 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In our present research, we synthesised new thiazolidine-2,4-diones (12–28). All the newly synthesised compounds were evaluated for antiproliferative and antibacterial activity. Antiproliferative evaluation was carried out using normal human skin fibroblasts and tumour cell lines: A549, HepG2, and MCF-7. The IC50 values were determined for tested compounds revealing antiproliferative activity. Moreover, safety index (SI) was calculated. Among all tested derivatives, the compound 18 revealed the highest antiproliferative activity against human lung, breast, and liver cancer cells. More importantly, the derivative 18 showed meaningfully lower IC50 values when compared to the reference substance, irinotecan, and relatively high SI values. Moreover, newly synthesised compounds were screened for the bacteria growth inhibition in vitro. According to our screening results, most active compound was the derivative 18 against Gram-positive bacteria. Therefore, it may be implied that the novel compound 18 appears to be a very promising agent for anticancer treatment.
Graphical Abstract
Introduction
Cancer is one of the most serious health problems in the world. It is the second cause of death after heart diseases worldwide. According to the World Cancer Report 2014, 8.2 million people died from cancer in 2012Citation1.
Lung, liver, stomach, and bowel cancer are the most common causes of human deaths worldwide, accounting for nearly a half of all cancer deaths. The five most common types of disease diagnosed in 2012 were lung, prostate, colorectal, stomach, and liver cancer among men; and breast, colorectal, lung, cervix, and stomach cancer among womenCitation1.
Despite enormous efforts aimed at the implementation of new treatment strategies of chemotherapeutic agents, treatment results in most cases are unsatisfactoryCitation2. Therefore, there is an urgent need to find new classes of substances with selective action against tumour cells.
Heterocyclic compounds play an important role in cancer therapy. Among them, the derivatives of thiazolidine-2,4-dione are foundCitation3–6. Researchers’ interest in the derivatives of thiazolidine-2,4-dione has increased recently, the main reason being a wide spectrum of biological properties shown by these derivatives. It has been confirmed by numerous reviews on the activity and mechanisms of action of thiazolidine-2,4-dionesCitation2,Citation7,Citation8.
Thiazolidine-2,4-diones are known as antidiabetic drugs and include rosiglitazone, pioglitazone, and darglitazone. Moreover, thiazolidine-2,4-dione derivatives possess biological activities such as aldose reductase inhibitoryCitation9, antibacterialCitation6,Citation10–13, antifungalCitation10, antitubercularCitation14, and anti-inflammatory activityCitation15, etc.
Regulation of both cell proliferation and pathways of apoptosis connected with cell death is important in understanding various diseases including malignanciesCitation16. Therefore, identification of regulators of the cell cycle and apoptosis stimulators is an attractive strategy to explore potential anticancer agentsCitation17.
There are several mechanisms of anticancer activity of thiazolidine-2,4-dione derivatives already discussed in the literature. The most known ones are induction of apoptosis, cell differentiation, and cell cycle arrestCitation8. The thiazolidine-2,4-dione derivatives showing anticancer activity are mainly derivatives modified in the position 5 of the thiazolidine-2,4-dione ring.
Patil et al. reported that 10 new derivatives of 5-benzylidenethiazolidine-2,4-dione had displayed a variable degree of antiproliferative activity against seven tumour cell lines. 2-{4-[(2,4-dioxo-1,3-thiazolidin-5-ylidene)methyl]phenoxy}-N-[3-(trifluoromethyl)phenyl]acetamide showed the most significant effect against breast cancer MCF-7 and leukaemia K562 cell linesCitation3.
New 5-substituted pyrazoline thiazolidine-4-one was tested for anticancer activity according to the NCI protocol. The most active compounds were the derivatives of 2-(2,4-dioxo-1,3-thiazolidin-5-ylidene)acetic acid, namely 5-{2-[5-(2-hydroxyphenyl)-3-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl]-2-oxoethylidene}-1,3-thiazolidine-2,4-dione and 5-{2-[5-(2-hydroxyphenyl)-3-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl]-2-oxoethylidene}-1,3-thiazolidine-2,4-dione. These derivatives displayed the highest inhibitory activity against leukaemia cells (HL-60 (TB)) and CNS cancer (SF-295)Citation5.
A number of derivatives of 2,4-dioxothiazolidine-5-acetic acid with the ring of 5-substituted-2-amino-1,3,4-thiadiazole showed cytotoxic effects in vitro against four human tumour cell lines (cervical carcinoma – HeLa, colorectal cancer – HT29, lung cancer – A549, and breast cancer – MCF-7). Among the 14 derivatives, 2-(2,4-dioxo-1,3-thiazolidin-5-yl)-N-[5-(3,4,5-trimethoxyphenyl)-1,3,4-thiadiazol-2-yl]acetamide showed significant inhibitory activity against the tested cell linesCitation6.
Havrylyuk et al. described 2,4-dioxothiazolidine-5-acetic derivatives with benzoxazole or benzothiazole substituents that showed in vitro cytotoxic activity against some tumour cell lines: leukaemia (HL-60(TB), K-562, MOLT-4), breast cancer (MDA-MB-231/ATCC), CNS cancer (SF-268), melanoma (LOX IMVI), and prostate cancer, all tested according to the NCI protocol. Among the tested compounds, three were the most active: 3-methoxy-4-(2,4-thiazolidinedione-5-acetoxy)benzylidenehydrazone of benzoxazole-2-thioacetic acid, 3-methoxy-4-(2,4-thiazolidinedione-5-acetoxy)benzylidenehydrazone of benzothiazole-2-thioacetic acid, and 4-(2,4-thiazolidinedione-5-acetoxy)benzylidenehydrazone of benzothiazole-2-thioacetic acidCitation4.
The aim of the present research was to synthesise new thiazolidine-2,4-dione derivatives and to evaluate in vitro their potential as anticancer and antibacterial agents. As thiosemicarbazoneCitation18–20 and acylhydrazone derivativesCitation20–24 present anticancer activity, which is similar to the activity of the above mentioned thiazolidine-2,4-diones, it was assumed that the structure modification of the thiazolidine-2,4-dione ring in position 5 by thiosemicarbazide and hydrazide derivatives can extend the biological activity of the new compounds.
