Abstract
A series of tetrahydropyrimidinethiones were synthesized from thiourea, β-diketones and aromatic aldehydes, such as p-tolualdehyde, p-anisaldehyde, o-tolualdehyde, salicylaldehyde and benzaldehyde. These cyclic thioureas showed good inhibitory action against acetylcholine esterase (AChE), butyrylcholine esterase (BChE), and human (h) carbonic anhydrase (CA) isoforms I and II. AChE and BChE inhibitions were in the range of 6.11–16.13 and 6.76–15.68 nM, respectively. hCA I and II were effectively inhibited by these compounds, with Ki values in the range of 47.40–76.06 nM for hCA I, and of 30.63–76.06 nM for hCA II, respectively. The antioxidant activity of the cyclic thioureas was investigated by using different in vitro antioxidant assays, including 1,1-diphenyl-2-picrylhydrazyl (DPPH·) radical scavenging, Cu2+ and Fe3+ reducing, and Fe2+ chelating activities.
Introduction
Recently, the synthesis of some pyrimidine thiones derivatives attracted great attention due to their biological and pharmaceutical properties including analgesic, anti-staphylococcal, antiviral, anti-tuberculosis, anti-fungal, and efficacy against some cardiovascular diseasesCitation1. A facile synthesis of these compounds includes Biginelli reaction, when aliphatic/aromatic aldehydes, urea/thiourea, acetylacetone, and various catalysts lead to the ring closure with the formation of the desired heterocycle. One major factor influencing the outcome and yields in the desired products is the selection of the catalyst for Biginelli’s reaction.Citation2 Synthesis of 3,4-dihydropyrimidine-2(1H) thiones in a simple and useful way is based on this one-pot, three-component condensation of aldehydes, methylene active compounds and thiourea in acidic media.Citation1,Citation2
The reversible hydration of carbon dioxide (CO2) to form bicarbonate (HCO3−) and protons (H+) is a simple but very important chemical reaction that can be performed by various classes of carbonic anhydrases (CAs, EC 4.2.1.1).Citation3–6 The interconversion of CO2 and HCO3− is spontaneously balanced to maintain the equilibrium between dissolved inorganic CO2, the highly unstable carbonic acid (H2CO3) and carbonate (CO32−) of which HCO3− is physiologically the most important species (due to the physiological pH values). Indeed, bicarbonate is a substrate for several carboxylation enzymes involved in biosynthetic pathways such as biosynthesis of urea, glucose, fatty acids and possibly nucleotidesCitation7–10. The reaction not only provides a means to control the physiological pH values, but also supplies HCO3− ions for various physiological processes or metabolic, biosynthetic pathways, as those mentioned aboveCitation11–14. So far, six unrelated genetic CA families have been discovered, namely, the α-, β-, γ-, δ-, ζ- and η-CAs. In many organisms, a large number of isoforms of the CA family are present, which possess specialized functions in various tissues and organsCitation15–19. These CA families are found in most living organisms. For example α-CAs are found in humans and other mammals and divided into four broad subgroups, which, in turn consist of several isoforms: the cytosolic CAs (CA I, II, III, VII, and XIII), mitochondrial CAs (CA VA and VB), secreted CA (CA VI), as well as membrane-associated CAs (CA IV, IX, XII, XIV and XV)Citation20–24. There are three additional, acatalytic CA isoforms (CA VIII, X, and XI) whose functions remain unclear. On the other hand, it was reported that β-CAs exist in most prokaryotes and plant chloroplasts, γ-CAs are present in methanogens and most bacteria. δ-CAs and ζ-CAs were found in diatomsCitation25. Recently, the η-CA family was identified in organisms of the genus Plasmodium. These enzymes previously thought to belong to the α-CA family, were shown to possess unique features such as their metal ion coordination patternCitation26–29, which motivates their designation as a new genetic family, i.e., the η-CAs. As such, CA inhibitors (CAIs) have a role as pharmaceutical agents in the treatment of various pathophysiological conditionsCitation30–33. CAIs possessing different inhibition mechanisms compared to the classical inhibitors were reported in the last yearsCitation34,Citation35. The interest in CAIs is mainly motivated by their pharmacological properties and clinical use as diuretics, antiglaucoma, antiobesity or antiepileptic agents, as well as anticancer agents and diagnostic toolsCitation36–38.
The neurotransmitter acetylcholine (ACh) mediates its physiological effects via a plethora of receptors expressed throughout the central nervous system and the peripheral nervous systemCitation39,Citation40. Elder persons suffering from Alzheimer’s disease (AD) have a low ACh level in the hippocampus and cortex, which is the main cause of ADCitation17,Citation41. Acetylcholinesterase (AChE, EC 3.1.1.7) and butyrylcholinesterase (BChE, EC 3.1.1.8) are serine hydrolases that catalyze the hydrolysis of acetylcholine, thus regulating cholinergic neurotransmissionCitation42–44. It is well known that both enzymes are two major forms of cholinesterases in mammalian tissues. AChE is highly active for the hydrolysis of acetylcholine (ACh) and can regulate the concentration of ACh, which plays a key role in memory and learning as a central neurotransmitterCitation45,Citation46. On the other hand, BChE is an endogenous enzyme synthesized by the liver, which serves as a catalyzer for the hydrolysis of esters in cholineCitation47. In general, AChE displays a greater affinity for ACh and thus has greater activity for its hydrolysis as compared to BChE. Studies indicate that in the case of AD, the level of AChE in certain brain regions is significantly reduced and the BChE level is progressively increased, which is responsible for the level of ACh. In fact, a portion of evidence suggests that the inhibition of BChE can raise ACh levels and improves cognition in AD. Currently, the most efficacious treatment approaches for AD are four cholinesterase inhibitors including tacrine, rivastigmine, donepezil and galantamineCitation48,Citation49.
All aerobic organisms have antioxidant defenses against free radicals and reactive oxygen species (ROS), including antioxidant enzymes and antioxidant food constituents to remove or repair the damaged moleculesCitation50–52. Antioxidant compounds can scavenge free radicals or ROS and increase shelf life by retarding the process of lipid peroxidation, which is one of the major reasons for deterioration of food and pharmaceutical products during processing and storageCitation53–55. Also, they protect the human body from hazardous effects of free radicals and ROS. They retard the progress of many chronic diseases as well as lipid peroxidation. Hence, a need has appeared to identify alternative safe sources of antioxidantsCitation56,Citation57. Antioxidants are often added to foods and pharmaceuticals for preservation from the radical chain reactions. They act by inhibiting the initiation and propagation steps of radical chain reactions. Thus they lead the termination of the reaction and delay the oxidation processCitation58,Citation59. At the present time, the most commonly used antioxidants are butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propylgallate, and tert-butyl hydroquinoneCitation60–62. However, BHA and BHT have been restricted by legislative rules due to doubts over their toxic and carcinogenic effectsCitation63,Citation64. Therefore, there is a growing interest in natural and safer antioxidants for food applications, and a growing trend in consumer preferences towards natural antioxidants, all of which have given impetus to the attempts to explore new and safer sources of antioxidantsCitation65–67.
