Abstract
Targeting EGFR has proven to be beneficial in the treatment of several types of solid tumours. So, a series of novel 2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio)-N-substituted acetamide 5–19 were synthesised from the starting material 4-(2-mercapto-4-oxobenzo[g]quinazolin-3(4H)-yl) benzenesulfonamide 4, to be evaluated as dual EGFR/HER2 inhibitors. The target compounds 5–19, were screened for their cytotoxic activity against A549 lung cancer cell line. The percentage inhibition of EGFR enzyme was measured and compared with erlotinib as the reference drug. Compounds 6, 8, 10, and 16 showed excellent EGFR inhibitory activity and were further selected for screening as dual EGFR/HER2 inhibitors. The four selected compounds showed IC50 ranging from 0.009 to 0.026 µM for EGFR and 0.021 to 0.069 µM for the HER2 enzyme. Compound 8 was found to be the most potent in this study with IC50 0.009 and 0.021 µM for EGFR and HER2, respectively.
Graphical Abstract
Introduction
Tyrosine kinases (TK) are involved in signalling transduction pathways which make them the candidates of choice for the treatment of cancerCitation1,Citation2. Epidermal growth factor receptor (EGFR) and human epidermal growth factor 2 (HER2) are members of the TK family. The EGFR family has four members: human epidermal growth factor receptor-2 (HER2; also known as erbB2) and its relatives HER1 (epidermal growth factor receptor; EGFR), HER3, and HER4Citation3. Each member of the epidermal growth factor receptor (EGFR) family plays a key role in normal development, homeostasis, and a variety of pathophysiological conditionsCitation4. Upon overexpression, they form a mono or heterodimers with other ERB receptors leading to activation of signalling pathways and hence tumour growthCitation5,Citation6. ERB inhibition can block TK phosphorylation leading to the loss of tumour regulatory functionsCitation5. The overexpression of EGFR and HER2 has been reported in a variety of solid tumours as non-small cell lung cancers (NSCLC)Citation7,Citation8. They are also accompanied with post-operative adverse, radiotherapy, and chemotherapy resistanceCitation9. So, it is more effective to dual target EGFR/HER2 rather than EGFR inhibition aloneCitation10.
Quinazolines are heterocyclic compounds having diverse biological activities including anticancer activityCitation11,Citation12. The wide reported biological activities of quinazoline derivative might have derived from the fact that its corresponding monocyclic counterpart, pyrimidines are being prebiotic in nature to living cells in biodiversity which made them be highly privileged motifs for the development of molecules of biological and pharmaceutical interestCitation3,Citation13.
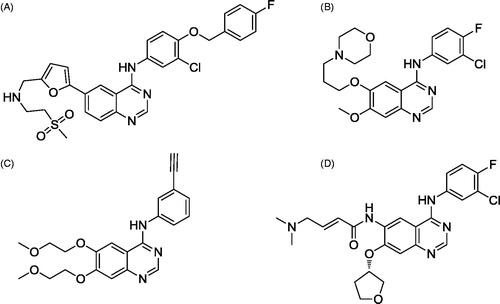
The 4-anilinoquinazoline is an important nucleus in targeting EGFR/HER2 dual inhibitors, the most common examples are lapatinib (Tykerb™) (A), used for patients with HER2 overexpression metastatic breast cancerCitation10,Citation14. Gefitinib (Iressa™) (ZD1839) (B), was approved for the treatment of patients with EGFR mutation-positive NSCLCCitation15. Erlotinib (Tarceva™) (C), the reference drug used in this study, is an EGFR inhibitor that is used in the therapy of advanced or metastatic NSCLC after the failure of at least one prior chemotherapyCitation15. And afatinib (Gilotrif™) (D), used for the treatment of cancers resistant to gefitinib and erlotinibCitation16,Citation17 (). They act through competitive binding to the ATP binding pocket of EGFR site, blocking EGFR downstream signalling required for tumour survival and proliferationCitation18,Citation19.
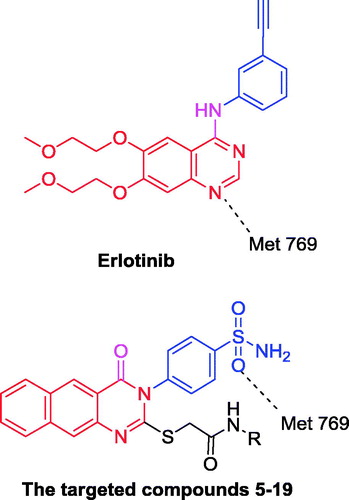
In order to design our targeted compounds, the essential requirements for EGFR receptor bearing quinazoline inhibitor (PDB ID: 1M17) were studiedCitation20 (). It was found that a hydrogen bond acceptor at N-1 of quinazoline ring interacts with Met 769Citation20. After docking of the targeted compounds, it is obvious that the oxygen of the sulfonamide group favours this interaction than the N-1 atom of the benzo[g]quinazolin and forms a hydrogen bond with Met 769.
