Abstract
1-(4-Methylsulfonyl)-2-thione-4-aryl-5-Z-6-methyl and oxyalkyl-imidazoles were synthesized from different tetrahydropyrimidinethiones and aryl sulfonyl chloride. These compunds were tested for metal chelating effects and to determine the phrase in which inhibition occured between two physiologically pertinent compunds and carbonic anhydrase (CA) isozymes I and II (hCA I and II), butyrylcholinesterase (BChE) and acetylcholinesterase (AChE). AChE was detected in high concentrations in the brain and red blood cells. BChE is another enzymes that is abundant available in the liver and released into the blood in a soluble form. Newly synthesized hetaryl sulfonamides exhibited impressive inhibition profiles with Ki values in the range of 1.42–6.58 nM against hCA I, 1.72–7.41 nM against hCA II, 0.20–1.14 nM against AChE and 1.55–5.92 nM against BChE. Moreover, acetazolamide showed Ki values of 43.69 ± 6.44 nM against hCA I and 31.67 ± 8.39 nM against hCA II. Additionally, tacrine showed Ki values of 25.75 ± 3.39 nM and 37.82 ± 2.08 against AChE and BChE, respectively.
Introduction
Pyridine thione compounds are the building blocks of drugs that improve cerebral blood flow (nimodipinum, nifedipinum (calcium channel blockers). At present, non-glycoside and nonadrenergic cardiotonics with a large range of therapeutic actions are actively being sought. Their synthetic analogs-amrinone, peroximon and milrinone- are widely used in intensive treatment. Furthermore, sulfonamides that contain pyrimidine moieties exhibit cytotoxic effects that permit their use as antiviral and anticancer drugs. However, these compounds are also potent bactericides. Their anti-microbial activity and effect on various microorganisms depends on the nature of the heterocycle and various functional groups. Therefore, the synthesis of new sulfonamides containing bicyclic compounds is relevant, particularly the reaction of aryl sulfonyl chlorides with heterocyclic amines. The influence of the heterocycle’s composition and the location of the amine are studied. Interestingly, the amino group’s position in the isoxazole greatly affects its reactivity. Within the reaction of sulphonic-acid chloride with 5-amino 3,4-dimethyl isoxazoleCitation1 and biphenyl isoxazoleCitation2, hetaryl sulfonamides were obtained in high yields. The reaction of sulfonyl chlorides with five-membered aminoheterocycles such as oxazoleCitation3 and pyrazole also proceed in high yield. However, the reaction of sulfonyl chlorides with benzoxazole requires a long boiling period in pyridine solutionCitation4.
The reaction of aryl sulfonyl chlorides with piperazines, connected via oxygen, sulfur, N-pyridyl or pyrimidine diyl moieties proceeds very easily. The influence of the radicals and functional groups on the reaction was not observedCitation5. These sulfonamides are used for treating diseases of the central nervous system and kidney function disorders. Despite the presence of bicinchoninic acid in the 4-amino benzenesulfonyl chloride moiety, the reaction of 2-amino-4,6-dimethylpyrimidine proceeds very easily to form sulfonamides that exhibit anti-inflammatory and analgesic activityCitation6.
It was observed that the reaction of acetamide benzenesulfonyl chloride in the presence of DMSO is easily connected with chitosan derivativesCitation7. These sulfonamides have antifungal activity against Alternaria Solani and Phomopsis asparage. Pyridazinyl sulfonamide derivatives obtained by the reaction of sulfonyl chlorides with pyridazinyl also have antimicrobial activityCitation8. Thus aminoheterocycle reactivity depends on the structure, location and presence of functional groups. Studies of aryl sulfonyl chloride reactions with heterocyclic and bicyclic amines are important for both obtaining new sulfonamide compounds and investigating their germicidal and other propertiesCitation8. It was discovered that the reaction of aryl sulfonyl chlorides in heterocyclic amine solvents results in the separation of hydrogen chloride when using freshly distilled pyridine. A similar separation of hydrogen chloride was also observed when aryl sulfonyl chlorides were reacted in ethyl alcohol or triethylamine solvent. New hetaryl sulfonamides were observed by continuing focused research on synthesing various classes of organic sulfur compounds and the studying their transformations.
Carbonic anhydrase (CA) enzymes (EC 4.2.1.1) are present in many living systems and play a role in a diversity of pathological and physiological effects including neurological disorders, fluid balance, pH regulation, bone resorption, carboxylation reactions, glaucoma, calcification, osteoporosis, cancer, tumorigenicity and the synthesis of bicarbonate (HCO3−)Citation9–13. CAs are a player main role in the physiology of coral calcificationCitation14. CA has active confidants in the gastric mucosa, brain, kidney, salivary glands, eye lens, pancreas, nerve myelin sheath, prostate and uterusCitation15. In addition, CA amounts are considerably decreased in the brain tissue of patients suffering from Alzheimer’s disease, proving an essential involvement of various human CA isozymes (hCA I, II, IV, and VII) in cognitive and learning functionsCitation16. CA inhibitors (CAIs) are classified into two principal groups: the unsubstituted sulfonamides and the metal complexing anions. Sulfonamides are very momentous CAIs and sulfonamide varieties are effective organic compounds in medicinal chemistryCitation17,Citation18. The detection of CA inhibition with sulfanilamide by Mann and Keilin was the beginning of these applications and many additional scientific discoveriesCitation19.
