Abstract
A set of novel quinolone–triazole conjugates (12–31) were synthesized in three steps in good yields starting from 2-phenylquinoline-4-carboxylic acid. All the intermediates, as well as the final 1,2,4-triazolyl quinolines were fully characterized by their detailed spectral analysis utilizing different techniques such as IR, 1H NMR, 13C NMR, and finally mass spectrometry. All the synthesized compounds were evaluated in vitro for their potential antibacterial activity and their preliminary safety profile was assessed through cytotoxicity assay. Additionally, six selected conjugates were evaluated for their antioxidative properties on the basis of density functional theory calculations, using radical scavenging assay (DPPH) and cellular antioxidant assay. The reported results encourage further investigation of selected compounds and are shading light on their potential pharmacological use.
Introduction
The development of resistance to currently used antibacterial therapy has obliged the scientists, chemists, and biologists, to further search for more effective agents with less or no side effects. This is even more relevant to the present day scenario as the primary and opportunistic bacterial and/or fungal infections still continue to escalate with the increased number of immune compromised patients (mostly due to the diseases such as AIDS, cancer, and also as a consequence of long-term therapy after transplants)Citation1. This is the reason why extensive research is going on around the world, to discover novel molecules to fight such infections.
Despite plenty of research on different heterocyclic molecules, the azole ring still remains interesting chemical fragment for the development of novel molecules especially in the antibacterial and antifungal therapeutic area. This is due to the fact that most of the existing azole derivatives possessed both bacterial and fungal static action and can be orally applied owing to favorable bioavailability. They possess broad spectrum of activities against most of the yeasts and filamentous fungiCitation2.
Among azoles, 1H-1,2,4-triazole derivatives are considered to be more interesting as they possess important pharmacological activities such as antifungalCitation3,Citation4, antiviralCitation5, antioxidantCitation6–8, anti-asthmaticCitation9, anticonvulsantCitation10, antidepressantCitation11, antithyroidCitation12, anti-HIVCitation13, anti-inflammatoryCitation14 and anticancerCitation15–17.
On the other hand, quinoline derivatives are known to possess diverse pharmacological properties such as antioxidantCitation18,Citation19, antibioticCitation20,Citation21, cardiovascularCitation22, anti-TBCitation23, antiplateletCitation24, anticancerCitation25,Citation26, receptor antagonistsCitation27, NK3 receptor antagonists-IICitation28, anti-inflammatoryCitation29, antimicrobialCitation30,Citation31, selective estrogen receptor modulators (SERMs)Citation32 and protein kinase inhibitorCitation33.
Keeping above in mind, we decided to synthesize conjugates of the above-mentioned moieties to study the biological profile of the resulting product. Genesis of our chemistry is derived from the fact that few earlier conjugates of 1,2,4-triazole and quinoline, where these molecules are linked via oxygen- or amide-containing linkers in a single frame, are known to possess biological activities like antimalarialCitation34, antimicrobialCitation35, anti tubercularCitation36, antitumorCitation37,Citation38 and antiviralCitation39. However, the literature data about molecules that contain both of these bioactive ligands directly linked/bonded to each other in a single molecular frame are unknown.
As a result of our research programs involving the synthesis of new bioactive moleculesCitation40–47, we report herein for the first time synthesis of a new set of directly coupled quinolone–triazole conjugates. We also assessed biological activity profiles of these conjugates, with particular emphasis on their antioxidative properties.
Methods
Chemistry
All chemicals were purchased from commercial suppliers (Merck, SD Fine, and Spectrochem) and used as such. Solvents used in extraction and purification were distilled prior to use. Products were purified by column chromatography using silica gel as an adsorbent. Melting points were determined on an electronic apparatus and are uncorrected. 1H and 13C NMR spectra were recorded at 400 MHz on a JEOL spectrometer (Tokyo, Japan) and at 300 MHz on a Bruker spectrometer (Billerica, MA) using CDCl3/DMSO-d6 as solvent and tetramethylsilane (TMS) as internal standard. TMS was used as a reference for both 1H and 13C NMR spectra. In 1H NMR abbreviations s, d, dd, t, q, and m represents singlet, doublet, double doublet, triplet, quartet, and multiplet respectively. Coupling constants J values are given in hertz and the chemical shifts are given in δ. Elemental analyses were performed on a PerkinElmer Series II, CHNS/O Analyzer 2400 (Waltham, MA). Mass spectra were recorded on JEOL-JMS-DX303 mass spectrometer (Tokyo, Japan).
General procedure for the synthesis of compounds 3–5
Indol-2,3-dione/5-fluoro-indol-2,3-dione/5-methyl-indol-2,3-dione (10 g) and NaOH (8.16 g) were stirred together in water (80 mL) in a round bottom flask. To the reaction mixture, acetophenone (8.16 g) was added and contents refluxed. Reaction was monitored on thin layer chromatography (TLC) and after its completion the reaction mixture was cooled and acidified with conc. HCl solution. The precipitate obtained was collected, washed, and dried to afford 3–5.
