?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The α- and β-class carbonic anhydrases (CAs, EC 4.2.1.1) from the pathogenic bacterium Vibrio cholerae, VchCAα, and VchCAβ, were investigated for their activation with natural and non-natural amino acids and amines. The most effective VchCAα activators were L-tyrosine, histamine, serotonin, and 4-aminoethyl-morpholine, which had KAs in the range of 8.21–12.0 µM. The most effective VchCAβ activators were D-tyrosine, dopamine, serotonin, 2-pyridyl-methylamine, 2-aminoethylpyridine, and 2-aminoethylpiperazine, which had KAs in the submicromolar – low micromolar range (0.18–1.37 µM). The two bacterial enzymes had very different activation profiles with these compounds, between each other, and in comparison to the human isoforms hCA I and II. Some amines were selective activators of VchCAβ, including 2-pyridylmethylamine (KA of 180 nm for VchCAβ, and more than 20 µM for VchCAα and hCA I/II). The activation of CAs from bacteria, such as VchCAα/β has not been considered previously for possible biomedical applications. It would be of interest to study in more detail the extent that CA activators are implicated in the virulence and colonisation of the host by such pathogenic bacteria, which for Vibrio cholerae, is highly dependent on the bicarbonate concentration and pH in the surrounding tissue.
1. Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are a superfamily of ubiquitous metalloenzymes with the catalytically active form represented by a metal hydroxide derivative acting as a potent nucleophile on CO2 (the physiological substrate) or other electrophiles (e.g. COS, CS2, esters, etc.)Citation1–15. CAs catalyse only one simple but physiologically highly relevant reaction, which is the reversible hydration of carbon dioxide to bicarbonate and protonsCitation5,Citation6,Citation13,Citation15. These enzymes are grouped in seven genetically distinct families, named α-, β-, γ-, δ-, ζ-, η- and ɵ-CAs, and although they share a low sequence similarity and protein three dimensional structure, all of them possess a high efficiency as catalysts for the transformation of the metabolically crucial gas CO2 into soluble products, HCO3− and H+ ionsCitation5,Citation6,Citation8,Citation13,Citation15–17. As a consequence, these enzymes are ubiquitous in all life kingdoms, being found in Archaea, Bacteria, and EukaryotesCitation1–5,Citation15. α-CAs are normally present in bacteria and eukaryotes, in which they have been thoroughly investigatedCitation1–5,Citation15. In fact many human (h) CAs, of the 15 diverse isoforms known to date, are drug targets for inhibitors acting as diuretics or agents for the treatment of glaucoma, epilepsy, obesity, tumorsCitation16–19, but recently they started to be considered as possible drug targets for neuropathic pain, cerebral ischemia, or arthritisCitation20,Citation21.
The metal ion from the CA active site is crucial for catalysis, and is coordinated by three His residues in the α-, γ-, δ-, and probably the θ-classes; by one His, and two Cys residues in β- and ζ-CAs or by two His and one Gln residues in the η-class, with the fourth ligand being a water molecule/hydroxide ion acting as nucleophile in the catalytic cycle of the enzymeCitation1–15. The rate determining step in the CA catalytic cycle is the formation of the metal hydroxide species of the enzyme from the acidic one in which a water molecule is coordinated as the fourth ligand to the metal centreCitation3–6,Citation9. This process is usually assisted by amino acid residues placed in the middle or at the rim of the active site, which can shuttle protons between the metal centre and the reaction medium by means of moieties possessing a pKa in the region of 6–8 pH units, such as imidazoles (from His residues), carboxylates (from Asp or Glu residues), etc.Citation3–6,Citation9. In α-CAs, the proton shuttle residues are His (e.g. His64 in isoforms, such as CA II, IV, VII, IX, etc.), or His clusters (His3, 4, 10, 15, and 64) placed at the amino terminal part of the protein and situated on the rim of the active site cavity, as demonstrated by X-ray crystal workCitation3–6,Citation9. In β-CAs, which are highly abundant in bacteria and plants, the identity of the proton shuttle residue is not well established although it seems that an Asp (or Glu) residue placed in the middle of the cavity has such a roleCitation22. Thus, compounds able to intervene in such proton transfer processes are known as CA activators (CAAs) and they were rather well investigated for mammalian α-CAsCitation23–30, but much less for bacterial such enzymes. In fact, whereas bacterial CA inhibitors (CAIs) were extensively studied, leading to a detailed understanding of the catalytic and inhibition mechanismsCitation15,Citation31–35, only a few studies are available on the bacterial CAAsCitation36. Recently, our groups described the biochemical properties of a α-, β-, and γ-CAs from the pathogenic bacterium Vibrio cholerae, responsible of choleraCitation37–43. These enzymes, called VchCAα/β/γ showed a significant catalytic activity for the physiologic CO2 hydration reaction to bicarbonate and protons (kcat 105 s−1)Citation37–43. Moreover, the study of the inhibition profiles with the classical CA inhibitors (sulphonamides and anions) revealed interesting structure–activity relationship for the interaction of these enzymes with inhibitorsCitation27–43, but no activation studies were reported so far. Here, we present the first activation study of two such enzymes, VchCAα/β, with a series of amino acid and amine derivatives. The main interest of this study is to understand whether CA activators are implicated in the virulence and colonisation of the host by this pathogenic bacterium, considering the fact that V. cholerae is highly dependent on the bicarbonate concentration and pH in the tissue which is colonised.
