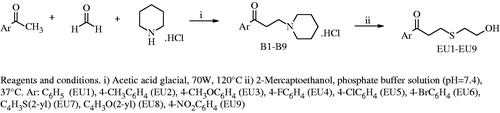
Abstract
A series of Mannich bases having piperidine moiety were reacted with 2-mercaptoethanol, leading to 1-aryl-3-piperidine-4-yl-1-propanone hydrochlorides. The cytotoxicity and carbonic anhydrase inhibitory activities of these new compounds were evaluated. Among the compounds, only one derivative, nitro substituent bearing EU9, showed an effective cytotoxicity, although weak tumor specificity against human oral malignant versus nonmalignant cells. The compound induced apoptosis in HSC-2 oral squamous cell carcinoma cells, but not in human gingival fibroblast. Chemical modifications of this lead are thus necessary to further investigate it as a drug candidate and to obtain compounds with a better activity profile.
Introduction
Cancer is the second cause of death after cardiovascular disorders. Although a great amount of improvements have been made in cancer chemotherapy, there is still need for new selective cytotoxic anticancer agents because of the problems available against the drugs that are on market such as gained resistance, low selectivity and stability. Mannich bases are an important group of compounds in medicinal chemistry and they may be synthesized by applying the reaction discovered by MannichCitation1–5. These compounds have a wide range of biological activities such as carbonic anhydrase (CA, EC 4.2.1.1) inhibitoryCitation1–3, cytotoxicCitation2–7, anti-inflammatoryCitation8 and anticonvulsant activitiesCitation9,10. The reported mechanism action of the Mannich bases are based on thiol alkylationCitation11–14, interaction with enzymes that are important for antioxidant mechanismsCitation7, inhibition of mitochondrial respirationCitation15,16, inhibition of topoisomerase enzymeCitation17 as well as tubulin polimerization inhibitionCitation18.
The CAs are that enzymes play important roles in physiological and pathological processesCitation19. Sixteen CA isoforms have been identified in mammals. Many CA isoforms take part in several vital biological processes such as electrolyte secretion, acid–base balance, ion transport and lipogenesis, ureagenesis and bone resorption. Inhibitors or activators of these enzymes have medical applications such as diuretics, in the treatment of glaucoma, neurological disorders including epilepsy and antitumor drugs targeting hypoxic tumors that overexpress some CA isoforms (e.g. CA IX and XII)Citation19–25.
The aim of this study was to synthesize 1-aryl-3-(2-hydroxyethylthio)-1-propanones starting from mono Mannich bases (1-aryl-3-piperidine-4-yl-1-propanone hydrochlorides) and to investigate their cytotoxic and CA inhibitory activities against human (h) isoforms hCA I and II.
Materials and methods
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were taken using a Varian spectrometer (Danbury, CT). Chemical shifts (δ) were reported in ppm. Melting points were determined using an Electrothermal 9100 (IA9100, Bibby Scientific Limited, Staffordshire, UK) instrument and are uncorrected. Mass spectra was taken using a MS (ESI–MS) VG Waters Micromass ZQ (Waters Corporation, Milford, MA). All reactions were carried out in CEM Discover Microwave Synthesis Systems (CEM, Matthews, NC).
Chemistry
Synthesis of 1-aryl-3-piperidin-4-yl-1-propanone hydrochlorides (B1–B9)
A mixture of the appropriate ketone, paraformaldehyde and piperidine hydrochloride in acetic acid (10 mL) was heated in microwave oven at 70 Watt and 120 °C for 20–65 min (Scheme 1). Reactions were monitored by thin-layer chromatography (TLC) using CHCl3:CH3OH (8:2 or 9:1) solvent system. When the reaction finished, reaction solvent was removed under vacuum and the crude solid was crystallized from suitable solvent such as CH3OH, CHCl3/CH3OH or CH3OH/Et2O to obtain B1–B9. Chemical structure of the compounds were confirmed by 1H NMR and melting points reported (data were not shown since they are reported in literatures).