CAs are biological catalysts that convert water and carbon dioxide (CO2) to bicarbonate (HCO3−) and a proton (H+)Citation20–23. This reaction is one of the fastest determined reactions (kcat, 106/s) in the environment and is required by animals and plants for survivalCitation24.
CAs have six distinct enzyme families: the α-, β-, γ-, δ-, ζ- and ŋ-CA. α-Cas (expressed in vertebrates and algae)Citation25 usually have monomer structures and occasionally a dimer form. β-CAs (in plants and prokaryotes)Citation25 have dimer, tetramers or octamer forms. γ-CAs (in archaea)Citation28 are trimers and the δ- and ζ-CAs (in marine diatoms)Citation25 are less well understood up to nowCitation26,Citation27. All human CAs (hCAs) belong to the α-class. Thus far, 16 isoenymes have been identifiedCitation28. All CA families are metalloenzymes, which have Cd2+, Zn2+ or Fe2+ at their active sitesCitation29. Different isoforms of CA have been recognized for their therapeutic effects toward several diseases. Therefore, the purpose of developing isoform-specific inhibitors is to develop new and improved treatmentsCitation30.
Cholinesterases (ChEs) have a role in catalyzing the hydrolysis of acetylcholine (ACh) into choline and acetic acid, a fundamental process for the restoration of the cholinergic neurotransmissionCitation31. Acetylcholinesterase (AChE; EC 3.1.1.7) and butyrylcholinesterase (BChE; EC 3.1.1.8) are among the various ChEsCitation31.
Acetylcholine (ACh) molecules are synthesized in pre-synaptic finals from choline and they are necessary for cholinergic neurotransmission in the peripheral nervous systems (PNS) and the central nervous system (CNS)Citation31. Alzheimer’s disease (AD) is characterized by dementia, memory loss and cognitive impairmentCitation32. Perception is affected in of derangements such as ADCitation32. An unusually low concentration of ACh can be create several neuropsychological and neuropsychiatric perturbations such as AD and Parkinson's diseaseCitation33. Generally, the treatment of AD is centralized on AChE inhibitors, such as rivastigmine, tacrine, galantamine and donepezilCitation34. AD is shown as a complex syndrome where various agents are responsible for its etiology such as tau protein aggregation, β-amyloid aggregation and low levels of AChCitation35. Additionally, AChE inhibitors are used in the treatment of multiple neuromuscular diseases, and they were implemented in the treatment of AD because AChE accelerates hydrolysis and enables the regulation of AChCitation36.
BChE is commonly referred to as plasma ChE and is also a nonspecific ChE enzyme that hydrolyzes several choline-based estersCitation36. BChE exhibits high activity levels in the intestine, liver, kidney, heart and lung; wheras high levels of AChE are present in the brain, muscle and erythrocyte membraneCitation36. Because BChE and AChE have up to 84% sequences similarity, they display similar activity in a variety of therapeutic applications and, thus, play an important role in medical scienceCitation26. In this study, we synthesized several new hetaryl sulfonamides (1–12), characterized their inhibition confidants against hCA I, hCA II, AChE and BChE and evaluated their inhibition confidants against acetazolamide (AZA), which is a clinical standard used as a CA inhibitor. Additionally, tacrine (TAC) was used as a standard for BChE and AChE inhibition.
Table 1. The synthesis route of hetaryl sulfonamides 1–12.
Experimental
General methodology
Synthesis of 1-(4-methylsulfonyl)-2-thione-4-aryl-5-Z-6-methyl and oxyalkyl-imidazoles (1–12)
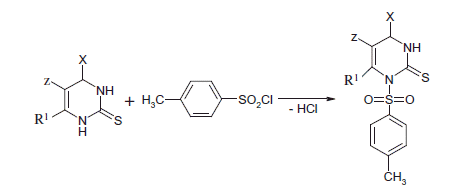
In this work, 0.01 mmol of the appropriate heterocyclic amine was dissolved in 10 mL of ethanol. Then, 0.01 mmol of aryl sulfonyl chloride and 0.011 mmol of triethylamine were slowly added to the solution. The mixture was heated at 70–80 °C for 1.5–2 h, cooled and diluted with acetone or water until crystals developed. The crystals were filtered and recrystallized from ethanol. The physical and chemical characteristics of the hetaryl sulfonamides are shown in and .
