Abstract
From the aerial parts of Thymus sibthorpii Bentham (Lamiaceae), five flavonoids apigenin (1), 7-methoxy-apigenin (2), naringenin (3), eriodictyol (4) and eriodictyol-7-glucoside (5), have been isolated together with caffeic acid methyl ester (6), rosmarinic acid (7) and rosmarinic acid methyl ester (8). The structures of the isolated compounds were established by spectroscopic methods. The extracts and the isolated compounds were tested for their free radical scavenging activity using the following in vitro assays: (i) interaction with the free stable radical of DPPH (1,1-diphenyl-2-picrylhydrazyl), (ii) inhibition of linoleic acid lipid peroxidation induced by the dihydrochloric acid of 2,2-azobis-2-amidinepropane (AAPH) and (iii) the scavenging activity of enzymatically produced superoxide anion. Their inhibitory activity toward soybean lipoxygenase was evaluated in vitro, using linoleic acid as a substrate. The antioxidant results of the extracts are discussed in terms of their constitution in phenolic compounds, which were determined following the Folin–Ciocalteu method.
Introduction
The genus Thymus (family Lamiaceae) is one of the eight most important genera as regards number of species within the Lamiaceae family. It is a perennial herbaceous plant indigenous to central and southern Europe and it is now widely cultivated as a tea, spice and herbal medicineCitation1. Thyme leaf tea is said to promote rest and sleep. It is widely used in folk medicine because of its anti-inflammatory, antirheumatic, digestive, antiulcer and antimicrobial activities in Europe. Its leaf is listed in the German and British Herbal Pharmacopeia and has been used as a stomachic, carminative, diuretic, urinary disinfectant and vermifuge. Additionally, based on its traditional medicinal use, thyme is proposed for the Monograph of EMA/HMPC to use in productive cough associated with coldCitation2.
Many studies refer to a sufficient antioxidant activity of Thymus extracts, due to its high concentration of phenolic compounds. There is a correlation between the antioxidant activity and the phenolic compounds concentration of the extractsCitation3. Thymus extracts behave as superoxide and peroxyl radical scavengers on the 2,2-diphenyl-1-picrylhydrazyl-hydrate (DPPH)Citation4–6 and 2,2-azobis-2-amidinepropane (AAPH) systemsCitation3.
Ethanolic, hexane and methanolic Thymus subfraction can be considered with antioxidative properties on the above systemsCitation6. Furthermore, the Thymus methanolic extract is mentioned as a stronger radical scavenger than others and standards BHT and BHA.Citation4 The polar subfraction of methanolic extract is a stronger radical scavenger than the nonpolar subfraction. An inhibitory efficiency of the polar subfraction on linoleic acid oxidation is also measured.Citation7
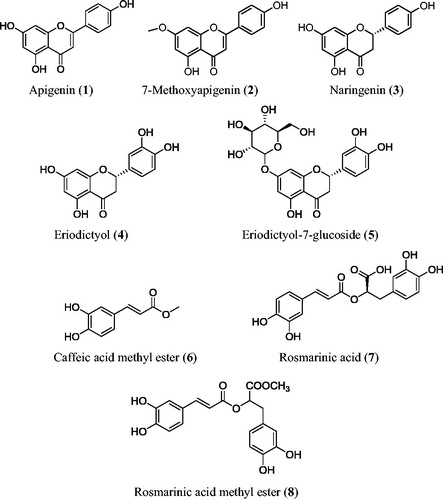
Another research was conducted to isolate the major components from the aerial parts of Thymus sibthorpii Benth – a morphologically variable species of the sect Serpyllum (Miller) BenthCitation8 and to evaluate their antioxidant and anti-inflammatory activities in vitro. Five flavonoids and three phenolic acids were isolated so far, by repeated chromatographic isolation of butanol soluble fraction. Their structures were elucidated as apigenin (1), 7-methoxy-apigenin (2), naringenin (3), eriodictyol (4) eriodictyol-7-glucoside (5), caffeic acid methyl ester (6), rosmarinic acid (7) and rosmarinic acid methyl ester (8), by the analysis of spectroscopic evidences. The group of flavonoids is long considered as antioxidant because it interferes in oxidative process with various mechanisms. Thus, flavonoids may inhibit enzymes either by suppressing free radical synthesis, by chelating element traces, by scavenging free radicals or by protecting the antioxidant defensesCitation9,Citation10.
