were prepared according to reported procedures.
Ethyl 2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetate (11)
A mixture of 6-methyl-1-phenyl-1,5-dihydropyrazolo[3,4-d]pyrimidin-4-one (10, 10 mmol, 2.26 g), ethyl chloroacetate (1.22 g, 10 mmol) and anhydrous potassium carbonate (1.38 g, 10 mmol) in dry acetone (20 mL) was heated under reflux for 6 h. After cooling, the reaction mixture was poured into ice-cold water. The separated solid was filtered, and crystallized from methanol to give pure compound 11. Physical and spectral data are listed below.
Yellow solid; Yield 71%; m.p. 115–116 °C; IR (KBr) 3062 (CH aromatic), 2924 (CH aliphatic), 1731 (C = O), 1549 (C = N) cm−1; 1H NMR (DMSO-d6) δ 1.24 (t, 3H, J = 7.2 Hz, CH3CH2), 2.59 (s, 3H, pyrimidine CH3), 4.20 (q, 2H, J = 7.2 Hz, CH2CH3), 4.95 (s, 2H, CH2O),7.40–7.45 (m, 1H, phenyl H-4), 7.56–7.59 (m, 2H, phenyl H-3, H-5), 8.05 (d, J = 7.5 Hz, 2H, phenyl H-2, H-6), 8.35 (s, 1H, pyrazole H-3); 13C NMR (CDCl3) δ 14.42, 23.92, 45.57, 61.89, 105.05, 122.21, 127.65, 129.73, 136.56, 138.53, 150.81, 157.49, 159.77, 168.37; EIMS (m/z) 312 (M+., 11.32%), 59 (100%). Anal.Calcd for C16H16N4O3: C, 61.53; H, 5.16; N, 17.94. Found: C, 61.55; H, 5.03; N, 18.13.
2-(6-Methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetohydrazide (12)
A mixture of compound (11, 3.12 g, 10 mmol), hydrazine hydrate (99.9%) (1 mL, 20 mmol) in ethanol (20 mL) was heated under reflux for 5 h. After cooling, the separated solid was filtered, dried and crystallized from acetone to afford the target compound12. Physical and spectral data are listed below.
White solid; Yield 62%; m.p. 245–246 °C; IR (KBr) 3439–3205 (br, NH and NH2), 3044 (CH aromatic), 2947 (CH aliphatic), 1652 (C = O), 1569 (C = N) cm−1; 1H NMR (DMSO-d6) δ 2.48 (s, 3H, CH3), 4.32 (s, 2H, NH2, D2O exchangeable), 4.76 (s, 2H, CH2O), 7.40–7.43 (m, 1H, phenyl H-4), 7.56–7.60 (m, 2H, phenyl H-3, H-5), 8.07 (d, J = 7.5 Hz, 2H, phenyl H-2, H-6), 8.32 (s, 1H, pyrazole H-3), 9.44 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 23.96, 44.82, 105.25, 122.17, 127.62, 129.76, 136.52, 138.58, 150.91, 157.82, 160.43, 166.62; EIMS (m/z) 298 (M+., 1.34%), 210 (100%). Anal.Calcd for C14H14N6O2: C, 56.37; H, 4.73; N, 28.17. Found: C, 56.62; H, 4.30; N, 28.00.
General procedure for preparation of 5-(aminopyrazol-1-yl) pyrazolo[3,4-d]pyrimidine derivatives (14a–d)
To a solution of 2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetohydrazide (12, 2.98 g, 10 mmol) in ethanol (20 mL), the appropriate ethoxymethylenemalononitrile or ethyl ethoxymethylenecyanoacetate derivative (13a–d, 10 mmol) was added and the reaction mixture was heated under reflux for 10 h. The reaction mixture was concentrated under reduced pressure and the product was left overnight in refrigerator. The separated solid was filtered and crystallized from acetic acid to afford the corresponding pyrazole derivatives 14a–d for which physical and spectral data are listed below.
5-Amino-1-[2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetyl]-1H-pyrazole-4-carbonitrile (14a)
66% yield; white crystals; m.p. 260–261 °C; IR (KBr disk) 3419, 3339 (forked, NH2), 2952 (C–H aliphatic), 2214 (C≡N), 1697 (C = O), 1602 (C = N); 1H NMR (DMSO-d6) 2.58 (s, 3H, pyrimidine CH3), 4.90 (s, 2H, CH2O), 7.37 (s, 1H, pyrazole H-3′), 7.40–7.44 (m, 3H, phenyl H-4 & NH2, D2O exchangeable), 7.56–7.60 (m, 2H, phenyl H-3, H-5), 8.06 (d, J = 7.6 Hz, 2H, phenyl H-2, H-6), 8.34 (s, 1H, pyrazole H-3);13C NMR (DMSO-d6) δ 23.87, 44.63, 105.23, 121.54, 122.11, 124.28, 127.55, 128.79, 129.75, 136.64, 138.65 150.87, 154.80, 157.54, 160.19, 166.44; MS (m/z): 374 (M+., 6.92%), 210 (100%); Anal. Calcd for C18H14N8O2: C, 57.75; H, 3.77; N, 29.93; Found: C, 57.60; H, 4.00; N, 29.68.