Table 2. Physical and chemical characteristics of hetaryl sulfonamides 1–12.
Biochemical studies
CA isoenzyme purification and inhibition studies
Affinity chromatography is an essential purification method because it offers high resolution, high selectivity and high capacity for protein purificationCitation37–40. Recently there has been a strong interest in two of the most important hCA isoforms that are particularly common proteins found in many tissues. Both cytosolic hCA isoforms were purified by the Sepharose-4B-l-tyrosine-sulfanilamide affinity segregation method using a single purification stepCitation41–43. In this study, the proteins presnt in the column eluates were spectrophotometrically determined at 280 nmCitation44. We then performed the sodium dodecyl sulfate-polyacrylamide gel electrophoresis method (SDS-PAGE) after purifying of the enzymesCitation45,Citation46. Upon visualizing the proteins by SDS-PAGE techniques, a single band was identifed for each isoenzymeCitation47. After spectrophotometrically purifying the samples, the protein concentrations were measured at 595 nm according to the Bradford techniqueCitation48–51. The Sepharose-4B-l-tyrosine-sulfanilamide affinity gel was cleaned with Tris-HCl (25 mM)/Na2SO4 (22 mM) at pH 8.7. Both CA isozymes were washed with NaCl (1.0 M)/Na2HPO4 (25 mM) at pH 6.3 and NaCH3COO (0.1 M)/NaClO4 (0.5 M) at pH 5.6, respectivelyCitation52,Citation53. The effects of the new hetaryl sulfonamides derivatives (1–12) were inwestigated by measuring the hydratase activity and determined in triplicate analysis at each concentration usedCitation54–56. In this study, the variations in activity were determined at 348 nm by measuring the conversion of the p-nitrophenylacetate substrate (NPA) to p-nitrophenolate (NP) and recording measurements in 3 min intervals at the room temperature (25 °C) by using a spectrophotometer (Shimadzu, UV-VIS Spectrophotometer, UVmini-1240, Kyoto, Japan)Citation57,Citation58.
AChE/BChE activity determination
The inhibitory effects of several new hetaryl sulfonamides (1–12) on AChE/BChE activity were recorded according to the spectrophotometric technique of Ellman et al.Citation59. Butyrylthiocholine iodide and acetylthiocholine iodide (BChI/AChI) were applicated as substrates for both repercussions. 5,5′-Dithio-bis(2-nitro-benzoic)acid (DTNB, D8130-1G, Sigma-Aldrich, Steinheim, Germany) was used for the measurement of the AChE/BChE reactions. In this study, 100 mL of Tris/HCl buffer (1 M, pH 8.0), and 10 mL of sample solution were dissolved in distilled water at varying concentrations and then 50 mL AChE/BChE (5.32 × 10 − 3 EU) solution was added and the reaction was incubated for 10 min at 25 °C. Then, 50 mL of DTNB (0.5 mM) was added. The activity was then initiated by the addition of 50 mL of AChI/BChICitation31.
Metal chelating activity
Metal chelating capacity of hetaryl sulfonamides 1–12 was determined according to Dinis et al.Citation60. The Fe2+-binding capacity of the hetaryl sulfonamides was spectrophotometrically recorded at 562 nm. Simultaneously, to a mixture of FeCl2 (0.1 mL, 0.6 mM), three concentrations (10–30 µg/mL) of hetaryl sulfonamides 1–12 in ethanol (0.4 mL) were added. The activities were initiated by the addition of ferrozine molecules (0.1 mL, 5 mM). Additionally, the reaction was mixed and maintained in a dark room for 10 min. Finally, the activities of the hetaryl sulfonamide mixture was recorded spectrophotometrically at 562 nmCitation41.
Results and discussion
Synthesis
The synthesis of 1-(4-methylsulfonyl)-2-thione-4-aryl-5-Z-6-methyl and oxyalkyl-imidazoles is reported in Scheme 1. The reaction of tetrahydropyrimidinethiones substituted with different heterocyclic amines and aryl sulfonyl chloride in the presence of triethylamine led to the desired new compounds (1–12). The reactions completed within 2.5–3.0 h at 70–80 °C. The newly synthesized compounds were crystalline and their structures were confirmed by spectral and physico-chemical methods, including IQ, 1H and 13C NMR spectroscopies.
Biochemistry
A variety of special isoforms from the CA family of zinc containig metalloenzymes been applied in the treatment of diseasesCitation61. Classical CAIs, generally sulfonamide-based compounds and their features, are known for their antiepileptic, antiobesity, antiglaucoma, anticancer and antineuropathic pain applicationsCitation25. Anticonvulsant drugs such as topiramate acetazolamide and zonisamide are recocnized CA inhibitorsCitation62. Classic CA enzymes, which are typically inhibited by compounds that contain a sulfonamide-based (SO2NH2) zinc-binding group (ZBG) or their bioisosteres such as sulfamides and sulfamates. Because CA II enzymes are the most physiologically abundant isoforms, they are often regarded as the predominant all-purpose isozymes of which inhibitions are to be eschewed. Recently, multiple types of compounds have been categorized as non-classical CAIs, including polyamines, phenols, coumarins, carboxylic acids and their derivatives, and also fullerenesCitation25. The hCA I and II isoenzyme inhibitory activity of the new hetaryl sulfonamides (1–12) is shown in . Cytosolic hCA I enzyme is found in the body and also could be observed in high concentrations in the erythrocytes and gastrointestinal tissuesCitation63.