Lipid peroxidation and biomolecular damage is a consequence of oxidative stress. Through flavonoids free radical scavenging activity, flavonoids may be considered as enzyme inhibitors of various enzymes like lipogygenaseCitation11, cyclooxygenase, xanthine oxidase, etcCitation12. Apparently, there is a correlation between structure and the type of inhibitionCitation11.
Position, structure and the total number of glycosides in a flavonoid is crucial for making a flavonoid a strong and effective antioxidant. Therefore, α-glycones give stronger antioxidants than their corresponding glycosides. As for the flavonoid chemical structure, those with a 3′-4′ catechol structure in B ring are stronger lipid peroxidation inhibitor through peroxyl, superoxide and peroxynitrite radical-scavenging activity. An unsaturated 2–3 bond in conjugation with a 4-oxo function gives strong antioxidants. Moreover, the presence of a 3-OH bond between A and B rings with the presence of 3′-4′ catechol, enhance the antioxidant activity of flavan-3-ols and flavon-3-ols whereas an O-methylation of hydroxyl groups of flavonoids decreases their radical-scavenging abilityCitation9.
The extracts, as well as the isolated compounds were tested for their free radical-scavenging activity using the following in vitro assays: (i) interaction with the free stable radical of DPPH (1,1-diphenyl-2-picrylhydrazyl), (ii) inhibition of linoleic acid lipid peroxidation with the dihydrochloric acid of AAPH and (iii) the scavenging activity of superoxide anion using the xanthine – xanthine oxidase system. Finally, their inhibitory activity toward soybean lipoxygenase was evaluated in vitro, using linoleic acid as a substrate. The antioxidant behavior of the extracts are discussed taking under consideration their constitution in phenolic compounds, which were determined with the method of Folin-Ciocalteu.
Materials and methods
Collection of plant material
Aerial parts of Thymus sibthorpii Bentham were collected when in flower from Chortiatis mountain, on June 2006. Voucher specimens have been deposited at the Herbaria of the Balkan Botanical Garden of Kroussia and the Laboratory of Pharmacognosy, University of Thessaloniki (AUTH).
Extraction and isolation
Air-dried aerial parts of the plant (381.3 g) were exhaustively extracted with petroleum ether (40–60°) (PE), dichloromethane (DM) and methanol (M). The methanol extract was concentrated and the residue redissolved in boiling water. The water-soluble fraction was filtered and extracted successively with hexane (HE), diethylether (ET), ethyl acetate (EA) and n-butanol (BU). CC of the residue of ET (3.42 g) over silica gel using DM-M mixtures followed by further purification on CC (Sephadex LH-20 using methanol as eluent) were obtained compounds 1 (2.4 mg), 2 (3.7 mg), 3 (0.4 mg), 4 (10.0 mg), 6 (4.7 mg) and 7 (87.8 mg). CC of the residue of EA (1.38 g) over silica gel using DM-M mixtures followed by further purification on CC (Sephadex LH-20 using methanol as eluent) were obtained compounds 2 (1.5 mg), 5 (5.3 mg), 7 (167.9 mg) and 8 (3.9 mg).
Chromatography
Column chromatography (CC): silica gel 60 (Merck Art. 9385), gradient elution with the solvents mixtures indicated in each case; Sephadex LH-20 (Pharmacia), elution with MeOH. TLC: Cellulose (Merck, Art. 5552). Solvents: acetic acid–water 15:85. Spray reagent: Neu reagentCitation13. Silica gel 60 F254s (Merck, Art. 5554); Solvents: mixture of CH2Cl2 with MeOH; mixture of CH2Cl2 with MeOH and water; mixture of cyclohexane with EtOAc; mixture of EtOAc with MeOH and water. Spray reagent: vanillin-H2SO4.
Spectroscopic data
UV spectra were recorded on a Hitachi U-2000 spectrophotometer, according to Mabry et al. (1970)Citation14. NMR: The 1H NMR spectra (300.0 MHz) were recorded in CD3OD using Bruker Avance 300 spectrometer. Chemical shifts are reported in δ (ppm) values relative to TMS.