2. Materials and methods
2.1. Materials
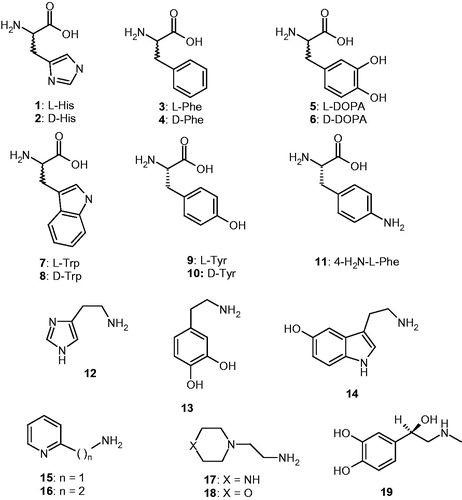
Amino acids and amines 1–19 were commercially available, highest purity reagents from Sigma-Aldrich, Milan, Italy.
2.2. CA enzyme activation assay
An Sx.18Mv-R Applied Photophysics (Oxford, United Kingdom) stopped-flow instrument has been used to assay the catalytic activity of various CA isozymes for CO2 hydration reactionCitation44. Phenol red (at a concentration of 0.2 mM) was used as indicator, working at the absorbance maximum of 557 nm, with 10 mM Hepes (pH 7.5) or Tris (pH 8.3) as buffers, 0.1 M Na2SO4 (for maintaining constant ionic strength), following the CA-catalysed CO2 hydration reaction for a period of 10 s at 25 °C. Activity of the α-CA was measured at pH 7.5 whereas that of the β-class enzyme at pH 8.3 in order to avoid the possibility that its active site is closedCitation40. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and activation constants. For each activator at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of activators (10 mM) were prepared in distilled-deionised water and dilutions up to 1 nM were done thereafter with the assay buffer. Activator and enzyme solutions were pre-incubated together for 15 min (standard assay at room temperature) prior to assay, in order to allow for the formation of the E–A complex. The activation constant (KA), defined similarly with the inhibition constant KI, can be obtained by considering the classical Michaelis–Menten equation (EquationEquation (1)(1)
(1) ), which has been fitted by non-linear least squares by using PRISM 3:
(1)
(1)
where [A]f is the free concentration of activator.
Working at substrate concentrations considerably lower than KM ([S] ≪KM), and considering that [A]f can be represented in the form of the total concentration of the enzyme ([E]t) and activator ([A]t), the obtained competitive steady-state equation for determining the activation constant is given by EquationEquation (2)(2)
(2) :
(2)
(2)
where v0 represents the initial velocity of the enzyme-catalysed reaction in the absence of activatorCitation23–30.
3. Results and discussion
The activators 1–19 were included in this study, as they were employed for investigations as CAAs against many classes of CAs, including the bacterial ones from Burkholderia pseudomallei, BpsCAβ/γCitation36c,Citation45. Both natural and non-natural amino acids and amines were included among the investigated compounds ().
