Abstract
4-(3-(4-Substituted-phenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl) benzenesulfonamides (9–16) were successfully synthesized and their chemical structures were confirmed by 1H NMR, 13C NMR, and HRMS spectra. Carbonic anhydrase I and II inhibitory effects of the compounds were investigated. Ki values of the compounds were in the range of 316.7 ± 9.6–533.1 ± 187.8 nM towards hCA I and 412.5 ± 115.4–624.6 ± 168.2 nM towards hCA II isoenzymes. While Ki values of the reference compound Acetazolamide were 278.8 ± 44.3 nM and 293.4 ± 46.4 nM towards hCA I and hCA II izoenzymes, respectively. Compound 14 with bromine and compound 13 with fluorine substituents can be considered as the leader compounds of the series because of the lowest Ki values in series to make further detailed carbonic anhydrase inhibiton studies.
Introduction
Carbonic anhydrases (CAs) are a ubiquitous metalloenzyme catalyzing the reversible conversion of carbon dioxide to bicarbonate. CAs are present in prokaryote and eukaryote life forms. The enzyme is found in many tissues such as gastrointestinal tract, nervous system, kidneys, lungs, skin, and eyes. CAs play key roles in a number of physiological and pathological processes such as ions and gas exchanges, pH regulation, photosynthesis, calcification, and biosynthetic reactions such as gluconeogenesis, lipogenesis, and ureagenesisCitation1. The mammalian enzymes belonging to α-CA family consist of 16 active members, in which several are cytosolic (CA I–III, CA VII, and CA XIII), five are membrane-bound (CA IV, CA IX, CA XII, CA XIV, and CA XV), two are mitochondrial (CA VA and VB), and one (CA VI) is secreted in saliva and milk. Out of 16 different isoforms of α-class of human-associated CAs, hCA I is found together with hCA II in erythrocytes. hCA II is the most widely distributed isoform in the eye, kidney, central nervous system, and inner ear, and is a drug target for clinically used diuretics, antiglaucoma drugs, and anticonvulsants. Targeting a particular CA is often associated with treatment of particular diseases such as CA II, IV, and XII are the targets for antiglaucoma agents; CA VA and VB for antiobesity agents; CA IX and XII for antitumour agents or diagnostic tools for imaging hypoxic tumoursCitation2–5.
The sulfonamides are an important class of the pharmaceutical compounds with a wide spectrum of biological activities such as anticancerCitation6,Citation7, CA inhibitoryCitation8,Citation9, antibacterialCitation10, antihypertensiveCitation11, antiinflammatoryCitation12, and antiprotozoalCitation13 activities among others.
On one hand, Acetazolamide (5-amino-1,3,4-thiadiazole-2-sulfonamide, AZA), which is used systematically to reduce intraocular pressure by lowering the humor formation in the eye, has sulfonamide moietyCitation14. Although AZA is in clinical use, it has several side effects such as numbness and tingling in the fingers and toes, taste alterations, blurred vision, kidney stones, and an increase in urinationCitation14.
On the other hand, pyrazole derivatives were registered in literature with several biological activities including antiepileptic, antidepressant, antiinflammatory, antimicrobial, antitubercular, anticancer, antibacterial, antioxidant, and CA inhibitory activitiesCitation7,Citation9,Citation15–17. In addition, several 2-pyrazoline derivatives are known with a broad range of pharmacological activities such as analgesic and antipyretic (Phenazone/Amidopyrene/Methampyrone), antiinflammatory (Azolid/Tandearil), insecticidal (Indoxacarb), and uricosuric (Anturane)Citation18.
The most extensively made modifications on 2-pyrazolines were diaryl/hetroaryl group substitutions at 3 and/or 5 positions. Celecoxib and Valdecoxib, which are in clinical use, were developed as cyclooxygenase-2 (COX-2) specific inhibitors. They are also known with their potent CA inhibitory activitiesCitation19. Both compounds possess a benzenesulfonamide group linked to a five-membered substituted heterocyclic ring. The presence of the –SO2NH2 moiety seems not to be necessary for COX-2 inhibition but it is essential for the CA inhibitionCitation19. These two drugs have shown interesting isoform selective CA inhibitory effects. Their X-ray crystal structures were in a complex form with hCA IICitation19.