Experimental
Chemistry
Materials and methods
Melting points were determined using Fisher-Johns apparatus (Fisher Scientific, Schwerte, Germany) and were not corrected. The 1H NMR and 13 C NMR spectra were recorded by a Bruker Avance 300 MHz instrument using DMSO-d6 as solvent and TMS as an internal standard. Chemical shifts were expressed as δ (ppm). MS using atmospheric pressure chemical ionisation (APCI) was recorded on a Bruker MicroTOF II mass spectrometer. APCI settings were as follows: vaporiser temperature, 350 °C; drying gas temperature, 180 °C; drying gas flow, 4 l/min; and nebuliser pressure, 2 bar. The purity of the compounds was checked by TLC on plates with silica gel Si 60 F254, produced by Merck Co. (Darmstadt, Germany). Elemental analyses were performed by AMZ 851 CHX analyser and the results were within ±0.4% of the theoretical value.
General procedure for the synthesis of hydrazones (12–21) or thiosemicarbazones (22–28)
Anhydrous ethanol (5–10 ml) was added to the mixture of 0.001 mol hydrazide (3-chlorobenzhydrazide, 2,4-dichlorobenzhydrazide), thiosemicarbazide or appropriate 4-substituted thiosemicarbazide, and 0.001 mol corresponding compoundsCitation5–11. Then, the mixture was heated under reflux till the dissolving of the substrates. The reflux lasted 5–15 min but in cases of compounds 13, 14, 17, 28 reflux lasted 1.5 h. After that, the mixture was cooled and precipitate was filtered off, dried, and crystallised from butanol or acetic acid.
3-{[2-(3-Chlorobenzoyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (12)
Yield 87%, m.p. 244–245 °C, 1H NMR δ ppm (DMSO-d6): 3.45–3.47 m (2H, CH–CH2); 4.86–4.90 m (1H, CH–CH2); 7.20–7.23 m, 7.51–7.70 m, 7.89 d, 7.97 s (8H, Ar, J = 7.5 Hz); 8.45 s (1H, CH=N); 12.03 s (1H, NHCO); 12.16 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 36.4 (CH2CH), 46.8 (CH2CH), 119.9, 123.9, 126.0, 127.0, 127.8, 130.7, 131.0, 132.2, 133.8, 135.8, 136.4, 147.6 (12 × Car.), 151.0 (CH=N), 162.3, 169.6 (2 × C=O), 172.7, 175.9 (2 × C=O, thiazolidine). MS (APCI) [M]+ m/z = 432.0441. Anal. calc. for C19H14ClN3O5S (431.85) (%): C 52.84; H 3.27; N 9.73. Found: C 52.80; H 3.24; N 9.70.
3-{[2-(2,4-Dichlorobenzoyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (13)
Yield 95%, m.p. 198–200 °C, 1H NMR δ ppm (DMSO-d6): 3.44–3.47 m (2H, CH–CH2); 4.85–4.90 m (1H, CH–CH2); 7.20–7.24 m, 7.52–7.66 m, 7.79 d (7H, Ar, J = 1.8 Hz); 8.27 s (1H, CH=N); 12.06 s (1H, NHCO); 12.15 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 36.4 (CH2CH), 46.9 (CH2CH), 120.1, 123.6, 123.9, 126.0, 128.0, 129.1, 129.8, 130.7, 131.2, 132.2, 136.1, 147.6 (12 × Car.), 151.0 (CH=N), 162.2, 169.6 (2 × C=O), 172.7, 176.0 (2 × C=O, thiazolidine). MS (APCI) [M]+ m/z = 466.0046. Anal. calc. for C19H13Cl2N3O5S (466.29) (%): C 48.94; H 2.81; N 9.01. Found: C 48.90; H 2.77; N 8.99.
4-{[2-(3-Chlorobenzoyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (14)
Yield 83%, m.p. 215–216 °C, 1H NMR δ ppm (DMSO-d6): 3.44–3.47 m (2H, CH–CH2); 4.85–4.89 m (1H, CH–CH2); 7.25 d, 7.81 d (4H, 4-O–C6H4, J = 8.7 Hz); 7.55–7.70 m, 7.89 d, 7.97 s (4H, Ar, J = 7.5 Hz); 8.46 s (1H, CH=N); 11.98 s (1H, NHCO); 12.16 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 36.4 (CH2CH), 46.8 (CH2CH), 122.7, 127.0, 127.8, 128.9, 131.0, 132.1, 132.6, 133.8, 135.8, 147.8 (12 × Car.), 151.9 (CH=N), 162.2, 169.4 (2 × C=O), 172.7, 176.0 (2 × C=O, thiazolidine). MS (APCI) [M]+ m/z = 432.0420. Anal. calc. for C19H14ClN3O5S (431.85) (%): C 52.84; H 3.27; N 9.73. Found: C 52.79; H 3.23; N 9.72.
4-{[2-(2,4-Dichlorobenzoyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (15)
Yield 61%, m.p. 196–198 °C, 1H NMR δ ppm (DMSO-d6): 3.44–3.47 m (2H, CH–CH2); 4.85–4.89 m (1H, CH–CH2); 7.25 d (2H, 4-O–C6H4, J = 8.7 Hz); 7.52–7.66 m, (2H, Ar); 7.78–7.82 m (3H, Ar); 8.28 s (1H, CH=N); 12.00 s (1H, NHCO); 12.16 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 36.4 (CH2CH), 46.8 (CH2CH), 122.7, 127.7, 128.0, 129.0, 131.2, 132.2, 132.4, 134.5, 135.7, 147.7 (12 × Car.), 151.9 (CH=N), 162.1, 169.4 (2 × C=O), 172.7, 175.9 (2 × C=O, thiazolidine). MS (APCI) [M]+ m/z = 465.9945. Anal. calc. for C19H13Cl2N3O5S (466.29) (%): C 48.94; H 2.81; N 9.01. Found: C 48.92; H 2.75; N 9.00.