5-Amino-3-methyl-1-[2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetyl]-1H-pyrazole-4-carbonitrile (14b)
61% yield; white crystals; m.p. 250–252 °C; IR (KBr disk) 3425, 3350 (forked, NH2), 2935 (C–H aliphatic), 2218 (C≡N), 1689 (C = O), 1601 (C = N); 1H NMR (DMSO-d6) 2.24 (s, 3H, pyrazole CH3), 2.62 (s, 3H, pyrimidine CH3), 5.52 (s, 2H, CH2O), 7.41–7.45 (m, 1H, phenyl H-4), 7.57–7.61 (m, 2H, phenyl H-3, H-5), 8.02–8.07 (m,4H, phenyl H-2, H-6 and NH2, D2O exchangeable), 8.36 (s, 1H, pyrazole H-3); 13C NMR (DMSO-d6) δ 16.22, 24.15, 43.22, 105.12, 122.82, 124.15, 127.62, 128.82, 129.60, 136.65, 138.49, 150.81, 154.70, 157.55, 160.22, 166.45; MS (m/z): 388 (M+., 93%), 77 (100%); Anal. Calcd for C19H16N8O2: C, 58.76; H, 4.15; N, 28.85 Found: C, 58.50; H, 4.12; N, 28.50.
Ethyl 5-amino-1-[2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetyl]-1H-pyrazole-4-carboxylate (14c)
82% yield; white crystals; m.p. 260–262 °C; IR (KBr disk) 3465, 3337 (forked, NH2), 3108 (C–H aromatic), 2931 (C–H aliphatic), 1710 (ester C = O), 1685 (C = O), 1564 (C = N); 1H NMR (DMSO-d6) δ 1.29 (t, J = 7.2 Hz, 3H, CH3CH2), 2.65 (s, 3H, pyrimidine CH3), 4.24 (q, 2H, J = 7.2 Hz, CH2CH3), 5.59 (s, 2H, CH2O), 7.41–7.45 (3, 3H, phenyl H-4 and NH2, D2O exchangeable), 7.57–7.61 (m, 2H, phenyl H-3, H-5), 7.94 (s, 1H, pyrazole H-3′), 8.07 (d, J = 8 Hz, 2H, phenyl H-2, H-6), 8.37 (s, 1H, pyrazole H-3); 13C NMR (DMSO-d6) δ 14.85, 24.03, 47.49, 59.93, 94.15, 105.05, 122.26, 127.68, 129.77, 136.59, 138.58, 144.58, 150.92, 153.34, 157.55, 160.17, 163.23, 169.87; MS (m/z): 421 (M+., 17%) 80 (100%); Anal. Calcd for C20H19N7O4: C, 57.00; H, 4.54; N, 23.27 Found: C, 56.62; H, 4.34; N, 23.54.
Ethyl 5-amino-3-methyl-1-[2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)-acetyl]-1H-pyrazole-4-carboxylate (14d)
White solid; Yield 62%; m.p. 264–265 °C; IR (KBr) 3443, 3333 (forked, NH2), 3064 (CH aromatic), 2927 (CH aliphatic), 1721 (ester C = O), 1694 (C = O), 1553 (C = N) cm−1; 1H NMR (DMSO-d6) δ 1.30 (t, J = 6.4 Hz, 3H, CH3CH2), 2.34 (s, 3H, pyrazole CH3), 2.64 (s, 3H, pyrimidine CH3), 4.25 (q, 2H, J = 6.4 Hz, CH2CH3), 5.56 (s, 2H, CH2O), 7.37 (s, 2H, NH2, D2O exchangeable), 7.41–7.45 (m, 1H, phenyl H-4), 7.58–7.61 (m, 2H, phenyl H-3, H-5), 8.07 (d, J = 7.2 Hz, 2H, phenyl H-2, H-6), 8.35 (s, 1H, pyrazole H-3); 13C NMR (DMSO-d6) δ 14.74, 15.03, 23.97, 47.43, 59.89, 92.90, 105.03, 122.31, 127.74, 129.78, 136.55, 138.50, 150.91, 153.97, 154.22, 157.62, 160.17, 163.88, 169.32; EIMS (m/z) 435 (M+., 6.71%), 40 (100%). Anal.Calcd for C21H21N7O4: C, 57.93; H, 4.86; N, 22.52. Found: C, 57.82; H, 4.90; N, 22.48.