In this respect, we designed novel compounds based on the benzo[g]quinazoline core and sulfonamide moiety. These derivatives were subjected to in vitro cytotoxic evaluation against A549, followed by EGFR inhibitory profile and measuring the IC50 towards both EGFR and HER2 in comparison with the reference drug erlotinib.
Materials and methods
Melting points (uncorrected) were determined in an open capillary on a Gallen Kamp melting point apparatus (Sanyo Gallen Kamp, UK). Pre-coated silica gel plates (Kieselgel 0.25 mm, 60 F254, Merck, Germany) were used for thin layer chromatography. A developing solvent system of chloroform/methanol (8:2) was used and the spots were detected by ultraviolet light. IR spectra (KBr discs) were recorded using a FT-IR spectrophotometer (Perkin Elmer, Waltham, MA). 1H-NMR spectra were scanned on an NMR spectrophotometer (Bruker AXS Inc., Switzerland), operating at 500 MHz for 1H- and 125.76 MHz for 13C. Chemical shifts are expressed in δ-values (ppm) relative to TMS as an internal standard, using DMSO-d6 as a solvent. Elemental analyses were done on a model 2400 CHNSO analyser (Perkin Elmer, Waltham, MA). All the values were within ±0.4% of the theoretical values. All reagents used were of AR grade.
Chemistry
4-(2-Mercapto-4-oxobenzo[g]quinazolin-3(4H)-yl) benzenesulfonamide (4)
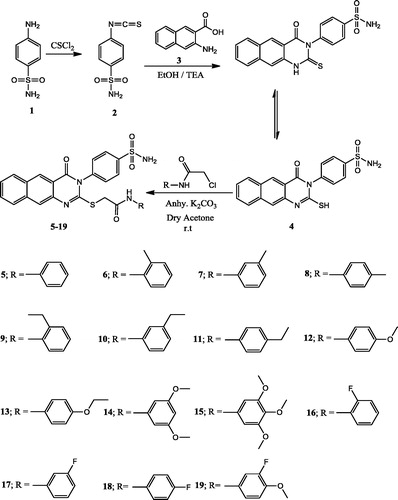
A mixture of 3-amino-2-naphthoic acid 3 (1.87 g, 0.01 mol) and 4-isothiocyanatobenzenesulfonamide 2 (2.14 g, 0.01 mol) in absolute ethanol (30 ml) containing 3 drops of triethylamine, was refluxed for 2 h, then left to cool. The solid product formed was collected by filtration and crystallised from ethanol to give 4.
Yield, 92%; m.p. 210.5 °C. IR: 3390, 3278 (NH2), 3068 (arom.), 1703 (CO), 1633 (CN), 1357, 1159 (SO2). 1H-NMR: 2.0 (s, 1H, SH), 7.5–8.1 (m, 10H, Ar-H), 8.7 (s, 2H, SO2NH2).Citation13C-NMR: 111.8, 116.5 (2), 126.3, 126.9, (2), 127.8, 129.8 (2), 130.0, 130.1, 130.2, 130.5, 135.7, 136.7, 144.1, 160.3, 176.0. MS m/z (%): 383 (M+) (9.22), 226 (100). Anal. Calcd. for C18H13N3O3S2 (383.44): C, 56.38; H, 3.42; N, 10.96. Found: C, 56.55; H, 3.65; N, 11.27.
2-(4-Oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio)-N-substituted acetamide (5–19)
General procedure
A mixture of 4 (3.83 g, 0.01 mol) and 2-chloro-N-substituted acetamide derivatives (0.01 mol) in dry acetone (50 ml) and anhydrous K2CO3 (0.5 g) was stirred at room temperature for 10 h, filtered and the product formed was crystallised from ethanol to give 5–19, respectively.
2-(4-Oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio)-N-phenylacetamide (5)
5: Yield, 80%; m.p. 278.9 °C. IR: 3291, 3265, 3140 (NH2, NH), 3053 (arom.), 2950, 2837 (aliph.), 1678, 1664 (2CO), 1598 (CN), 1398, 1157 (SO2). 1H-NMR: 4.2 (s, 2H, CH2), 7.5–8.7 (m, 15H, Ar–H), 8.8 (s, 2H, SO2NH2), 9.0 (s, 1H, NH). Citation13C-NMR: 27.2, 119.3, 119.9 (2), 120.6 (2), 125.7, 126.8 (2), 127.0, 127.2, 127.9 (2), 128.7, 128.9, 129.9, 130.0, 131.1, 133.6, 135.0, 136.2, 137.8, 143.2, 160.6, 161.7, 167.4. MS m/z (%): 516 (M+) (20.47), 362 (100). Anal. Calcd. for C26H20N4O4S2 (519.59): C, 60.45; H, 3.90; N, 10.85. Found: C, 60.12; H, 3.64; N, 10.58.