Data in indicate that L-Tyr (at 10 µM concentration) is an effective activator because this amino acid enhances the kcat values for all enzymes considered (hCA I, II, and VchCAα/β). Moreover, KM remains unchanged by addition of L-Tyr, which has been the case for all CAAs that have been investigated so far, including those belonging to vertebrates (α-class enzymes) and microorganisms (enzymes belonging to various CA genetic families)Citation23–30,Citation45. L-Tyr was a nanomolar activator for the α-class enzymes (hCA I and II) with KAs in the range of 11–20 nMCitation23 and a micromolar activator for VchCAα/β, with KAs of 6.15–8.21 µM. It should be mentioned that due to its high efficacy as activator, L-Tyr induced an increase of the kinetic constant of 2.66 times compared to the uncatalysed rate for the α-CA and of 4.85 times for the β-CA from V. cholerae. This is the most significant kinetic effect observed so far any activator that has been identified for these enzymes to date, and L-Tyr is in fact not even the most effective activator of VchCAα/β evidenced here (see below).
Table 1. Activation of human carbonic anhydrase (hCA) isozymes I, II, and VchCAα/β with L-Tyr, at 25 °C, for the CO2 hydration reactionCitation44.
Amino acids and amines 1–19 () previously investigated as CAAs of human (α-class CAs) and few bacterial enzymes, showed significant activating effects against VchCAα/β, as observed from data of , in which the activation constants (KAs) of these compounds against four CAs are presented. The following structure-activity relationship (SAR) can be evidenced from the data of :
Table 2. Activation constants of hCA I, hCA II and the bacterial CAs VchCAγ/β with amino acids and amines 1–19. Data for hCA I and II are from Ref.Citation23.
(i) The α-class bacterial enzyme was activated by amino acids and amines 1–19 in the micromolar range (KAs of 8.21–71.9 µM), and is thus much less sensitive to activation compared to the human CA isoforms belonging to the same class, hCA I and II, because some of these compounds acted as nanomolar activators. However, a distinct SAR could be observed for these CAAs even if their potency is not very high. The most effective VchCAα activators were L-Tyr 9, histamine 12, serotonin 14, and 4-aminoethyl-morpholine 18, which had KAs in the range of 8.21–12.0 µM. The remaining amines and amino acids were less effective CAAs, with KAs in the range of 19.4–71.9 µM. The stereochemistry of the amino acid derivatives influenced the activation potency, with the D-enantiomers being generally more effective than the L-ones (for His, Phe, DOPA, and Trp), whereas the reverse situation is true for Tyr, case in which the L-enantiomer was 4.6 times more effective at activation than the D-enantiomer (). In some cases, the amines were more effective activators compared to the amino acids structurally related to them, e.g. histamine was more effective compared to L/D-His, whereas dopamine was less effective compared to L/D-DOPA. The least effective activators were the pyridyl-amine derivatives 15 and 16. All these data demonstrate that relatively small differences in the scaffold of the activator induce important differences in the activation efficacy, obviously due to the fact that the structural diversity of these compounds induces diverse interactions with amino acid residues from the active site in the enzyme-activator (E-A) complex.
(ii) VchCAβ was more sensitive to activation with the amines and amino acids investigated here, which showed KAs in the range of 0.18–20.3 µM (). The most effective activators were D-Tyr 10, dopamine 13, serotonin 14, 2-pyridyl-methylamine 15, 2-aminoethylpyridine 16, and 2-aminoethylpiperazine 17, which showed activation constants in the submicromolar – low micromolar range, of 0.18–1.37 µM. Apart D-Tyr, all of these most effective activators are amines. Another subset of derivatives, such as 4–9, 11, 12, 18, and 19 were slightly less effective CAAs with KAs in the range of 4.18–12.8 µM. They include both amino acid and amine derivatives. The least effective activators were L/D-His and L-Phe, with KAs in the range of 15.4–20.3 µM. Again, generally D-enantiomers of the amino acids were generally more effective activators compared to the L-enantiomers (for His, Phe, DOPA, and Tyr), whereas in the case of Trp, the L-enantiomer was a better activator compared to the D one ().