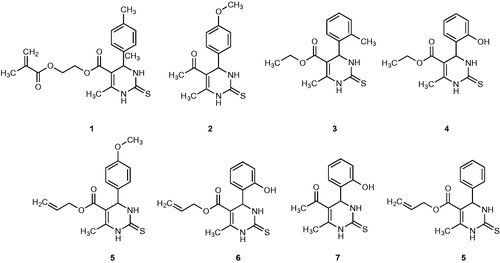
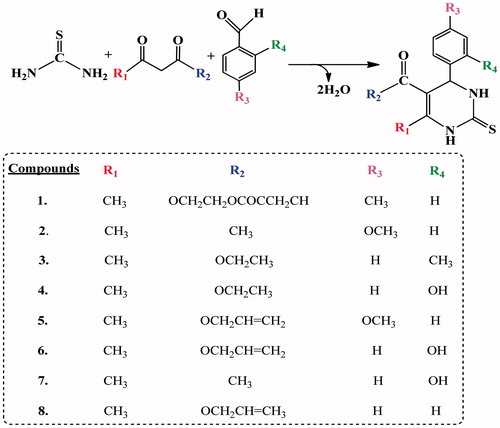
The interest on the synthesis of new such heterocyclic compounds, containing multifunctional nitrogen and sulfur atoms within the ring, and their application as antimicrobial and antioxidant agentsCitation68, prompted us to design the new cyclic thioureas (1–8), which were obtained for the first time by us based on using trifluoroacetic acid (TFAA) as catalyst ().
In the present study we describe the design, synthesis and evaluation of some of cyclic thioureas (1–8) as effective acetylcholinesterase, butyrylcholinesterase, human carbonic anhydrase inhibitor and antioxidant agents. Another main goal of this study, is compared their activities to related standard compounds including tacrine (for acetylcholinesterase and butyrylcholinesterase), acetazolamide (for human carbonic anhydrase isoenzymes I and II) and BHA, BHT, α-tocopherol and trolox (for antioxidant activities).
Experimental
Chemistry
Synthesis of 2-(methacryloyloxy)ethyl 6-methyl-2-thioxo-4- (p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (1)
Thiourea (0.76 g, 0.01 mol) was added to the solution of acetylacetone (5 mL) in ethyl alcohol (1 mL) and then 2-(methacryloyloxy) ethyl acetoacetate (1.91 mL, 0.01 mol) was added to the solution dropwise. After the mixture was stirred for 5 minutes, p-tolualdehyde (1.18 mL, 0.01 mol) was added and it was stirred for 30 min. Then TFAA (0.02 mL) was added to the reaction mixture and it was stirred by heating at 70–75 °C. The progress of the reaction was controlled by Sulifol UV 254 plate. After determining the full completion of reaction, solution was evaporated and is cleansed in ethyl alcohol solution. The white crystalline having the melting temperature of 212 °C is obtained. The yield is 1.8 g.
Eluent-ethanol: hexane (5:2). 1H NMR (300 MHz, (DMSO-d6, δ): 2.27 (s, 3H, CH3); 4.49 (d, 2H, CH2O); 5.06–5.17 (dd, 2H, CH2); 5.78 (m, 1H, =CH); 7.25 (d, 2H, 2CH-Ar); 7.30 (t, 2H, 2CH-Ar); 7.77 (s, 1H, NH); 9.26 (1H, NH). Citation13C NMR (75 MHz, DMSO-d6) 18.34, 54.32, 64.18, 99.25, 117.43, 126.70, 127.79, 128.91, 133.43, 145.10, 149.56, 152.53, 165.38. IR ν, sm−1: 3175 (NH), 1709 (C=O), 1092 (C=S), 756, 852, 974, 1230 (CH), 1608 (C=C).
Synthesis of 1-(4-(4-methoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)ethanone (2)
Synthesis of 1-(4-(4-methoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)ethanone (2) was realized according to the general procedure at 2.1 according to the literatureCitation69. 1H NMR data are in agreement with data given in the literatureCitation70. Citation13C NMR (75 MHz, DMSO-d6) 10.01, 19.33, 21.11, 30.19, 48.26, 54.01, 126.82, 129.50, 137.64, 141.76, 152.58, 194.78. IR ν, sm−1: 1810–1951, 3000–3100 (Ar), 1493 (Ar C-H), 1614 (Ar C-C), 703 (CH), 1675, 1703 (C=O), 3257 (NH), 1236 (C=S).
Synthesis of ethyl 6-methyl-2-thioxo-4-(o-tolyl)-1,2,3,4- tetrahydropyrimidine-5-carboxylate (3)
Compound 3 was also synthesized fallowing the procedure above and its 1H- and 13C-NMR data are in agreement with data given in the literatureCitation71.
Synthesis of ethyl 4-(2-hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4)
Synthesized according to the general procedure at given aboveCitation72. 1H NMR data are in agreement with data given in the literatureCitation72. 13C NMR (75 MHz, DMSO-d6) 14, 15, 48, 61, 104, 115, 121, 122, 128, 154, 160, 167, 180. IR ν, sm−1: 3370–3040 (NH), 1709 (C=O), 1092 (C=S), 756, 852, 974, 1230 (CH), 1608 (C=C).
Synthesis of allyl 4-(4-methoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (5)
Compound 5 was synthesized according to the procedure described above (“Synthesis of 2-(methacryloyloxy)ethyl 6-methyl-2-thioxo-4-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (1)” section). White crystalline. M.p: 198 °C. 1H NMR (300 MHz, (DMSO-d6, δ): 1.79 (s, 3H, CH3); 2.22 (s, 3H, CH3); 4.19 (d, 2H, CH2O); 5.12 (d, 1H, CH-Ar); 5.63–5.92 (s-s, 2H, =CH2); 7.20 (m, 5H, 5CH-Ar); 9.75 (s, 1H, NH); 9.19 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6) 15.5, 54.6, 55.8, 67.8, 106, 114, 116, 127, 133, 135, 158, 167, 180. IR ν, sm−1: 3370–3040 (NH), 1635–1630 (C=O), 1092 (C=S), 756, 852, 974, 1230 (CH), 1607–1608 (C=C).