Table 3. The inhibition profiles of a dozen 1-(4-methylsulfonyl)-2-thione-4-aryl-5-Z-6-methyl and oxyalkyl-imidazoles 1–12 against human carbonic anhydrase isoenzymes I and II (hCA I and II), acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes.
The low cytosolic isoform hCA I was measured for each new hetaryl sulfonamides (1–12), and the resulting Ki values ranged between 1.42 ± 0.24–6.58 ± 2.01 nM. (). In addition, acetazolamide (AZA), which is kbown to exhibit mediocre activity as an hCA I inhibitör, showed a Ki of 43.69 ± 6.44 nM. Likewise, the strongest hCA I inhibition level was obseved for hetaryl sulfonamide 6, with a Ki value of 1.42 ± 0.24 nM. The inhibitory activity of hetaryl sulfonamides (1–12) was also assessed against the low cytosolic hCA I isoform, the higher activity cytosolic hCA II isoenzyme and the AChE enzyme. Interestingly, these hetaryl sulfonamide compounds were determined to be effective due to their ferrous ions (Fe2+)-chelating effectsCitation63–70.
The importance of an isoenzyme and its effects on the physiologically dominant cytosolic hCA II enzyme varies by the disease target. For hCA II, new hetaryl sulfonamides 1–12 had Ki values in the range of 1.72 ± 0.62–7.41 ± 2.32 nM. Hetaryl sulfonamide 3 demonstrated the best inhibition profile, with a Ki value of 1.72 ± 0.62 nM. In addition, acetazolamide (AZA, 5-aceta-mido-1,3,4-thiadiazole-2-sulfonamide), a clinical standard used in this study as a medium strength CA II inhibition for this isoenzyme, exhibited a stable level of inhibition at 31.67 ± 8.39 nM.
A significant cause contributing to the onset of AD is the reduced amount of ACh and other enzymes responsible for its synthesis and reduction in the brain tissueCitation71. Generally, a person who has suffered from AD will exhibit lower amounts of ACh (i.e., less than 0.20 µM ACh)Citation71. Recently, Wei and coworkers have determined the levels of ACh using carbon dotsCitation70. Schistosome AChE plays a significant role in limiting this action and other reactions by inhibiting the AChE emulator ligand causibg receptor desensitizationCitation71. AChE can increase the deposition of aging β-amyloid plaques in aging brain tissueCitation71. It has been recorded that the use of AChE inhibitors can strongly reduce some of the cognitive symptoms of AD and other behavioral traitsCitation71. Recently, Chen and his coworkers recorded a sensor for determining the activity of AChE using a changed BSA preserved clusterCitation72. During the blood lodging steps of schistosomes, AChE is available on the parasite stratum membraneCitation73. Effective BChE and AChE inhibitors can also be used for AD therapy. Thus far, the typical drugs for treating AD on the market -including rivastigmine, tacrine, donepezil and galantamine- are BChE or AChE inhibitorsCitation72,Citation64. AChE orginated in the brain and in erythrocyte cells with higher prudaction levels and it is an important enzyme for neural devicesCitation73. The activity (%)-[Hetaryl sulfonamides] graphs were plotted and the IC50 values of each hetaryl sulfonamide against AChE were computed after appropriate each dilutionCitation73. Accordingly, the manufacturing of new inhibitors is important for thedeveloping improved therapies to treat AD. The inhibitory effects of hetaryl sulfonamides 1–12 on AChE and BChE are shown in . In this study, the recently synthesized compounds exhibited strong inhibitory activity against AChE, with Ki values ranging from 0.20 ± 0.05 nM to 1.14 ± 0.26 nM. In addition, tacrine as a standard inhibitor for AChE exhibited a Ki value of 25.75 ± 3.39 nM. Based on these results, the inhibition of AChE by hetaryl sulfonamides 1–12 is significantly stronger than that of tacrine, a standard AD medication.