2-Phenylquinoline-4-carboxylic acid (3). White solid. Yield: 14.72 g, 87%, m.p. 208–210 °C. IR (KBr) νmax: 3442, 2465, 1953, 1705, 1601, 1550, 1448, 1354, 1259, 1204, 1082, 894, 781, 760, 732, 699 cm−1. 1H NMR (δ, DMSO-d6, 400 MHz): 11.38 (brs, 1H, –COOH), 8.79 (d, 1H, J = 8.80 Hz), 8.47 (s, 1H), 8.29–8.27 (m, 2H), 8.19 (d, 1H, J = 8.80 Hz), 7.85–7.66 (m, 2H), 7.59–7.52 (m, 3H). 13C NMR (δ, DMSO-d6, 100 MHz): 167.50, 155.73, 148.50, 138.01, 137.03, 129.64, 129.56, 128.64, 127.28, 126.97, 125.39, 123.61, 119.28. Mass Spectral data, TOF MS ES+ m/z (%): 250 (M++1). Anal. Calcd for C16H11NO2: C, 77.10; H, 4.45; N, 5.62. Found: C, 77.14; H, 4.42; N, 5.60.
General procedure for the synthesis of compounds 6–8
Prepared compound 3/4/5 (10 g) was added to abs. ethanol (150 mL) in a flask, followed by conc. H2SO4 (5 mL). The resulting reaction mixture was refluxed and completion of reaction was monitored by TLC. The reaction mixture was cooled and poured over crushed ice in a beaker. The resulting contents were rendered alkaline by adding sufficient amount of ammonia solution. The mixture was then extracted thrice with diethyl ether. The combined ethereal solution was dried over anhydrous sodium sulfate and the solvent was removed by distillation to get the desired compounds 6/7/8.
Ethyl 2-phenylquinoline-4-carboxylate (6). Yellow color oil. Yield: 9.90 g, 89%. IR (film) νmax: 2983, 1724, 1623, 1594, 1513, 1338, 1248, 1230, 1193, 1143, 1119, 1037, 827, 749 cm−1. 1H NMR (δ, CDCl3, 400 MHz): 8.73 (d, 1H, J = 8.72 Hz), 8.38 (s, 1H), 8.23–8.19 (m, 3H), 7.76 (t, 1H, J = 7.32 Hz), 7.62 (t, 1H, J = 7.32 Hz), 7.56–7.52 (m, 3H), 4.53 (q, 2H, –COOCH2CH3), 1.50 (t, 3H, –COOCH2CH3). 13C NMR (δ, CDCl3, 100 MHz): 166.45, 156.72, 149.22, 138.84, 136.07, 130.28, 129.85, 129.68, 128.91, 127.71, 127.46, 125.38, 123.98, 120.19, 61.90, 14.32. Mass Spectral data, TOF MS ES+ m/z (%): 278 (M++1). Anal. Calcd for C18H15NO2: C, 77.96; H, 5.45; N, 5.05. Found: C, 78.02; H, 5.48; N, 5.02.
General procedure for the synthesis of compounds 9–11
A mixture of compounds 6–8 (5 g) and hydrazine hydrate (1.31 mL) was heated at 50–60 °C temperature with constant stirring. The solid that separated out on cooling was filtered and crystallized to give their corresponding carbohydrazide 9–11.
2-Phenylquinoline-4-carbohydrazide (9). White crystalline solid. Yield: 4.46 g, 94%, m.p. 214–216 °C. IR (KBr) νmax: 3385, 2917, 2366, 1701, 1606, 1483, 1449, 1360, 1287, 1246, 1229, 1149, 1039, 932, 889, 830, 754 cm−1. 1H NMR (δ, DMSO-d6, 400 MHz): 10.03 (s, 1H, –NH), 8.31 (d, 1H, J = 7.8 Hz), 8.25–8.23 (m, 2H), 8.14 (m, 1H), 8.03 (m, 1H), 7.78–7.74 (m, 2H), 7.60–7.48 (m, 3H), 4.52 (brs, 2H, –NH2). 13C NMR (δ, DMSO-d6, 100 MHz): 166.35, 155.71, 147.92, 140.76, 138.24, 129.55, 129.47, 129.43, 128.36, 127.02, 126.69, 125.05, 123.36, 116.84. Mass Spectral data, TOF MS ES+ m/z (%): 264 (M++1). Anal. Calcd for C16H13N3O: C, 72.99; H, 4.98; N, 15.96. Found: C, 73.04; H, 5.01; N, 15.97.
General procedure for the synthesis of compounds 12–26
The synthesized carbohydrazides 9–11 (200 mg) and substituted benzaldehyde (92 mg) were dissolved in 10 mL of glacial acetic acid in a round bottom flask. To the mixture, ammonium acetate (84 mg) was added. The reaction mixture was stirred for a period of 6–8 h at room temperature. The progress of the reaction was monitored by TLC. After completion, the reaction mixture was poured into ice-cold water and neutralized with ammonia. The precipitated product was filtered, washed with water, and crystallized from chloroform/methanol to give the desired product.