Synthesis of allyl 4-(2-hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (6)
Compound 6 was synthesized as described above the section “Synthesis of 1-(4-(4-methoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)ethanone (2)”. M.p: 210 °C. 1H NMR (300 MHz, (DMSO-d6, δ): 1.75 (s, 3H, CH3); 4.67 (d, 2H, CH2O); 5.21–5.41 (dd, 2H, =CH2); 5.89 (m, 1H, =CH); 6.78 (d, 1H, CH-Ar); 6.90 (d, 1H, CH-Ar); 7.19 (t, 2H, CH-Ar); 7.65 (s, 1H, NH); 9.64 (s, 1H, NH); 9.83 (H, OH). 13C NMR (75 MHz, DMSO-d6) 24.43, 29.79, 48.16, 65.28, 83.51, 117.01, 118.26, 120.96, 125.84, 129.12, 132.71, 151.06, 155.00, 168.57. IR ν, sm−1: 1618, 1560, 1526 (Ar), 3112 (CH2 = C), 785 (Ar-CH), 1722, 1701 (C(O)R), 1377, 1370 (CH3), 1651 (C=C), 3242 (NH), 1314 (C-N).
Synthesis of 1-(4-(2-hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)ethanone (7) and Allyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (8)
The compounds 7 and 8 were also synthesized according to above procedure and their 1H- and 13C-NMR data are in agreement with data given in the literatureCitation73,Citation74.
Enzymes studies
CA isoenzymes (hCA I, and II) were purified by sepharose-4B-l-tyrosine-sulfanilamide affinity chromatography in a single purification stepCitation75. Sepharose-4B-l-tyrosine-sulfanilamide was prepared according to a reported methodCitation76. Thus, pH of the solution was adjusted to 8.7, using solid Tris. Then, supernatant was transferred to the previously prepared Sepharose-4B-l-tyrosine-sulphanilamide affinity columnCitation77. Subsequently, the proteins from the column were spectrophotometrically determined at 280 nmCitation78. For determination of the purity of the hCA isoenzymes, sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)Citation79, having 10 and 3% acrylamide as an eluent and packing gel, respectively, with 0.1% SDSCitation80 was performed, through which a single band was observed for each isoenzyme.
CA isoenzymes activities were determined according to the methods described by Verpoorte et al.Citation81 and the methods reported previouslyCitation82. Absorbance change at 348 nm from p-nitrophenylacetate (NPA) to p-nitrophenolate (NP) was recorded by 3 min intervals at the room temperature (25 °C) using a spectrophotometer (Shimadzu, UV-VIS Spectrophotometer, UVmini-1240, Kyoto, Japan). Quantity of the protein was measured spectrophotometrically at 595 nm during the purification steps according to the Bradford methodCitation83 as reported previously. Bovine serum albumin was used as a standard protein. An activity (%)-[Cyclic thioureas] graph was depicted to determine the inhibition effect of each cyclic thioureas. For Ki values, three different cyclic thioureas were tested. NPA was used as a substrate at five different concentrations, and Lineweaver–Burk curvesCitation84 were drawn as described previouslyCitation85.
In the third part of this study, the inhibitory effects of cyclic thioureas (1–8) on AChE and BChE activities were determined according to the Ellman test.Citation86 Acetylthiocholine iodide (AChI) or butyrylthiocholine iodide (BChI) were used as substrates for both reactions. 5,5′-Dithio-bis(2-nitro-benzoic)acid (DTNB) was used for the measurement of the AChE/BChE activities. Briefly, 100 mL of Tris/HCl buffer (1.0 M and pH 8.0), 10 mL of cyclic thioureas (1–8) solution dissolved in deionized water at different concentrations and 50 mL AChE/BChE (5.32 10–3 EU) solution were mixed and incubated for 10 min at 25 °C. Then a portion of DTNB (50 mL, 0.5 mM) was added. Subsequently, the reaction was initiated by the addition of 50 mL of AChI/BChI (10 mM). The hydrolysis of these AChI/BChI was monitored spectrophotometrically by the formation of yellow 5-thio-2-nitrobenzoate anion as the result of the reaction of DTNB with thiocholine, released by the enzymatic hydrolysis of AChI/BChI, at a wavelength of 412 nmCitation48. For determination of the effects of cyclic thioureas (1–8) on AChE/BChE, cyclic thioureas (1–8) concentrations were added into the reaction solution. AChE/BChE activities were measured, and an experiment in the absence of drug was used as control. The IC50 values were obtained from activity (%) versus cyclic thioureas (1–8) concentration plots. For determination of Ki values in the media with cyclic thioureas (1–8) as inhibitor, different ACh/BCh concentrations were used as substrates.
Determination of antioxidant activities
Fe3+ reducing ability of cyclic thioureas (1–8), Fe3+(CN−) 6 to Fe2+(CN−)6 reduction method was usedCitation87. Briefly, various concentrations of cyclic thioureas (1–8) (10–30 μg/mL) in 0.75 mL of deionized H2O were added with 1.25 mL of phosphate buffer (0.2 M, pH 6.6) and 1.25 mL of potassium ferricyanide [K3Fe(CN)6] (1%)Citation88. Then, the solution was incubated at 50 °C during 20 min. After incubation period, trichloroacetic acid was added (1.25 mL, 10%). Lastly, a portion of FeCl3 (0.5 mL, 0.1%) was transferred to this mixture and the absorbance value was enrolled at 700 nm in a spectrophotometerCitation89,Citation90. According to the obtained results, when reduction capability increases, absorbance indicates greater valueCitation91.
Cu2+ reducing capability was performed according to the previous studiesCitation92–94. For this purpose, aliquots of CuCl2 solution (0.25 mL, 0.01 M), ethanolic neocuproine solution (0.25 mL, 7.5 × 10−3 M) and NH4Ac buffer solution (0.25 mL, 1.0 M) were transferred to a test tube, which contains cyclic thioureas (1–8) at different concentrations (10–30 μg/mL). Total volume was completed with distilled H2O to 2 mL and shaken vigorously. Absorbance of samples was recorded at 450 nm after 30 minCitation95.
Metal chelating ability of cyclic thioureas (1–8) was predicted according to previous studiesCitation96–98. Fe2+-binding capacity of cyclic thioureas (1–8) was spectrophotometrically recorded at 562 nm. In brief, to a mixture of FeCl2 (0.1 mL, 0.6 mM) cyclic thioureas (1–8) were added at three different concentrations (10–20 μg/mL) in methanol (0.4 mL). The reactions were started by Ferrozine (3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid sodium salt) solution addition (0.1 mL, 5 mM). After that, the solution was mixed and incubated at room temperature for 10 min. Finally, absorbance value of the mixture was quantified spectrophotometrically at 562 nm versus blank sampleCitation99,Citation100.