The BChE enzyme play a major role as an ACh hydrolyzing enzyme in environmental mammalian systemsCitation37. The BChE enzyme has a particular role in cholinergic neurotransmission and it has been a key factor in ADCitation37. An improved Ellman methodCitation59 was defined to quantify the activity of BChE. Currently, the most commonly prescribed cholinesterase inhibitors are galantamine, donepezil and rivastigmineCitation61. Donepezil and galantamine are short-lived reversible competitive inhibitors, whereas rivastigmine actively reacts with ChE. However, rivastigmine has an equal affinity for both the BChE and AChE enzymes, which makes it a very momentous drug currently prescribed for the therapeutic treatment of ADCitation41. It has also been shown that phenothiazines inhibit ChEs, especially BChE. In this study, hetaryl sulfonamides 1–12 inhibited BChE, with Ki values in the range of 1.55 ± 0.44–5.92 ± 1.63 nM. Additionally, hetaryl sulfonamide 10 was potent BChE inhibitor (Ki: 1.55 ± 0.44 nM). Morever, all of the hetaryl sulfonamides displaed higher BChE inhibition activity than tacrine (Ki: 37.82 ± 2.08 nM).
The metal chelating procedure used in this study is also an antioxidant technique that is based on the absorbance measurement of the ferrous ion (Fe2+)-ferrozine molecule after subsequent treatment of a Fe2+ solution with the experimental sampleCitation74–79. The ferrozine–Fe2+ molecule created a red chromophore with an absorbance that was measured at 562 nmCitation80–82. A weak antioxidant method for iron chelation would be strongly underestimated in low concentrations. The metal-chelating process was considerable since it decreased the concentration of the catalyzing transition metal in lipid peroxidationCitation83–85. EDTA is a potent metal chelator; therefore, this compound was recorded as a standard metal chelator operative in this testCitation86,Citation87. In addition, it was determined that the IC50 values for hetaryl sulfonamide compounds 1–12 were calculated in the range of 43.31–196.11 µg/mL (). Moreover, the IC50 values corresponding to to the Fe2+ ion-chelating method of the positive control samples – like trolox, α-tocopherol, BHA, BHT and EDTA – were determined to be in the range of 9.36–76.96 mg/mL. A lower IC50 value corresponds to a higher Fe2+ ion-binding capacityCitation88–90.
Table 4. Determination of the half maximal concentrations (IC50, μg/mL) of Fe2+ chelating of several new hetaryl sulfonamides 1–12 and standard compounds including BHA, BHT, α-Tocopherol, Trolox and EDTA.
Conclusion
In this work, we focus on new hetaryl sulfonamides 1–12, which exhibited effective inhibition profiles against BChE and AChE enzymes and hCA isoforms. We also defined the AZA data, as this standard sulfonamide inhibitor exhibits anticonvulsant properties. In addition, the IC50 values of the compounds were studied; the best inhibitor was compound 8 toward hCA II. Both the BuChE and AChE enzymes hydrolyze ACh and demonstrate retained AChE levels in AD patients. In this work, the AChEIs have been a primary drug target for regulating AD at the symptomatic level. Human AChE and BuChE also have 65% amino acid sequence similarity. Furthermore, these compounds showed effective metal chelating activity in the presence of Fe2+ ions and ferrozine.
Acknowledgements
S.H. Alwasel would like to thank the Distinguished Scientist Fellowship Program at King Saud University for financial support.
Disclosure statement
The authors declare that there is no conflict of interest.
References
- Chang MP, Ruju BC. USA Patent 6541492, appl. 27.12.2003, publ. 01.04.2003.
- Polniazek RO, Wang X, Pandit CR. USA Patent 6515130, appl. 24.08.1998, publ. 04.02.2003.
- Taritsuka K, Ocasio M, Yamamoto N, Seishi K. Application 63-44534 (Japan), appl. 11.08.2006, K-Ni 61-189020, publ. 23.02.2008.
- Tosheva M, Antonova A. Department of Chemistry of Sophiya University 2005;91:149–52.
- Braje WM, Haupt A, Labirch W, et al. USA Patent 7320979, appl. 13.04.2004, publ. 22.01.2008.
- Novikov MV, Michalov AM, Konishev ME, et al. Pat Russia 2364594, appl. 09.01.2008, publ. 20.08.2009.
- Zhimli Z, Rong C, Ronga X. Synthesis and antifungal properties of sulfanilamide derivatives of chitosan. Carbohydrate Res 2007;342:2390–5.
- Mohammed MJ. Bulg Chem Commun 2007;39:152–8.
- Gülçin I, Beydemir S. Phenolic compounds as antioxidants: carbonic anhydrase isoenzymes inhibitors. Mini Rev Med Chem 2013;13:408–30.
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitorsand their therapeutic potential. Exp Opin Ther Pat 2000;10:575–600.
- Gocer H, Akıncıoğlu A, Göksu, et al. Carbonic anhydrase inhibitory properties of phenolic sulfonamides derived from dopamine related compounds. Arab J Chem 2014. [Epub ahead of print]. doi: 10.1016/j.arabjc.2014.08.005
- Taslimi P, Gulcin I, Ozgeris B, et al. The human carbonic anhydrase isoenzymes I and II (hCA I and II) inhibition effects of trimethoxyindane derivatives. J Enzyme Inhib Med Chem 2016;31:152–7.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50.