4-{[2-(3-Chlorobenzoyl)hydrazinylidene]methyl}-2-methoxyphenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (16)
Yield 92%, m.p. 220–222 °C, 1H NMR δ ppm (DMSO-d6): 3.42–3.44 m (2H, CH–CH2); 3.85 s (3H, OCH3); 4.84–4.88 m (1H, CH–CH2); 7.21 d, 7.31–7.34 m, 7.48–7.70 m, 7.87–7.90 m, 7.97 s (7H, Ar, J = 8.1 Hz); 8.44 s (1H, CH=N); 12.00 s (1H, NHCO); 12.14 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 36.1 (CH2CH), 46.9 (CH2CH), 56.4 (OCH3), 110.5, 121.1, 123.6, 127.0, 127.8, 131.0, 132.1, 133.8, 133.9, 135.9, 141.0, 148.1 (12 × Car.), 151.5 (CH=N), 162.2, 168.8 (2 × C=O), 172.7, 175.8 (2 × C=O, thiazolidine). MS (APCI) [M]+ m/z = 462.0488. Anal. calc. for C20H16ClN3O6S (461.88) (%): C 52.01; H 3.49; N 9.10. Found: C 52.00; H 3.47; N 9.06.
4-{[2-(2,4-Dichlorobenzoyl)hydrazinylidene]methyl}-2-methoxyphenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (17)
Yield 87%, m.p. 186–188 °C, 1H NMR δ ppm (DMSO-d6): 3.41–3.44 m (2H, CH–CH2); 3.84 s (3H, OCH3); 4.84–4.87 m (1H, CH–CH2); 7.20 d, 7.32 dd, 7.48–7.58 m, 7.64 d, 7.78 d (6H, Ar, J1=8.4 Hz, J2 = 1.8 Hz); 8.25 s (1H, CH=N); 12.01 s (1H, NHCO); 12.16 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 36.0 (CH2CH), 46.9 (CH2CH), 56.4 (OCH3), 110.7, 121.1, 123.6, 128.0, 129.8, 131.2, 132.2, 133.6, 134.5, 135.7, 141.0, 148.0 (12 × Car.), 151.5 (CH=N), 162.2, 168.8 (2 × C=O), 172.7, 175.8 (2 × C=O, thiazolidine). MS (APCI) [M]+ m/m/z = 496.0087. Anal. calc. for C20H15Cl2N3O6S (496.32) (%): C 48.40; H 3.05; N 8.47. Found: C 48.37; H 3.03; N 8.44.
2-{[2-(3-Chlorobenzoyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-ylidene)acetate (18)
Yield 88%, m.p. 243–245 °C, 1H NMR δ ppm (DMSO-d6): 7.16 s (1H, CH=); 7.34–7.46 m, 7.52–7.68 m, 7.84–7.99 m (8H, Ar); 8.51 s (1H, CH=N); 11.94 s (1H, NHCO); 12.95 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 116.5 (CH = C), 123.5, 126.6, 127.0, 127.5, 127.8, 131.0, 131.8, 132.2, 133.8, 134.3, 135.7, 143.1 (12 × Car.), 145.7 (CH=C), 148.9 (CH=N), 162.1, 164.3 (2 × C=O), 166.6, 169.3 (2 × C=O, thiazolidine). MS (APCI) [M]+ m/z = 430.0207. Anal. calc. for C19H12ClN3O5S (429.83) (%): C 53.09; H 2.81; N 9.78. Found: C 53.06; H 2.82; N 9.79.
3-{[2-(3-Chlorobenzoyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-ylidene)acetate (19)
Yield 91%, m.p. 282–283 °C, 1H NMR δ ppm (DMSO-d6): 7.08 s (1H, CH=); 7.31–7.34 m, 7.54–7.70 m, 7.88–7.97 m (8H, Ar); 8.47 s (1H, CH=N); 12.05 s (1H, NHCO); 12.98 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 116.6 (CH = C), 120.0, 123.7, 124.0, 126.0, 127.0, 127.9, 130.7, 131.0, 132.2, 133.8, 135.7, 136.4 (12 × Car.), 147.5 (CH=C), 150.8 (CH=N), 162.3, 164.3 (2 × C=O), 166.9, 169.6 (2 × C=O, thiazolidine). MS (APCI) [M]+ m/z = 430.0212. Anal. calc. for C19H12ClN3O5S (429.83) (%): C 53.09; H 2.81; N 9.78. Found: C 53.05; H 2.77; N 9.74.
4-{[2-(3-Chlorobenzoyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-ylidene)acetate (20)
Yield 93%, m.p. 278–279 °C, 1H NMR δ ppm (DMSO-d6): 7.08 s (1H, CH=); 7.37 d, 7.55–7.69 m, 7.82–7.91 m, 7.97 s (8H, Ar, J = 8.4 Hz); 8.48 s (1H, CH=N); 12.00 s (1H, NHCO); 12.98 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 116.7 (CH = C), 122.6, 127.0, 127.8, 128.9, 131.0, 132.1, 132.9, 133.8, 135.8, 147.7 (12 × Car.), 145.4 (CH=C), 151.7 (CH=N), 162.2, 164.1 (2 × C=O), 166.5, 169.4 (2 × C=O, thiazolidine). MS (APCI) [M]+ m/z = 430.0210. Anal. calc. for C19H12ClN3O5S (429.83) (%): C 53.09; H 2.81; N 9.78. Found: C 53.13; H 2.84; N 9.76.
4-{[2-(3-Chlorobenzoyl)hydrazinylidene]methyl}-2-methoxyphenyl (2,4-dioxo-1,3-thiazolidin-5-ylidene)acetate (21)
Yield 93%, m.p. 246–248 °C, 1H NMR δ ppm (DMSO-d6): 3.86 s (3H, OCH3); 7.09 s (1H, CH=); 7.30–7.38 m, 7.52–7.70 m, 7.88–7.90 m, 7.97 s (7H, Ar); 8.47 s (1H, CH=N); 12.02 s (1H, NHCO); 12.98 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 56.5 (OCH3), 115.8 (CH = C), 110.5, 121.1, 123.6, 127.0, 127.8, 131.0, 132.1, 133.8, 134.2, 135.8, 140.6, 148.0 (12 × Car.), 146.2 (CH=C), 151.4 (CH=N), 162.2, 163.7 (2 × C=O), 166.6, 169.4 (2 × C=O, thiazolidine). MS (APCI) [M]+ m/z = 460.0306. Anal. calc. for C20H14ClN3O6S (459.86) (%): C 52.24; H 3.07; N 9.14. Found: C 52.22; H 3.02; N 9.10.