2-(4-Oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio)-N-o-tolylacetamide (6)
6: Yield, 86%; m.p. 255.5 °C. IR: 3261, 3267, 3192 (NH2, NH), 3053 (arom.), 2936, 2877 (aliph.), 1691, 1660 (2CO), 1568 (CN), 1325, 1157 (SO2). 1H-NMR: 2.2 (s, 3H, CH3), 4.3 (s, 2H, CH2), 6.8–8.2 (m, 14H, Ar–H), 8.8 (s, 2H, SO2NH2), 10.3 (s, 1H, NH). Citation13C-NMR: 21.6, 30.1, 116.8, 117.0 (2), 119.4, 120.2, 123.4, 124.6 (2), 125.0, 126.6, 127.4 (2), 128.1, 129.1, 129.9, 131.0, 131.1, 136.8, 138.4, 138.5, 138.8, 142.8, 161.3, 165.0, 165.9. MS m/z (%): 530 (M+) (9.21), 366 (100). Anal. Calcd. for C27H22N4O4S2 (530.62): C, 61.12; H, 4.18; N, 10.56. Found: C, 60.87; H, 3.80; N, 10.22.
2-(4-Oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio)-N-m-tolylacetamide (7)
7: Yield, 79%; m.p. 246.5 °C. IR: 3290, 3220, 3170 (NH2, NH), 3093 (arom.), 2976, 2912 (aliph.), 1691, 1664 (2CO), 1610 (CN), 1330, 1159 (SO2). 1H-NMR: 2.2 (s, 3H, CH3), 4.2 (s, 2H, CH2), 7.1–8.2 (m, 14H, Ar–H), 8.8 (s, 2H, SO2NH2), 10.3 (s, 1H, NH). Citation13C-NMR: 21.3, 30.0, 119.4, 119.7, 119.8, 123.4 (2), 126.6, 127.4, 128.1 (2), 128.8, 129.4, 129.6 (2), 129.7, 129.8, 131.0, 132.9, 133.3, 136.4, 136.8, 139.1, 145.8, 155.4, 164.8, 165.7. MS m/z (%): 530 (M+) (2.93), 91 (100). Anal. Calcd. for C27H22N4O4S2 (530.62): C, 61.12; H, 4.18; N, 10.56. Found: C, 61.44; H, 4.52; N, 10.88.
2-(4-Oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio)-N-p-tolylacetamide (8)
8: Yield, 75%; m.p. 318.0 °C. IR: 3302, 3255, 3132 (NH2, NH), 3089 (arom.), 2920, 2861 (aliph.), 1691, 1668 (2CO), 1604 (CN), 1332, 1161 (SO2). 1H-NMR: 2.1 (s, 3H, CH3), 4.3 (s, 2H, CH2), 7.0–8.2 (m, 14H, Ar–H), 8.8 (s, 2H, SO2NH2), 9.7 (s, 1H, NH). Citation13C-NMR: 18.3, 30.1, 119.4, 123.5 (2), 125.4 (2), 125.5, 125.9 (2), 126.1, 126.4 (2), 127.4 (2), 128.8, 129.4, 130.7, 131.0, 132.3, 136.0, 136.5, 139.1, 142.9, 161.4, 165.3, 166.1. MS m/z (%): 530 (M+) (32.72), 106 (100). Anal. Calcd. for C27H22N4O4S2 (530.62): C, 61.12; H, 4.18; N, 10.56. Found: C, 61.32; H, 4.37; N, 10.70.
N-(2-ethylphenyl)-2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio) acetamide (9)
9: Yield, 88%; m.p. 299.1 °C. IR: 3392, 3273, 3191 (NH2, NH), 3051 (arom.), 2966, 2833 (aliph.), 1693, 1653 (2CO), 1568 (CN), 1354, 1157 (SO2). 1H-NMR: 1.1 (t, 3H, CH3 ethyl), 2.6 (q, 2H, CH2 ethyl), 4.3 (s, 2H, CH2), 6.9–8.2 (m, 14H, Ar–H), 8.8 (s, 2H, SO2NH2), 10.7 (s, 1H, NH). Citation13C-NMR: 15.9, 28.6 (2), 117.1, 119.0 (2), 123.4, 123.5, 123.8, 126.6, 127.4 (2), 128.1, 128.8, 129.1 (2), 129.2, 129.4, 129.9, 131.0 (2), 136.8, 138.9, 139.1, 144.8, 155.4, 161.3, 165.9. MS m/z (%): 544 (M+) (22.41), 360 (100). Anal. Calcd. for C28H24N4O4S2 (544.64): C, 61.75; H, 4.44; N, 10.29. Found: C, 61.48; H, 4.11; N, 10.04.