1-[2-(6-Methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetyl]-[1,2]diazetidin-3-one (15)
A mixture of 2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetohydrazide (12, 2.98 g, 10 mmol), chloroacetyl chloride (0.8 mL, 10 mmol) and anhydrous potassium carbonate (1.38 gm, 10 mmol) in dry dimethylformamide was stirred at room temperature for 24 h. The reaction mixture was poured into ice-cold water and the separated product was filtered, dried and crystallized from benzene to give compound 15. Physical and spectral data are listed below.
White solid; Yield 55%; m.p. 272–273 °C; IR (KBr) 3432 (NH), 3053 (CH aromatic), 2942 (CH aliphatic), 1692 (2C = O), 11599 (C = N) cm−1; 1H NMR (DMSO-d6) δ 2.60 (s, 3H, CH3), 4.15 (s, 2H, diazetidinone CH2), 4.91 (s, 2H, CH2O), 7.42–7.45 (m, 1H, phenyl H-4), 7.57–7.61 (m, 2H, phenyl H-3, H-5), 8.06 (d, J = 7.5 Hz, 2H, phenyl H-2, H-6), 8.34 (s, 1H, pyrazole H-3), 10.51 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 23.85, 41.21, 44.64, 105.19, 122.16, 127.16, 129.76, 136.60, 138.58, 150.86, 157.60, 160.20, 165.51, 166.24; EIMS (m/z) 338 (M+., 5.97%), 77 (100%). Anal.Calcd for C16H14N6O3: C, 58.80; H, 4.17; N, 24.84. Found: C, 58.92; H, 4.00; N, 24.50.
5-Methyl-2-[2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetyl]-2,4-dihydropyrazol-3-one (16)
A mixture of hydrazide (12, 2.98 g, 10 mmol), ethyl acetoacetate (1.30 g, 10 mmol) in ethanol (20 mL) was heated under reflux for 12 h. After cooling, the reaction mixture was poured onto ice-water, the separated solid was filtered, dried and crystallized from dioxane to afford compound 16. Physical and spectral data are listed below.
White solid; Yield 68%; m.p. 260–261 °C; IR (KBr) 3069 (CH aromatic), 2942 (CH aliphatic), 1685, 1668 (2C = O), 1560 (C = N) cm−1; 1H NMR (DMSO-d6) δ 2.65 (s, 3H, pyrimidine CH3), 2.71 (s, 3H, pyrazolone CH3), 5.08 (s, 2H, pyrazolone COCH2), 5.40 (s, 2H, CH2O), 7.42–7.45 (m, 1H, phenyl H-4), 7.57–7.61 (m, 2H, phenyl H-3, H-5), 8.06 (d, J = 7.5 Hz, 2H, phenyl H-2, H-6), 8.36 (s, 1H, pyrazole H-3); 13C NMR (DMSO-d6) δ 23.92, 24.25, 37.16, 45.70, 105.18, 122.21, 127.66, 129.77, 136.64, 138.58, 149.85, 150.90, 157.42, 157.67, 160.15, 167.51; EIMS (m/z) 364 (M+., 12%), 77 (100%). Anal.Calcd for C18H16N6O3: C, 59.34; H, 4.43; N, 23.06. Found: C, 59.50; H, 4.40; N, 23.35.
1-(3,5-Dimethylpyrazol-1-yl)-2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)-ethanone (17)
A mixture of hydrazide (12, 2.98 g, 10 mmol), and acetylacetone (10 mmol, 1 g) in acetic acid (10 mL) was heated under reflux for 6 h. After cooling, the reaction mixture was poured onto ice-water. The colorless powder obtained was crystallized from ethanol to give compound 17 for which physical and spectral data are listed below.
White crystals; Yield 55%; m.p. 189–190 °C; IR (KBr disk) 3079 (C–H aromatic), 2898 (C–H aliphatic), 1692 (C = O), 1609 (C = N); 1H NMR (DMSO-d6) δ 2.58 (s, 3H, pyrimidine CH3), 2.73 (s, 3H, pyrazole CH3), 2.78 (s, 3H, pyrazole CH3), 4.88 (s, 2H, CH2O), 6.65 (s, 1H, pyrazole H-4′), 7.40–7.43 (m, 1H, phenyl H-4), 7.56–7.60 (m, 2H, phenyl H-3, H-5), 8.06 (d, J = 7.5 Hz, 2H, phenyl H-2, H-6), 8.34 (s, 1H, pyrazole H-3);13C NMR (DMSO-d6) δ 14.12, 15.17, 23.90, 45.51, 105.10, 112.5, 114.4, 121.78, 122.24, 127.67, 129.76, 136.55, 138.52, 150.83, 157.58, 159.88, 169.82; MS (m/z): 362 (M+., 8.5%) 173 (100%); Anal. Calcd for C19H18N6O2: C, 62.97; H, 5.01; N, 23.19; Found: C, 62.86; H, 5.00; N, 23.10.