3-{[2-(Phenylcarbamothioyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (22)
Yield 86%, m.p. 209–211 °C, 1H NMR δ ppm (DMSO-d6): 3.42–3.45 m (2H, CH–CH2); 4.84–4.88 m (1H, CH–CH2); 7.17–7.25 m, 7.36–7.56 m, 7.74–7.78 m (9H, Ar); 8.16 s (1H, CH=N); 10.17 s, 11.89 s (NHCSNH); 12.16 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 36.4 (CH2CH), 46.8 (CH2CH), 120.3, 123.8, 126.0, 126.4, 126.7, 128.6, 130.4, 136.3, 139.5, 142.2 (12 × Car.), 151.0 (CH=N), 169.6 (C=O), 172.7, 175.9 (2 × C=O, thiazolidine), 176.7 (C=S). MS (APCI), fragment ion (m/z): 355.0727; 336.0097; 277.0273; 179.0266; 157.9907; 136.0225; 94.0657. Anal. calc. for C19H16N4O4S2 (428.48) (%): C 53.26; H 3.76; N 13.08. Found: C 53.29; H 3.77; N 13.09.
3-[{2-[(4-Methylphenyl)carbamothioyl]hydrazinylidene}methyl]phenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (23)
Yield 80%, m.p. 194–196 °C, 1H NMR δ ppm (DMSO-d6): 2.32 s (3H, CH3); 3.42–3.45 m (2H, CH–CH2); 4.83–4.87 m (1H, CH–CH2); 7.18 d, 7.39–7.51 m, 7.73–7.77 m (8H, Ar, J = 8.4 Hz); 8.15 s (1H, CH=N); 10.10 s, 11.84 s (NHCSNH); 12.16 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 21.1 (CH3), 36.4 (CH2CH), 46.8 (CH2CH), 120.2, 123.7, 126.4, 126.6, 129.0, 130.4, 135.1, 136.3, 136.9, 142.0 (12 × Car.), 151.0 (CH=N), 169.6 (C=O), 172.7, 175.9 (2 × C=O, thiazolidine), 176.8 (C=S). MS (APCI) [M]+ m/z = 443.0791. Anal. calc. for C20H18N4O4S2 (442.51) (%): C 54.29; H 4.10; N 12.66. Found: C 54.23; H 3.95; N 12.63.
4-[(2-Carbamothioylhydrazinylidene)methyl]phenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (24)
Yield 66%, m.p. 208–210 °C, 1H NMR δ ppm (DMSO-d6): 3.42–3.45 m (2H, CH–CH2); 4.84–4.88 m (1H, CH–CH2); 7.17 d, 7.88 d (4H, Ar, J = 8.7 Hz); 8.05 s (2H, NH2); 8.22 s (1H, CH=N); 11.46 s (NHCS); 12.16 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 36.4 (CH2CH), 46.8 (CH2CH), 122.4, 129.0, 132.7, 141.6 (6 × Car.), 151.5 (CH=N), 169.4 (C=O), 172.7, 175.9 (2 × C=O, thiazolidine), 178.5 (C=S). MS (APCI) [M]+ m/z = 353.0346. Anal. calc. for C13H12N4O4S2 (352.39) (%): C 44.31; H 3.43; N 15.90. Found: C 44.27; H 3.38; N 15.86.
4-{[2-(Phenylcarbamothioyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (25)
Yield 84%, m.p. 159–162 °C, 1H NMR δ ppm (DMSO-d6): 3.43–3.46 m (2H, CH–CH2); 4.85–4.89 m (1H, CH–CH2); 7.19–7.24 m, 7.38 t, 7.56 d (7H, Ar, J = 7.5 Hz); 7.99 d (2H, 4-O–C6H4, J = 8.7 Hz); 8.17 s (1H, CH=N); 10.15 s, 11.86 s (NHCSNH); 12.16 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 36.5 (CH2CH), 46.8 (CH2CH), 122.4, 125.9, 126.5, 128.5, 129.4, 132.5, 139.5, 142.3 (12 × Car.), 151.7 (CH=N), 169.4 (C=O), 172.7, 175.9 (2 × C=O, thiazolidine), 176.5 (C=S). MS (APCI), fragment ion (m/z): 338.3411; 284.2929; 257.2465; 149.0230. Anal. calc. for C19H16N4O4S2 (428.48) (%): C 53.26; H 3.76; N 13.08. Found: C 53.22; H 3.77; N 13.08.
2-Methoxy-4-{[2-(phenylcarbamothioyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-yl)acetate (26)
Yield 88%, m.p. 182–184 °C, 1H NMR δ ppm (DMSO-d6): 3.40–3.43 m (2H, CH–CH2); 3.85 s (3H, OCH3); 4.83–4.87 m (1H, CH–CH2); 7.15–7.25 m, 7.36–7.47 m, 7.54–7.58 m, 7.67 d (8H, Ar, J = 1.8 Hz); 8.14 s (1H, CH=N); 10.12 s, 11.90 s (NHCSNH); 12.13 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 36.1 (CH2CH), 46.9 (CH2CH), 56.7 (OCH3), 111.5, 121.4, 123.3, 126.0, 126.7, 128.6, 133.7, 139.6, 140.8, 142.6 (12 × Car.), 151.4 (CH=N), 168.8 (C=O), 172.7, 175.8 (2 × C=O, thiazolidine), 176.6 (C=S). MS (APCI) [M]+ m/z = 458.0958. Anal. calc. for C20H18N4O5S2 (458.51) (%): C 52.39; H 3.96; N 12.22. Found: C 52.40; H 3.95; N 12.21.
4-{[2-(Phenylcarbamothioyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-ylidene)acetate (27)
Yield 84%, m.p. 199–202 °C, 1H NMR δ ppm (DMSO-d6): 7.06 s (1H, CH=); 7.19–7.41 m, 7.56 d (7H, Ar, J = 8.1 Hz); 8.02 d (2H, 4-O–C6H4, J = 8.7 Hz); 8.18 s (1H, CH=N); 10.16 s, 11.88 s (NHCSNH); 12.72 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 116.6 (CH = C), 122.3, 125.9, 126.5, 128.5, 129.4, 132.8, 139.5, 142.2 (12 × Car.), 145.6 (CH=C), 151.6 (CH=N), 164.2 (C=O), 166.8, 169.5 (2 × C=O, thiazolidine), 176.6 (C=S). MS (APCI), fragment ion (m/z): 353.0611; 333.9962; 275.0140; 155.9762; 136.0231; 94.0658. Anal. calc. for C19H14N4O4S2 (426.47) (%): C 53.51; H 3.31; N 13.14. Found: C 53.47; H 3.25; N 13.11.