N-(3-ethylphenyl)-2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio) acetamide (10)
10: Yield, 81%; m.p. 201.3 °C. IR: 3273, 3155, 3103 (NH2, NH), 3087 (arom.), 2929, 2870 (aliph.), 1680, 1666 (2CO), 1616 (CN), 1334, 1159 (SO2). 1H-NMR: 1.0 (t, 3H, CH3 ethyl), 2.6 (q, 2H, CH2 ethyl), 4.2 (s, 2H, CH2), 7.3–8.2 (m, 14H, Ar–H), 8.8 (s, 2H, SO2NH2), 9.6 (s, 1H, NH). Citation13C-NMR: 14.6, 24.1, 30.0, 119.4, 123.5, 126.4, 126.5 (2), 126.6, 126.7, 127.4 (2), 128.1, 128.9, 129.1 (2), 129.4, 129.9, 131.1, 135.3, 135.8, 136.9, 138.4, 142.9, 145.9, 155.4, 161.4, 166.4. MS m/z (%): 544 (M+) (10.35), 121 (100). Anal. Calcd. for C28H24N4O4S2 (544.64): C, 61.75; H, 4.44; N, 10.29. Found: C, 61.99; H, 4.69; N, 9.95.
N-(4-ethylphenyl)-2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio) acetamide (11)
11: Yield, 89%; m.p. 238.2 °C. IR: 3336, 3210, 3153 (NH2, NH), 3099 (arom.), 2986, 2844 (aliph.), 1691, 1662 (2CO), 1612 (CN), 1377, 1161 (SO2). 1H-NMR: 1.1 (t, 3H, CH3 ethyl), 2.6 (q, 2H, CH2 ethyl), 4.2 (s, 2H, CH2), 7.1–8.2 (m, 14H, Ar-H), 8.8 (s, 2H, SO2NH2), 10.3 (s, 1H, NH). Citation13C-NMR: 16.1, 28.0, 31.1, 119.4, 119.8 (2), 119.9 (2), 123.4, 126.6 (2), 127.4 (2), 128.1, 128.4 (2), 128.8, 129.3, 131.0, 131.1, 136.6, 136.8, 137.0, 142.8, 145.9, 155.4, 161.3, 164.8. MS m/z (%): 544 (M+) (4.83), 154 (100). Anal. Calcd. for C28H24N4O4S2 (544.64): C, 61.75; H, 4.44; N, 10.29. Found: C, 61.50; H, 4.21; N, 10.02.
N-(4-methoxyphenyl)-2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio) acetamide (12)
12: Yield, 78%; m.p. 318.0 °C. IR: 3412, 3290, 3138 (NH2, NH), 3055 (arom.), 2971, 2833 (aliph.), 1692, 1681 (2CO), 1627 (CN), 1338, 1161 (SO2). 1H-NMR: 1.1 (s, 3H, CH3), 3.8 (s, 3H, CH2), 7.1–8.2 (m, 14H, Ar–H), 8.8 (s, 2H, SO2NH2), 10.5 (s, 1H, NH). Citation13C-NMR: 18.2, 30.0, 56.5, 108.2 (2), 115.6, 119.4 (2), 123.4 (2), 126.6, 127.4 (2), 128.1, 128.8 (2), 129.4, 129.8, 131.0, 131.1, 136.8, 139.1, 145.8, 150.3, 155.3, 161.3, 165.9. MS m/z (%): 546 (M+) (51.12), 122 (100). Anal. Calcd. for C27H22N4O5S2 (546.62): C, 59.33; H, 4.06; N, 10.25. Found: C, 59.04; H, 3.85; N, 9.91.
N-(4-ethoxyphenyl)-2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio) acetamide (13)
13: Yield, 88%; m.p. 266.7 °C. IR: 3367, 3271, 3130 (NH2, NH), 3049 (arom.), 2978, 2916 (aliph.), 1695, 1651 (2CO), 1602 (CN), 1398, 1153 (SO2). 1H-NMR: 1.1 (s, 3H, CH3), 4.0 (s, 2H, CH2), 4.3 (q, 2H, OCH2), 7.0–8.2 (m, 14H, Ar–H), 8.8 (s, 2H, SO2NH2), 10.6 (s, 1H, NH). Citation13C-NMR: 16.1, 30.9, 68.0, 119.4 (2), 119.7, 119.8 (2), 123.4 (2), 123.9, 124.3 (2), 126.6, 127.4 (2), 128.1, 128.8, 129.2, 129.3, 131.0, 136.8, 138.9, 142.8, 155.3, 161.3, 165.1, 166.0. MS m/z (%): 560 (M+) (6.44), 361 (100). Anal. Calcd. for C28H24N4O5S2 (560.64): C, 59.98; H, 4.31; N, 9.99. Found: C, 59.61; H, 4.04; N, 9.69.