DPPH· radical scavenging activity was performed according to our previous studiesCitation101–103. The solution of DPPH· was prepared daily, stored in a flask coated with aluminum foil and kept in the dark at 4 °C. In brief, fresh solution of DPPH· (0.1 mM) was prepared in ethanolCitation104. Then, 1.5 mL of each cyclic thiourea (1–8) in ethanol was added an aliquot of this solution (0.5 mL) (10–30 μg/mL). These mixtures were mixed vigorously and incubated in the dark for 30 min. Finally the absorbance value was recorded at 517 nm in a spectrophotometerCitation105.
Results and discussion
The synthesis of compounds 1–8 was reported in Scheme 1. The synthesis of cyclic thioureas related to the title compounds has been reported in the literatureCitation69–73. By a similar approach, the reaction of substituted p-tolualdehyde, p-anisaldehyde, o-tolualdehyde, salicylaldehyde and benzaldehyde with methylene active compounds such as β-diketones and thiourea in the presence of TFAA led to the desired cyclic thioureas (1–8). The three-component condensation reactions occurred within 2.5–3.0 h, at 60–75 °C. The synthesized compounds were crystalline and their structures were confirmed by IR, 1H-, and 13C-NMR spectroscopy techniques and elemental analysis.
Valence vibrations of NH bond in IR spectrum of synthesized compounds (1–8) were observed in 3370–3040 cm−1 regions. Valence vibrations of the C=O bond in acetyl group are compatible with 1635–1630 cm−1 band.
Only some functional groups differ in their appearance. Carbon atoms in molecule in the Citation13C NMR spectrum of the same compounds have the following signals according to their electronic densities: 24, 29, 37, 51, 86, 117, 122, 125, 129, 132, 141, 151, 179 (C=S), 205 (C=O) ppm. It is known from the reaction mechanism that, enol form of aceto-acetic ether takes part in the reaction.
Biological activities
Here, we report the inhibition effect of cyclic thioureas (1–8) on two catalytically active hCA I, and II as well as against AChE, and BChE. Also, antioxidant activity of cyclic thioureas (1–8) was studied using four different antioxidant methods, including 1,1-diphenyl-2-picrylhydrazyl (DPPH·) radical scavenging, Cu2+ and Fe3+ reducing, and Fe2+ chelating activities. We discovered nanomolar inhibition against these metabolic enzymes. Cyclic thioureas 1, 4 and 8, which possesses a phenolic moiety in its scaffolds demonstrated effective antioxidant activities. The enzymes inhibition and antioxidant activities data of cyclic thioureas (1–8) reported here are shown in , and the following comments can be drawn from these data:
The cytosolic isoenzymes CA I, and II are examined in this study. Cytosolic hCA I isoenzyme is ubiquitously expressed in body, and available in high concentrations in blood and gastrointestinal tractCitation106. It was demonstrated that CA I is involved in retinal and cerebral edema. Also, inhibition of CA I could be a valuable tool for fighting the conditionCitation107. It is generally accepted that if Ki value of a tested compound is less than 50 μM (Ki > 50 μM), that compound is considered to be inactive against hCA ICitation108. The results presented in indicate that cyclic thioureas (1–8) had effective inhibition profile against slow cytosolic hCA I isoform, and cytosolic dominant rapid hCA II isoenzyme. The cytosolic hCA I isoenzyme was inhibited by cyclic thioureas (1–8) in low nanomolar levels, the Ki of which differed between 47.40 ± 4.43 and 77.68 ± 3.69 nM. On the other hand, acetazolamide (AZA), considered being a broad-specificity CA inhibitor owing to its widespread inhibition of CAs, showed Ki value of 289.22 ± 2.60 nM against hCA I. Among the inhibitors, 1-(4-(2-hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)ethanone (7) was found to be the best hCA I inhibitor with Ki of 47.40 ± 4.43 nM. The hCA I inhibition effects of all cyclic thioureas (1–8) were found to be the greater than that of acetazolamide, which was a clinically standard CA inhibitor.
CA II, which generally exists in red blood cells in lower concentrations in mammals, has approximately ten times higher activity compare with CA I.Citation109 The hCA II is not only a very effective catalyst for interconversion between CO2 and HCO3Citation110, it also shows some catalytic versatility, participating in several other hydrolytic processes, which presumably involve nonphysiological substratesCitation111. Against the physiologically dominant isoform hCA II, cyclic thioureas (1–8) demonstrated Kis varying from 30.63 ± 7.62 to 76.06 ± 3.15 nM (). Among which the cyclic thiourea 2 was the best hCA II inhibitor (Ki: 30.63 ± 7.62 nM). Thus, these cyclic thioureas (1–8) demonstrated high hCA II inhibition. They are probably interact with the distinct hydrophilic and hydrophobic halves of the CA II active site. The compound 2 [1-(4-(4-Methoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)ethanone] shown the most inhibition effect with Ki value of 30.63 ± 7.62 nM against cytosolic CA II. CA isoenzymes are physiological very important enzymes. Recently, very intense studies were performed on this subject.
AChE is localized at cholinergic synapses in vertebrates and regulates neurotransmission through rapid hydrolysis of the neurotransmitter ACh into choline and acetate. Cholinesterase inhibitors, including carbamates, have gained much attention since they have been successfully used in the treatment of a number of diseases involving cholinergic dysfunction. A number of different types of cholinesterase inhibitors are known and have been found to affect these enzymes in a variety of waysCitation112. In our study, cyclic thioureas (1–8) were investigated for their ability to inhibit AChE, which was the primary cholinesterase in the body. According to our data, inhibitory effects of these cyclic thioureas (1–8) revealed a significant elevation in the case of AChE. AChE inhibitors inhibit the AChE from breaking down ACh, thereby increasing both the level and duration of action of the neurotransmitter ACh. Generally, these compounds showed higher inhibition and higher lipophilicity. Considering the results, all cyclic thioureas (1–8) expressed significantly higher inhibition activity. All of cyclic thioureas (1–8) derivatives had significantly higher AChE inhibitory activity than that of standard AChE inhibitors such as Tacrine. Furthermore, the Ki values of cyclic thioureas (1–8) and standard compound (tacrine) are summarized in . As can be seen in the results obtained from , AChE was effectively inhibited by cyclic thioureas (1–8), with Ki values in the range of 6.11 ± 9.32 to 16.13 ± 56.1 nM. However, all of cyclic thioureas (1–8) had almost similar inhibition profiles. The most active one is 1-(4-(2-hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)ethanone (7) and showed a Ki value of 6.11 ± 9.32 nM. These results clearly indicate that newly synthesized compounds as well as future similar derivatives may function as drugs for the treatment of AD.