(iii) There are important differences in activation efficacy of these amino acids and amines against the two bacterial enzymes, with the β-class one being much more sensitive to activation compared to the α-class. There are also important differences of the activation profiles of these compounds for the bacterial and human CAs, which is a rather important observation as this may lead to isoform-selective activators. However, for this small panel of activators, the human CAs were generally much better activated compared to the bacterial enzymes, with few exceptions, such as the activity of 13–17 for VchCAβ which was much more susceptible to be activated compared to hCA I, II, and VchCAα. This observation demonstrates that it may be possible to design bacterial CA – selective activators.
4. Conclusions
The first activation study of two CAs from the bacterial pathogen Vibrio cholerae is reported here, with a series of amino acid and amine derivatives. The most effective VchCAα activators were L-tyrosine, histamine, serotonin, and 4-aminoethyl-morpholine, which had KAs in the range of 8.21–12.0 µM. The most effective VchCAβ activators were D-tyrosine, dopamine, serotonin, 2-pyridyl-methylamine, 2-aminoethylpyridine, and 2-aminoethylpiperazine, which showed activation constants in the submicromolar – low micromolar range, KAs of 0.18–1.37 µM. The two bacterial enzymes had very different activation profiles with these compounds, between them, and also when compared to the human isoforms hCA I and II. Some amines were VchCAβ – selective activators. The activation of CAs from bacteria, such as VchCAα/β, was never considered up until now for possible biomedical applications. It would be of interest to study in more detail whether CA activators may contribute to processes connected with the virulence and colonisation of the host by such pathogenic bacteria, which as Vibrio cholerae, is highly dependent on the bicarbonate concentration in the tissue.
Disclosure statement
The authors do not declare any conflict of interest.
Additional information
Funding
References
- Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;12:4421–68.
- (a) Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J Enzyme Inhib Med Chem 2013;28:229–30. (b) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- (a) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88. (b) Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- (a) Capasso C, Supuran CT. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32. (b) Del Prete S, Vullo D, Fisher GM, et al. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum–the η-carbonic anhydrases. Bioorg Med Chem Lett 2014;24:4389–96.
- (a) Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72. (b) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- (a) Scozzafava A, Briganti F, Mincione G, et al. Carbonic anhydrase inhibitors: synthesis of water-soluble, aminoacyl/dipeptidyl sulfonamides possessing long-lasting intraocular pressure-lowering properties via the topical route. J Med Chem 1999;42:3690–700. (b) Puccetti L, Fasolis G, Vullo D, et al. Carbonic anhydrase inhibitors. Inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with Schiff’s bases incorporating chromone and aromatic sulfonamide moieties, and their zinc complexes. Bioorg Med Chem Lett 2005;15:3096–101.
- (a) Gul HI, Yamali C, Yesilyurt F, et al. Microwave-assisted synthesis and bioevaluation of new sulfonamides. J Enzyme Inhib Med Chem 2017;32:369–74. (b) Gulçin İ, Abbasova M, Taslimi P, et al. Synthesis and biological evaluation of aminomethyl and alkoxymethyl derivatives as carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase inhibitors. J Enzyme Inhib Med Chem 2017;32:1174–82.
- (a) Briganti F, Mangani S, Orioli P, et al. Carbonic anhydrase activators: X-ray crystallographic and spectroscopic investigations for the interaction of isozymes I and II with histamine. Biochemistry 1997;36:10384–92. (b) Clare BW, Supuran CT. Carbonic anhydrase activators. 3: structure-activity correlations for a series of isozyme II activators. J Pharm Sci 1994;83:768–73. (c) Ilies M, Scozzafava A, Supuran CT. Carbonic anhydrase activators. In: Supuran CT, Scozzafava A, Conway J, eds. Carbonic anhydrase – its inhibitors and activators. Boca Raton (FL): CRC Press; 2004. 317–52 p.
- (a) Scozzafava A, Menabuoni L, Mincione F, Supuran CT. Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyamino − polycarboxylate tails and of their metal complexes possessing long-lasting, topical intraocular pressure-lowering properties. J Med Chem 2002;45:1466–76. (b) Pacchiano F, Aggarwal M, Avvaru BS, et al. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb) 2010;46:8371–3. (c) Carta F, Scozzafava A, Supuran CT. Sulfonamides: a patent review (2008–2012). Expert Opin Ther Pat 2012;22:747–58.