Antioxidant assays
Each in vitro experiment was performed at least in triplicate, and the standard deviation of absorbance was less than 10% of the mean. In all cases, significant difference from control: *p < 0.1 **p < 0.01 (Student’s t-test).
Determination of total phenolic content
The concentration of total phenolic compounds in the fractions was determined spectrophotometrically using Folin–Ciocalteu reagent following the previously described methodCitation15 with few modifications. A certain amount (100 μl) from the stock solution of the sample was mixed with 500 μl of Folin–Ciocalteu phenol reagent and 400 μl of 7.5% sodium carbonate solution. The mixture was shaken thoroughly and incubated for 1.5 h at 30 °C protected from light. The absorbance of the blue color produced was measured with a spectrophotometer at 765 nm. The concentration of total phenolic compounds for each extract was calculated on the basis of a standard curve obtained using gallic acid as a standard (12 serial 2-fold dilutions to give arrange of 0.01–0.001 mg/ml in triplicate). Results were expressed as milligrams of gallic acid equivalent (GAE) per 100 g of dried weight.
Interaction of the extracts and the tested compounds with 1,1-diphenyl-2-picryl-hydrazyl (DPPH) stable free radical
DPPH assay test is very useful in the micromolar range, since it demands minutes to hours for both lipophilic and hydrophilic samples. Many studies have been previously reported concerning the antioxidant activity of natural substances tested with DPPHCitation16. In the presence of an antioxidant, which can donate an electron to DPPH, the purple color is typical of the free DPPH radical decays and can be followed spectrophotometrically (517 nm). This interaction indicates its radical scavenging ability in an iron-free system.
A 10 and a 20 μl sample from the stock solution of the extracts (approximately 2.5 mg in 1 ml DMSO) or 10 μl of the isolated compounds (final concentration 50 μM) were dissolved in absolute ethanol to a final volume of 1 ml in which 1 ml DPPH (0.1 mM, in absolute ethanol) was added. The reaction mixture was kept at room temperature. The optical density (OD) of the solution was measured at 517 nm, after 20 and 60 min. The optical densities of the samples in the absence of DPPH were subtracted from the corresponding OD with DPPH. The % reduction values were determined and compared to appropriate standards.Citation17
Inhibition of linoleic acid peroxidation induced by the dihydrochloric acid of 2,2-azobis-2-amidinepropane (AAPH)
This assay can be used to follow oxidative changes and to understand the contribution of each tested compound. The water soluble azo compound AAPH (2,2′-azobis-(2-amidinopropane)-dihydrochloride) is used as a free radical initiator for in vitro studies of free radical production. Production of conjugated dienehydroperoxide by the oxidation of linoleic acid sodium salt in an aqueous solution is monitored at 234 nm. This assay can be used to follow oxidative changes and to understand the contribution of each tested compound. An amount of 10 μl of the 16 mM sodium linoleate solution was added to the UV cuvette containing 0.93 ml of 0.05 M phosphate buffer, pH 7.4, prethermostated at 37 °C. The oxidation reaction was initiated at 37 °C under air by the addition of 50 μl of 40 mM AAPH solution. Oxidation was carried out in the presence of samples (extracts 10 μl or isolated compounds 10, 50, 100 μM) in the assay without antioxidant, and lipid peroxidation was measured in the presence of the same level of DMSO. The rate of oxidation at 37 °C was monitored by recording the increase in absorption at 234 nm caused by conjugated dienehydroperoxidesCitation18. Trolox has been tested as reference compoundCitation19.
Scavenging activity of superoxide anion using the xanthine–xanthine oxidase system
The superoxide anion was generated enzymatically by the xanthine–xanthine oxidase system and measured by the nitroblue tetrazolium (NBT) methodCitation20. To the reaction mixture in phosphate buffer pH 7.4 (0.1 M) containing xanthine, NBT, the tested isolated compounds (using two different concentrations: 10 μM and 100 μΜ) and xanthine oxidase (at final concentration 0.07 U/mL) were added. After incubating for 10 min at room temperature, the absorbance was recorded at 560 nm. Caffeic acid was evaluated as reference compoundCitation21.