2-Phenyl-4-[5-(4-hydroxyphenyl)-4H-[1,2,4]-triazol-3-yl]quinoline (13). Cream-colored solid. Yield: 254 mg, 92%, m.p. 270–272 °C. IR (KBr) νmax: 3257, 2925, 1654, 1606, 1585, 1545, 1513, 1365, 1267, 1235, 1166, 845, 766 cm−1. 1H NMR (δ, DMSO-d6, 400 MHz): 12.08 (s, 1H, >NH, D2O exchangeable), 10.06 (s, 1H, –OH, D2O exchangeable), 8.36–8.33 (m, 2H), 8.27–8.25 (m, 2H), 8.20–8.16 (m, 3H), 7.85 (m, 1H), 7.67 (d, 2H, J = 7.1 Hz), 7.57 (d, 2H, J = 7.5 Hz), 6.88 (d, 2H, J = 7.8 Hz). 13C NMR (δ, DMSO-d6, 100 MHz): 162.56, 159.71, 155.77, 149.14, 147.89, 145.17, 143.53, 141.46, 138.05, 130.34, 129.96, 129.60, 128.91, 127.15, 125.13, 123.47, 117.14, 115.79. Mass spectral data, TOF MS ES+ m/z (%): 365 (M++1). Anal. Calcd for C23H16N4O: C, 75.81; H, 4.43; N, 15.38. Found: C, 75.94; H, 4.48; N, 15.39.
General procedure for the synthesis of compounds 27–31
The synthesized carbohydrazides 9–11 (200 mg) and heterocyclic aldehyde (78 mg) were dissolved in 10 mL of glacial acetic acid in a round bottom flask. To the mixture, ammonium acetate (84 mg) was added. The reaction mixture was stirred for a period of 6–8 h at room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was poured into ice-cold water and neutralized with ammonia. The precipitated product was filtered, washed with water, and crystallized from chloroform/methanol to give the desired product.
2-Phenyl-4-[5-(furan-2-yl)-4H-[1,2,4]-triazol-3-yl]quinoline (27). White solid. Yield: 230 mg, 88%, m.p. 198–200 °C. IR (KBr) νmax: 3182, 3052, 2925, 1655, 1623, 1591, 1544, 1349, 1284, 1270, 1158, 938, 765 cm−1. 1H NMR (δ, DMSO-d6, 400 MHz): 12.16 (s, 1H, >NH, D2O exchangeable), 8.34–8.30 (m, 2H), 8.25–8.22 (m, 2H), 8.15 (m, 1H), 7.85–7.82 (m, 2H), 7.67 (m, 1H), 7.56–7.52 (m, 3H), 6.97 (m, 1H), 6.56 (m, 1H). 13C NMR (δ, DMSO-d6, 100 MHz): 162.94, 155.65, 148.96, 147.98, 144.48, 140.65, 138.37, 129.77, 129.45, 128.48, 126.88, 126.77, 123.67, 123.26, 124.87, 119.21, 116.87. Mass spectral data, TOF MS ES+ m/z (%): 339 (M++1). Anal. Calcd for C21H14N4O: C, 74.54; H, 4.17; N, 16.56. Found: C, 74.60; H, 4.20; N, 16.58.
In vitro biological screening
Antibacterial activity assay
Bacterial strains, Staphylococcus aureus (American Type Culture Collection (Manassas, VA; ATCC), 29213), Streptococcus pneumoniae (ATCC, 49619), Streptococcus pyogenes (ATCC, 700294), Haemophilus influenzae (ATCC, 49247), and Escherichia coli (ATCC, 25922), were purchased from ATCC and utilized to evaluate antibacterial activity of compounds.
Antibacterial activity was determined by the standard broth microdilution method with azithromycin as comparator. Minimum inhibitory concentrations (MICs) were established according to guidelines of the Clinical Laboratory Standards InstituteCitation48 with the exception that lysed blood was substituted by 5% horse serum for Streptococcus medium. Double dilutions of tested compounds were prepared in 128–0.5 μg/mL concentration range within microplate wells. Bacteria were grown on appropriate agar plates (Becton Dickinson, Franklin Lakes, NJ). Inocula were prepared by direct colony suspension method and microplates were inoculated with 5 × 104 CFU/well. Results were determined by visual inspection after 20-h incubation at 37 °C.
Cytotoxicity assays
A549 human lung adenocarcinoma cell line (ATCC, CCL-185), HepG2 human hepatocellular carcinoma cell line (ATCC, HB-8065), MDA-MB-231 human breast adenocarcinoma cell line (ATCC, HTB-26), PC-3 human prostate adenocarcinoma cell line (ATCC, CRL-1435), and THP-1 human acute monocytic leukemia cell line (ATCC, TIB-202) were purchased from ATCC. Cell lines were maintained in complete DMEM/F12 medium (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany; D8437), or complete RPMI1640 (Sigma, R7388) for THP-1 cells, supplemented with 10% Fetal Bovine Serum (Sigma, F7524) at 37 °C in 5% CO2 atmosphere.
Cytotoxicity assay was performed using MTS Cell Titer 96 AQueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, WI; G3580)Citation49. Double dilutions of tested compounds were prepared in 100–0.2 μM concentration range within microplate wells. 5 × 104 cells were added per well and incubated overnight at 37 °C in 5% CO2 atmosphere. 15 μL of MTS reagent was dispensed per well and plates incubated for 1–4 h at 37 °C in 5% CO2 atmosphere. The absorbance was recorded at 490 nm using Wallac Victor2 microplate reader (PerkinElmer Life and Analytical Sciences, Turku, Finland). Results were analyzed in GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA).