Recently, our research group focused on the synthesis of the compounds having 2-pyrazoline and sulfonamide pharmacophores in a single molecule and investigated their several bioactivities such as cytotoxic and/or CA inhibitory activities based on the results of previous studiesCitation7–9,Citation17. In the present study, it was aimed to synthesize 4-[3–(4-substitutedphenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl] benzenesulfonamides to investigate their carbonic anhydrase inhibitory effects on hCA I and II isoenzymes.
Experimental
Materials and methods
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were obtained using a Varian Mercury Plus spectrometer (Varian Inc., Palo Alto, CA). Chemical shifts (δ) were reported in parts per million (ppm). HRMS spectra of the compounds were taken by liquid chromatography ion trap-time of flight tandem mass spectrometer (Shimadzu, Kyoto, Japan) equipped with an electrospray ionization (ESI) source, operating in both positive and negative ionization mode. Shimadzu’s LCMS Solution software (Shimadzu, Kyoto, Japan) was used for data analysis. Melting points were determined on Buchi 530 (Buchi Labortechnik AG, Flawil, Switzerland).
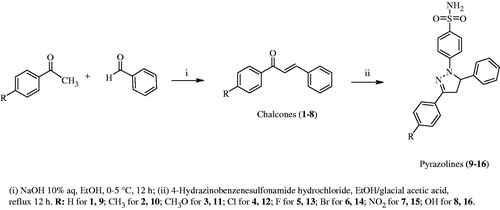
General procedure for the synthesis of chalcones (1–8, Scheme 1)
An aqueous solution of NaOH (10%, 10 mL) was added into the ethanol (6 mL) solution of benzaldehyde (20.0 mmol) and a suitable acetophenone (20.0 mmol). The mixture was stirred overnight at room temperature and it was then poured on ice-water (100 ml). The mixture was neutralized with a solution of HCl (10%). The colored precipitate formed was filtered and crystallized from methanol-water (1–8). The yields of the chalcones were in the range of 34–82% [1 (39%), 2 (57%), 3 (82%), 4 (79%), 5 (71%), 6 (42%), 7 (34%), 8 (37%)]Citation7,Citation9,Citation17,Citation20–22.
General procedure for the synthesis of pyrazolines (9–16, Scheme 1)
The mixture of a suitable chalcone (1.0 mmol) and 4-hydrazinobenzenesulfonamide hydrochloride (1.1 mmol) was dissolved in ethanol, and then catalytic amount of glacial acetic acid was addedCitation7,Citation9,Citation17. The mixture was refluxed for 12 h. Reactions were followed by thin layer chromotography (TLC). After the reaction was stopped, some of the solvent was removed under vacuum and the mixture was stirred for 12 h at room temperature. The obtained solid was filtered, dried at room temperature and crystallized from methanol–ether.
4-(3,5-Diphenyl-4,5-dihydro-1H-pyrazol-1-yl)benzenesulfonamide (9)
Mp 202–204 °C. 2.81 g (78%). 1H NMR (400 MHz, DMSO-d6, ppm) δ = 7.77 (d, 2H, J = 8.1 Hz), 7.57 (d, 2H, J = 8.1 Hz), 7.45–7.31 (m, 5H), 7.25–7.22 (m, 3H), 7.06 (d, 2H, J = 8.4 Hz), 7.00 (s, 2H, NH2), 5.63 (dd, 1H, J = 12.1, 5.1 Hz), 3.96 (dd, 1H, J = 17.8, 12.1 Hz), 3.16 (dd, 1H, J = 17.8, 5.1 Hz); 13C NMR (100 MHz, DMSO-d6, ppm) δ = 150.3, 146.6, 142.3, 133.7, 132.4, 130.0, 129.8, 129.4, 128.3, 127.8, 126.8, 126.4, 112.7, 63.0, 43.7; HRMS (ESI-MS): calcd. for C21H20N3O2S [M + H]+ 378.1271; found 378.1265.