Soybean lipoxygenase inhibition
The bioassay was performed according to a previously described procedureCitation17. All samples (extracts 10 μl or isolated compounds 10 and 100 μΜ) were initially dissolved in DMSO (approximately 2.5 mg in 1 ml DMSO for the extracts). The incubation mixture (final volume 1 ml) consisted of 10 μl or 1 μl of the test sample, 100 μl of sodium linolate (0.1 mM) and 0.2 μl of the enzyme solution (1/9 × 10−4, w/v in saline) in buffer tris pH 9. After incubation at room temperature for 3 min, the conversion of the sodium linoleate to 13-hydroperoxylinoleic acid was recorded at 234 nm and compared with an appropriate standard inhibitor (Nordihydroguaeretic acid).
Results and discussion
Phytochemical analysis
The present study was carried out on the aerial parts of Thymus sibthorpii Bentham (Lamiaceae) a taxa native of Greece, Bulgaria and TurkeyCitation22. The crude extracts of the aerial parts of T. sibthorpii Bentham afforded five flavonoids apigenin (1),Citation23 7-methoxy-apigenin (2),Citation24 naringenin (3),Citation25 eriodictyol (4)Citation26 and eriodictyol-7-glucoside (5),Citation27 together with caffeic acid methyl ester (6),Citation28 rosmarinic acid (7) and rosmarinic acid methyl ester (8)Citation29,Citation30. The structures of the isolates were given in . The spectroscopic data (UV-Vis and 1H NMR) of all the compounds are given in the Supplementary Material.
Total phenol and flavonoid content
The total polyphenol content was 5.4 mg of GAE/gr extract () gives information of the extracts total phenolic content (1.5, 3.5, 0.4 mg of GAE/g extract, respectively). The total GAE phenolic content was 141.00 ± 0.90 μg/mg (14.1%, w/w)Citation7. M (methanol) extract was the most rich in phenols followed by EA (ethyl acetate).
Table 1. Polyphenol content of different plant extracts.
Bioactivity
The present study was an attempt to isolate compounds from Thymus sibthorpii Bentham and to test them as potent antioxidants, using the DPPH, AAPH and xanthine/xanthine oxidase assays and as LOX inhibitors. All the isolated compounds are known to be widely distributed in Lamiaceae familyCitation31.
Various extracts of the plant were tested on DPPH and LOX assay. DPPH assay provides a good oxidative reagent to describe the antioxidant profile of the Thymus extracts, as well as of the isolated compounds.
All the plant extracts were tested for their antioxidant activity using the DPPH assay. The reducing activity seems to be time and concentration dependent . Methanol (M), Et2O (ET), EtOAc (EA), butanol (BU) and water (W) extracts, immediately interact with the free radical (20 min) on both concentrations. These results are correlated with the findings of , in which the total phenolics are presented. On a similar study referring to Thymus spathulifolius (Hausskn. and Velen.) the methanolic extract DPPH had an IC50 of 16.15 ± 0.5 μg/ml, which was close to the synthetic antioxidant, BHT, (19.8 ± 0.5 μg/ml).
Table 2: % Interaction of different plant extracts with DPPH.
In , the % interaction values of the isolated compounds (final concentration 50 μM) are given with DPPH. Compound 1 (apigenin) exerted 50% antioxidant activity () after 60 min comparable to the results published to another study of Zeghad and Merghem, 2013Citation32.
Table 3. Percentage soybean LOX inhibitory activity of extracts.
Table 4. Percentage interaction of isolated compounds with DPPH.
Compounds 4 (eriodictyol), 5 (eriodictyol-7-glucoside), 6 (caffeic acid methyl ester), 7 (rosmarinic acid) and 8 (rosmarinic acid methyl ester) were found to highly interact with the free radical.
AAPH is a water-soluble azo compound, which is used as a free radical generator, on lipid peroxidation assays, therefore, in screening antioxidants’ efficiencyCitation21,33–35. All the isolated compounds were tested in three different concentrations (10 μM, 50 μM and 100 μM) for their ability to inhibit linoleic acid peroxidation. The results from compounds – antilipid peroxidation activity – are given in . Compound 4 (eriodictyol) was the strongest inhibitor with 100% inhibition at 50 μM. Compound 1 (apigenin) and 3 (naringenin) did not exhibit any inhibition, compounds 2 (7-methoxy apigenin) and 6 (caffeic acid methyl ester) exhibited a moderate inhibition at 100 μM, whereas compounds 5 (eriodictyol-7- glucoside), 7 (rosmarinic acid) and 8 (rosmarinic acid methyl ester) totally inhibited linoleic acid peroxidation at 100 μΜ.