Synthesis of 1-aryl-3-(2-hydroxyethylthio)-1-propanones (EU1-EU9, Scheme 1)
A mixture of 2-mercaptoethanol and a suitable compound of 1-aryl-3-piperidin-4-yl-1-propanone hydrochloride (B1–B9) in a phosphate buffer solution (5 mL, pH = 7.4) was shaken at 37 °C. Reactions were monitored by TLC. When the reaction was stopped, reaction content was extracted with CHCl3 (3 × 10 mL) and then with distilled water (3 × 10 mL). Organic phase was dried on anhydrous sodium sulfate. Solvent was removed under vacuum. Crude compounds were purified by column chromatography on silica gel 60 (70–230 mesh) using ethylacetate:hexane (8:2) solvent system as a mobile phase to obtain a suitable compound of EU.
During the synthesis of EU2, EU3, EU5, EU8 and EU9 Mannich bases used (B2, B3, B5, B8 and B9) did not consumed in the reaction medium, although reactions were continued for 77–96 h. The compounds EU2, EU3, EU5 and EU6 were solid, while the compounds EU1, EU7, EU8 and EU9 were viscous liquid. EU4 was solid at +4 °C, while it was viscous liquid at room temperature. Experimental and spectral details of EU1–EU9 are presented in and , respectively.
Table 1. Experimental data of the compounds EU1–EU9.
Table 2. Spectral data of the compounds EU1–EU9.
Biological activity
Cytotoxicity assay
The compounds were assayed toward human oral squamous cell carcinoma cell lines (HSC-2, HSC-3, HSC-4), human promyelocytic leukemic cell line (HL-60) and human oral normal mesenchymal cells (gingival fibroblast (HGF), pulp cells (HPC) and periodontal ligament fibroblast (HPLF)) based on a literature procedure with some minor modificationsCitation26–28. In brief, cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) except the HL-60 cells that were cultured in RPMI 1640 medium supplemented with 10% FBS. Varying concentrations of the compound in dimethylsulfoxide were added to the medium and incubated at 37 °C for 48 h. The viable cell numbers were determined by the MTT method except for HL-60 cells, the viable cell number of which was counted with a hemocytometer after staining with 0.15% trypan blue. The 50% cytotoxic concentration (CC50) value was determined from the growth curves plotted at different concentrations of each compounds in triplicate wells. Calculation of tumor-specificity (TS) index: The TS value was calculated by dividing the mean CC50 value of each compound against normal cells to mean CC50 value against OSCC.
Immunoblot analysis
Primary antibodies against cPARP were purchased from Cell Signaling Technology (Danvers, MA), and the primary antibody against actin was purchased from Sigma-Aldrich (St. Louis, MO). The horseradish peroxidase-conjugated secondary anti-mouse immunoglobulin G (IgG) and anti-rabbit IgG antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The HSC-2 and HGF cells were cultured in six well plates for 24 h and then incubated with the compound for 24 h. The cells were scraped with a rubber policeman and collected in 10 × cell lysis buffer (Cell Signaling Technology, Beverly, MA) supplemented with 1 mM phenylmethanesulfonyl fluoride plus one tablet of protease inhibitor cocktail (Complete, EDTA-free; Roche Diagnostics GmbH, Mannheim, Germany). Aliquots of the lysates (50 μg protein) were subjected to SDS-polyacrylamide gel electrophoresis, followed by immunoblotting with primary antibodies against cPARP and actin (employed as a loading control) and secondary anti-IgG antibodies, as previously describedCitation29.
Carbonic anhydrase inhibition assay
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity by using the method of KhalifahCitation30. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled and deionized water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by nonlinear least-squares methods using PRISM (www.graphpad.com), and nonlinear least squares methods, values representing the mean of at least three different determinations, as described earlier by usCitation31. All enzymes used were recombinant, produced in E. coli and the cell pellets were lysed and enzyme was purified through affinity chromatography using pAMBS resin as reported earlierCitation24,32–34.
Results and discussion
In this study, the designed compounds, 1-aryl-3-(2-hydroxyethylthio)-1-propanones, were successfully synthesized by the reaction of a suitable Mannich base, 1-aryl-3-amino-1-propenone hydrochloride, with 2-mercaptoethanol in phosphate buffer solution (PBS) (pH = 7.4) at 37 °C. Chemical structures of the compounds were confirmed by 1H NMR, 13C NMR and HRMS. EU2, EU3, EU5, EU6 and EU8 were reported for the first time, while EU1Citation35, EU4 and EU7 and EU9Citation36 had been reported before. The compounds were synthesized with the yield of 19–89%. EU2, EU3, EU5 and EU6 were solid, while EU1, EU7, EU8 and EU9 were viscous liquid. EU4 was solid at +4 °C, while it was viscous liquid at room temperature.