DPPH-free radical scavenging assay
The DPPH (1,1-diphenyl-1,2-picryl hydrazyl) (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) method was used to determine the free radical scavenging activity of compoundsCitation50. Dilutions of tested compounds and ascorbic acid as a standard antioxidant comparator were prepared in 1000–1 μg/mL concentration range. 1 mL of compound solution was added to 1 mL of freshly prepared DPPH solution (3.9 mg/50 mL ethanol) and the reaction mixture incubated in the dark at room temperature for 20 min. Absorbance (A) was measured at 517 nm using Analytik Jena UV Winaspect Specrod PC 250 spectrophotometer (Analytic Jena AG, Jena, Germany). Inhibition of the DPPH radical by the compounds was calculated according to the following formula:
Where A0 is the absorbance of the control and A1 is the absorbance of the sample. The results are averages of three measurements. The EC50 value, compound concentration to reduce 50% of the DPPH, was calculated using GraphPad Prism software.
Cellular antioxidant activity (CAA) assay
OxiSelect Cellular Antioxidant Assay Kit (Cell Biolabs Inc., San Diego, CA; STA-349) was used to assess antioxidant activity of the compounds within a cell in a standard cell culture environment. The assay employs cell-permeable fluorogenic probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), which is diffused into cells, deacetylated by cellular esterases and oxidized by free radicals to fluorescent 2′,7′-dichloro-dihydrofluorescein (DCF), with the fluorescence intensity being proportional to the reactive oxygen species levels within the cell cytosolCitation51. The assay was performed according to manufacturer’s instructions. In brief, HepG2 cells were seeded at 5 × 104 per well in 96-well black microplates and incubated overnight at 37 °C in 5% CO2 atmosphere. The medium was removed and wells washed with sterile phosphate-buffered saline (PBS). Double dilutions of compounds were prepared in 500–8 μM concentration range in cell medium (50 μL) and DCFH-DA probe was added to wells (50 μL). The cells were incubated 1 h at 37 °C in 5% CO2 atmosphere. After the medium was removed and cells washed with sterile PBS, free radical initiator solution was added to all wells (100 μL) and fluorescence read using a fluorescence microplate reader (Victor 2 Wallac, PerkinElmer Life and Analytical Sciences, Turku, Finland) (excitation 480 nm/emission 530 nm). The readouts were saved in increments of 15 min for a total of 180 min. Results were analyzed in GraphPad Prism software and quantified as IC50 values.
Density functional theory (DFT) calculations
The reaction enthalpies BDE, IP, PA, and ETE and corresponding free energies (Table 2 in Supplementary Material) were calculated by a known methodCitation52. These reaction parameters were calculated by applying the DFT model (U)B3LYP/6-31 + G(d, p) as implemented in software Gaussian 03 (Gaussian, Inc., Wallingford, CT)Citation53. Calculations were performed for the gas and aqueous phases. Equilibrium geometries in unionized and anionic closed-shell ground electronic states as well as of corresponding radical and radical cation open-shell doublet ground electronic states were fully optimized in the gas phase. The minima were confirmed by no imaginary vibrational frequencies at temperature of 298.15 K and pressure of 1 atm. The free energies of solvation ΔG*hyd for all studied molecular species at 1 M standard state in water were determined at the gas phase equilibrium geometries by using integral equation formalism of polarizable continuum model (IEFPCM) of solvation with Bondi radii and tight SCF convergence criterionCitation54. The free energies (Table 2 in Supplementary Material) were calculated by employing corresponding thermodynamic cyclesCitation55. Lipophilicity values logP were calculated by OpenBabelCitation56.
Results and discussion
Chemistry
The synthetic route employed for the preparation of a set of novel quinoline-triazole conjugates (12–31) is shown in Scheme 1. 2-Phenylquinoline-4-carboxylic acid (3) was converted into its ethyl ester 6 by using absolute ethanol in the presence of concentrated sulfuric acid. The ester was purified and characterized by its IR spectrum which showed characteristic absorption band at 1724 cm−1 for ester group. Its 1H NMR spectrum displayed signals at δ 1.50 (t, 3H) for –COOCH2CH3 and at δ 4.53 (q, 2H) for –COOCH2CH3. Compound 6 on treatment with hydrazine hydrate gave 2-phenylquinoline-4-carbohydrazide (9), which exhibited characteristic absorption band at 3385 cm−1 for NHNH2 stretching in its IR spectrum. Further its 1H NMR did not show any signal corresponding to the ester group indicating thereby complete conversion. Final confirmation of structure of compound 9 came from its mass spectrum, which showed M++1 signal at m/z 264. The hydrazide obtained was cyclocondensed with 4-hydroxybenzaldehyde in the presence of ammonium acetate in glacial acetic acid at room temperature to finally give a cream-colored solid compound characterized as 13 (Scheme 1). Its 1H NMR spectrum showed two characteristic D2O exchangeable broad signals at δ 12.08 and δ 10.06 integrating for one proton each, indicating the presence > NH and –OH protons, respectively. In IR spectrum, absorption band at 1654 cm−1 indicated the presence of imido bond (C=N) which was further supported by the absence of –CH=N-proton in 1H NMR spectrum thus confirming that cyclization has taken place. The other protons of the phenyl group and quinoline skeleton were observed at δ 8.36–8.33 (m, 2H), 8.27–8.25 (m, 2H), 8.20–8.16 (m, 3H), 7.85 (m, 1H), 7.67 (d, 2H, J = 7.1 Hz), 7.57 (d, 2H, J = 7.5 Hz), 6.88 (d, 2H, J = 7.8 Hz). The 13C NMR spectrum showed all expected characteristic signals. On the basis of above analysis, and mass spectrum compound 13 was characterized as 2-phenyl-4-[5-(4-hydroxyphenyl)-4H-[1,2,4]-triazol-3-yl]quinoline.