2-Methoxy-4-{[2-(phenylcarbamothioyl)hydrazinylidene]methyl}phenyl (2,4-dioxo-1,3-thiazolidin-5-ylidene)acetate (28)
Yield 93%, m.p. 186–187 °C, 1H NMR δ ppm (DMSO-d6): 3.86 s (3H, OCH3); 7.08 s (1H, CH=); 7.20–7.28 m, 7.39 t, 7.48–7.58 m, 7.71 d (8H, Ar, J1=7.5 Hz, J2=1.5 Hz); 8.17 s (1H, CH=N); 10.14 s, 11.92 s (NHCSNH); 12.94 s (1H, NH, thiazolidine), 13 C NMR δ ppm (DMSO-d6): 56.7 (OCH3), 115.9 (CH = C), 111.5, 121.4, 123.4, 126.0, 126.7, 128.6, 134.1, 139.6, 140.5, 142.5 (12 × Car.), 146.0 (CH=C), 151.4 (CH=N), 163.7(C=O), 166.5, 169.4 (2 × C=O, thiazolidine), 176.6 (C=S). MS (APCI), fragment ion (m/z): 383.0639; 302.0948; 150.0544; 136.0216; 94.0655. Anal. calc. for C20H16N4O5S2 (456.49) (%): C 52.62; H 3.53; N 12.27. Found: C 52.63; H 3.54; N 12.30.
Bioassays
Antiproliferative activity in vitro
The experiment was carried out using normal human skin fibroblasts (BJ) and tumour cell lines: human lung carcinoma (A549), human hepatocellular carcinoma (HepG2), and human breast adenocarcinoma (MCF-7). All cell lines were obtained from ATCC (American Type Culture Collection, England, UK) and cultured in the appropriate, recommended by ATCC, culture medium, additionally supplemented with 10% foetal bovine serum and antibiotics: 100 U/ml penicillin and 100 µg/ml streptomycin. The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Antiproliferative activity of the new compounds was assessed by, commonly used for this purpose, colorimetric MTT assay. Cells with active mitochondrial dehydrogenases reduced intracellularly the tetrazolium dye into purple formazan, which is proportional to the number of viable cells. The cells were seeded in 96-multiwell plates in 100 μl of the appropriate complete culture medium at a concentration of 7 × 103 cells/well in the case of BJ cells (normal fibroblasts), 1 × 104 cells/well in the case of A549 cells (lung cancer), 1.5 × 104 cells/well in the case of HepG2 cells (liver cancer), and 2 × 104 cells/well in the case of MCF-7 cells (breast cancer) so as to obtain 60–70% confluent culture after 24 h of incubation at 37 °C – the cells are then in the log (logarithmic) phase, during a period of active proliferation, but still have an unoccupied space to divide. Afterwards, the culture medium was discarded and replaced with 100 µl of different concentrations of new tested compounds and reference antiproliferative agent: irinotecan. The stock solutions of the compounds were prepared in DMSO, and solvent control was being tested concurrently to exclude toxicity attributed to DMSO. Ten serial two-fold dilutions using culture medium were performed to prepare different concentrations of the tested compounds, and the highest tested concentration was 200 µg/ml. Untreated cells (maintained in the complete culture medium) served as a negative control. After exposing cells for 48 h to the tested derivatives, the MTT assay was performed as described earlierCitation25. Three independent experiments (n = 3) were performed in a quadruplicate. The obtained data were analysed using GraphPad Prism 5, version 5.03 software (La Jolla, CA). The IC50 values were determined for tested compounds revealing antiproliferative activity. Moreover, safety index (SI) was calculated using the following formula:
Microbiological tests
Newly synthesised compounds’ antibacterial activities were tested in vitro against six aerobic bacteria reference strains: three Gram-positive (Staphylococcus aureus ATCC 25923, methicillin-resistant S. aureus MIKROBANK 14.001 reference strain from National Medicines Institute in Warsaw, and Staphylococcus epidermidis ATCC 12228) and three Gram-negative (Escherichia coli ATCC 25922, Proteus mirabilis ATCC 12453, and Pseudomonas aeruginosa ATCC 9027).
Bacterial cell suspensions equivalent to 0.5 McFarland (1.5 × 108 CFU/ml) were prepared in 0.85% sterile saline. Tested compounds’ stock solutions (50 mg/ml) were dissolved in dimethyl sulphoxide (DMSO). DMSO final concentration did not inhibit bacteria growth.
Bacteria strain susceptibility to tested agents was determined by broth microdilution method. Broth microdilution method was carried out in accordance with Clinical and Laboratory Standards Institute recommendations and was used as reference methodCitation26. Mueller–Hinton broth containing two-fold dilutions of the tested substances (at concentrations ranging from 3.91 to 1000 µg/ml) was used to determine their minimal inhibitory concentration (MIC) which is the lowest concentration of an antibacterial that will inhibit the visible growth of a microorganism after 18 h of incubation). Mueller–Hinton broth is recommended as the medium of choice for susceptibility testing of commonly isolated, rapidly growing aerobic, or facultative organisms. Gentamicin (KRKA, Slovenia), an aminoglycoside antibiotic active against a wide range of human bacterial infections, mostly Gram-negative bacteria including Pseudomonas, Proteus, Serratia, and the Gram-positive Staphylococcus, was used as a control antibacterial agent (at concentration from 0.12 µg to 1000 µg).
Tested compounds two-fold dilutions in Mueller–Hinton broth without bacteria were blank control wells (incubated on equal conditions). Polystyrene trays containing 96 wells were filled with serial two-fold Mueller–Hinton broth dilutions of tested agents. Next, the broth was inoculated with 20 μl of 0.5 McFarland microbial suspension (previously diluted 1:10 by Mueller–Hinton broth). Total volume per each well was 200 μl. After incubation (at 35 °C for 18 h), bacterial cultures’ optical density was measured by microplate reader (BioTek Synergy H4 Hybrid Reader) at 600 nm. The MIC values were determined by the comparison of broth bacterial culture OD600 containing tested concentrations of examined substances to blank control wells.