Table 5. % Inhibition of linoleic acid peroxidation induced by the isolated compounds.
The ability of the tested compounds to scavenge the superoxide anion generated by the xanthine/xanthine oxidase system is showed in . Compounds 4 (eriodictyol) and 8 (rosmarinic acid methyl ester) presented a moderate inhibition at 10 μM concentration. Compounds 1 (apigenin), 5 (eriodictyol-7-glucoside), 6 (caffeic acid methyl ester) and 7 (rosmarinic acid) at concentration 100 μM, moderate inhibited the enzyme.
Table 6. Percentage soybean LOX inhibitory activity of the isolated compounds.
Table 7. Superoxide anion scavenging activity % of the isolated compounds.
All extracts and the isolated compounds were tested as possible LOX inhibitors. Lipoxygenase is a key enzyme in inflammation in the arachidonic acid metabolism to leukotrienes. In , the % inhibitions of the tested extracts (Chloroform extract 48.3%, Hexane extract 36.8% and Et2O 27.1%) are given. In , the inhibitory activities of the isolated compounds at 10 μM and 100 μM concentration are given. Compounds 3 (naringenin), 6 (caffeic acid methyl ester), 7 (rosmarinic acid) and 8 (rosmarinic acid methyl ester) moderate inhibited LOX at 10 μM. Higher inhibition is observed at 100 μM. The inhibition values for compounds 1 (apigenin), 2 (7-methoxy-apigenin) and 5 (eriodictyol-7-glucoside) at 100 μM were found to be moderate inhibitors, whereas compound 4 (eriodictyol) did not present any inhibition at both concentrations.
Conclusions
Five flavonoids have been isolated from the aerial parts of Thymus sibthorpii Bentham (Lamiaceae). Both the extracts and the isolated compounds have been evaluated for their biological activity. Most of the extracts presented significant antioxidant activity in DPPH test. Among the isolated compounds, caffeic acid methyl ester (6) and rosmarinic acid (7) presented the highest antioxidant activity as well the strongest inhibitory activity against lipoxygenase. On the other hand, eriodictyol (4) was found to stronger inhibit the lipid peroxidation of linoleic acid. Further studies will be needed to clarify the antioxidant profile of Thymus sibthorpii Bentham and the isolated compounds.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Supplementary material available online
IENZ_1222583_Supplementary_Material.pdf
Download PDF (584.8 KB)Funding
The authors are grateful to Dr. N. Krigas (Scientific collaborator of Department of Crop Production to Technological Education Institute of Thessaloniki for the identification of the plant material and to Dr. C. Litinas (Professor of Department of Chemistry, AUTH) for recording NMR spectra. This study was financially supported by the GSRT (015-ɛ 5838. 14–03-06).
References
- Schwarz K, Ernst H, Ternes W. Evaluation of antioxidative constituents from thyme. J Sci Food Agric 1996;70:217–23
- EMA/HMPC, 2013. Assessment report on Thymus vulgaris L., Thymus zygis Loefl. ex. L., herba. 342334/2013. London: European Medicines Agency/Committee on Herbal Medicinal Products. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2014/06/WC500167810.pdf
- Soares JR, Dinis TC, Cunha AP, Almeida LM. Antioxidant activities of some extracts of Thymus zygis. Free Radic Res 1997;26:469–78
- Roby MHH, Sarhan MA, Selim KAH, Khalel KI. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Industr Crops Prod 2013;43:827–31
- Fatma G, Mouna BF, Mondher M, Ahmed L. In-vitro assessment of antioxidant and antimicrobial activities of methanol extracts and essential oil of Thymus hirtus sp. algeriensis. Lipids Health Dis 2014;13:114
- Tabti L, Dib M, El A, et al. Antioxidant and antifungal activity of extracts of the aerial parts of Thymus capitatus (L.) Hoffmanns against four phytopathogenic fungi of Citrus sinensis. Jundishapur J Nat Pharm Prod 2014;9:49–54