N-(3,4-dimethoxyphenyl)-2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio) acetamide (14)
14: Yield, 72%; m.p. above 398 °C. IR: 3412, 3314, 3200 (NH2, NH), 3100 (arom.), 2961, 2841 (aliph.), 1678, 1645 (2CO), 1560 (CN), 1394, 1157 (SO2). 1H-NMR: 3.6 (s, 6H, 2OCH3), 4.1 (s, 2H, CH2), 7.3–8.0 (m, 13H, Ar–H), 8.8 (s, 2H, SO2NH2), 11.8 (s, 1H, NH). Citation13C-NMR: 28.9, 59.2 (2), 87.4, 110.3, 112.6, 119.3, 121.8 (2), 123.4, 126.7 (2), 127.4, 128.1 (2), 129.4, 129.8, 130.6, 131.0, 136.8, 137.9, 139.1, 142.8, 145.9, 155.3 (2), 161.3, 166.3. MS m/z (%): 576 (M+) (0.98), 151 (100). Anal. Calcd. for C28H24N4O6S2 (576.64): C, 58.32; H, 4.20; N, 9.72. Found: C, 58.68; H, 4.49; N, 9.98.
2-(4-Oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio)-N-(3,4,5-trimethoxyphenyl)acetamide (15)
15: Yield, 83%; m.p. 285.8 °C. IR: 3343, 3315, 3181 (NH2, NH), 3081 (arom.), 2954, 2857 (aliph.), 1690, 1684 (2CO), 1613 (CN), 1388, 1167 (SO2). 1H-NMR: 3.6, 3.7 (2 s, 9H, 3OCH3), 4.1 (s, 2H, CH2), 7.0–8.2 (m, 12H, Ar–H), 8.8 (s, 2H, SO2NH2), 10.3 (s, 1H, NH). Citation13C-NMR: 27.8, 56.1 (2), 60.5, 97.3 (2), 119.4, 123.4 (2), 126.7, 127.4 (2), 128.0, 128.8 (2), 129.4, 129.9, 131.1 (2), 134.0, 135.5, 136.8, 139.1, 142.8, 153.2 (2), 155.4, 161.3, 165.8. MS m/z (%): 606 (M+) (33.04), 433 (100). Anal. Calcd. for C29H26N4O7S2 (606.67): C, 57.41; H, 4.32; N, 9.24. Found: C57.71; H, 4.63; N, 9.48.
N-(2-fluorophenyl)-2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio) acetamide (16)
16: Yield, 72%; m.p. 264.4 °C. IR: 3431, 3302, 3196 (NH2, NH), 3086 (arom.), 2955, 2862 (aliph.), 1691, 1676 (2CO), 1624 (CN), 1396, 1163 (SO2). 1H-NMR: 4.3 (s, 2H, CH2), 6.9–8.2 (m, 14H, Ar–H), 8.8 (s, 2H, SO2NH2), 10.8 (s, 1H, NH). Citation13C-NMR: 30.0, 106.7, 115.5, 119.4, 123.4 (2), 126.6 (2), 127.4 (2), 128.1, 128.8, 129.3 (2), 129.8, 130.9 (2), 131.0, 131.1, 136.8, 139.1, 145.9, 155.2, 161.3, 165.4, 166.5. MS m/z (%): 534 (M+) (14.23), 94 (100). Anal. Calcd. for C26H19FN4O4S2 (534.58): C, 58.42; H, 3.58; N, 10.48. Found: C, 58.11; H, 3.23; N, 10.21.
N-(3-fluorophenyl)-2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio) acetamide (17)
17: Yield, 66%; m.p. 251.5 °C. IR: 3381, 3320, 3211 (NH2, NH), 3075 (arom.), 2963, 2844 (aliph.), 1692, 1681 (2CO), 1613 (CN), 1390, 1166 (SO2). 1H-NMR: 4.2 (s, 2H, CH2), 7.0–8.2 (m, 14H, Ar–H), 8.8 (s, 2H, SO2NH2), 10.6 (s, 1H, NH). Citation13C-NMR: 31.1, 115.4, 115.5, 119.4 (2), 123.4 (2), 126.6, 127.4 (2), 128.1, 128.8 (2), 129.3, 129.8, 130.9, 131.0, 136.8, 139.1 (2), 140.6, 145.9, 155.2, 161.3, 163.5, 165.4. MS m/z (%): 534 (M+) (28.19), 94 (100). Anal. Calcd. for C26H19FN4O4S2 (534.58): C, 58.42; H, 3.58; N, 10.48. Found: C, 58.69; H, 3.93; N, 10.13.
N-(4-fluorophenyl)-2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio) acetamide (18)
18: Yield, 75%; m.p. 258.0 °C. IR: 3267, 3212, 3154 (NH2, NH), 3099 (arom.), 2976, 2833 (aliph.), 1678, 1654 (2CO), 1614 (CN), 1327, 1153 (SO2). 1H-NMR: 4.1 (s, 2H, CH2), 6.8–8.2 (m, 14H, Ar–H), 8.8 (s, 2H, SO2NH2), 10.2 (s, 1H, NH). Citation13C-NMR: 27.9, 114.9 (2), 119.4 (2), 121.2, 123.4 (2), 126.7, 127.4 (2), 128.1, 128.8 (2), 128.4, 129.9, 131.0, 131.1 (2), 132.4, 136.8, 145.8, 155.1, 155.4, 161.3, 165.4. MS m/z (%): 534 (M+) (21.09), 94 (100). Anal. Calcd. for C26H19FN4O4S2 (534.58): C, 58.42; H, 3.58; N, 10.48. Found: C, 58.60; H, 3.75; N, 10.91.