Among the nine compounds reported here, derivatives EU1, EU2, EU3, EU4, EU7 and EU8 were cytotoxic above 400 μM toward both cancer and normal cells. Therefore, the calculation of tumor-specific (TS) value was practically impossible. EU5 and EU6 showed very weak tumor specificity (TS ≥ 1.2). EU9 showed the highest TS value (TS ≥ 1.3), and one-order higher cytotoxicity against both cancer and normal cells as compared with the other derivatives. All compounds had lower TS values than a popular anticancer drugs 5-Fluorouracil (5-FU) and Melphalan ().
Table 3. Cytotoxic activities of the compounds EU1–EU9.
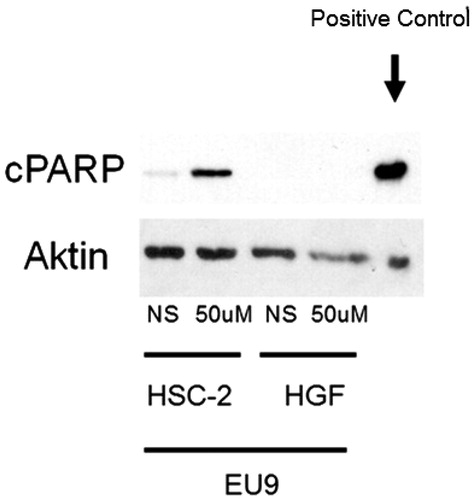
Most of the cytotoxic compounds induce apoptosisCitation37,38. The breaks in single-strand DNA may be repaired by poly(ADP-ribose)polymerase (PARP1)Citation39. This is the reason why PARP1 test was done by using the compound EU9. EU9 at 50 μM concentration (CC50) induced apoptosis (assessed by the cleavage of PARP1) in HSC-2 (OSCC), but not in HGF cells (normal cells) ().
Figure 1. Effect of the compound EU9 on HSC-2 cancer cells and HGF normal cells after. 24 h. (NS means no stimulation (control)).
All compounds inhibited hCA I (23–30%) and hCA II (25–27%) isoenzymes with similar percentages. There was not selectivity toward any of hCA isoenzymes. Inhibitions of these izoenzymes by the compounds studied were lower than the reference compound ().
Table 4. Inhibition percentages of hCA I and II by the compounds EU1–EU9.
Conclusion
Several new compounds are reported here, being obtained by reaction of a suitable Mannich base, 1-aryl-3-amino-1-propenone hydrochloride, with 2-mercaptoethanol. The 1-aryl-3-(2-hydroxyethylthio)-1-propanone investigated here showed rather low tumor-specificity although they induced apoptosis, suggesting that apoptosis-inducing activity itself does not guarantee the antitumor effects. Chemical modifications of the best compound detected here, EU9, are thus necessary to further evaluate such derivatives for their biological activity, as cytotoxic agents or CA inhibitorsCitation40–43.
Declaration of interest
This study was supported by Ataturk University (BAP Project Number: 2010/166). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
Professor Gul HI thanks Dr. Murat Sukuroglu from Gazi University for HRMS.