- (a) Akocak S, Lolak N, Vullo D, et al. Synthesis and biological evaluation of histamine Schiff bases as carbonic anhydrase I, II, IV, VII, and IX activators. J Enzyme Inhib Med Chem 2017;32:1305–12. (b) Angeli A, Vaiano F, Mari F, et al. Psychoactive substances belonging to the amphetamine class potently activate brain carbonic anhydrase isoforms VA, VB, VII, and XII. J Enzyme Inhib Med Chem 2017;32:1253–9. (c) Licsandru E, Tanc M, Kocsis I, et al. A class of carbonic anhydrase I – selective activators. J Enzyme Inhib Med Chem 2017;32:37–46.
- (a) Kumar R, Sharma V, Bua S, et al. Synthesis and biological evaluation of benzenesulphonamide-bearing 1,4,5-trisubstituted-1,2,3-triazoles possessing human carbonic anhydrase I, II, IV, and IX inhibitory activity. J Enzyme Inhib Med Chem 2017;32:1187–94. (b) Del Prete S, Perfetto R, Rossi M, et al. A one-step procedure for immobilising the thermostable carbonic anhydrase (SspCA) on the surface membrane of Escherichia coli. J Enzyme Inhib Med Chem 2017;32:1120–8. (c) Stanica L, Gheorghiu M, Stan M, et al. Quantitative assessment of specific carbonic anhydrase inhibitors effect on hypoxic cells using electrical impedance assays. J Enzyme Inhib Med Chem 2017;32:1079–90.
- (a) Zengin Kurt B, Sonmez F, Durdagi S, et al. Synthesis, biological activity and multiscale molecular modeling studies for coumaryl-carboxamide derivatives as selective carbonic anhydrase IX inhibitors. J Enzyme Inhib Med Chem 2017; 32:1042–52. (b) Nocentini A, Vullo D, Del Prete S, et al. Inhibition of the β-carbonic anhydrase from the dandruff-producing fungus Malassezia globosa with monothiocarbamates. J Enzyme Inhib Med Chem 2017;32:1064–70. (c) Del Prete S, Vullo D, Osman SM, et al. Anion inhibitors of the β-carbonic anhydrase from the pathogenic bacterium responsible of tularemia, Francisella tularensis. Bioorg Med Chem 2017;25:4800–4.
- (a) Perfetto R, Del Prete S, Vullo D, et al. Cloning, expression and purification of the α-carbonic anhydrase from the mantle of the Mediterranean mussel, Mytilus galloprovincialis. J Enzyme Inhib Med Chem 2017;32:1029–35. (b) Abdoli M, Angeli A, Bozdag M, et al. Synthesis and carbonic anhydrase I, II, VII, and IX inhibition studies with a series of benzo[d]thiazole-5- and 6-sulfonamides. J Enzyme Inhib Med Chem 2017;32:1071–8. (c) De Simone G, Langella E, Esposito D, et al. Insights into the binding mode of sulphamates and sulphamides to hCA II: crystallographic studies and binding free energy calculations. J Enzyme Inhib Med Chem 2017;32:1002–11.
- (a) Supuran CT, Capasso C. Carbonic Anhydrase from Porphyromonas Gingivalis as a Drug Target. Pathogens 2017;6:E30. (b) Capasso C, Supuran CT. Inhibition of bacterial carbonic anhydrases as a novel approach to escape drug resistance. Curr Top Med Chem 2017;17:1237–48. (c) Supuran CT, Capasso C. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. J Enzyme Inhib Med Chem 2016;31:1254–60.
- Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat 2013;23:681–91.
- Masini E, Carta F, Scozzafava A, Supuran CT. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Pat 2013;23:705–16.
- Scozzafava A, Supuran CT, Carta F. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat 2013;23:725–35.
- (a) Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Pat 2013;23:737–49. (b) Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:E48.
- (a) Supuran CT. Carbonic anhydrase inhibition and the management of neuropathic pain. Expert Rev Neurother 2016;16:961–8. (b) Di Cesare Mannelli L, Micheli L, Carta F, et al. Carbonic anhydrase inhibition for the management of cerebral ischemia: in vivo evaluation of sulfonamide and coumarin inhibitors. J Enzyme Inhib Med Chem 2016;31:894–9.