N-(3-fluoro-4-methoxyphenyl)-2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio) acetamide (19)
19: Yield, 79%; m.p. 253.0 °C. IR: 3268, 3209, 3143 (NH2, NH), 3078 (arom.), 2965, 2845 (aliph.), 1691, 1679 (2CO), 1562 (CN), 1348, 1161 (SO2). 1H-NMR: 4.0 (s, 3H, OCH3), 4.3 (s, 2H, CH2), 7.1–8.2 (m, 13H, Ar–H), 8.8 (s, 2H, SO2NH2), 10.3 (s, 1H, NH). Citation13C-NMR: 30.0, 61.0, 115.8, 115.9, 116.0, 119.4, 123.4 (2), 124.3, 125.8 (2), 126.3, 127.4 (2), 128.8, 129.4, 131.0, 136.8 (2), 139.1 (2), 142.8, 145.9, 153.0, 161.3, 165.6, 166.7. MS m/z (%): 564 (M+) (17.92), 407 (100). Anal. Calcd. for C27H21FN4O5S2 (564.61): C, 57.44; H, 3.75; N, 9.92. Found: C, 57.11; H, 3.46; N, 9.67.
Biological evaluation
MTT cytotoxicity assay
A549 lung cancer cells (obtained from VACSERA, Cairo, Egypt) were obtained from American Type Culture Collection, cells were cultured using DMEM (Dulbecco's Modified Eagle's Medium) (Invitrogen/Life Technologies) supplemented with 10% foetal bovine serum (Hyclone), 10 μg/ml of insulin (Sigma), and 1% penicillin–streptomycin. The 96-well plate was incubated for 24 h before the MTT assay. Then, briefly rinse the cell layer with 0.25% (w/v) Trypsin, 0.53 mM EDTA solution. Add reconstituted MTT in an amount equal to 10% of the culture medium volume. Incubate for 2–4 h. Measure absorbance at a wavelength of 570 nm. The IC50 values were calculated according to the equation for Boltzmann sigmoidal concentration–response curve using the nonlinear regression fitting models (GraphPad, Prism, GraphPad Software Inc., La Jolla, CA).
In vitro enzymatic activity assay
EGFR and HER2 kinase kit were purchased from Invitrogen. The experiments were performed according to the manufacturer's instructions. Briefly, EGFR (PV3872), 0.200 mg/ml and HER2 (PV3366), 0.192 mg/ml were used. Six concentration gradients were set for all the tested compounds in DMSO. An ATP solution and a kinase/peptide mixture were prepared right before use. The solutions on the plate were mixed thoroughly, and the plate was incubated for 1 h at room temperature. After that, 5 ml of the developing solution was added to each well. The plate was incubated for 1 h at room temperature and then read by ELISA Reader (PerkinElmer, Waltham, MA). Curve fitting and data presentations were performed using Graph Pad Prism 5.0. Every experiment was repeated three times. Data represented as means ± SD from three independent experiments.