BChE is the major ACh hydrolyzing enzyme in peripheral mammalian systems. It can either reside in the circulation or adhere to cells and tissues and protect them from anticholinesterases, including insecticides and poisonous nerve gases. Some cholinesterase inhibitors such as rivastigmine and phenserine readily cross the blood-brain barrier to inhibit cholinesterases in the central nervous system, leading to improved cognition in dementiasCitation113. It is well known phenothiazines are known to inhibit cholinesterases, especially BChECitation114. In the present study, cyclic thioureas (1–8) inhibited BChE in ranging of 6.76 ± 0.59–22.44 ± 5.71 nM. Cyclic thiourea (6) was the best BChE inhibitor (Ki: 6.76 ± 0.59 nM). However, all cyclic thioureas (1–8) demonstrated higher BChE inhibition activity than that of Tacrine (Ki: 22.49 ± 7.59 nM).
Reducing capability of cyclic thioureas (1–8) was evaluated by reduction of Fe4[(CN)6]3 to Fe4[(CN)6]2Citation115. In this technique, the presence of reductants would result in the reduction of ferric ions (Fe3+) to ferrous ions (Fe2+)Citation116. Addition of free Fe3+ to the reduced molecule brings about the formation of intensive Perl’s Prussian blue complex, Fe4[Fe(CN−)6]3, which has a strong absorbance at 700 nmCitation117. Fe3+ reducing assay gets advantage of an electron chain reaction where a ferric salt is utilized as an oxidantCitation118. In addition, the yellow color of the tested mixture changes into diverse tons of green and blue by the ability of the compounds. However, the effective reducing ability was only demonstrated by cyclic thioureas, which possesses phenolic hydroxyl group in their scaffold (1, 4 and 5). Cu2+ reducing capability of cyclic thioureas (1–8) and positive controls at the same concentration (30 μg/mL) showed the following order: BHA (2.046 ± 0.039; r2: 0.9722) >α-Tocopherol (1.715 ± 0.014; r2: 0.9981) > BHT (1.398 ± 0.007; r2: 0.9718) > Trolox (1.266 ± 0.011; r2: 0.9828) > 1 (0.816 ± 0.006; r2: 0.9683) ≈ 5 (0.806 ± 0.014; r2: 0.9938) > 4 (0.671 ± 0.009; r2: 0.9877) ().
The CUPRAC method is a rapid, simple, cost-effective, selective, steady and versatile antioxidant assay useful for a wide variety of phenolic compounds. CUPRAC reactions are essentially complete within 30 min. Cu2+ reducing power of cyclic thioureas (1–8) and positive controls are shown in . A positive relationship was found between Cu2+ reducing power and different concentration of cyclic thioureas (1–8). It was detected that Cu2+ reducing capacity of these compounds was addicted to different concentration (10–30 μg/mL). Cu2+ reducing capability of cyclic thioureas (1–8) and positive controls at the same concentration (30 μg/mL) showed the following order: BHA (2.126 ± 0.004; r2: 0.9870) > BHT (1.709 ± 0.003; r2: 0.9822) > α-Tocopherol (1.202 ± 0.009; r2: 0.9940) ≈ Trolox (1.177 ± 0.014; r2: 0.9917) > 4 (0.768 ± 0.017; r2: 0.9921) > 1 (0.507 ± 0.011; r2: 0.9918) ≈ 5 (0.479 ± 0.003; r2: 0.9787). There was a positive control between Fe3+ and Cu2+ reducing abilities.
DPPH test is generally used as the substrate to gauge free radical scavenging effectiveness of antioxidant moleculesCitation119. It is based on the reduction of a DPPH solution in alcohol in the source of a hydrogen-donating antioxidant, owing to the formation of non-radical form DPPH-H by the reactionCitation120. Cyclic thioureas (1–8) have the ability to reduce steady radical DPPH to yellow-colored DPPH-H. defines a crucial decrement (p < 0.01) in the concentration of DPPH radical owing to the scavenging capability of cyclic thioureas (1–8) and reference radical scavenging agents like Trolox, α-Tocopherol, BHT and BHA. IC50 values were found as 21.65 μg/mL (0.9686) for trolox, 28.87 μg/mL (0.9924) for α-Tocopherol, 30.13 μg/mL (0.9622) for BHA, 40.76 μg/mL (0.9881) for BHT, 69.30 μg/mL (0.9760) for 4, 76.15 μg/mL (0.9938) for 5, and 86.62 μg/mL (0.9743) for 1. A lower EC50value showed a higher DPPH radical scavenging activityCitation121. DPPH exposed an absorbance at 517 nm, which vanished after acceptation of an electron or hydrogen radical from an antioxidant compound to become a steadier diamagnetic moleculeCitation122.
On the other hand, cyclic thioureas (1–8) had also effective Fe2+ ions chelating effect. The distinction between different concentrations of cyclic thioureas (1–8) (10–30 μg/mL) and the control value was fixed to be statistically important (p < 0.01). Furthermore, it is found that IC50 values for compounds (1–8) varied between 33.02–100.01 μg/mL (). Whereas, IC50 values belonging to Fe2+ ions chelating capacity of positive controls like BHT, BHA, α-Tocopherol, Trolox, and was found in ranging from 11.01 μg/mL to 31.50 μg/mL. A lower EC50 value reflects a higher Fe2+ ions binding activity. These results clearly introduce that Fe2+ ions chelating effect of cyclic thioureas (1–8) was close to trolox, α-tocopherol, BHA and BHT. Fe2+ ions are the most efficient pro-oxidants in pharmacology systems and food. Ferrozine can create complexes with Fe2+. In the presence of Fe2+ chelating compounds, Ferrozine-Fe2+ complex formation is a broken down, resulting in a decrease in the red color of Ferrozine-Fe2+ complexCitation123.
Table 1. Human carbonic anhydrase isoenzymes I and II, AChE and BChE enzymes inhibition values of compounds 1–8.
Table 2. Fe3 + and Cu2 + reducing abilities of cyclic thioureas 1–8.
Table 3. 1,1-Diphenyl-2-picrylhydrazyl (DPPH•) radical scavenging, and ferrous ion (Fe2+) chelating activities of cyclic thioureas (1–8).
Conclusion
The cyclic thioureas (1–8) used in the present study demonstrated effective inhibition profiles against hCA isoforms, AChE and BChE enzymes. Additionally, these compounds demonstrated effective antioxidant activities using by different bioassays. These compounds identified their potential CA isoenzymes, and AChE and BChE enzyme inhibitors. In this study, nanomolar level of Ki values was observed for all cyclic thioureas (1–8) and these compounds can be selective inhibitor of both cytosolic CA I and II isoenzymes and AChE and BChE enzymes. Also, they can be used as novel antioxidants in applications including pharmaceutical industry.
Declaration of interest
The authors have declared no conflict of interest.
IG and SA would like to extend his sincere appreciation to the Research Chairs Program at King Saud University for funding this research.