- (a) Margheri F, Ceruso M, Carta F, et al. Overexpression of the transmembrane carbonic anhydrase isoforms IX and XII in the inflamed synovium. J Enzyme Inhib Med Chem 2016;31:60–3. (b) Bua S, Di Cesare Mannelli L, Vullo D, et al. Design and synthesis of novel nonsteroidal anti-inflammatory drugs and carbonic anhydrase inhibitors hybrids (NSAIDs-CAIs) for the treatment of rheumatoid arthritis. J Med Chem 2017;60:1159–70.
- (a) Tu C, Rowlett RS, Tripp BC, et al. Chemical rescue of proton transfer in catalysis by carbonic anhydrases in the beta- and gamma-class. Biochemistry 2002;41:15429–35. (b) Smith KS, Ingram-Smith C, Ferry JG. Roles of the conserved aspartate and arginine in the catalytic mechanism of an archaeal beta-class carbonic anhydrase. J Bacteriol 2002;184:4240–5.
- (a) Temperini C, Scozzafava A, Supuran CT. Carbonic anhydrase activation and the drug design. Curr Pharm Des 2008;14:708–15. (b) Isik S, Guler OO, Kockar F, et al. Saccharomyces cerevisiae β-carbonic anhydrase: inhibition and activation studies. Curr Pharm Des 2010;16:3327–36.
- (a) Temperini C, Scozzafava A, Vullo D, Supuran CT. Carbonic anhydrase activators. Activation of isozymes I, II, IV, VA, VII, and XIV with l- and d-histidine and crystallographic analysis of their adducts with isoform II: engineering proton-transfer processes within the active site of an enzyme. Chemistry 2006;12:7057–66. (b) Temperini C, Scozzafava A, Vullo D, Supuran CT. Carbonic anhydrase activators. Activation of isoforms I, II, IV, VA, VII, and XIV with L- and D-phenylalanine and crystallographic analysis of their adducts with isozyme II: stereospecific recognition within the active site of an enzyme and its consequences for the drug design. J Med Chem 2006;49:3019–27. (c) Temperini C, Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase activators: kinetic and X-ray crystallographic study for the interaction of D- and L-tryptophan with the mammalian isoforms I-XIV. Bioorg Med Chem 2008;16:8373–8.
- (a) Temperini C, Innocenti A, Scozzafava A, et al. Carbonic anhydrase activators: L-Adrenaline plugs the active site entrance of isozyme II, activating better isoforms I, IV, VA, VII, and XIV. Bioorg Med Chem Lett 2007;17:628–35. (b) Temperini C, Scozzafava A, Puccetti L, Supuran CT. Carbonic anhydrase activators: X-ray crystal structure of the adduct of human isozyme II with L-histidine as a platform for the design of stronger activators. Bioorg Med Chem Lett 2005;15:5136–41. (c) Temperini C, Scozzafava A, Supuran CT. Carbonic anhydrase activators: the first X-ray crystallographic study of an adduct of isoform I. Bioorg Med Chem Lett 2006;16:5152–6.
- (a) Vullo D, Nishimori I, Innocenti A, et al. Carbonic anhydrase activators: an activation study of the human mitochondrial isoforms VA and VB with amino acids and amines. Bioorg Med Chem Lett 2007;17:1336–40. (b) Pastorekova S, Vullo D, Nishimori I, et al. Carbonic anhydrase activators: activation of the human tumor-associated isozymes IX and XII with amino acids and amines. Bioorg Med Chem 2008;16:3530–6. (c) Nishimori I, Onishi S, Vullo D, et al. Carbonic anhydrase activators. The first activation study of the human secretory isoform VI. Bioorg Med Chem 2007;15:5351–7.
- (a) Parkkila S, Vullo D, Puccetti L, et al. Carbonic anhydrase activators: activation of isozyme XIII with amino acids and amines. Bioorg Med Chem Lett 2006;16:3955–9. (b) Vullo D, Innocenti A, Nishimori I, et al. Carbonic anhydrase activators: activation of the human isoforms VII (cytosolic) and XIV (transmembrane) with amino acids and amines. Bioorg Med Chem Lett 2007;17:4107–12. (c) Vullo D, Nishimori I, Scozzafava A, Supuran CT. Carbonic anhydrase activators: activation of the human cytosolic isozyme III and membrane-associated isoform IV with amino acids and amines. Bioorg Med Chem Lett 2008;18:4303–7.