- Güney M, Coşkun A, Topal F, et al. Oxidation of cyanobenzocycloheptatrienes: synthesis, photooxygenation reaction and carbonic anhydrase isoenzymes inhibition properties of some new benzotropone derivatives. Bioorg Med Chem 2014;22:3537–43.
- Tafreshi NK, Lloyd MC, Bui MM, et al. Carbonic anhydrase IX as an imaging and therapeutic target for tumors and metastases. Subcell Biochem 2014;75:221–54.
- Lomelino CL, Supuran CT, McKenna R. Non-classical ınhibition of carbonic anhydrase. Int J Mol Sci 2016;17:1150.
- Takakura M, Yokomizo A, Tanaka Y, et al. Carbonic anhydrase I as a new plasma biomarker for prostate cancer. ISRN Oncol 2012;2012:768190.
- Vullo D, De Luca V, Del Prete S, et al. Sulfonamide inhibition studies of the c-carbonic anhydrase from the Antarctic cyanobacterium Nostoc commune. Bioorg Med Chem 2015;23:1728–34.
- Winum JY, Scozzafava A, Montero JL, Supuran CT. Design of zinc binding functions for carbonic anhydrase inhibitors. Curr Pharm Des 2008;14:615–21.
- Scozzafava A, Passaponti M, Supuran CT, Gulçin I. Carbonic anhydrase inhibitors: guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:586–91.
- Arabaci B, Gülçin I, Alwasel S. Capsaicin: a potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2015;19:10103–14.
- ArasHisar Ş, Hisar O, Beydemir Ş, et al. Effect of vitamin E on carbonic anhydrase enzyme activity in rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Acta Vet Hung 2004;52:413–22.
- Maccallini C, Di Matteo M, Vullo D, et al. Indazole, pyrazole, and oxazole derivatives targeting nitric oxide synthases and carbonic anhydrases. ChemMedChem 2016;11:1695–9.
- Hanf E, Böhmer A, Zinke M, et al. Carbonic anhydrases are producers of S-nitrosothiols from inorganic nitrite and modulators of soluble guanylyl cyclase in human platelets. Amino Acids 2016;48:1695–706.
- Özgeris B, Goksü S, Köse Polat L, et al. Acetylcholinesterase and carbonic anhydrase inhibitory properties of novel urea and sulfamide derivatives incorporating dopaminergic 2-aminotetralin scaffolds. Bioorg Med Chem 2016;24:2318–29.
- Boztaş M, Çetinkaya Y, Topal M, et al. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxy-bromophenol derivatives incorporating cyclopropane moieties. J Med Chem 2015;58:640–50.
- Taslimi P, Gülçin I, Öztaşkın N, et al. The effects of some bromophenols on human carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2016;31:603–7.
- Gülçin I, Scozzafava A, Supuran CT, et al. Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase, and carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2016;31:1698–702.
- Scozzafava A, Kalın P, Supuran CT, et al. The impact of hydroquinone on acetylcholine esterase and certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:941.
- Özbey F, Taslimi P, Gülçin İ, et al. Synthesis of diaryl ethers with acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase inhibitory actions. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.1080/14756366.2016.1189422.
- Garibov E, Taslimi P, Sujayev A, et al. Synthesis of 4,5-disubstituted-2-thioxo-1,2,3,4- tetrahydropyrimidines and investigation of their acetylcholinesterase, butyrylcholinesterase, carbonic anhydrase I/II inhibitory and antioxidant activities. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.1080/14756366.2016.1198901.
- Haider A, Inam W, Khan SA, et. al. β-Glucan attenuated scopolamine induced cognitive impairment via hippocampal acetylcholinesterase inhibition in rats. Brain Res 2016;1644:141–8.
- Mathew MS, Baksi A, Pradeep T, et al. Choline-induced selective fluorescence quenching of acetylcholinesterase conjugated Au@BSA clusters. Biosen Bioelect 2016;81:68–74.
- Gülçin İ, Beydemir Ş, Büyükokuroğlu ME. In vitro and in vivo effects of dantrolene on carbonic anhydrase enzyme activities. Biol Pharm Bull 2004;27:613–16.
- Danish Rizvi SM, Shaikh S, Naaz D, et al. Kinetics and molecular docking study of an anti-diabetic drug glimepiride as acetylcholinesterase ınhibitor: ımplication for Alzheimer’s disease-diabetes dual therapy. Neurochem Res 2016;41:1475–82.
- Bertucci A, Moya A, Tambutté S, et al. Carbonic anhydrases in anthozoan corals – a review. Bioorg Med Chem 2013; 21:1437–50.
- Akıncıoğlu A, Akıncıoğlu H, Gülçin I, et al. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg Med Chem 2015;23:3592–602.