Scheme 1. Synthesis of quinolone–triazole conjugates (12nju). Reagents and conditions: ai = NaOH, H2O, ref lux, 4–6 h; b–= abs. C2H5OH, conc. H2SO4, ref lux, 18–20 h; c8 = N2H4 H2O, heat, 50–60 °C, 4–6 h; d–= aldehyde, glc. CH3COOH, CH3COONH4, 6–8 h at room temperature.
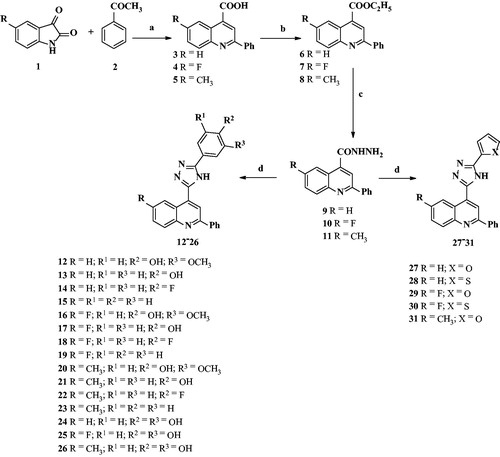
Similar set of the above-described reactions were repeated with substituted aryl and hetero aromatic ring aldehydes to obtain corresponding desired compounds.
Biological activity
All final products (12–31) have been profiled in vitro, in terms of their antibacterial activity and cytotoxicity. The cytotoxicity of a compound is closely associated with potential adverse effects on particular cells, tissues, or organs of drugs intended for human use.
The antibacterial activity was determined against five different bacterial species: two gram-negative (E. coli, H. influenzae) and three gram-positive (S. aureus, S. pneumoniae, S. pyogenes). However, neither of the compounds showed substantial and significant antibacterial activity (Supplementary Table S1). Although molecules containing quinoline and 4H-1,2,4-triazole fragments are often recognized in vitro as potent antibacterialsCitation57 this has not to be general caseCitation58. In our case for 2-phenyl 4H-1,2,4-triazole substituted quinolines, no antibacterial activity has been detected. This may be either due to the specific unfavorable structural factors such as site of substitutionCitation57 or unfavorable physicochemical propertiesCitation59.
The antibacterials are generally hydrophilic molecules, while these derivatives are very lipophilic with clogP values around 5 or higher. Absence or very weak of antibacterial activity generally indicates the potential of specific compounds for their long-term use, for example as anti-inflammatory agents in the treatment of chronic disease, without potential risks to induce resistance.
The cytotoxic effect of compounds was evaluated on five different human cell lines (A549, HepG2, MDA-MB-231, PC-3, and THP-1) using MTS test (). The test quantifies metabolic activity of cells by measuring their metabolism through the released NADH levels, thus indicating whether a compound impairs any of the key cellular metabolic pathways.
Table 1. The results of compound cytotoxicity screening expressed as IC50 values in μM.
The cytotoxicity of a compound is closely associated with its potential adverse drug effects. In addition, MTS test is very often used for the estimation of antiproliferative capacity of a compound when followed through longer period of time. The results of compound cytotoxicity screening, here performed during 24 h, are shown in . 5-Aryl 4H-1,2,4-triazolyl compounds 13, 17, 21, and 26 displayed significant cytotoxic effect on THP-1 cell line. Derivatives 25 as well as 27–30 showed weak cytotoxic effect on THP-1 cells. Only compounds 17 and 26 were additionally cytotoxic for HepG2 cell line. With respect to these data, all the other compounds could be considered suitable for further in vitro profiling in cellular assays.
The cytotoxicity of the active compounds toward the most sensitive THP-1 cell-line is determined by substituents at position 5 of the triazole ring. The observed cytotoxic activity may be attributed to the presence of hydrogen-bond accepting centers at specific positions in these substituents, the chemical feature that is absent in the inactive analogs.
In addition, 5-aryl 4H-1,2,4-triazolyl derivatives which moderately inhibited metabolic activity in the most sensitive cell line (THP-1) are (poly)phenolic compounds. It is also well known that many polyphenols possess antioxidant features since they can directly scavenge-free radicals by donating H-atom and/or modulate activities of various proteins included directly or indirectly in free radical productionCitation60. Therefore, some of the 5-aryl 4H-1,2,4-triazolyl derivatives have been tested for their antioxidant activity.
At first, assuming that free hydroxyl (OH) group of the active compounds () can donate hydrogen atom to a free radical, the radical scavenging activity of these derivatives was estimated in silico by using common approachCitation52. Derivatives 12, 16, and 20 containing guaiacyl-like group were also included in computations. Values of the calculated parameters gas-phase bond dissociation enthalpy (BDEg) and aqueous bond dissociation free energy (BDFEaq) were used for comparing radical scavenging capacities of our polyphenolic derivatives mutually as well as with corresponding natural polyphenols (). As expected, compounds 24, 25, and 26 with catechol fragment have stronger radical scavenging ability than compounds 13, 17, and 21 with para-phenyl substituent and compounds 12, 16, and 20 with guaiacyl-like moietyCitation52. Compounds within each of these three subgroups have mutually similar radical scavenging capacities. Additionally, the order of radical scavenger capacities of the three subgroups corresponds to the order of the natural polyphenols quercetin, apigenin, and tamarixetin containing analogous polyphenolic fragments (). The obtained density functional theory (DFT) results indicate that for our compounds, the radical scavenging activities of (poly)phenolic fragments are quite independent on the rest of their structures.