- (a) Innocenti A, Hilvo M, Parkkila S, et al. Carbonic anhydrase activators. Activation of the membrane-associated isoform XV with amino acids and amines. Bioorg Med Chem Lett 2009;19:3430–3. (b) Supuran CT, Dinculescu A, Balaban AT. Carbonic anhydrase activators. Part 5. CA II activation by 2,4,6-trisubstituted pyridinium cations with 1-(ω-aminoalkyl) side chains. Rev Roum Chim 1993;38:343–9. (c) Supuran CT, Barboiu M, Luca C, et al. Carbonic anhydrase activators. Part 14. Synthesis of mono- and bis- pyridinium salt derivatives of 2-amino-5-(2-aminoethyl)- and 2-amino-5-(3-aminopropyl)-1,3,4-thiadiazole, and their interaction with isozyme II. Eur J MedChem 1996;31:597–606. (d) Ilies MA, Banciu MD, Ilies M, et al. Carbonic anhydrase activators. Part 17. Synthesis and activation study of a series of 1-(1,2,4-triazole-(1H)-3-yl)-2,4,6-trisubstituted-pyridinium salts against isozymes I, II and IV. Eur J MedChem 1997;32:911–18.
- (a) Ilies M, Banciu MD, Ilies MA, et al. Carbonic anhydrase activators: design of high affinity isozymes I, II, and IV activators, incorporating tri-/tetrasubstituted-pyridinium-azole moieties. J Med Chem 2002;45:504–10. (b) Dave K, Scozzafava A, Vullo D, et al. Pyridinium derivatives of histamine are potent activators of cytosolic carbonic anhydrase isoforms I, II and VII. Org Biomol Chem 2011;9:2790–800. (c) Dave K, Ilies MA, Scozzafava A, et al. An inhibitor-like binding mode of a carbonic anhydrase activator within the active site of isoform II. Bioorg Med Chem Lett 2011;21:2764–8.
- (a) Scozzafava A, Supuran CT. Carbonic anhydrase activators: human isozyme II is strongly activated by oligopeptides incorporating the carboxyterminal sequence of the bicarbonate anion exchanger AE1. Bioorg Med Chem Lett 2002;12:1177–80. (b) Scozzafava A, Supuran CT. Carbonic anhydrase activators: high affinity isozymes I, II, and IV activators, incorporating a beta-alanyl-histidine scaffold. J Med Chem 2002;45:284–91. (c) Abdo MR, Vullo D, Saada MC, et al. Carbonic anhydrase activators: activation of human isozymes I, II and IX with phenylsulfonylhydrazido l-histidine derivatives. Bioorg Med Chem Lett 2009;19:2440–3. (d) Saada MC, Montero JL, Vullo D, et al. Carbonic anhydrase activators: gold nanoparticles coated with derivatized histamine, histidine, and carnosine show enhanced activatory effects on several mammalian isoforms. J Med Chem 2011;54:1170–7. (e) Zhang Y, Legrand YM, Petit E, et al. Dynamic encapsulation and activation of carbonic anhydrase in multivalent dynameric host matrices. Chem Commun (Camb) 2016;52:4053–5.
- Vullo D, Kumar RSS, Scozzafava A, et al. Sulphonamide inhibition studies of the β-carbonic anhydrase from the bacterial pathogen Clostridium perfringens. J Enzyme Inhib Med Chem 2018;33:31–6.
- Aspatwar A, Hammarén M, Koskinen S, et al. β-CA-specific inhibitor dithiocarbamate Fc14-584B: a novel antimycobacterial agent with potential to treat drug-resistant tuberculosis. J Enzyme Inhib Med Chem 2017;32:832–40.
- (a) Modak JK, Liu YC, Supuran CT, Roujeinikova A. Structure-activity relationship for sulfonamide inhibition of Helicobacter pylori α-carbonic anhydrase. J Med Chem 2016;59:11098–109. (b) Cau Y, Mori M, Supuran CT, Botta M. Mycobacterial carbonic anhydrase inhibition with phenolic acids and esters: kinetic and computational investigations. Org Biomol Chem 2016;14:8322–30.