4-(5-Phenyl-3-(p-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)benzenesulfonamide (10)
Mp 224–225 °C. 3.36 g (95%). 1H NMR (400 MHz, DMSO-d6, ppm) δ = 7.66 (d, 2H, J = 8.1 Hz), 7.56 (d, 2H, J = 9.1 Hz), 7.34–7.30 (m, 2H), 7.25–7.22 (m, 5H), 7.04 (d, 2H, J = 8.8 Hz), 6.99 (s, 2H, NH2), 5.59 (dd, 1H, J = 12.1, 5.1 Hz), 3.93 (dd, 1H, J = 17.6, 12.1 Hz), 3.14 (dd, 1H, J = 17.6, 5.1 Hz), 2.32 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6, ppm) δ = 150.4, 146.6, 142.4, 139.8, 133.5, 130.0, 129.8, 129.7, 128.3, 127.8, 126.8, 126.4, 112.6, 62.9, 43.8, 21.7; HRMS (ESI-MS): calcd. for C22H22N3O2S [M + H]+ 392.1427; found 392.1410.
4-(3-(4-Methoxyphenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)benzenesulfonamide (11)
Mp 204–206 °C. 2.91 g (85%). 1H NMR (400 MHz, DMSO-d6, ppm) δ = 7.71 (d, 2H, J = 8.8 Hz), 7.56 (d, 2H, J = 8.8 Hz), 7.34–7.30 (m, 2H), 7.25–7.22 (m, 3H), 7.03 (d, 2H, J = 8.8 Hz), 7.01–6.97 (m, 2H), 6.99 (s, 2H, NH2), 5.56 (dd, 1H, J = 12.1, 5.1 Hz), 3.92 (dd, 1H, J = 17.6, 12.1 Hz), 3.78 (s, 3H, OCH3), 3.13 (dd, 1H, J = 17.6, 5.1 Hz); 13C NMR (100 MHz, DMSO-d6, ppm) δ = 160.9, 150.3, 146.8, 142.4, 133.3, 129.8, 128.4, 128.2, 127.8, 126.4, 125.0, 114.9, 112.4, 62.9, 55.9, 43.9; HRMS (ESI-MS): calcd. for C22H22N3O3S [M + H]+ 408.1376; found 408.1370.
4-(3-(4-Chlorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)benzenesulfonamide (12)
Mp 196–198 °C. 2.75 g (81%). 1H NMR (400 MHz, DMSO-d6, ppm) δ = 7.77 (d, 2H, J = 8.4 Hz), 7.58 (d, 2H, J = 9.0 Hz), 7.48 (d, 2H, J = 8.4 Hz), 7.34–7.31 (m, 2H), 7.25–7.22 (m, 3H), 7.07 (d, 2H, J = 9.0 Hz), 7.02 (s, 2H, NH2), 5.63 (dd, 1H, J = 12.1, 5.1 Hz), 3.94 (dd, 1H, J = 17.8, 12.1 Hz), 3.16 (dd, 1H, J = 17.8, 5.1 Hz); 13C NMR (100 MHz, DMSO-d6, ppm) δ = 149.2, 146.4, 142.2, 134.4, 134.0, 131.4, 129.8, 129.4, 128.4, 128.3, 127.8, 126.4, 112.8, 63.2, 43.5; HRMS (ESI-MS): calcd. for C21H19ClN3O2S [M + H]+ 412.0881; found 412.0873.
4-(3-(4-Fluorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)benzenesulfonamide (13)
Mp 202–204 °C. 3.03 g (87%). 1H NMR (400 MHz, DMSO-d6, ppm) δ = 7.82 (dd, 2H, J = 8.8, 5.5 Hz), 7.57 (d, 2H, J = 9.1 Hz), 7.34–7.22 (m, 7H), 7.05 (d, 2H, J = 9.1 Hz), 6.99 (s, 2H, NH2), 5.62 (dd, 1H, J = 12.1, 5.1 Hz), 3.95 (dd, 1H, J = 17.9, 12.1 Hz), 3.17 (dd, 1H, J = 17.9, 5.1 Hz); 13C NMR (100 MHz, DMSO-d6, ppm) δ = 163.4 (d, 1J = 247 Hz), 149.5, 146.6, 142.3, 133.8, 129.8, 129.1 (d, 4J = 3 Hz), 129.0 (d, 3J = 9 Hz), 128.3, 127.8, 126.4, 116.4 (d, 2J = 21 Hz), 112.7, 63.2, 43.8; HRMS (ESI-MS): calcd. for C21H19FN3O2S [M + H]+ 396.1177; found 396.1166.