References
- Liu X, Lu M, Lu T. An efficient protocol for the synthesis of 3,4-dihydropyrimidine-2-(1H)-ones catalyzed by functionalized ionic liquid [DDPA][HSO4]. Chiang Mai J Sci 2011;38:263–9. [Patternmatch]
- Atwal KS, Rovnyak GC, O’Reilly BC, et al. 4-dihydropyrimidines. Synthesis of selectively functionalized 2-hetero-1,4- dihydropyrimidines. J Org Chem 1989;54:5898–907
- Akdemir A, Güzel-Akdemir O, Karalı N, Supuran CT. Isatin analogs as novel inhibitors of Candida spp. β-carbonic anhydrase enzymes. Bioorg Med Chem 2016;24:1648–52
- Artunç T, Çetinkaya Y, Göçer H, et al. Synthesis of 4-[2-(3,4-dimethoxybenzyl)cyclopentyl]-1,2-dimethoxybenzene derivatives and evaluations of their carbonic anhydrase isoenzymes inhibitory effects. Chem Biol Drug Des 2016;87:594–607
- Taslimi P, Gülçin İ, Öztaşkın N, et al. The effects of some bromophenols on human carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2016;31:603–7
- Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat 2013;23:681–91
- Del Prete S, De Luca V, Supuran CT, Capasso C. Protonography, a technique applicable for the analysis of η-carbonic anhydrase activity. J Enzyme Inhib Med Chem 2015;30:920–4
- Gül Hİ, Kucukoglu K, Yamali C, et al. Synthesis of 4-(2-substituted hydrazinyl)benzenesulfonamides and their carbonic anhydrase inhibitory effects. J Enzyme Inhib Med Chem 2016;31:568–73
- Del Prete S, Vullo D, De Luca V, et al. Comparison of the sulfonamide inhibition profiles of the α-, β- and γ-carbonic anhydrases from the pathogenic bacterium. Bioorg Med Chem Lett 2016;26:1941–6
- Gocer H, Aslan A, Gülçin İ, Supuran CT. Spirobisnaphthalenes effectively inhibit carbonic anhydrase. J Enzyme Inhib Med Chem 2016;31:503–7
- Capasso C, Supuran CT. Anti-infective carbonic anhydrase inhibitors: a patent and literature review. Expert Opin Ther Pat 2013;23:693–704
- Gocer H, Topal F, Topal M, et al. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J Enzyme Inhib Med Chem 2016;31:441–7
- Taslimi P, Gulcin İ, Ozgeris B, et al. The human carbonic anhydrase isoenzymes I and II (hCA I and II) inhibition effects of trimethoxyindane derivatives. J Enzyme Inhib Med Chem 2016;31:152–7
- Özgeris B, Göksu S, Polat Köse L, et al. Acetylcholinesterase and carbonic anhydrase inhibitory properties of novel urea and sulfamide derivatives incorporating dopaminergic 2-aminotetralin scaffolds. Bioorg Med Chem 2016;24:2318–29
- Ceruso M, Antel S, Scozzafava A, Supuran CT. Synthesis and inhibition potency of novel ureido benzenesulfonamides incorporating GABA as tumor-associated carbonic anhydrase IX and XII inhibitors. J Enzyme Inhib Med Chem 2016;31:205–11
- Scozzafava A, Kalın P, Supuran CT, et al. The impact of hydroquinone on acetylcholine esterase and certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:941–6
- Akıncıoğlu A, Akıncıoğlu H, Gülçin I, et al. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg Med Chem 2015;23:3592–602
- Aksu K, Nar M, Tanç M, et al. Synthesis and carbonic anhydrase inhibitory properties of sulfamides structurally related to dopamine. Bioorg Med Chem 2013;21:2925–31
- Yıldırım A, Atmaca U, Keskin A, et al. N-Acylsulfonamides strongly inhibit human carbonic anhydrase isoenzymes I and II. Bioorg Med Chem 2015;23:2598–605
- Ameis HM, Drenckhan A, Freytag M, et al. Carbonic anhydrase IX correlates with survival and is a potential therapeutic target for neuroblastoma. J Enzyme Inhib Med Chem 2016;31:404–9
- Boztaş M, Çetinkaya Y, Topal M, et al. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxy-bromophenol derivatives incorporating cyclopropane moieties. J Med Chem 2015;58:640–50
- De Simone G, Alterio V, Supuran CT. Exploiting the hydrophobic and hydrophilic binding sites for designing carbonic anhydrase inhibitors. Expert Opin Drug Disc 2013;8:793–810
- Scozzafava A, Passaponti M, Supuran CT, Gülçin İ. Carbonic anhydrase inhibitors: guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:586–91
- Göçer H, Akıncıoğlu A, Göksu S, et al. Carbonic anhydrase and acetylcholinesterase inhibitory effects of carbamates and sulfamoylcarbamates. J Enzyme Inhib Med Chem 2015;30:316–20
- Supuran CT. Carbonic anhydrases. Bioorg Med Chem 2013;21:1377–8
- Arabaci B, Gülçin İ, Alwasel S. Capsaicin: a potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2015;19:10103–14
- Del Prete S, Vullo D, Fisher GM, et al. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum-the η-carbonic anhydrases. Bioorg Med Chem Lett 2014;24:4389–96
- Akbaba Y, Bastem E, Topal F, et al. Synthesis and carbonic anhydrase inhibitory effects of novel sulfamides derived from 1-aminoindanes and anilines. Arch Pharm (Weinheim) 2014;347:950–7
- Göksu S, Naderi A, Akbaba Y, et al. Carbonic anhydrase inhibitory properties of novel benzylsulfamides using molecular modeling and experimental studies. Bioorg Chem 2014;56:75–82
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Supuran CT, Capasso C. The η-class carbonic anhydrases as drug targets for antimalarial agents. Expert Opin Ther Targets 2015;19:551–63
- Güney M, Coşkun A, Topal F, et al. Oxidation of cyanobenzocycloheptatrienes: synthesis, photooxygenation reaction and carbonic anhydrase isoenzymes inhibition properties of some new benzotropone derivatives. Bioorg Med Chem 2014;22:3537–43
- Topal M, Gülçin İ. Rosmarinic acid: a potent carbonic anhydrase isoenzymes inhibitor. Turk J Chem 2014;38:894–902
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60
- Çetinkaya Y, Göçer H, Gülçin İ, Menzek A. Synthesis and carbonic anhydrase isoenzymes inhibitory effects of brominated diphenylmethanone and its derivatives. Arch Pharm (Weinheim) 2014;347:354–9
- Bozdag M, Carta F, Vullo D, et al. Dithiocarbamates with potent inhibitory activity against the Saccharomyces cerevisiae β-carbonic anhydrase. J Enzyme Inhib Med Chem 2016;31:132–6
- Akıncıoğlu A, Topal M, Gülçin İ, Göksu S. Novel sulfamides and sulfonamides incorporating tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch Pharm 2014;347:68–76
- Çetinkaya Y, Göçer H, Göksu S, Gülçin İ. Synthesis and carbonic anhydrase isoenzymes I and II inhibitory effects of novel benzylamine derivatives. J Enzyme Inhib Med Chem 2014;29:168–74