Table 2. Comparison of calculated gas-phase (g) bond dissociation enthalpies (BDE) and corresponding aqueous (aq) free energies (all given in kJ/mol) as well as estimated lipophilicity values logP of the 5‐aryl-4H‐1,2,4‐triazolyl derivatives and natural polyphenols. Experimentally determined radical scavenging (DPPH assay) and cellular antioxidant (CAA) activities are also presented.
The DPPH assay provides an easy and rapid in vitro method commonly used to evaluate antioxidant activity of natural plant extracts and chemical compounds which act as free radical scavengers. The activity of six selected compounds assessed using the DPPH assay demonstrates considerable radical scavenging activity (). Ascorbic acid was used as a comparator in this assay, yielding IC50 value of 9.67 μg/mL. Four compounds displayed radical scavenging capabilities comparable to ascorbic acid, with the highest DPPH-scavenging activity shown by compound 25 (IC50 value of 3.57 μg/mL).
There are many chemical assays used to quantify radical scavenging activity of compounds. However, their efficacy to predict in vivo antioxidant activity is modest since they do not address critical issues such as uptake, distribution, and metabolism of antioxidants which may significantly impact their bioavailability, stability, and tissue retention. In addition, in such a way, it is not possible to assess indirect antioxidant activities of compounds attainable in the cellCitation60. With respect to all this, cellular antioxidant assay (CAA) serves as a more biologically relevant method for assessing the antioxidant activity of compounds in vivo. CAA was employed to test antioxidant activity of 6 selected compounds in HepG2 cell line (). Catecholic compounds 24 and 25 displayed considerable antioxidant activity in cellular system with IC50 values of 114 μM and 101 μM, respectively. Quercetin was used as a comparator in this assay exhibiting IC50 value of 31 μM.
Conclusions
The novel quinolone–triazole conjugates were synthesized in three steps starting from 2-phenylquinoline-4-carboxylic acid. The acid was converted into carbohydrazide derivative via its ethyl ester. Carbohyrazide on cyclocondensation using ammonium acetate in acetic acid in the presence of aryl/hetero aryl aldehyde gave finally the target molecules. These novel compounds were evaluated in vitro for their potential antibacterial activity. No significant antibacterial activity has been observed, qualifying these compounds for additional development as promising leads in other therapeutic areas where chronic, long-term application is required. Moreover, when their preliminary safety profile was assessed through cytotoxicity assays on five different cell lines, we have seen potential for further cell-based assays profiling since only moderate inhibition of cell-metabolism activity has been observed on the most sensitive THP-1 cell line. And finally, based on DFT calculations, six selected conjugates were evaluated for their antioxidative properties on radical scavenging assay (DPPH) and CAA. A plausible way to increase antibacterial activity would be to decrease lipophilicity. For example, by synthetizing derivatives without phenyl ring at position 2 (which will reduce clogP by 2 or 3 units). However, his approach needs to be further investigated.
Nevertheless, the obtained results in all these assays are advocating in terms that additional synthesis of new derivatives and further investigations in this therapeutic area might provide interesting and potentially promising results that can finally be applied for enriching our knowledge and experience in the development of new chemical leads with this specific biological activity.
Declaration of interest
The authors declare no conflict of interest.
This work was supported by the research grants received from the University of Delhi, India (number DRCH/R&D/2010-13) and from the Croatian Science Foundation, Croatia (number 5467).
Supplementary material available online
IENZ_1190714_Supplementary_Material.pdf
Download PDF (931.7 KB)Acknowledgements
M.C. and M.V. are thankful to UGC and K.K. to CSIR New Delhi (India) for research fellowships. Additionally, V.S. is grateful to the University of Zagreb Computing Centre SRCE for supporting the computer cluster where computations were done.