- Aksu K, Nar M, Tanc¸ M, et al. Synthesis and carbonic anhydrase inhibitory properties of sulfamides structurally related to dopamine. Bioorg Med Chem 2013;21:2925–31.
- Yıldırım A, Atmaca U, Keskin A, et al. N-Acylsulfonamides strongly inhibit human carbonic anhydrase isoenzymes I and II. Bioorg Med Chem 2015;23:2598–605.
- Kucuk M, Gulcin İ. Purification and characterization of the carbonic anhydrase enzyme from Black Sea trout (Salmo trutta Labrax Coruhensis) kidney and inhibition effects of some metal ions on enzyme activity. Environ Toxicol Pharmacol 2016;44:134–9.
- Aksu K, Topal F, Gülçin I, et al. Acetylcholinesterase inhibitory and antioxidant activities of novel symmetric sulfamides derived from phenethylamines. Arch Pharm 2015;348:446–55.
- Gocer H, Akıncıoğlu A, Öztaskın N, et al. Synthesis, antioxidant, and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine-related compounds. Arch Pharm 2013;346:783–92.
- Artunç¸ T, Çetinkaya Y, Gocer H, et al. Synthesis of 4-[2-(3,4- dimethoxybenzyl)cyclopentyl]-1,2-dimethoxybenzene derivatives and evaluations of their carbonic anhydrase isoenzymes inhibitory effects. Chem Biol Drug Des 2016;87:594–607.
- Beydemir Ş, Gülçin İ, Küfrevioğlu Öİ, Çiftçi M. Glucose 6-phosphate dehydrogenase: in vitro and in vivo effects of dantrolene sodium. Pol J Pharmacol 2003;55:787–92.
- Göksu S, Naderi A, Akbaba Y, et al. Carbonic anhydrase inhibitory properties of novel benzylsulfamides using molecular modeling and experimental studies. Bioorg Chem 2014;56:75–82.
- Gül HI, Tugrak M, Sakagami H, et al. Synthesis and bioactivity studies on new 4-(3-(4-substitutedphenyl)-3a,4-dihydro-3H-indeno[1,2-c]pyrazol-2-yl) benzenesulfonamides. J Enzyme Inhib Med Chem 2016;31:1619–24.
- Coban TA, Beydemir S, Gülçin I, Ekinci D. The effect of ethanol on erythrocyte carbonic anhydrase isoenzymes activity: an in vitro and in vivo study. J Enzyme Inhib Med Chem 2008;23:266–70.
- Coban TA, Beydemir S, Gülçin I, Ekinci D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol Pharm Bull 2007;30:2257–61.
- Darvesh S, Darvesh KV, McDonald RS, et al. Carbamates with differential mechanism of inhibition toward acetylcholinesterase and butyrylcholinesterase. J Med Chem 2008;51:4200–12.
- Şentürk M, Gülçin I, Beydemir S, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9.
- Atasever A, Özdemir H, Gülçin I, Kufrevioglu OI. One-step purification of lactoperoxidase from bovine milk by affinity chromatography. Food Chem 2013;136:864–70.
- Nar M, Çetinkaya Y, Gülçin I, Menzek A. (3,4-Dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives as carbonic anhydrase isoenzymes inhibitors. J Enzyme Inhib Med Chem 2013;28:402–6.
- Innocenti A, Özturk Sarıkaya SB, Gülçin I, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I–XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–64.
- Göksu H, Topal M, Keskin A, et al. 9,10-Dibromo-N-aryl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones: synthesis and ınvestigation of their effects on carbonic anhydrase ısozymes I, II, IX, and XII. Arch Pharm 2016;349:466–74.
- Gökcen T, Gülçin I, Özturk T, et al. A class of sulfonamides as carbonic anhydrase I and II inhibitors. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.1080/14756366.2016.1198900.
- Oktay K, Polat Köse L, Şendil K, et al. The synthesis of (Z)-4-Oxo- 4-(arylamino)but-2-enoic acids derivatives and determination of theirs inhibition properties against human carbonic anhydrase I, and II isoenzymes. J Enzyme Inhib Med Chem 2016;31:939–45.
- Hisar O, Beydemir S, Gülçin I, et al. The effects of melatonin hormone on carbonic anhydrase enzyme activity in rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Turk J Vet Anim Sci 2005;29:841–5.
- Hisar O, Beydemir Ş, Gülçin İ, et al. Effect of low molecular weight plasma inhibitors of rainbow trout (Oncorhyncytes mykiss) on human erythrocytes carbonic anhydrase-II isozyme activity in vitro and rat erythrocytes in vivo. J Enzyme Inhib Med Chem 2005;20:35–9.
- Ellman GL, Courtney KD, Andres V, Featherston RM. A new and rapid colorimetric deermination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994;315:161–9.
- Sujayev A, Polat Köse L, Garibov E, et al. Synthesis of N-alkyl (aril)-tetra pyrimidine thiones and investigation of their human carbonic anhydrase I and II inhibitory effects. J Enzyme Inhib Med Chem 2016;31:1192–97.