- Sokmen A, Gulluce M, Akpulat A, et al. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 2004;15:627–34
- Morales R. Synopsis of the genus Thymus L. in the Mediterranean area. Lagascalia 1997;19:249–62
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J 2013;2013:162750
- Haraguchi H, Saito T, Ishikawa H, et al. Antiperoxidative components in Thymus vulgaris. Planta Med 1996;62:217–21
- Ribeiro D, Freitas M, Tomé SM, et al. Inhibition of LOX by flavonoids: a structure-activity relationship study. Eur J Med Chem 2014;24:137–45
- Czaplińska M, Czepas J, Gwoździński K. Structure, antioxidative and anticancer properties of flavonoids. Post Biochem 2012;58:235–44
- Neu R. Chelate von diarylborsauren mit aliphatischen oxyalkylaminen als reagenzien fur den nachweis von oxyphenyl-benzo-γ-pyronen. Die Naturwissenchaften 1957;44:181–3
- Mabry JT, Markham RK, Thomas BM. The systemtic identification of flavonoids. Berlin, Heidelberg, New York: Springer-Verlag; 1970
- Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 2001;49:5165–70
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol 2011;48:412–22
- Kontogiorgis C, Hadjipavlou-Litina D. Synthesis and anti-inflammatory activity of coumarin derivatives. J Med Chem 2005;48:6400–8
- Fenda C. Effect of rhus coriaria L. (Anacardiaceae) on superoxide radical scavenging and xanthine oxidase activity. J Enzyme Inhib Med Chem 2003;18:59
- Kontogiorgis CA, Savvoglou K, Hadjipavlou-Litina DJ. Antiinflammatory and antioxidant evaluation of novel coumarin derivatives. J Enzyme Inhib Med Chem 2006;21:21–9
- Kontogiorgis C, Hadjipavlou-Litina D. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidant agents. J Enzyme Inhib Med Chem 2003;18:63–9
- Liegeois C, Lermusieau G, Collin S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2′-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J Agri Food Chem 2000;48:1129
- Davis PH. Flora of Turkey and the East Aegean Islands. Edinburg: University Press; 366
- Li R, Fang N, Mabry TJ. Flavonoids from Brickellia scoparia. J Nat Prod 1986;49:732–3
- Ayatollahi SA, Shojaii A, Kobarfard F, et al. Two flavones from Salvia leriaefolia. Iran J Pharm Res 2009;8:179–84
- Ibrahim AS. Sulfation of naringenin by Cunninghamella elegans. Phytochemistry 2000;53:209–12
- Harborne JB. The flavonoids, advances in research since 1986. London, New York: Chapman and Hall; 1994
- Ragab EA, Hosny M, Kadry HA, Ammar HA. Flavanone glycosides from Gleditsia caspia. J Nat Prod 2010;3:35–46
- Chang SW, Kim KH, Lee IK, et al. Phytochemical constituents of Bistorta manshuriensis. Nat Prod Sci 2009;15:234–40
- Woo ER, Piao MS. Antioxidative constituents from Lycopus lucidus. Arch Pharm Res 2004;27:173–6
- Wang M, Li JG, Rangarajan M, et al. Antioxidative phenolic compounds from sage (Salvia officinalis). J Agric Food Chem 1998;46:4869–73
- Viuda-Martos M, Perez-Alvarez JA, Fernandez-Lopez J. Lamiaceae herbs: a potential ingredient to functional foods In: Vattem DA, Maitin V, eds. Functional foods, nutraceuticals and natural products: concepts and applications. Lancaster, PA: DEStech Publications, Inc; 2015
- Zeghad N, Merghem R. Antioxidant and antibacterial activities of Thymus vulgaris L. Med Arom Plant Res J 2013;1:5–11
- Noguchi N, Takahashi M, Tsuchiya J, et al. Action of 21-aminosteroid U74006F as an antioxidant against lipid peroxidation. Biochem Pharmacol 1998;55:785–91
- Liu ZQ, Yu W, Liu ZL. Antioxidative and prooxidative effects of coumarin derivatives on free radical initiated and photosensitized peroxidation of human low-density lipoprotein. Chem Phys Lipids 1999;103:125–35
- Rice-Evans C, Miller N. Total antioxidant status in plasma and body fluids. Meth Enzymol 1994;234:279–93