Ethyl 4-(2-N′-[2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetyl]hydrazineacetylamino)benzoate (19)
A mixture of 2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetohydrazide (12, 2.98 g, 10 mmol), and ethyl 4-(2-chloroacetamido)benzoate (18, 2.41 g, 10 mmol) in absolute ethanol (30 mL) was heated under reflux for 8 h. After cooling, the formed precipitate was filtered, washed with ethanol and crystallized from ethanol to give compound 19. Physical and spectral data are listed below.
White crystals; Yield 76%; m.p. 189–190 °C; IR (KBr disk) 3441–3352 (3NH), 3064 (C–H aromatic), 2980 (C–H aliphatic), 1720–1689 (3C = O), 1562 (CN); 1H NMR (DMSO-d6) δ 1.32 (t, J = 6.4 Hz, 3H, CH3CH2), 2.58 (s, 3H, pyrimidine CH3), 4.30 (q, 2H, J = 6.4 Hz, CH2CH3), 4.76 (s, 2H, CH2O), 5.44 (s, 2H, CH2CO), 7.36–7.43 (m, 1H, phenyl H-4), 7.58–7.62 (m, 2H, phenyl H-3, H-5), 7.79 (s, 1H, NH, D2O exchangeable), 7.90 (d, J = 6.8 Hz, 2H, ethyl benzoate H-3, H-5), 7.97 (d, J = 6.8 Hz, 2H, ethyl benzoate H-2, H-6), 8.08 (d, J = 7.5 Hz, 2H, phenyl H-2, H-6), 8.32 (s, 1H, pyrazole H-3), 10.60 (s, 1H, NH, D2O exchangeable), 12.30 (s, 1H, NH, D2O exchangeable);13C NMR (DMSO-d6) δ 14.65, 24.12, 45.72, 45.91, 61.05, 105.27, 122.11, 122.22, 127.64, 129.74, 130.64, 136.54, 138.45, 142.99, 150.92, 157.75, 160.27, 161.57, 165.80, 168.80, 169.74; MS (m/z): 503 (M+., 1.32%), 192 (100%); Anal. Calcd for C25H25N7O5: C, 59.64; H, 5.00; N, 19.47; Found: C, 59.70; H, 5.20; N, 19.50.
Benzoic acid N′-[2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetyl]-hydrazide (20)
A mixture of hydrazide (12, 2.98 g, 10 mmol) and benzoyl chloride (1.40 g, 10 mmol) in absolute ethanol (30 mL) was heated under reflux for 6 h. After cooling, the formed precipitate was filtered and crystallized from butanol to give compound 20. Physical and spectral data are listed below.
White crystals; Yield 86%; m.p. 238–239 °C; IR (KBr disk) 3444–3256 (2NH), 3055 (C–H aromatic), 1693 (2C = O), 1599 (C = N); 1H NMR (DMSO-d6) δ 2.66 (s, 3H, pyrimidine CH3), 4.97 (s, 2H, CH2O), 7.40–7.44 (m, 1H, phenyl H-4), 7.49–7.52 (m, 1H, benzoyl H-4), 7.57–7.61 (m, 4H, phenyl H-3, H-5 and benzoyl H-3, H-5), 7.87 (d, J = 6.8 Hz, 2H, benzoyl H-2, H-6), 8.07 (d, J = 7.5 Hz, 2H, phenyl H-2, H-6), 8.34 (s, 1H, pyrazole H-3), 10.45 (s, 1H, NH, D2O exchangeable), 10.51 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 23.91, 44.69, 105.27, 122.12, 127.56, 127.90, 128.08, 128.98, 129.76, 132.42, 136.67, 138.68, 150.90, 157.58, 160.29, 166.00, 166.81; MS (m/z): 402 (M+., 5.86%),77 (100%); Anal. Calcd for C21H18N6O3: C, 62.68; H, 4.51; N, 20.88; Found: C, 62.50; H, 4.66; N, 20.50.
N′-4-chlorophenylaminocarbonyl-2-(6-Methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetohydrazide (21)
A mixture of hydrazide (12, 2.98 g, 10 mmol) and 4-chlorophenyl isocyanate (1.53 g, 10 mmol) in dioxane (20 mL) was heated under reflux for 4 h. The obtained solid was filtered, washed with dioxane, dried and crystallized from acetic acid to give compound 21.