Results and discussion
Chemistry
Scheme 1 reports the synthetic pathway utilised to obtain the targeted compounds 5–19, from the reaction of 4-isothiocyanatobenzenesulfonamide 2 with 3-amino-2-naphthoic acid 3Citation21 in ethanol containing triethylamine to yield the 4-(2-mercapto-4-oxobenzo[g]quinazolin-3(4H)-yl) benzenesulfonamide 4. 1H-NMR of 4 revealed a singlet at 2.0 ppm attributed to the SH. Citation13C-NMR exhibited two signals at 160.3 and 176.0 ppm attributed to C–SH and CO, respectively. The reaction of 4 with 2-chloro-N-substituted acetamide in dry acetone and anhydrous K2CO3 gave the corresponding 2-(4-oxo-3-(4-sulfamoylphenyl)-3,4-dihydrobenzo[g]quinazolin-2-ylthio)-N-substituted acetamide 5–19. IR of 5 revealed bands at 1678, 1664 cm−1 for the CO groups. 1H-NMR displayed singlet at 4.2 ppm attributed to CH2 and another singlet at 9.0 ppm for the NH proton. Citation13C-NMR exhibited signal at 27.2 ppm for the CH2, and another signal at 167.4 ppm due to the CO acetamide. 1H-NMR of 6–8 displayed three singlets at the range of 2.1–2.2 ppm attributed to CH3 group, 4.2–4.3 ppm for the CH2, and 9.7–10.3 ppm for the NH proton. Citation13C-NMR of 6–8 exhibited new signals in the range of 18.3–21.6 ppm for the CH3, 30.0–30.1 ppm for the CH2, and another signal at 165.7–166.1 ppm due to the CO acetamide. 1H-NMR of 9–11 displayed triplet at the range of 1.0–1.1 ppm attributed to CH3 ethyl, the quartet at 2.6 ppm for the CH2 ethyl, singlet at the range of 4.2–4.3 ppm for the CH2, and a singlet at 9.6–10.7 ppm for the NH proton. Citation13C-NMR of 9–11 exhibited new signals in the range of 14.6–16.1 ppm for the CH3 ethyl, 24.1–28.6 ppm for the CH2 ethyl, 28.6–31.1 ppm for the CH2, and 164.8–166.4 ppm due to the CO acetamide. 1H-NMR of 12 and 13 displayed singlet at 1.1 ppm attributed to CH3, singlet at the range of 3.8–4.0 ppm for the CH2, the quartet at 4.1–4.3 ppm due to OCH2, and a singlet at 10.5–10.6 ppm for the NH proton. Citation13C-NMR of 12 and 13 exhibited new signals in the range of 16.1–18.2 ppm for the CH3, 30.0–30.9 ppm for the CH2, 56.5–68.0 ppm for the OCH2, and another signal at 165.9–166.0 ppm due to the CO acetamide. 1H-NMR of 14 displayed three singlets at 3.6 ppm due to the 2OCH3, 4.1 ppm attributed to CH2 and at 11.8 ppm for the NH proton. Citation13C-NMR of 14 exhibited signal at 28.9 ppm for the CH2, 59.2 ppm for the 2OCH3, and at 166.3 ppm due to the CO acetamide. 1H-NMR of 15 displayed two singlets at 3.6 and 3.7 ppm due to the 3OCH3, singlet at 4.1 ppm attributed to CH2 and singlet at 10.3 ppm for the NH proton. Citation13C-NMR of 15 exhibited signal at 27.8 ppm for the CH2, 56.1, 60.5 ppm for the 3OCH3, and at 165.8 ppm due to the CO acetamide. 1H-NMR of 16–18 displayed two singlets at the range of 4.1–4.3 ppm for the CH2, and 10.2–10.6 ppm for the NH proton. Citation13C-NMR of 16–18 exhibited new signals in the range of 27.9–31.1 ppm for the CH2, and another signal at 165.4–166.5 ppm due to the CO acetamide. Citation13C-NMR of 16 displayed a signal at 155.2 ppm attributed to the C–F carbon at the ortho position, while for 17 and 18 the C–F carbon appeared at 163.5 and 161.3 ppm due to its presence at the meta and para position, respectively. 1H-NMR of 19 displayed three singlets at 4.0 ppm attributed to OCH3, 4.3 ppm for CH2 and 10.3 ppm for the NH proton. Citation13C-NMR of 19 exhibited signal at 30.0 ppm for the CH2, 61.0 ppm for the OCH3 and another signal at 166.7 ppm due to the CO acetamide.
Biological evaluation
In vitro cytotoxic activity against A549
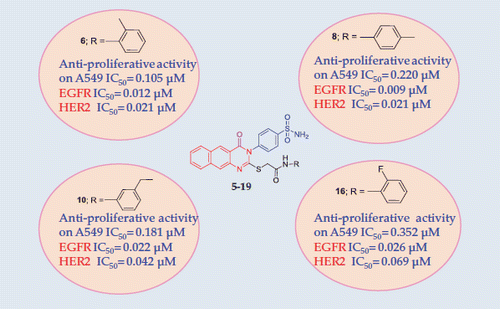
The newly synthesised compounds were evaluated for their in vitro cytotoxic activity through MTT cytotoxicity assay against human Lung (A549) cancer cell line, and erlotinib was used as the reference drug. In a closer look to , we can see that compounds 5–19 cytotoxicity ranges from 0.105 to 1.992 µM, in comparison with erlotinib (IC50= 0.727 µM). Compounds 6, 8, 10, and 14–16 were more active than the reference drug, with IC50 ranging from 0.105 to 0.711 µM. the O-methyl derivative 6 was the most active followed by the m-ethyl 10, the p-methyl 8, the O-fluoro 16, the 3,4,5-trimethoxy 15 and the 3,5-dimethoxy derivative 14 (IC50 values 0.105, 0.181, 0.220, 0.352, 0.545, and 0.711 µM, respectively). The EGFR inhibitory activity of the tested compounds 5–19 was measured. Results showed that most of the tested compounds have high inhibitory activity ranging from 90.21% to 34.37%. Compounds 6, 8, 10, and 16 showed the highest inhibition percentages ranging from 90.21% to 84.19%.
Table 1. EGFR inhibitory activity and anti-proliferative activity against A549 cell line.