4-(3-(4-Bromophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)benzenesulfonamide (14)
Mp 190–192 °C. 2.86 g (90%). 1H NMR (400 MHz, DMSO-d6, ppm) δ = 7.71 (d, 2H, J = 8.8 Hz), 7.62 (d, 2H, J = 8.8 Hz), 7.57 (d, 2H, J = 9.0 Hz), 7.34–7.31 (m, 2H), 7.25–7.22 (m, 3H), 7.06 (d, 2H, J = 9.0 Hz), 7.01 (s, 2H, NH2), 5.63 (dd, 1H, J = 12.2, 5.3 Hz), 3.94 (dd, 1H, J = 17.8, 12.2 Hz), 3.16 (dd, 1H, J = 17.8, 5.3 Hz); 13C NMR (100 MHz, DMSO-d6, ppm) δ = 149.3, 146.4, 142.2, 134.0, 132.4, 131.7, 129.8, 128.7, 128.4, 127.8, 126.4, 123.1, 112.8, 63.2, 43.4; HRMS (ESI-MS): calcd. for C21H19BrN3O2S [M + H]+ 456.0376; found 456.0364.
4-(3-(4-Nitrophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)benzenesulfonamide (15)
Mp 216–218 °C. 2.74 g (82%). 1H NMR (400 MHz, DMSO-d6, ppm) δ = 8.25 (d, 2H, J = 8.6 Hz), 7.99 (d, 2H, J = 8.6 Hz), 7.61 (d, 2H, J = 8.8 Hz), 7.35–7.32 (m, 2H), 7.26–7.24 (m, 3H), 7.14 (d, 2H, J = 8.8 Hz), 7.06 (s, 2H, NH2), 5.74 (dd, 1H, J = 12.4, 5.3 Hz), 4.01 (dd, 1H, J = 17.8, 12.4 Hz), 3.24 (dd, 1H, J = 17.8, 5.3 Hz); 13C NMR (100 MHz, DMSO-d6, ppm) δ = 148.3, 147.7, 145.8, 141.9, 138.8, 134.9, 129.9, 128.5, 127.8, 127.5, 126.4, 124.7, 113.4, 63.7, 43.1; HRMS (ESI-MS): calcd. for C21H19N4O4S [M + H]+ 423.1122; found 423.1120.
4-(3-(4-Hydroxyphenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)benzenesulfonamide (16)
Mp 184–186 °C. 0.77 g (22%). 1H NMR (400 MHz, DMSO-d6, ppm) δ = 9.86 (s, 1H, OH), 7.61 (d, 2H, J = 8.8 Hz), 7.54 (d, 2H, J = 8.8 Hz), 7.33–7.30 (m, 2H), 7.24–7.21 (m, 3H), 6.99 (d, 2H, J = 9.1 Hz), 6.97 (s, 2H, NH2), 6.81 (d, 2H, J = 8.8 Hz), 5.54 (dd, 1H, J = 11.7, 5.1 Hz), 3.89 (dd, 1H, J = 17.6, 11.7 Hz), 3.10 (dd, 1H, J = 17.6, 5.1 Hz); 13C NMR (100 MHz, DMSO-d6, ppm) δ = 159.5, 150.6, 146.8, 142.5, 133.1, 129.8, 128.5, 128.2, 127.8, 126.4, 123.4, 116.2, 112.3, 62.7, 43.9; HRMS (ESI-MS): calcd. for C21H20N3O3S [M + H]+ 394.1220; found 394.1206.