White crystals; Yield 81%; m.p. 160–161 °C; IR (KBr disk) 3435–3276 (3NH), 1728, 1691 (2C = O)1593 (C = N); 1H NMR (DMSO-d6) δ 2.67 (s, 3H, pyrimidine CH3), 5.20 (s, 2H, CH2O), 7.32–7.42 (m, 4H, phenyl H-4 and chlorophenyl H-2, H-6 and NH, D2O exchangeable), 7.49–7.60 (m, 4H, phenyl H-3, H-5 and chlorophenyl H-3, H-5), 8.06 (d, J = 7.5 Hz, 2H, phenyl H-2, H-6), 8.38 (s, 1H, pyrazole H-3), 10.40 (s, 1H, NH, D2O exchangeable), 11.07 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ 24.11, 45.06, 105.14, 122.17, 123.08, 127.62, 128.52 129.26, 129.76, 136.70, 136.84, 138.61, 150.88, 152.58, 157.78, 160.33, 169.01; MS (m/z): 451 (M+., 1.13%),154 (100%); Anal. Calcd for C21H18ClN7O3: C, 55.82; H, 4.02; N, 21.70; Found: C, 55.50; H, 4.00; N, 21.50.
Biological evaluation
COX-1/COX-2 inhibition colorimetric assay
The in vitro inhibition of ovine COX-1/COX-2 was measured using an enzyme immuno assay (EIA) kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions and as reported beforeCitation27.
In vivo anti-inflammatory activity
Animals
Male Wister albino rats with average body weight of 120–150 g were used in the experiments. The animals were kept under controlled environment, humidity 60 ± 10%, light period of 12 h/day and temperature 27 ± 2° C with access to food and water. The experimental procedures were carried out in compliance with the Institutional Animal Ethics Committee regulations. All experiments were performed in the morning according to the guidelines for the care of laboratory animals.
Carrageenan-induced rat paw edema
The anti-inflammatory activity of the synthesized compounds was determined in vivo by carrageenan-induced paw edema method in ratsCitation28. Rats were divided into 12 groups of 3 animals each. The first group was administered with vehicle; the second one was administrated with celecoxib (50 mg/kg), while the remaining groups were administrated with test compounds (14a–d–21, 50 mg/kg, and one group per one compound). One hour after administration of vehicle, test compounds or celecoxib, paw edema was induced by subcutaneous injection of 1% carrageenan in saline (0.05 mL/rat) into the left hind paw of each rat. Paw thickness of each rat was measured after 3 h of carrageenan injection, and then the change in thickness and the % inhibition of paw edema were calculated.
Additionally, the ED50 values for the most potent derivatives (14a–d, 16, 17 and 21) were calculated using at least three different doses (three rats per group for each dose) and paw thickness of each rat was measured after 3 h of carrageenan injection according to the reported procedureCitation27.
Ulcerogenic liability study
Ulcerogenic liability for the most biologically active synthesized compounds (14a–d, 16, 17, 21) and celecoxib was evaluated using the previously reported proceduresCitation29. Thirty rats were used in this study, divided into 10 groups and fasted for 18 h before drug administration. The control group received the vehicle (2.5% Tween 80). Other groups were received test compounds or celecoxib as a reference drug at a dose of 50 mg/kg. After 2 h, animals were fed. Rats were given the required dose orally for three successive days. After 2 h of the last dose, rats were sacrificed; the stomach of each rat was removed and then, opened along the greater curvature and rinsed with saline. In order to examine the stomach, it was stretched by pins on a corkboard. The gastric mucosa was carefully inspected for the occurrence of ulcers with the aid of an illuminated magnifying lens (l0x), and ulcer index was calculated according to the method described by Cho and OgleCitation28. Lesions were counted and measured along the greater diameter using transparent ruler. Every five hemorrhagic spots were considered equivalent to 1 mm of ulcer. The ulcer index (mm) was calculated from the sum of the total length of ulcers and hemorrhagic spots in each stomach.
Statistical analysis
Significant difference among groups was assessed using one-way ANOVA followed by Dunnett’s test. The results were expressed as mean ± standard error (SEM). Differences were considered significant at *p > 0.05, **p > 0.01 and ***p >0.001.
Results and discussion
Chemistry
A group of 1-phenylpyrazolo[3,4-d]pyrimidine derivatives (14a–d–21) were synthesized using the reaction sequence illustrated in Scheme 1. Accordingly, the reaction of 6-methyl-1-phenyl-1,5-dihydropyrazolo[3,4-d]pyrimidin-4-one (10) with ethyl chloroacetate in presence of anhydrous potassium carbonate provided the corresponding ethyl acetate ester 11, which upon reaction with hydrazine hydrate yielded the key intermediate 2-(6-methyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)acetohydrazide (12) in 62% yield. Cyclization of the hydrazide 12 with the appropriate ethoxymethylenemalononitrile or ethyl ethoxymethylenecyanoacetate derivative (13a–d) gave the respective 5-aminopyrazole derivatives 14a–d in high yields (61–82%). Reaction of hydrazide 12 with chloroacetyl chloride and anhydrous potassium carbonate in dimethylformamide yielded the cyclic diazetidin-3-one derivative 15 rather than the open chloroacetyl derivative. Formation of 15 was confirmed by IR, 1H NMR, 13C NMR and elemental analyses. Also, mass spectrum confirmed the formation of 15 through i) presence of peak at m/z = 338, which corresponds to molecular ion peak of 15, ii) absence of any peaks at m/z = 374 or 376, which corresponds to M+. or M + 2 of the open form, respectively, and iii) absence of any isotopic peaks attributed to chlorine atom of the open form.