- Topal M, Gocer H, Topal F, et al. Antioxidant, antiradical and anticholinergic properties of cynarin purified from the illyrian thistle (Onopordum illyricum L.). J Enzyme Inhib Med Chem 2016;31:266–75
- Polat Köse L, Gülçin İ, Gören AC, et al. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind Crops Prod 2015;74:712–21
- Öztaşkın N, Çetinkaya Y, Taslimi P, et al. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg Chem 2015;60:49–57
- Gülçin İ, Scozzafava A, Supuran CT, et al. The effect of caffeic acid phenethyl ester (CAPE) metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, glutathione s-transferase, lactoperoxidase and carbonic anhydrase ısoenzymes I, II, IX and XII. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. doi: http://dx.doi.org/10.3109/14756366.2015.1094470
- Gülçin İ, Scozzafava A, Supuran CT, et al. Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase, and carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. doi: https://doi.org/http://dx.doi.org/10.3109/14756366.2015.1135914
- Yılmaz S, Akbaba Y, Özgeriş B, et al. Synthesis and inhibitory properties of some carbamates on carbonic anhydrase and acetylcholine esterase. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: https://doi.org/http://dx.doi.org/10.3109/14756366.2016.1149477
- Zhou G, Wang F, Wang H, et al. Colorimetric and fluorometric assays based on conjugated polydiacetylene supramolecules for screening acetylcholinesterase and its inhibitors. ACS Appl Mater Interfaces 2013;5:3275–80
- Sujayev A, Garibov E, Taslimi P, et al. Synthesis of some tetrahydropyrimidine-5-carboxylates, determination of their metal chelating effects and inhibition profiles against acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: http://dx.https://doi.org/doi:10.3109/14756366.2016.1156104
- Aksu K, Topal F, Gulcin İ, et al. Acetylcholinesterase inhibitory and antioxidant activities of novel symmetric sulfamides derived from phenethylamines. Arch Pharm 2015;348:446–55
- Mao F, Li J, Wei H, et al. Tacrine–propargylamine derivatives with improved acetylcholinesterase inhibitory activity and lower hepatotoxicity as a potential lead compound for the treatment of Alzheimer’s disease. J Enzyme Inhib Med Chem 2015;30:995–1001
- Göçer H, Akıncıoğlu A, Öztaşkın N, et al. Synthesis, antioxidant, and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine-related compounds. Arch Pharm (Weinheim) 2013;346:783–92
- Gülçin İ. Antioxidant properties of resveratrol: a structure-activity insight. Innov Food Sci Emerg 2010;11:210–18
- Gülçin İ. Antioxidant activity of L-adrenaline: a structure-activity insight. Chem Biol Interact 2009;179:71–80
- Köksal E, Gülçin İ, Öztürk Sarıkaya SB, Bursal E. On the in vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem 2009;24:395–405
- Çakmakçı S, Topdaş EF, Kalın P, et al. Antioxidant capacity and functionality of oleaster (Elaeagnus angustifolia L.) flour and crust in a new kind of fruity ice cream. Int J Food Sci Technol 2015;50:472–81
- Sehitoglu MH, Han H, Kalin P, et al. Pistachio (Pistacia vera L.) gum: a potent inhibitor of reactive oxygen species. J Enzyme Inhib Med Chem 2015;30:264–9
- Kalın P, Gülçin İ, Gören AC. Antioxidant activity and polyphenol content of Vaccinium macrocarpon. Rec Nat Prod 2015;9:496–502
- Topal F, Topal M, Gocer H. Antioxidant activity of taxifolin: an activity-structure relationship. J Enzyme Inhib Med Chem 2016;31:674–83
- Işık M, Korkmaz M, Bursal E, et al. Determination of antioxidant properties of Gypsophila bitlisensis. Int J Pharmacol 2015;11:366–71
- Bursal E, Köksal E, Gülçin İ, et al. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC-MS/MS. Food Res Int 2013;51:66–74
- Gülçin İ, Elmastaş M, Aboul-Enein HY. Antioxidant activity of clove oil-A powerful antioxidant source. Arab J Chem 2012;5:489–99
- Gülçin İ. Antioxidant activity of food constituents: an overview. Arch Toxicol 2012;86:345–91
- Çetinkaya Y, Göçer H, Menzek A, Gülçin İ. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch Pharm (Weinheim) 2012;345:323–34
- Gülçin İ, Beydemir S, Topal F, et al. Apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst. and kit. J Enzyme Inhib Med Chem 2012;27:587–94
- Göçer H, Gülçin İ. Caffeic acid phenethyl ester (CAPE): correlation of structure and antioxidant properties. Int J Food Sci Nutr 2011;62:821–5
- Bursal E, Gülçin İ. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int 2011;44:1482–9
- Gülçin İ, Topal F, Çakmakçı R, et al. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J Food Sci 2011;76:C585–93
- Gülçin İ, Topal F, Oztürk Sarikaya SB, et al. Polyphenol contents and antioxidant properties of medlar (Mespilus germanica L.). Rec Nat Prod 2011;5:158–75
- Köksal E, Bursal E, Dikici E, et al. Antioxidant activity of Melissa officinalis leaves. J Med Plants Res 2011;5:217–22
- Brands M, Endermann R, Gahlmann R, et al. Dihydropyrimidinones a new class of anti-staphylococcal antibiotics. Bioorg Med Chem Lett 2003;13:241–5
- Wang DC, Guo HM, Qu GR. Efficient, green, solvent-free synthesis of 3,4-dihydropyrimidin-2(1h)-ones via biginelli reaction catalyzed by Cu(NO3)2·3H2O. Synthetic Commun 2010;40:1115–22
- Hussein WM, Fatahala SS, Mohamed ZM, et al. Synthesis and kinetic testing of tetrahydropyrimidine-2-thione and pyrrole derivatives as inhibitors of the metallo-β-lactamase from Klebsiella pneumonia and Pseudomonas aeruginosa. Chem Biol Drug Des 2012;80:500–5
- Li N, Chen XH, Song J, et al. Highly enantioselective organocatalytic biginelli and biginelli-like condensations: reversal of the stereochemistry by tuning the 3,3′-disubstituents of phosphoric acids. J Am Chem Soc 2009;131:15301–10
- Ahmed B, Khan RA, Habibullah Keshari M. An improved synthesis of biginelli-type compounds via phase-transfer catalysis. Tetrahedron Lett 2009;50:2889–92
- Liu J, Wu F, Chen L, et al. Evaluation of dihydropyrimidin-(2H)-one analogues and rhodanine derivatives as tyrosinase inhibitors. Bioorg Med Chem Lett 2011;21:2376–9
- Lacotte P, Puente C, Ambroise Y. Synthesis and evaluation of 3,4-dihydropyrimidin-2(1h)-ones as sodium iodide symporter inhibitors. Chem Med Chem 2013;8:104–11