Results and discussions
Chemistry
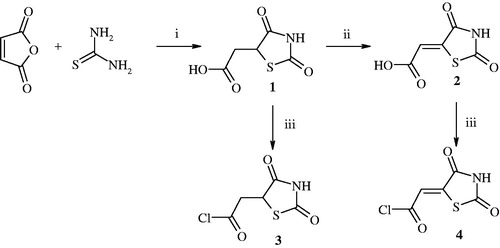
2-(2,4-Dioxothiazolidin-5-yl)acetic chlorideCitation3 and 2-(2,4-dioxothiazolidin-5-ylidene)acetic chlorideCitation4 were used as a starting material for the synthesis of target compounds. Those acetic chloridesCitation3,Citation4 were synthesised by the reaction of 2-(2,4-dioxothiazolidin-5-yl)acetic acid or 2-(2,4-dioxothiazolidin-5-ylidene)acetic acid with thionyl chloride in anhydrous 1,4-dioxane medium. In turn, 2-(2,4-dioxothiazolidin-5-yl)acetic acidCitation1 was prepared from thiourea and maleic anhydride in the presence of concentrated hydrochloric acidCitation27. 2-(2,4-dioxothiazolidin-5-ylidene)acetic acidCitation2 was obtained by the reaction of compound 1 with bromine in the presence of acetic acidCitation28. The reactions are shown in .
Figure 1. Synthesis of 2-(2,4-dioxothiazolidin-5-yl)acetyl chloride and 2-(2,4-dioxothiazolidin-5-ylidene)acetyl chloride. Reagent and conditions: (i) HCl, reflux; (ii) Br2, CH3COOH, reflux; (iii) SOCl2, DMF, 1,4-dioxane, reflux 1 h.
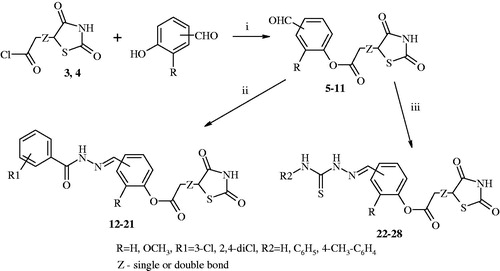
The next step of the synthesis was obtaining corresponding formylphenyl 2-(2,4-dioxothiazolidin-5-yl)acetate and formylphenyl 2-(2,4-dioxothiazolidin-5-ylidene)acetateCitation5–11. Compounds 5–11 were prepared from acetic acid chloridesCitation3,Citation4 and a series of hydroxybenzaldehydes (namely salicylaldehyde, 3-hydroxybenzaldehyde, 4-hydroxybenzaldehyde, and vanillin). The reaction was provided in anhydrous 1,4-dioxane medium in the presence of anhydrous pyridine by previously described methodCitation4,Citation29. Then, compounds 5–11 reacted with benzhydrazide derivatives (3-chlorobenzhydrazide and 2,4-dichlorobenzhydrazide), and thiosemicarbazide or 4-substituted-3-thiosemicarbazide derivatives (4-phenyl-3-thiosemicarbazide and 4-(4-methylphenyl)-3-thiosemicarbazide) in the presence of anhydrous ethanol. The result of this reaction was obtaining target compounds: hydrazonesCitation12–21 and thiosemicarbazonesCitation22–28. The synthesis of these compoundsCitation12–28 was achieved through an efficient synthetic route outlined in .
Figure 2. Synthesis of target compounds (12–28) 2-(2,4-dioxothiazolidin-5-yl)acetic and 2-(2,4-dioxothiazolidin-5-ylidene)acetic acid derivatives. Reagent and conditions: (i) pyridine, 1,4-dioxane, rt, after 2 h acidified of solution of hydrochloric acid; (ii) 3-chlorobenzhydrazide or 2,4-dichlorobenzhydrazide, anhydrous ethanol, reflux; (iii) corresponding 4-substituted thiosemicarbazide derivatives, anhydrous ethanol, reflux.
The structure of the new derivatives was confirmed by an elemental analysis, 1H NMR and 13 C NMR and MS spectra. In 1H NMR spectra, the protons of CH=N group of all the newly synthesised compounds showed a singlet signal at δ ∼ 8.14–8.51 ppm. The protons of NHCSNH group resonated as two singlets in the range of 10.10–10.17 ppm and 11.84–11.92 ppm correspondingly. The protons of NH group of the thiazolidine ring appeared in the 12.13–12.16 ppm region for compounds (12–17 and 22–26) and 12.72–12.98 ppm region for compounds as singletsCitation18–21,Citation27,Citation28.
The presence of all carbon atoms for compoundsCitation12–28 was confirmed by 13 C NMR spectra. For compounds 12–17 and 22–26 that are the derivatives of 2-(2,4-dioxothiazolidin-5-yl)acetic acid, carbon signal of two C=O group of the thiazolidine ring appeared in the 172.7 and 175.8–176.0 ppm regions. Signals of two C=O group of the thiazolidine ring for the 2-(2,4-dioxothiazolidin-5-ylilidene)acetic acid derivatives 18–21, 27, and 28 were visible at δ∼ 166.5–166.8 ppm and 169.3–169.6 ppm ranges, respectively. The detailed results of 1H NMR and 13 C NMR spectra and MS are presented in the experimental part.
Molecular weight of compounds 12–21, 23, 24, and 26 were confirmed by the mass spectra. In cases of compounds 22, 25, 27, and 28, a molecular ion was not observed in the mass spectra. The fragmentation was presented in the experimental part and ways of fragmentation were depicted in the supplementary material.