References
- Zitouni GT, Kaplancıklı ZA, Yıldız MT, et al. Synthesis and antimicrobial activity of 4-phenyl/cyclohexyl-5-(1-phenoxyethyl)-3-[N-(2-thiazolyl)acetamido]thio-4H-1,2,4-triazole derivatives. Eur J Med Chem 2005;40:607–13
- Aher NG, Pore VS, Mishra NN, et al. Synthesis and antifungal activity of 1,2,3-triazole containing fluconazole analogues. Bioorg Med Chem Lett 2009;19:759–63
- Tsukuda Y, Shiratori M, Watanabe H, et al. Modeling, synthesis and biological activity of novel antifungal agents. Bioorg Med Chem Lett 1998;8:1819–24
- Roberts J, Schock K, Marino S, Andriole VT. Efficacies of two new antifungal agents, the triazole Ravuconazole and the Echinocandin LY-303366, in an experimental model of invasive aspergillosis. Antimicrob Agents Chemother 2000;44:3381–8
- Kini GD, Robins RK, Avery TL. Synthesis and antitumor activity of ribavirin imidates. A new facile synthesis of ribavirin amidine (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamidine hydrochloride). J Med Chem 1989;32:1447–9
- Aswathanarayanappa C, Bheemappa E, Bodke YD, et al. Synthesis and evaluation of antioxidant properties of novel 1,2,4-triazole-based Schiff base heterocycles. Arch Pharm 2013;346:922–30
- Pokuri S, Singla RK, Bhat VG, Shenoy GG. Insights on the antioxidant potential of 1, 2, 4-triazoles: synthesis, screening & QSAR studies. Curr Drug Metab 2014;15:389–97
- Maddila S, Kumar AS, Gorle S, et al. Synthesis and antioxidant activity of 1,2,4-triazole linked thieno[2,3-d]pyrimidine derivatives. Lett Drug Des Discov 2014;10:186–93
- Naito Y, Akahoshi F, Takeda S, et al. Synthesis and pharmacological activity of triazole derivatives inhibiting Eosinophilia. J Med Chem 1996;39:3019–29
- Kamboj VK, Verma PK, Danda A, Ranjan S. 1,2,4-Triazole derivatives as potential scaffold for anticonvulsant activity. Cent Nerv Syst Agents Med Chem 2015;15:17–22
- Deng XQ, Song MX, Zheng Y, Quan ZS. Design, synthesis and evaluation of the antidepressant and anticonvulsant activities of triazole containing quinolinones. Eur J Med Chem 2014;73:217–24
- Takaoka M, Manabe S, Yamoto T, et al. Comparative study of goitrogenic actions of 3-substituted 1,2,4-triazoles in rats. J Vet Med Sci 1994;56:341–6
- Patel NB, Khan IH, Pannecouque C, Clercq ED. Anti-HIV, antimycobacterial and antimicrobial studies of newly synthesized 1,2,4-triazole clubbed benzothiazoles. Med Chem Res 2013;22:1320–9
- Sarigol D, Baran AU, Tel BC, et al. Novel thiazolo[3,2-b]-1,2,4-triazoles derived from naproxen with analgesic/anti-inflammatory properties: synthesis, biological evaluation and molecular modeling studies. Bioorg Med Chem 2015;23:2518–28
- Goss PE, Strasser-Weippl K. Aromatase inhibitors for chemoprevention. Best Pract Res Clin Endocrinol Metab 2004;18:113–30
- Santen JR. Inhibition of aromatase: insights from recent studies. Steroids 2003;68:559–67
- Clemons M, Coleman RE, Verma S. Aromatase inhibitors in the adjuvant setting: bringing the gold to a standard. Cancer Treat Rev 2004;30:325–32
- Orhan PM, Tekiner B, Suzen S. Recent studies of antioxidant quinoline derivatives. Mini Rev Med Chem 2013;13:365–72
- Savegnago L, Vieira AI, Seus N, et al. Synthesis and antioxidant properties of novel quinolone-chalcogenium compounds. Tet Lett 2013;54:40–4
- Lam KH, Gambari R, Lee KKH, et al. Preparation of 8-hydroxyquinoline derivatives as potential antibiotics against Staphylococcus aureus. Bioorg Med Chem Lett 2014;24:367–70
- Bringmann G, Reichert Y, Kane V. The total synthesis of streptonigrin and related antitumor antibiotic natural products. Tetrahedron 2004;60:3539–74
- Sircar I, Haleen SJ, Burke SE, Barth H. Synthesis and biological activity of 4-(diphenylmethyl)-α-[(4-quinolinyloxy)methyl]-1-piperazineethanol and related compounds. J Med Chem 1992;35:4442–9
- Senthilkumar P, Dinakaran M, Yogeeswari P, et al. Synthesis and antimycobacterial activities of novel 6-nitroquinolone-3-carboxylic acids. Eur J Med Chem 2009;44:345–58
- Ko TC, Hour MJ, Lien JC, et al. Synthesis of 4-alkoxy-2-phenylquinoline derivatives as potent antiplatelet agents. Bioorg Med Chem Lett 2001;11:279–82
- Mikata Y, Mika Y, Shun-ichiro O, et al. Effect of side chain location in (2-aminoethyl)aminomethyl-2-phenylquinolines as antitumor agents. Bioorg Med Chem Lett 1998;8:1243–8
- Zhang L, Sun F, Li Y, et al. Rapid synthesis of iminosugar derivatives for cell based in situ screening: discovery of “Hit” compounds with anticancer activity. Chem Med Chem 2007;2:1497–594
- Bromidge SM, Bertani B, Borriello M, et al. 6-[2-(4-Aryl-1-piperazinyl)ethyl]-2H-1,4-benzoxazin-3(4H)-ones: dual-acting 5-HT1 receptor antagonists and serotonin reuptake inhibitors. Bioorg Med Chem Lett 2008;18:5653–6
- Zhang J, Chiang FI, Wu L, et al. Surprising alteration of antibacterial activity of 5′′-modified neomycin against resistant bacteria. J Med Chem 2008;51:7563–73