- Atasever A, Özdemir H, Gülçin İ, Küfrevioğlu Öİ. One-step purification of lactoperoxidase from bovine milk by affinity chromatography. Food Chem 2013;136:864–70
- Şentürk M, Gülçin İ, Beydemir Ş, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9
- Öztürk Sarıkaya SB, Gülçin İ, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des 2010;75:515–20
- Gülçin İ, Küfrevioğlu Öİ, Oktay M. Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibitory effects of some chemicals on enzyme activity. J Enzyme Inhib Med Chem 2005;20:297–302
- Aydin B, Gülcin I, Alwasel SH. Purification and characterization of polyphenol oxidase from Hemşin apple (Malus communis L.). Int J Food Propert 2015;18:2735–45
- Ozturk Sarikaya SB, Sisecioglu M, Cankaya M, et al. Inhibition profile of a series of phenolic acids on bovine lactoperoxidase enzyme. J Enzyme Inhib Med Chem 2015;30:479–83
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9
- Akbaba Y, Akıncıoğlu A, Göçer H, et al. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J Enzyme Inhib Med Chem 2014;29:35–42
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66
- Nar M, Çetinkaya Y, Gülçin İ, Menzek A. (3,4-Dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives as carbonic anhydrase isoenzymes inhibitors. J Enzyme Inhib Med Chem 2013;28:402–6
- Ellman GL, Courtney KD, Andres V, Featherston RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95
- Gülçin İ, Huyut Z, Elmastaş M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem 2010;3:43–53
- Gülçin İ, Bursal E, Şehitoğlu HM, et al. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol 2010;48:2227–38
- Gülçin İ. Antioxidant activity of eugenol: a structure-activity relationship study. J Med Food 2011;14:975–85
- Gülçin İ, Kirecci E, Akkemik E, et al. Antioxidant and antimicrobial activities of an aquatic plant: duckweed (Lemna minor L.). Turk J Biol 2010;34:175–88
- Şerbetçi Tohma H, Gülçin İ. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int J Food Propert 2010;13:657–71
- Balaydın HT, Gülçin İ, Menzek A, et al. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J Enzyme Inhib Med Chem 2010;25:685–95
- Gülçin İ, Elias R, Gepdiremen A, et al. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: Cepharanthine and fangchinoline. J Enzyme Inhib Med Chem 2010;25:44–53
- Talaz O, Gülçin İ, Göksu S, Saracoglu N. Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorg Med Chem 2009;17:6583–9
- Gülçin İ, Elias R, Gepdiremen A, et al. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.). Wood Sci Technol 2009;43:195–212
- Ak T, Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174:27–37
- Köksal E, Gülçin İ. Antioxidant activity of cauliflower (Brassica oleracea L.). Turk J Agric For 2008;32:65–78
- Gülçin İ, Oktay M, Köksal E, et al. Antioxidant and radical scavenging activities of uric acid. Asian J Chem 2008;20:2079–90
- Gülçin İ, Tel AZ, Kirecci E. Antioxidant, antimicrobial, antifungal and antiradical activities of Cyclotrichium niveum (Boiss.) Manden and Scheng. Int J Food Propert 2008;11:450–71
- Gülçin İ. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J Enzyme Inhib Med Chem 2008;23:871–6
- Gülçin İ, Elmastas M, Aboul-Enein HY. Determination of antioxidant and radical scavenging activity of basil (Ocimum basilicum) assayed by different methodologies. Phytother Res 2007;21:354–61
- Gülçin İ. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 2007;32:431–8
- Gülçin İ, Elias R, Gepdiremen A, Boyer L. Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur Food Res Technol 2006;223:759–67
- Elmastaş M, Gülçin İ, Beydemir Ş, et al. A study on the in vitro antioxidant activity of juniper (Juniperus communis L.) seeds extracts. Anal Lett 2006;39:47–65
- Elmastas M, Türkekul İ, Öztürk L, et al. The antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta). Comb Chem High Thr Scr 2006;9:443–8
- Öztürk Sarıkaya SB, Topal F, Şentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62
- Innocenti A, Gülçin İ, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Antioxidant polyphenol natural products effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–3
- Innocenti A, Öztürk Sarıkaya SB, Gülçin İ, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–64
- Şentürk M, Gülçin İ, Daştan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11
- Çoban TA, Beydemir Ş, Gülçin İ, Ekinci D. The effect of ethanol on erythrocyte carbonic anhydrase isoenzymes activity: an in vitro and in vivo study. J Enzyme Inhib Med Chem 2008;23:266–70
- Çoban TA, Beydemir Ş, Gülçin İ, Ekinci D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol Pharm Bull 2007;30:2257–61
- Darvesh S, Darvesh KV, McDonald RS, et al. Carbamates with differential mechanism of inhibition toward acetylcholinesterase and butyrylcholinesterase. J Med Chem 2008;51:4200–12
- Debord J, Merle L, Bollinger JC, Dantoine T. Inhibition of butyrylcholinesterase by phenothiazine derivatives. J Enzyme Inhib Med Chem 2002;17:197–202
- Gülçin İ, Daştan A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J Enzyme Inhib Med Chem 2007;22:685–95
- Gülçin İ, Elias R, Gepdiremen A, et al. A comparative study on the antioxidant activity of fringe tree (Chionanthus virginicus L.) extracts. Afr J Biotechnol 2007;6:410–8
- Gülçin İ. Antioxidant and antiradical activities of L-carnitine. Life Sci 2006;78:803–11
- Koksal E, Bursal E, Gulcin İ, et al. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by LC-MS/MS. Int J Food Propert. [Epub ahead of print]. doi: http://dx.doi.org/10.1080/10942912.2016.1168438
- Gülçin İ, Beydemir Ş, Şat İG, Küfrevioğlu Öİ. Evaluation of antioxidant activity of cornelian cherry (Cornus mas L.). Acta Aliment Hung 2005;34:193–202
- Gülçin İ, Alici HA, Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull 2005;53:281–5
- Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med 2004;70:561–3
- Gülçin İ, Küfrevioğlu Öİ, Oktay M, Büyükokuroğlu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol 2004;90:205–15
- Gülçin İ, Şat İG, Beydemir Ş, et al. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem 2004;87:393–400
- Gülçin İ, Şat İG, Beydemir Ş, Küfrevioğlu Öİ. Evaluation of the in vitro antioxidant properties of extracts of broccoli (Brassica oleracea L.). Ital J Food Sci 2004;16:17–30