Biology
Antiproliferative evaluation
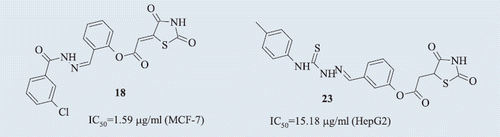
Among all the new compounds tested, only 16, 18, 20, 21, 22, and 23 derivatives revealed antiproliferative activity against tumour cell lines (). Compound 16 exhibited slight anticancer activity only against human breast adenocarcinoma cells (MCF-7). However, IC50 (concentration causing inhibition of cell proliferation by 50%) of compound 16 determined using normal human skin fibroblasts (BJ) was higher than IC50 of compound 16 estimated using MCF-7 cells, and the SI was relatively high and >1. Note that the higher SI (SI > 1) is, the safer compound is (less cytotoxic against normal cells). Also, agents revealing SI < 1 are considered unsafe and highly cytotoxic. The slight modification of the structure of compound 16 (changing a single bond in the position 5 of the thiazolidine-2,4-dione to the double bond) resulted in a new compound 21 that revealed broader antiproliferative activity, also against human hepatocellular carcinoma cells (HepG2). However, the SI value of compound 21 was low, indicating its high cytotoxicity against normal cells. Compound 20, an analogue of the derivative 21 characterised by the lack of a methoxy group in a 4-formylphenyl, showed antiproliferative activity only against HepG2 cells. However, similar to compound 21, the derivative 20 revealed low SI value. Compound 18, characterised by the change in the configuration of formylphenyl (from 4-formyl-phenyl to 2-formyl-phenyl), appeared to be the most promising antiproliferative agent among all tested compounds. Compound 18 exhibited high antiproliferative activity against all tumour cell lines used in the experiment (lung, breast, and liver cancer) and high SI values (>1). Moreover, the derivative 18 showed meaningfully lower IC50 values when compared to the reference agent, irinotecan, which is an inhibitor of topoisomerase I and is commonly used in cancer treatment. Compound 18 revealed the highest antiproliferative activity against human breast adenocarcinoma cells (MCF-7). In the case of MCF-7 cells, the IC50 value of compound 18 was 13-fold lower (1.59 µg/ml) than IC50 value of irinotecan (21.08 µg/ml), whereas the SI value of compound 18 was three-fold higher when compared to the reference substance. In the case of human hepatocellular carcinoma (HepG2) and human lung carcinoma (A549) cells, the IC50 values of compound 18 were also very low: 3.86 µg/ml (four-fold lower compared to irinotecan) and 8.08 µg/ml (five-fold lower compared to irinotecan), respectively. Comparing the activity of compound 18 to the activity of structurally similar 2,4-dioxothiazolidine-5-acetic acid derivatives synthesised by Alegaon et al.Citation6, it was observed that compound 18 showed higher activity against MCF-7 and A549 tumour cells. In their research, IC50 of tumour cell lines MCF-7 and A549 for the most active derivative 2-(2,4-dioxo-1,3-thiazolidin-5-yl)-N-[5-(3,4,5-trimethoxyphenyl)-1,3,4-thiadiazol-2-yl]acetamide was 15.28 and 12.7 µg/ml, respectively.
Table 1. Antiproliferative activity of novel thiazolidine-2,4-dione derivatives and irinotecan used as a reference substance.
The derivatives of 3-formylphenyl 2-(2,4-dioxo-1,3-thiazolidin-5-yl)acetate with the 4-phenyl-3-thiosemicarbazide (compound 22) and 4-(4-methylphenyl)-3-thiosemicarbazide structure (compound 23) also exhibited antiproliferative activity against all three tumour cell lines (A549, HepG2, and MCF-7), but only IC50 of compound 23, determined using A549 cells (lung cancer), was lower compared to the reference substance. However, the derivative 23 showed antiproliferative activity against liver cancer cells (HepG2) comparable to irinotecan and with relatively high SI value (1.66), indicating that this new compound has a great potential to be used as an anticancer drug. The IC50 values of compound 22, determined using all three cell lines, were relatively high. Nevertheless, the SI values of compound 22 were >1, indicating its high safety.
Antibacterial evaluation
Antibacterial activity of the newly synthesised compoundsCitation12–28 in vitro was screened for the bacteria growth inhibition using broth microdilution method (). Reference strains of Gram-negative and Gram-positive bacterial species were used. According to our screening results, some of the tested compounds showed potential antibacterial activity against Gram-positive bacteria. Compounds 12, 13, 15, 17, and 25 exhibited similar MIC values for S. aureus ATCC 25923, methicillin-resistant S. aureus MIKROBANK 14.001, and S. epidermidis ATCC 12228 strains. The MIC values ranged from 250 µg/ml to 500 µg/ml. Compound 27 inhibited all the tested staphylococci reference strains in the concentration of 125 µg/ml. Compound 23 showed antibacterial activity in the concentration of 125 µg/ml for S. aureus ATCC 25923, methicillin-resistant S. aureus MIKROBANK 14.001 strains and exhibited better antibacterial activity against S. epidermidis ATCC 12228 strain (MIC value was 62.5 µg/ml). The same MIC value (62.5 µg/ml) was estimated for compound 18 towards S. aureus ATCC 25923, while MIC value for methicillin-resistant S. aureus MIKROBANK 14.001 and S. epidermidis ATCC 12228 strains was 125 µg/ml which indicates a lower antibacterial activity of compound 18 in comparison with the activity in case of S. aureus ATCC 25923.
Table 2. Newly synthesised compounds’ influence on the Gram-positive bacterial strains growth – MIC (μg/ml) values.
The new compounds have no inhibitory effect on Gram-negative bacterial strains, with the exception of compound 18 which inhibited E. coli ATCC 25922 growth in the concentration of 125 µg/ml (the value is not placed in the ). MIC values obtained for gentamicin, an antibiotic active against Gram-negative bacteria and Gram-positive Staphylococcus spp., were from 0.12 to 2.0 µg/ml, based on our results.
Relying on the obtained results, it may be assumed that some of the modifications introduced to the thiazolidine-2,4-dione may result in the synthesis of new compounds with a broad biological activity spectrum inter alia anticancer and antibacterial properties. Such outcome was observed especially in the case of the change in the configuration of formylphenyl (from 4-formyl-phenyl to 2-formyl-phenyl) in compound 18 and introduction of the 4-(4-methylphenyl)-3-thiosemicarbazide structure into compound 23. The above-mentioned compounds revealed anticancer as well as antibacterial activity. However, they showed relatively high MIC values; thus, their application just as antibacterial agents is limited. However, it should be noted that compounds 18 and 23 proved to possess very promising anticancer properties, so the extension of their activity towards antibacterial properties may be an additional advantage as cancer patients have a reduced immunity and are particularly sensitive to bacterial infections.