Scheme 1. Reagents and conditions: (a) ethyl chloroacetate, acetone, K2CO3, reflux, 6 h; (b) hydrazine hydrate, ethanol, reflux, 5 h; (c) CR(OC2H5)=C(CN)X, ethanol, 10 h; (d) chloroacetyl chloride, DMF, K2CO3, RT, overnight; (e) ethyl acetoacetate, ethanol, reflux, 12 h; (f) acetylacetone, CH3COOH, reflux, 6 h; (g) 4-COOC2H5-C6H4-NHCOCH2Cl, ethanol, reflux, 8 h; (h) benzoyl chloride, ethanol, reflux, 6 h; (i) 4-chlorophenylisocyanate, dioxane, reflux, 4 h.
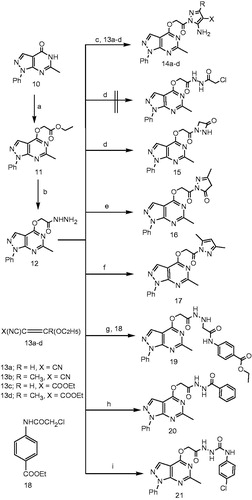
Moreover, while reacting the hydrazide 12 with ethyl acetoacetate gave the pyrazol-3-one derivative 16, reaction of 12 with acetylacetone yielded the 4,5-dimethylpyrazole derivative 17. Also, compound 12 was coupled with ethyl 4-(2-chloroacetamido)benzoate (18) to yield the corresponding ethyl benzoate ester 19. Finally, the key intermediate 12 was reacted with benzoyl chloride and 4-chlorophenyl isocyanate to give the target 1-phenylpyrazolo[3,4-d]pyrimidine derivatives 20 (86% yield) and 21 (81% yield), respectively.
Biological evaluation
In vitro COX inhibition assay
The potency of the synthesized target compounds 14a–d–21 as COX inhibitors was determined as the concentration causing 50% inhibition (IC50) for ovine COX enzyme using an enzyme immunoassay (EIA) kit. Also, the COX-2 selectivity indexes (SI values) that is defined as IC50 (COX-1)/IC50 (COX-2) were calculated and compared with that of celecoxib (4) as a COX-2-selective reference drug. The results showed that the target compounds (14a–d–21) exhibited a broad range (moderately potent to weakly potent) of COX-1 (IC50 = 3.97–10.11 μM), and (moderately potent to highly potent) COX-2 (IC50 = 0.56–5.89 μM range, see data in ), inhibitory activities. All the tested compounds had more potential in inhibiting COX-2 isozyme than COX-1 isozyme. Compounds having pyrazolyl moiety (5-aminopyrazole derivatives 14a–d, pyrazol-3-one derivative 16 and 4,5-dimethylpyrazole derivative 17) in a hybrid structure with the pyrazolo[3,4-d]pyrimidine scaffold were generally less potent inhibitors of COX-1, more potent inhibitors of COX-2 and in turn more COX-2 selective (COX-2 S.I. = 3.11–11.99) than compounds having the other moieties (15, 19, 20 and 21) (COX-2 S.I. = 1.38–5.60). The 4,5-dimethylpyrazole derivative 17 was the most potent COX-2 inhibitor (IC50 = 0.56 μM), while the 5-aminopyrazole derivative 14b was the most COX-2 selective (S.I. = 11.99) in comparison with the reference COX-2 selective drug celecoxib (COX-2 IC50 = 1.11 μM, S.I. = 6.61).
Table 1. In vitro COX-1 and COX-2 inhibition of 1-phenylpyrazolo[3,4-d]pyrimidine derivatives (14a–d–21) and celecoxib.
In vivo anti-inflammatory activity
The anti-inflammatory activity of the prepared 1-phenylpyrazolo[3,4-d]pyrimidine derivatives (14a–d–21) were evaluated using carrageenan-induced rat paw edema assay. Each compound was administered orally (50 mg/kg) immediately prior to the induction of inflammation by carrageenan subcutaneous injection. The anti-inflammatory activity was then calculated based on paw-volume changes at 3 h after carrageenan injection as presented in .
Table 2. Anti-inflammatory activities for 50 mg/kg dose of 1-phenylpyrazolo[3,4-d]pyrimidine derivatives (14a–d–21), (ED50, μmol/kg) of most potent derivatives (14a–d, 16, 17 and 21) and celecoxib.