Biological activity
Carbonic anhydrase enzyme assay
The purification of cytosolic CA isoenzymes (CA I and II) were previously described with a simple one-step method by a Sepharose-4B-L tyrosine-sulfanilamide affinity chromatographyCitation23. The protein quantity in the column effluents was determined spectrophotometrically at 280 nm. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was applied with a Bio-Rad Mini Gel system (Mini-PROTEAN Tetra System, Guangdong, China) after purification of both CA isoenzymesCitation24,Citation25. Briefly, it was performed in acrylamide for the running (10%) and the stacking gel (3%) contained SDS (0.1%), respectively. Activities of CA I and II isoenzymes were determined according to the esterase method by Verpoorte et al.Citation26. The increase in absorbance of reaction medium was spectrophotometrically recorded at 348 nm (UV–VIS Spectrophotometer, Shimadzu, UVmini-1240, Kyoto, Japan). Also, the quantity of protein was determined at 595 nm according to Bradford methodCitation27. Bovine serum albumin was used as standard protein. The IC50 values were obtained from activity (%) versus compounds plots. For calculation of Ki values, three different concentrations were used. The Lineweaver–Burk curves were drawn and calculations were realisedCitation28.
Results and discussion
The compounds designed were successfully synthesized. The chemical structures of the compounds were confirmed by 1H NMR, 13C NMR, and HRMS spectra. The CA inhibition effects of the compounds were evaluated towards hCA I and hCA II isoenzymes. The inhibition values are presented in . As shown in , IC50 values were in the range of 402.9–554.8 nM towards hCA I, while they were in the range of 458.6–620.4 nM towards hCA II. The IC50 values of the reference compound AZA towards hCA I and hCA II were 985.8 nM and 489.4 nM, respectively. All compounds had lower IC50 value than AZA toward hCA I, while the compounds 11, 14, and 16 had lower IC50 value than AZA towards hCA II. According to IC50 values of the compounds, chlorine-bearing compound 12 and bromine-bearing compound 14 were the most effective compounds towards hCA I while it was hydroxy derivative 16 towards hCA II.
Table 1. Human CA isoenzymes (hCA I and II) inhibition value of the compounds (9–16) by the esterase method with 4-nitrophenyl acetate as substrate.
When Ki values of the compounds were considered, Ki values of the compounds were in the range of 316.7 ± 9.6–533.1 ± 187.8 nM towards hCA I, while Ki values were 412.5 ± 115.4–624.6 ± 168.2 nM towards hCA II. The Ki values of reference compound AZA were 278.8 ± 44.3 nM and 293.4 ± 46.4 nM towards hCA I and hCA II, respectively. When Ki values of the compounds were considered, 12 with chlorine, 14 with bromine, and 15 with nitro substituents, which have very close Ki values, are the leader compounds of series towards hCA I, while 14 was the leader compound of the series towards hCA II because of the lowest Ki values. All compounds were less effective than AZA on both hCA I and II isoenzymes, since Ki value of the compound had higher values than AZA’s.
Any substitution rather than hydrogen at the para position of phenyl ring decreased Ki value of the substituted compound toward hCA I by comparing with 9, which is non-substituted derivative. This means that substitution at the para position of phenyl ring was a useful modification to increase the effect of compound toward hCA I. Exception was 11, which is a methoxy-substituted derivative. The effect of any substituent on phenyl ring to Ki value of substituted compound towards hCA II isoenzyme was similar to hCA I’s. It means that any type of substituent on phenyl ring rather than hydrogen was a useful modification to increase the compounds effect on hCA II by lowering the Ki values without exception.
Conclusion
In conclusion, the compounds 12, 14, and 15 towards hCA I and 14 towards hCA II seem to be the leader compounds of the series for further investigations.
Disclosure statement
The authors report that they have no conflicts of interest and are responsible for the contents and writing of the paper. The authors H. I. G. and E. M. thank to Ataturk University for the financial support. This research work was supported by Ataturk University Research Found, Turkey (Project No: 2013/289).
References
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–89.
- Supuran CT. Carbonic anhydrases as drug targets – an overview. Curr Top Med Chem 2007;7:825–33.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Scozzafava A, Menabuoni L, Mincione F, Supuran CT. Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyamino-polycarboxylate tails and of their metal complexes possessing long-lasting, topical intraocular pressure-lowering properties. J Med Chem 2002;45:1466–76.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- Ghorab MM, Alsaid MS, Al-Dosary MS, et al. Design, synthesis and anticancer activity of some novel thioureido-benzenesulfonamides incorporated biologically active moieties. Chem Cent J 2016;10:1–13.