EGFR and HER2 inhibition
The IC50 values for the most potent compounds 6, 8, 10, and 16 were determined on both EGFR and HER2 enzymes. Compound 8 was found to be the most potent on both EGFR and HER2 with IC50 values of 0.009 and 0.021 µM, respectively. Followed by compound 6 with IC50 values of 0.012 and 0.021 µM. Compound 10 showed IC50 values of 0.022 and 0.044 µM and compound 16 of 0.026 and 0.069 µM. Erlotinib IC50 values were 0.047 and 0.071 µM towards EGFR and HER2 enzymes, respectively ().
Table 2. Inhibition activities of the most potent compounds against EGFR and HER2.
Conclusion
In summary, a novel series of benzo[g]quinazolin bearing sulfonamide was designed and synthesised. All the compounds showed very potent anticancer activity against A549 lung cancer cell line and high inhibition percentage towards EGFR in A549 cancer cells. Compounds 6, 8, 10, and 16 that showed the highest cytotoxic activity and inhibition percentages were further screened against EGFR and HER2 enzymes. The IC50 values for those four compounds were found to be better than that of erlotinib against both EGFR and HER2 enzymes. Compound 8 was found to be the most potent on both EGFR and HER2 with IC50 values of 0.009 and 0.021 µM, respectively. Followed by compound 6 with IC50 values of 0.012 and 0.021 µM.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The authors are thankful to the Deanship of the Scientific Research and Research Centre, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
References
- Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer 2004;4:361–70.
- Tao X-X, Duan Y-T, Chen L-W, et al. Design, synthesis and biological evaluation of pyrazolyl-nitroimidazole derivatives as potential EGFR/HER-2 kinase inhibitors. Bioorg Med Chem Lett 2016;26:677–83.
- Ajani OO, Obafemi CA, Nwinyi OC, Akinpelu DA. Microwave assisted synthesis and antimicrobial activity of 2-quinoxalinone-3-hydrazone derivatives. Bioorg Med Chem 2010;18:214–21.
- Kennedy SP, Hastings JF, Han JZ, Croucher DR. The under-appreciated promiscuity of the epidermal growth factor receptor family. Front Cell Dev Biol 2016;4:88–99.
- Reid A, Vidal L, Shaw H, de Bono J. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu). Eur J Cancer 2007;43:481–9.
- Abdelgawad MA, Bakr RB, Alkhoja OA, Mohamed WR. Design, synthesis and antitumor activity of novel pyrazolo [3, 4-d] pyrimidine derivatives as EGFR-TK inhibitors. Bioorg Chem 2016;66:88–96.
- Marshall C. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 1995;80:179–85.
- Hirsch F, Varella-Garcia M, Cappuzzo F. Predictive value of EGFR and HER2 overexpression in advanced non-small-cell lung cancer. Oncogene 2009;28:S32–7.
- Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res 2001;7:2958–70.
- Johnston S, Leary A. Lapatinib: a novel EGFR/HER2 tyrosine kinase inhibitor for cancer. Drugs Today 2006;42:441–53.
- Zhang C, Li W. Regioselective synthesis of 6-aryl-benzo [h][1, 2, 4]-triazolo [5, 1-b] quinazoline-7, 8-diones as potent antitumoral agents. Bioorg Med Chem Lett 2013;23:5002–5.
- Elkamhawy A, Farag AK, Viswanath ANI, et al. Targeting EGFR/HER2 tyrosine kinases with a new potent series of 6-substituted 4-anilinoquinazoline hybrids: design, synthesis, kinase assay, cell-based assay, and molecular docking. Bioorg Med Chem Lett 2015;25:5147–54.
- Tu Y, Wang C, Xu S, et al. Design, synthesis, and docking studies of quinazoline analogues bearing aryl semicarbazone scaffolds as potent EGFR inhibitors. Bioorg Med Chem 2017;25:3148–57.
- Yin S, Tang C, Wang B, et al. Design, synthesis and biological evaluation of novel EGFR/HER2 dual inhibitors bearing a oxazolo [4, 5-g] quinazolin-2 (1H)-one scaffold. Eur J Med Chem 2016;120:26–36.
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 2004;101:13306–11.
- Schuler M, Yang J-H, Park K, et al. Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: phase III randomized LUX-Lung 5 trial. Ann Oncol 2016;27:417–23.
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595–605.
- Wang C, Sun Y, Zhu X, et al. Novel quinazoline derivatives bearing various 4‐aniline moieties as potent EGFR inhibitors with enhanced activity against NSCLC cell lines. Chem Biol Drug Des 2016;87:635–43.
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005;5:341–54.
- Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem 2002;277:46265–72.
- Casini A, Scozzafava A, Mincione F, et al. Carbonic anhydrase inhibitors: water-soluble 4-sulfamoylphenylthioureas as topical intraocular pressure-lowering agents with long-lasting effects. J Med Chem 2000;43:4884–92.