The obtained in vivo data was compatible with the in vitro results consequently, compounds having pyrazolyl ring (14a–d, 16 and 17) showed higher edema inhibition percentage activities (34–68%), while the other derivatives (15, 19, 20 and 21) showed lower edema inhibition percentage activities (17–46%) in comparison with celecoxib (72%).
Moreover, the ED50 values for the most potent derivatives (14a–d, 16, 17 and 21) were calculated in comparison with celecoxib. The seven derivatives showed good anti-inflammatory activities (ED50 = 87.9–170.1 μmol/kg), especially the 5-aminopyrazole derivative (14c, ED50 = 87.9 μmol/kg) was more potent than celecoxib (ED50 = 91.9 μmol/kg).
Ulcerogenic liability
The most potent anti-inflammatory compounds (14a–d, 16, 17 and 21) were subjected to ulcerogenic liability in comparison with celecoxib, low ulcerogenic reference drug, which was reportedCitation18,Citation22 by our group to be about seven folds less ulcerogenic than ibuprofen as traditional NSAID and the results are shown in . It was clear that the tested compounds caused variable ulceration effect (ulcer index = 0.33–4.0) comparable to that of celecoxib (ulcer index = 0.33). The pyrazol-3-one derivative (16) and the acetohydrazide (21) were the least ulcerogenic derivatives showing the same ulcerogenic potential of celecoxib. The low ulcerogenic potential of 16 and 21 (ulcer index = 0.33) could be attributed to its low potency against COX-1 isozyme (IC50 = 7.50, 9.74 μM, respectively) in addition to its good COX-2 selectivity indices (S.I. = 10.12, 5.60). Similarly, the relatively high ulcerogenic potential of 3,5-dimethylpyrazole derivatives 14c, 14d (ulcer index = 3.0, 4.0 respectively) was attributed to their considerable potency against COX-1 isozyme (IC50 = 4.11 and 4.51 μM, respectively) in addition to their relatively low COX-2 selectivity indices (S.I. = 3.11, 5.24).
Table 3. Ulcerogenic effect for most potent 1-phenylpyrazolo[3,4-d]pyrimidine derivatives (14a–d, 16, 17 and 21) and celecoxib.
Conclusion
A new series of 1-phenylpyrazolo[3,4-d]pyrimidine derivatives 14a–d–21 were synthesized for its evaluation as COX inhibitors, anti-inflammatory agents and ulcerogenic liability. Structure-activity data acquired and biological studies showed that (i) all compounds were more potential in inhibiting COX-2 than COX-1, (ii) Compounds having pyrazolyl moiety in a hybrid structure with the pyrazolo[3,4-d]pyrimidine scaffold 14a–d, 16, 17 were generally more potent inhibitors of COX-2 and in turn more COX-2 selective than compounds having the other moieties, (iii) Compounds having pyrazolyl moiety 14a–d, 16, 17 in addition to the acetohydrazide derivative 21 showed good anti-inflammatory activity, especially 14c, which was more potent than celecoxib and compound 21 that had approximately 77% of celecoxib potency, (iv) both the compounds 16 and 21 showed the same ulceration effect of the low ulcerogenic reference drug (celecoxib) and (v) coupling of the pyrazolyl moiety with pyrazolo[3,4-d]pyrimidine scaffold in one hybrid structure offers a potential drug design concept for the development of NSAIDs that have good anti-inflammatory activity and low adverse ulcerogenic side effects.
Declaration of interest
The authors have declared no conflict of interest.
References
- Modi CM, Mody SK, Patel HB, et al. Toxicopathological overview of analgesic and anti-inflammatory drugs in animals. J Appl Pharm Sci 2012;2:149–57.
- Charlier C, Michaux C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem 2003;38:645–59.
- Crofford LJ, Wilder RL, Ristimäki A, et al. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Invest 1994;93:1095
- Pairet M, Engelhardt G. Distinct isoforms (COX-1 and COX-2) of cyclooxygenase: possible physiological and therapeutic implications. Fundam Clin Pharmacol 1996;10:1–15.
- Kulkarni S, Singh V. Positioning dual inhibitors in the treatment of pain and inflammatory disorders. Inflammopharmacology 2008;16:1–15.
- Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J 1998;12:1063–73.
- Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol 2006;38:1654–61.
- McGettigan P, Henry D. Current problems with non-specific COX inhibitors. Curr Pharm Des 2000;6:1693–724.
- Jackson LM, Hawkey CJ. COX-2 selective nonsteroidal anti-inflammatory drugs: do they really offer any advantages? Drugs 2000;59:1207–16.
- Greenberg J, Fisher M, Kremer J, et al. The COX-2 inhibitor market withdrawals and prescribing patterns by rheumatologists in patients with gastrointestinal and cardiovascular risk. Clin Exp Rheumatol 2009;27:395.
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954–9.