- Gul HI, Tugrak M, Sakagami H, et al. Synthesis and bioactivity studies on new 4-(3-(4-substitutedphenyl)-3a,4-dihydro-3H-indeno[1,2-c]pyrazol-2-yl) benzenesulfonamides. J Enzyme Inhib Med Chem 2016;31:1619–24.
- Gul HI, Kucukoglu K, Yamali C, et al. Synthesis of 4-(2-substituted hydrazinyl)benzenesulfonamides and their carbonic anhydrase inhibitory effects. J Enzyme Inhib Med Chem 2016;31:568–73.
- Mete E, Comez B, Gul HI, et al. Synthesis and carbonic anhydrase inhibitory activities of new thienyl-substituted pyrazoline benzenesulfonamides. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.1080/14756366.2016.1181627.
- Chen L, Yang D, Pan Z, et al. Synthesis and antimicrobial activity of the hybrid molecules between sulfonamides and active antimicrobial pleuromutilin derivative. Chem Biol Drug Des 2015;86:239–45.
- Mujeeb Ur R, Rathore A, Siddiqui AA, et al. Synthesis and characterization of quinazoline derivatives: search for hybrid molecule as diuretic and antihypertensive agents. J Enzyme Inhib Med Chem 2014;29:733–43.
- Chen Z, Wang ZC, Yan XQ, et al. Design, synthesis, biological evaluation and molecular modeling of dihydropyrazole sulfonamide derivatives as potential COX-1/COX-2 inhibitors. Bioorg Med Chem Lett 2015;25:1947–51.
- Anusha S, Sinha A, Babu Rajeev CP, et al. Synthesis, characterization and in vitro evaluation of novel enantiomerically-pure sulfonamide antimalarials. Org Biomol Chem 2015;13:10681–90.
- Kasimogullari R, Bulbul M, Gunhan H, Guleryuz H. Effects of new 5-amino-1,3,4-thiadiazole-2-sulfonamide derivatives on human carbonic anhydrase isozymes. Bioorg Med Chem 2009;17:3295–301.
- Naim MJ, Alam O, Nawaz F, et al. Current status of pyrazole and its biological activities. J Pharm Bioallied Sci 2016;8:2–17.
- Khan MF, Alam MM, Verma G, et al. The therapeutic voyage of pyrazole and its analogs: a review. Eur J Med Chem 2016;120:170–201.
- Kucukoglu K, Oraf F, Aydin T, et al. Synthesis, cytotoxicity and carbonic anhydrase inhibitory activities of new pyrazolines. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.1080/14756366.2016.1217852.
- Rahman MA, Siddiqui AA. Pyrazoline derivatives: a worthy insight into the recent advances and potential pharmacological activities. Int J Pharm Sci Drug Res 2010;2:165–75.
- Marini AM, Maresca A, Aggarwal M, et al. Tricyclic sulfonamides incorporating benzothiopyrano[4,3-c]pyrazole and pyridothiopyrano[4,3-c]pyrazole effectively inhibit alpha- and beta-carbonic anhydrase: X-ray crystallography and solution investigations on 15 isoforms. J Med Chem 2012;55:9619–29.
- Dimmock JR, Kandepu NM, Hetherington M, et al. Cytotoxic activities of Mannich bases of chalcones and related compounds. J Med Chem 1998;41:1014–26.
- Yamali C, Tugrak M, Gul HI, et al. The inhibitory effects of phenolic Mannich bases on carbonic anhydrase I and II isoenzymes. J Enzyme Inhib Med Chem 2016;31:1678–81.
- Tugrak M, Yamali C, Sakagami H, Gul HI. Synthesis of mono Mannich bases of 2-(4-hydroxybenzylidene)-2,3-dihydroinden-1-one and evaluation of their cytotoxicities. J Enzyme Inhib Med Chem 2016;31:818–23.
- Akincioglu A, Topal M, Gulcin I, Goksu S. Novel sulphamides and sulfonamides incorporating the tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch Pharm (Weinheim) 2014;347:68–76.
- Senturk M, Gulcin I, Dastan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11.
- Bayram E, Senturk M, Kufrevioglu OI, Supuran CT. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg Med Chem 2008;16:9101–5.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66.