- (a) Supuran CT. Bortezomib inhibits bacterial and fungal β-carbonic anhydrases. Bioorg Med Chem 2016; 24:4406–9. (b) Supuran CT. Legionella pneumophila carbonic anhydrases: underexplored antibacterial drug targets. Pathogens 2016;5:E44.
- Annunziato G, Angeli A, D’Alba F, et al. Discovery of new potential anti-infective compounds based on carbonic anhydrase inhibitors by rational target-focused repurposing approaches. ChemMedChem 2016;11:1904–14.
- (a) Vullo D, De Luca V, Scozzafava A, et al. The first activation study of a bacterial carbonic anhydrase (CA). The thermostable α-CA from sulfurihydrogenibium yellowstonense YO3AOP1 is highly activated by amino acids and amines. Bioorg Med Chem Lett 2012;22:6324–7. (b) Innocenti A, Zimmerman SA, Scozzafava A, et al. Carbonic anhydrase activators: activation of the archaeal beta-class (Cab) and gamma-class (Cam) carbonic anhydrases with amino acids and amines. Bioorg Med Chem Lett 2008;18:6194–8. (c) Vullo D, Del Prete S, Osman SM, et al. Comparison of the amine/amino acid activation profiles of the β- and γ-carbonic anhydrases from the pathogenic bacterium Burkholderia pseudomallei. J Enzyme Inhib Med Chem 2018;33:25–30.
- (a) Del Prete S, Isik S, Vullo D, et al. DNA cloning, characterization, and inhibition studies of an α-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. J Med Chem 2012;55:10742–8. (b) Del Prete S, De Luca V, Scozzafava A, et al. Biochemical properties of a new α-carbonic anhydrase from the human pathogenic bacterium, Vibrio cholerae. J Enzyme Inhib Med Chem 2014;29:23–7.
- (a) Vullo D, Isik S, Del Prete S, et al. Anion inhibition studies of the α-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem Lett 2013;23:1636–8. (b) Ceruso M, Del Prete S, Alothman Z, et al. Sulfonamides with potent inhibitory action and selectivity against the α-carbonic anhydrase from Vibrio cholerae. ACS Med Chem Lett 2014;5:826–30.
- (a) Alafeefy AM, Ceruso M, Al-Tamimi AM, et al. Quinazoline-sulfonamides with potent inhibitory activity against the α-carbonic anhydrase from Vibrio cholerae. Bioorg Med Chem 2014;22:5133–40. (b) Carta F, Osman SM, Vullo D, et al. Poly(amidoamine) dendrimers show carbonic anhydrase inhibitory activity against α-, β-, γ- and η-class enzymes. Bioorg Med Chem 2015;23:6794–8.
- (a) Ferraroni M, Del Prete S, Vullo D, et al. Crystal structure and kinetic studies of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Acta Crystallogr D Biol Crystallogr 2015;71:2449–56. (b) Del Prete S, Vullo D, De Luca V, et al. Sulfonamide inhibition studies of the β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem 2016;24:1115–20.
- (a) Vullo D, Del Prete S, De Luca V, et al. Anion inhibition studies of the β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem Lett 2016;26:1406–10. (b) Del Prete S, Vullo D, De Luca V, et al. Comparison of the sulfonamide inhibition profiles of the α-, β- and γ-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem Lett 2016;26:1941–6.
- (a) Del Prete S, Vullo D, De Luca V, et al. Anion inhibition profiles of α-, β- and γ-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem 2016;24:3413–17. (b) Mohamed MA, Abdel-Aziz AA, Sakr HM, et al. Synthesis and human/bacterial carbonic anhydrase inhibition with a series of sulfonamides incorporating phthalimido moieties. Bioorg Med Chem 2017;25:2524–9.
- (a) De Vita D, Angeli A, Pandolfi F, et al. Inhibition of the α-carbonic anhydrase from Vibrio cholerae with amides and sulfonamides incorporating imidazole moieties. J Enzyme Inhib Med Chem 2017;32:798–804. (b) Angeli A, Abbas G, Del Prete S, et al. Acyl selenoureido benzensulfonamides show potent inhibitory activity against carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg Chem 2017;75:170–2.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- Vullo D, Del Prete S, Osman SM, et al. Burkholderia pseudomallei γ-carbonic anhydrase is strongly activated by amino acids and amines. Bioorg Med Chem Lett 2017;27:77–80.