- Shishoo CJ, Pathak US, Rathod IS, et al. Synthesis and pharmacological evaluation of some novel 5-aryl-6-arylamino-l-phenylpyrazolo [3, 4-d] pyrimidin-4 (5H)-ones as analgesic and antiinflammatory agents. Ind J Chem Sec B 1999;38:684–95.
- Yadava U, Singh M, Roychoudhury M. Pyrazolo[3,4-d]pyrimidines as inhibitor of anti-coagulation and inflammation activities of phospholipase A 2: insight from molecular docking studies. J Biol Phys 2013;39:419–38.
- Raffa D, Maggio B, Plescia F, et al. Pyrazolo[3,4-d]pyrimidine derivatives as COX-2 selective inhibitors: synthesis and molecular modelling studies. Arch Pharm (Weinheim) 2009;342:321–6.
- Yewale SB, Ganorkar SB, Baheti KG, Shelke RU. Novel 3-substituted-1-aryl-5-phenyl-6-anilinopyrazolo[3,4-d]pyrimidin-4-ones: docking, synthesis and pharmacological evaluation as a potential anti-inflammatory agents. Bioorg Med Chem Lett 2012;22:6616–20.
- Abdelazeem AH, Abdelatef SA, El-Saadi MT, et al. Novel pyrazolopyrimidine derivatives targeting COXs and iNOS enzymes; design, synthesis and biological evaluation as potential anti-inflammatory agents. Eur J Pharm Sci 2014;62:197–211.
- Abdellatif KRA, Lamie PF, Omar HA. 3-Methyl-2-phenyl-1-substituted-indole derivatives as indomethacin analogs: design, synthesis and biological evaluation as potential anti-inflammatory and analgesic agents. J Enzyme Inhib Med Chem 2016;31:318–24.
- Abdellatif KRA, Abdelall EKA, Fadaly WA, Kamel GK. Synthesis, cyclooxygenase inhibition and anti-inflammatory evaluation of novel diarylheterocycles with a central pyrazole, pyrazoline or pyridine ring. Med Chem Res 2015;24:2632–44.
- Abdellatif KRA, Elshemy HAH, Salama SA, Omar HA. Synthesis, characterization and biological evaluation of novel 4′-fluoro-2′-hydroxy-chalcones derivatives as antioxidant, anti-inflammatory and analgesic agents. J Enz Inhib Med Chem 2015;30:484–91.
- Abdellatif KRA, Chowdhury MA, Velázquez C, et al. Celecoxib prodrugs possessing a diazen-1-ium-1,2-diolate nitric oxide donor moiety: synthesis, biological evaluation and nitric oxide release studies. Bioorg Med Chem Lett 2010;20:4544–9.
- Chowdhury MA, Abdellatif KRA, Dong Y, et al. Synthesis and biological evaluation of salicylic acid and N-acetyl-2-carboxybenzenesulfonamide regioisomers possessing a N-difluoromethyl-1,2-dihydropyrid-2-one pharmacophore: dual inhibitors of cyclooxygenases and 5-lipoxygenase with anti-inflammatory activity. Bioorg Med Chem Lett 2009;19:6855–61.
- Abdellatif KRA, Abdelall EKA, Fadaly WA, Kamel GK. Synthesis, cyclooxygenase inhibition, anti-inflammatory evaluation and ulcerogenic liability of new 1,3,5-triarylpyrazoline and 1,5-diarylpyrazole derivatives as selective COX-2 inhibitors. Bioorg. Med Chem Lett 2016;26:406–12.
- Devarakonda M, Doonaboina R, Vanga S, et al. Synthesis of novel 2-alkyl-4-substituted-amino-pyrazolo [3, 4-d] pyrimidines as new leads for anti-bacterial and anti-cancer activity. Med Chem Res 2013;22:1090–101.
- Jones RG. Reactions of orthoesters with active methylene compounds. J Am Chem Soc 1952;74:4889–91.
- Heravi MM, Nami N, Seifi N, et al. Microwave-assisted synthesis of substituted pyrazoles and pyrazolo [3, 4-d] thiopyrimidines. Phosphorus Sulfur 2006;181:591–9.
- Behbehani H, Ibrahim HM. 4-Thiazolidinones in heterocyclic synthesis: synthesis of novel enaminones, azolopyrimidines and 2-Arylimino-5-arylidene-4-thiazolidinones. Molecules 2012;17:6362–85.
- Praveen Rao P, Amini M, Li H, et al. Design, synthesis, and biological evaluation of 6-substituted-3-(4-methanesulfonylphenyl)-4-phenylpyran-2-ones: a novel class of diarylheterocyclic selective cyclooxygenase-2 inhibitors. J Med Chem 2003;46:4872–82.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 1962;111:544–7.
- Cho CH, Ogle CW. Cholinergic-mediated gastric mast cell degranulation with subsequent histamine H1-and H2-receptor activation in stress ulceration in rats. Eur J Pharmacol 1979;55:23–33.