- Elliott JM, Carling RW, Chicchi GG, et al. N′,2-Diphenylquinoline-4-carbohydrazide based NK3 receptor antagonists II. Bioorg Med Chem Lett 2006;16:5752–6
- Cuny GD, Robin M, Ulyanova NP, et al. Structure–activity relationship study of acridine analogs as haspin and DYRK2 kinase inhibitors. Bioorg Med Chem Lett 2010;20:3491–4
- Mathew V, Keshavayya J, Vaidya VP, Giles D. Studies on synthesis and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. Eur J Med Chem 2007;42:823–40
- Rizk OH, Mahran MA, El-Khawass SM, et al. Synthesis of some new antimicrobial thiadiazolyl and oxadiazolyl quinoline derivatives. Med Chem Res 2005;14:260–73
- Hoekstra WJ, Patel HS, Liang X, et al. Discovery of novel quinoline-based estrogen receptor ligands using peptide interaction profiling. J Med Chem 2005;48:2243–7
- Havaldar FH, Patil AR. Syntheses of 1, 2, 4 triazole derivatives and their biological activity. Eur J Chem 2008;5:347–54
- Pandey SK, Singh A, Nizamuddin A. Antimicrobial studies of some novel quinazolinones fused with [1,2,4]-triazole, [1,2,4]-triazine and [1,2,4,5]-tetrazine rings. Eur J Med Chem 2009;44:1188–97
- Upadhayaya RS, Kulkarni GM, Vasireddy NR, et al. Design, synthesis and biological evaluation of novel triazole, urea and thiourea derivatives of quinoline against Mycobacterium tuberculosis. Bioorg Med Chem 2009;17:4681–92
- Rashad AE, El-Sayed WA, Mohamed AM, Ali MM. Synthesis of new quinoline derivatives as inhibitors of human tumor cells growth. Arch Pharm (Weinheim) 2010;343:440–8
- Upadhayaya RS, Vandavasi JK, Kardile RA, et al. Novel quinoline and naphthalene derivatives as potent antimycobacterial agents. Eur J Med Chem 2010;45:1854–67
- Wang Z, Wu B, Kuhen KL, et al. Synthesis and biological evaluations of sulfanyltriazoles as novel HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg Med Chem Lett 2006;16:4174–7
- Panda SS, Jain SC. Synthesis and QSAR studies of some novel disubstituted 1,2,4-triazoles as antimicrobial agents. Med Chem Res 2014;23:848–61
- Panda SS, Jain SC. New trifluoromethyl quinolone derivatives: synthesis and investigation of antimicrobial properties. Bioorg Med Chem Lett 2013;23:3225–9
- Vashist M, Kushwaha K, Kaushik R, Jain SC. Synthesis of medicinally important quinazolines decorated with 1,4-disubstituted-1,2,3-triazoles using CuSO4·5H2O-Et3N catalytic system. RSC Adv 2014;4:23679–84
- Kushwaha K, Kaushik N, Lata Jain SC. Design and synthesis of novel 2H-chromen-2-one derivatives bearing 1,2,3-triazole moiety as lead antimicrobials. Bioorg Med Chem Lett 2014;27:1795–801
- Kushwaha K, Sakhuja R, Jain SC. Synthesis and antimicrobial activity of novel bis-azaphenothiazines. Med Chem Res 2013;22:4459–67
- Verbanac D, Jain SC, Jain N, et al. An efficient and convenient microwave-assisted chemical synthesis of (thio)xanthones with additional in vitro and in silico characterization. Bioorg Med Chem 2012;20:3180–5
- Panda SS, Malik R, Chand M, Jain SC. Synthesis and antimicrobial activity of some new 4-triazolylmethoxy-2H-chromen-2-one derivatives. Med Chem Res 2012;21:3750–6
- Sakhuja R, Panda SS, Khanna L, et al. Design and synthesis of spiro[indole-thiazolidine]spiro[indole-pyrans] as antimicrobial agents. Bioorg Med Chem Lett 2011;21:5465–9
- Clinical and Laboratory Standards Institute (CLSI) (formerly known as National Committee on Clinical Laboratory Standards–NCCLS) (http://www.clsi.org/) 2009;11:32–40. Available from: http://www.cdc.gov/meningitis/lab-manual/chpt11-antimicrobial-suscept-testing.pdf
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 1995;28:25–30
- Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 2007;55:8896–907
- Stepanić V, Gall TK, Lučić B, et al. Bond dissociation free energy as a general parameter for flavonoid radical scavenging activity. Food Chem 2013;141:1562–70
- Frisch MJ, Trucks GW, Schlegel HB, et al. Gaussian 03, Revision C.02. Wallingford (CT): Gaussian Inc.; 2004. Available from: http://www.gaussian.com/g_misc/g03/citation_g03.htm
- Cancès MT, Mennucci B, Tomasi J. A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 1997;107:3032–41
- Alongi KS, Shields GC. Theoretical calculations of acid dissociation constants: a review. Annu Rep Comput Chem 2010;6:113–38
- O’Boyle NM, Banck M, James CA. Open Babel: an open chemical toolbox. J Cheminform 2011;3:33–46
- Eswaran S, Adhikari AV, Shetty NS. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur J Med Chem 2009;44:4637–47
- Patel RV, Park SW. Access to a new class of biologically active quinoline based 1,2,4-triazoles. Eur J Med Chem 2014;71:24–30
- O’Shea R, Moser HE. Physicochemical properties of antibacterial compounds: implications for drug discovery. J Med Chem 2008;51:2878–1
- López-Alarcón C, Denicola A. Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Anal Chim Acta 2013;763:1–10