- Topal M, Gülçin İ. Rosmarinic acid: a potent carbonic anhydrase isoenzymes inhibitor. Turk J Chem 2014;38:894–902.
- Gülçin I, Küfrevioğlu ÖI, Oktay M. Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibitory effects of some chemicals on enzyme activity. J Enzyme Inhib Med Chem 2005;20:297–302.
- Huyut Z, Beydemir S, Gulcin İ. In vitro and in vivo inhibitory effects of some phenolic compounds on the activities of carbonic anhydrase. J Enzyme Inhib Med Chem 2016;31:1234–40.
- Turan B, Şendil K, Şengül E, et al. The synthesis of some β-lactams and investigation of their metal-chelating activity, carbonic anhydrase and acetylcholinesterase inhibition profiles. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.3109/14756366.2016.1170014.
- Gülçin I, Elias R, Gepdiremen A, et al. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.). Wood Sci Technol 2009;43:195–212.
- Talaz O, Gülçin I, Göksu S, Saracoğlu N. Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorg Med Chem 2009;17:6583–9.
- Gülçin I, Elias R, Gepdiremen A, et al. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: cepharanthine and fangchinoline. J Enzyme Inhib Med Chem 2010;25:44–53.
- Kalın P, Gülçin I, Gören AC. Antioxidant activity and polyphenol content of Vaccinium macrocarpon. Rec Nat Prod 2015;9:496–502.
- Bursal E, Köksal E, Gülçin I, et al. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC-MS/MS. Food Res Int 2013;51:66–74.
- Yılmaz S, Akbaba Y, Özgeriş B, Et al. Synthesis and inhibitory properties of some carbamates on carbonic anhydrase and acetylcholine esterase. J Enzyme Inhib Med Chem 2016;31:1484–91.
- Ozmen OD, Yamali C, Gül Hİ, et al. Inhibitory effects of isatin mannich bases on carbonic anhydrases, acetylcholinesterase and butyrylcholinesterase. J Enzyme Inhib Med Chem 2016;31:1498–501.
- Sujayev A, Garibov E, Taslimi P, et al. Synthesis of some tetrahydropyrimidine-5-carboxylates, determination of their metal chelating effects and inhibition profiles against acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase. J Enzyme Inhib Med Chem 2016;31:1531–9.
- Öztaşkin N, Çetinkaya Y, Taslimi P, et al. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg Chem 2015;60:49–57.
- Gülçin I, Elmastas M, Aboul-Enein HY. Antioxidant activity of clove oil-a powerful antioxidant source. Arab J Chem 2012;5:489–99.
- Gülçin I, Beydemir S, Topal F, et al. Apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst. and kit. J Enzyme Inhib Med Chem 2012;27:587–94.
- Bursal E, Gülçin I. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int 2011;44:1482–9.
- Gülçin I, Huyut Z, Elmasta¸s M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem 2010;3:43–53.
- Gülçin I, Topal F, Çakmakçı R, et al. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J Food Sci 2011;76:C585–93.
- Gülçin I, Topal F, Öztürk Sarikaya SB, et al. Polyphenol contents and antioxidant properties of medlar (Mespilus germanica L.). Rec Nat Prod 2011;5:158–75.
- Gülçin I. Antioxidant activity of eugenol: a structure-activity relationship study. J Med Food 2011;14:975–85.
- Köksal E, Bursal E, Dikici E, et al. Antioxidant activity of Melissa officinalis leaves. J Med Plants Res 2011;5:217–22.
- Şerbetçi TH, Gülçin I. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int J Food Propert 2010;13:657–71.
- Gülçin I, Kirecci E, Akkemik E, et al. Antioxidant and antimicrobial activities of an aquatic plant: Duckweed (Lemna minor L.). Turk J Biol 2010;34:175–88.
- Gülçin I. Antioxidant properties of resveratrol: a structure-activity insight. Innov Food Sci Emerg 2010;11:210–18.
- Köksal E, Gülçin I, Öztürk Sarıkaya SB, Bursal E. On the in vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem 2009; 24:395–405.
- Gülçin I, Daştan A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J Enzyme Inhib Med Chem 2007;22:685–95.
- Gülçin İ, Beydemir Ş, Şat İG, Küfrevioğlu Öİ. Evaluation of antioxidant activity of cornelian cherry (Cornus mas L.). Acta Aliment Hung 2005;34:193–202.
- Topal F, Topal M, Gocer H, et al. Antioxidant activity of taxifolin: an activity-structure relationship. J Enzyme Inhib Med Chem 2016;31:674–83.
- Topal M, Gocer H, Topal F, et al. Antioxidant, antiradical and anticholinergic properties of cynarin purified from the illyrian thistle (Onopordum illyricum L.). J Enzyme Inhib Med Chem 2016;31:266–75.