References
- Yamali C, Tugrak M, Gul HI, et al. The inhibitory effects of phenolic Mannich bases on carbonic anhydrase I and II isoenzymes. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.3109/14756366.2015.1126715
- Bilginer S, Unluer E, Gul HI, et al. Carbonic anhydrase inhibitors. Phenols incorporating 2- or 3-pyridyl-ethenylcarbonyl and tertiary amine moieties strongly inhibit Saccharomyces cerevisiae β-carbonic anhydrase. J Enzyme Inhib Med Chem 2014;29:495–9
- Gul HI, Yamali C, Yasa AT, et al. Carbonic anhydrase inhibition and cytotoxicity studies of Mannich base derivatives of thymol. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.3109/14756366.2016.1140755
- Gul HI, Das U, Pandit B, Li PK. Evaluation of the cytotoxicity of some mono-Mannich bases and their corresponding azine derivatives against androgen-independent prostate cancer cells. Arzneimittelforschung 2006;56:850–4
- Gul HI, Gul M, Erciyas E. Toxicity of some bis Mannich bases and corresponding piperidinols in the brine shrimp (Artemia salina) bioassay. J Appl Toxicol 2003;23:53–7
- Gul HI, Yerdelen KO, Das U, et al. Synthesis and cytotoxicity of novel 3-aryl-1-(3′-dibenzylaminomethyl-4′-hydroxyphenyl)-propenones and related compounds. Chem Pharm Bull 2008;56:1675–81
- Gul M, Mete E, Atalay M, et al. Cytotoxicity of 1-aryl-3-buthylamino-1-propanone hydrochlorides against Jurkat and L6 cells. Arzneimittelforschung 2009;59:364–9
- Suleyman H, Gul HI, Gul M, et al. Anti-inflammatory activity of bis(3-aryl-3-oxo-propyl)methylamine hydrochloride in rat. Biol Pharm Bull 2007;30:63–7
- Gul HI, Calis U, Vepsalainen J. Synthesis and evaluation of anticonvulsant activities of some bis Mannich bases and corresponding piperidinols. Arzneimittelforschung 2002;52:863–9. [Patternmatch]
- Gul HI, Calis U, Vepsalainen J. Synthesis of some mono-Mannich bases and corresponding azine derivatives and evaluation of their anticonvulsant activity. Arzneimittelforschung 2004;54:359–64
- Gul M, Atalay M, Gul HI, et al. The effects of some Mannich bases on heat shock proteins HSC70 and GRP75, and thioredoxin and glutaredoxin levels in Jurkat cells. Toxicol In Vitro 2005;19:573–80
- Gul M, Gul HI, Das U, Hanninen O. Biological evaluation and structure-activity relationships of bis-(3-aryl-3-oxo-propyl)-methylamine hydrochlorides and 4-aryl-3-arylcarbonyl-1-methyl-4-piperidinol hydrochlorides as potential cytotoxic agents and their alkylating ability towards cellular glutathione in human leukemic T cells. Arzneimittelforschung 2005;55:332–7. [Patternmatch]
- Gul M, Gul HI, Hanninen O. Effects of Mannich bases on cellular glutathione and related enzymes of Jurkat cells in culture conditions. Toxicol In Vitro 2002;16:107–12
- Gul M, Gul HI, Vepsalainen J, et al. Effect of acetophenone derived Mannich bases on cellular glutathione level in Jurkat cells. A possible mechanism of action. Arzneimittelforschung 2001;51:679–82. [Patternmatch]
- Kucukoglu K, Gul HI, Cetin-Atalay R, et al. Synthesis of new N,N'-bis[1-aryl-3-(piperidine-1-yl)propylidene]hydrazine dihydrochlorides and evaluation of their cytotoxicity against human hepatoma and breast cancer cells. J Enzyme Inhib Med Chem 2014;29:420–6
- Kucukoglu K, Gul M, Atalay M, et al. Synthesis of some Mannich bases with dimethylamine and their hydrazones and evaluation of their cytotoxicity against Jurkat cells. Arzneimittelforschung 2011;61:366–71
- Canturk P, Kucukoglu K, Topcu Z, et al. Effect of some bis Mannich bases and corresponding piperidinols on DNA topoisomerase I. Arzneimittelforschung 2008;58:686–91
- Mete E, Gul HI, Canturk P, et al. Biological activity of 1-aryl-3-phenethylamino-1-propanone hydrochlorides and 3-aroyl-4-aryl-1-phenethyl-4-piperidinols on PC-3 cells and DNA topoisomerase I enzyme. Z. Naturforsch C J Biosci 2010;65:647–52
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60
- Winum JY, Supuran CT. Recent advances in the discovery of zinc-binding motifs for the development of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2015;30:321–4
- Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors: synthesis of sulfonamides incorporating dtpa tails and of their zinc complexes with powerful topical antiglaucoma properties. Bioorg Med Chem Lett 2001;11:575–82
- Bozdag M, Carta F, Vullo D, et al. Dithiocarbamates with potent inhibitory activity against the Saccharomyces cerevisiae β-carbonic anhydrase. J Enzyme Inhib Med Chem 2016;31:132–6
- Ceruso M, Antel S, Scozzafava A, Supuran CT. Synthesis and inhibition potency of novel ureido benzenesulfonamides incorporating GABA as tumor-associated carbonic anhydrase IX and XII inhibitors. Enzyme Inhib Med Chem 2016;31:205–11
- Ameis HM, Drenckhan A, Freytag M, et al. Carbonic anhydrase IX correlates with survival and is a potential therapeutic target for neuroblastoma. J Enzyme Inhib Med Chem 2016;31:404–9
- Sakagami H, Shimada C, Kanda Y, et al. Effects of 3-styrylchromones on metabolic profiles and cell death in oral squamous cell carcinoma cells. Toxicol Rep 2015;2:1281–90
- Tugrak M, Yamali C, Sakagami H, Gul HI. Synthesis of mono Mannich bases of 2-(4-hydroxybenzylidene)-2,3-dihydroinden-1-one and evaluation of their cytotoxicities. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. doi: 10.3109/14756366.2015.1070263
- Bilginer S, Gul HI, Mete E, et al. 1-(3-aminomethyl-4-hydroxyphenyl)-3-pyridinyl-2-propen-1-ones: a novel group of tumour-selective cytotoxins. J Enzyme Inhib Med Chem 2013;28:974–80
- Umemura N, Zhu J, Mburu YK, et al. Defective NF-κB signaling in metastatic head and neck cancer cells leads to enhanced apoptosis by double-stranded RNA. Cancer Res 2012;72:45–55
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73. [Patternmatch]
- Buzás GM, Supuran CT. The history and rationale of using carbonic anhydrase inhibitors in the treatment of peptic ulcers. In memoriam Ioan Puşcaş (1932-2015). J Enzyme Inhib Med Chem 2016;31:527–33
- Ozensoy Guler O, Capasso C, Supuran CT. A magnificent enzyme superfamily: carbonic anhydrases, their purification and characterization. J Enzyme Inhib Med Chem 2016;31:689–94
- Taslimi P, Gülçin İ, Öztaşkın N, et al. The effects of some bromophenols on human carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2016;31:603–7
- Kose LP, Gülçin İ, Özdemir H, et al. The effects of some avermectins on bovine carbonic anhydrase enzyme. J Enzyme Inhib Med Chem 2016;31:773–8
- Gul HI, Ojanen T, Hanninen O. Antifungal evaluation of bis Mannich bases derived from acetophenones and their corresponding piperidinols and stability studies. Biol Pharm Bull 2002;25:1307–10
- Godwin AR, Pedone E, Choi J, et al. Conjugated biological molecules, their preparation, and novel reagents for conjugating biological molecules. PCT Int Appl 2005. WO 2005007197 A2 20050127
- Tsurusawa M, Saeki K, Fujimoto T. Differential induction of apoptosis on human lymphoblastic leukemia Nalm-6 and Molt-4 cells by various antitumor drugs. Int J Hematol 1997;66:79–88
- Gunji H, Kharbanda S, Kufe D. Induction of internucleosomal DNA fragmentation in human myeloid leukemia cells by 1-beta-D-arabinofuranosylcytosine. Cancer Res 1991;51:741–3
- Malanga M, Althaus FR. The role of poly(ADP-ribose) in the DNA damage signaling network. Biochem. Cell Biol 2005;83:354–64
- De Luca V, Del Prete S, Vullo D, et al. Expression and characterization of a recombinant psychrophilic γ-carbonic anhydrase (NcoCA) identified in the genome of the Antarctic cyanobacteria belonging to the genus Nostoc. J Enzyme Inhib Med Chem 2016;31:810–17
- Yılmaz Ö, Özbaş Turan S, Akbuğa J, et al. Synthesis of pro-apoptotic indapamide derivatives as anticancer agents. J Enzyme Inhib Med Chem 2015;30:967–80
- Vullo D, Isik S, Bozdag M, et al. 7-Amino-3,4-dihydro-1H-quinolin-2-one, a compound similar to the substituted coumarins, inhibits α-carbonic anhydrases without hydrolysis of the lactam ring. J Enzyme Inhib Med Chem 2015;30:773–7
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32