Conclusion
Conducted research resulted in the synthesis of new thiazolidine-2,4-dione derivatives exhibiting antiproliferative activity. Among all tested derivatives, compound 18, characterised by the change in the configuration of formylphenyl from 4-formylphenyl to 2-formylphenyl, revealed the highest antiproliferative activity against human lung, breast, and liver cancer cells. More importantly, the derivative 18 showed meaningfully lower IC50 values when compared to the reference substance, irinotecan, and relatively high SI values. Moreover, compound 18 is characterised by antibacterial activity against some Gram-positive and Gram-negative strains. It is a very important quality as cancer patients have reduced immunity and are particularly sensitive to all kinds of infections, bacterial infections in particular. Therefore, it may be implied that the novel compound 18 appears to be a very promising agent for anticancer treatment.
IENZ_1387543_Supplementary_Material.pdf
Download PDF (4.4 MB)Disclosure statement
The authors declare that there are no conflicts of interest.
References
- Stewart BW, Wild CP. World Cancer Report 2014. Available from: http://www.who.int/cancer/publications/WRC_2014/en/
- Asati V, Mahapatra DK, Bharti SK. Thiazolidine-2,4-diones as multi-targeted scaffold in medicinal chemistry: potential anticancer agents. Eur J Med Chem 2014;87:814–33.
- Patil V, Tilekar K, Mehendale-Munj S, et al. Synthesis and primary cytotoxicity evaluation of new 5-benzylidene-2,4-thiazolidinedione derivatives. Eur J Med Chem 2010;45:4539–44.
- Havrylyuk DYa, Lesyk RB, Zimenkovsky BS, Pachovsky VYu. Synthesis and anticancer studying of 4-(2,4-thiazolidinedione-5-acetoxy)benzylidenehydrazones of benzazol-2-thioacetic acids. Farm Zh (Kiev) 2006;2:53–8.
- Havrylyuk D, Zimenkovsky B, Vasylenko O, et al. Synthesis and biological activity evaluation of 5-pyrazoline substituted 4-thiazolidinones. Eur J Med Chem 2013;66:228–37.
- Alegaon SG, Alagawadi KR. New thiazolidinedione-5-acetic acid amide derivatives: synthesis, characterization and investigation of antimicrobial and cytotoxic properties. Med Chem Res 2012;21:816–24.
- Chadha N, Bahia MS, Kaur M, Silakari O. Thiazolidine-2,4-dione derivatives: programmed chemical weapons for key protein targets of various pathological conditions. Bioorg Med Chem 2015;23:2953–74.
- Jain VS, Vora DK, Ramaa CS. Thiazolidine-2,4-diones: progress towards multifarious applications. Bioorg Med Chem 2013;21:1599–620.
- Rakowitz D, Maccari R, Ottana R, Vigorita MG. In vitro aldose reductase inhibitory activity of 5-benzyl-2,4-thiazolidinediones. Bioorg Med Chem 2006;14:567–74.
- Aneja DK, Lohan P, Arora S, et al. Synthesis of new pyrazolyl-2, 4-thiazolidinediones as antibacterial and antifungal agents. Org Med Chem Lett 2011;1:15.
- Bozdağ-Dündar O, Özgen Ö, Menteşe A, et al. Synthesis and antimicrobial activity of some new thiazolyl thiazolidine-2,4-dione derivatives. Bioorg Med Chem 2007;15:6012–7.
- Heerding DA, Christmann LT, Clark TJ, et al. New benzylidenethiazolidinediones as antibacterial agents. Bioorg Med Chem Lett 2003;13:3771–3.
- Zimenkovskii BS, Kutsyk RV, Lesyk RB, et al. Synthesis and antimicrobial activity of 2,4-dioxothiazolidine-5-acetic acid amides. Pharm Chem J 2006;40:303–6.
- Pattan S, Kedar M, Pattan J, et al. Synthesis and evaluation of some novel 2,4-thiazolidinedione derivatives for antibacterial, antitubercular and antidiabetic activities. Indian J Chem 2012;51B:1421–5.
- Ceriello A. Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes Metab Res Rev 2008;24:14–26.
- Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood 2005;106:408–18.
- Cai SX, Nguyen B, Jia S, et al. Discovery of substituted N-phenyl nicotinamides as potent inducers of apoptosis using a cell- and caspase-based high throughput screening assay. J Med Chem 2003;46:2474–81.
- Hu K, Yang Z-H, Pan S-S, et al. Synthesis and antitumor activity of liquiritigenin thiosemicarbazone derivatives. Eur J Med Chem 2010;45:3453–8.
- Krishnan K, Prathiba K, Jayaprakash V, et al. Synthesis and ribonucleotide reductase inhibitory activity of thiosemicarbazones. Bioorg Med Chem Lett 2008;18:6248–50.
- Patel DH, Divatia SM, Clercq E. Synthesis of some novel thiosemicarbazone derivatives having anticancer, anti-HIV as well as antibacterial activity. Indian J Chem 2013;44:535–45.
- Cui Z, Li Y, Ling Y, et al. New class of potent antitumor acylhydrazone derivatives containing furan. Eur J Med Chem 2010;45:5576–84.
- Rodrigues FAR, Oliveira ACA, Cavalcanti BC, et al. Biological evaluation of isoniazid derivatives as an anticancer class. Sci Pharm 2014;82:21–8.
- Yu X, Shi L, Ke S. Acylhydrazone derivatives as potential anticancer agents: synthesis, bio-evaluation and mechanism of action. Bioorg Med Chem Lett 2015;25:5772–6.
- Naveen Kumar HS, Parumasivam T, Jumaat F, et al. Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med Chem Res 2014;23:269–79.
- Przekora A, Czechowska J, Pijocha D, et al. Do novel cement-type biomaterials reveal ion reactivity that affects cell viability in vitro? Cent Eur J Biol 2014;9:277–89.
- Clinical Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard M7 – A10. Wayne, PA; 2009.
- Lesyk RB, Zimenkovsky BS, Golota SM, Leb'yak MM. Synthesis of 2,4-dioxothiazolidine-5-acetic acid and its amides – perspective synthons for obtaining combinatorial libraries of biologically active substances. Farm Zh (Kiev) 2001;5:57–62.
- Deghenghi R, Daneault G. Orotic acid and its analogues: part II. On the alkaline rearrangement of 5-carboxymethylidenehydantoin. Can J Chem 1960;38:1255–60.
- Lesyk RB, Zimenkovsky BS. Synthesis of potential biologically active substances on the base of 5-carboxymethylidene-2,4-thiazolidinedione. Farm Zh (Kiev) 2002;4:64–8.
