Abstract
The two β-carbonic anhydrases (CAs, EC 4.2.1.1) from the pathogenic bacterium Brucella suis, BsuCA1 and BsuCA2, were investigated for their inhibition profile with a series of pyridine-3-sulphonamide derivatives incorporating 4-hetaryl moieties. BsuCA1 was effectively inhibited by these sulphonamides with inhibition constants ranging between 34 and 624 nM. BsuCA2 was less sensitive to these inhibitors, with KIs in the range of 62 nM - > 10 µM. The nature of the 4-substituent present on the pyridine ring was the main factor influencing the inhibitory profile against both isoforms, with 4-halogenophenylpiperazin-1-yl and 3,4,5-trisubstituted-pyrazol-1-yl derivatives showing the most effective inhibition. Some of these sulphonamides were most effective bacterial CA than human (h) CA I and II inhibitors, making them selective for the prokaryotic enzymes. Investigation of bacterial CA inhibitors may be relevant for finding antibiotics with a new mechanism of action compared to the clinically used agents for which substantial drug resistance emerged.
1. Introduction
Bacteria encode for at least three genetic families of the metalloenzyme carbonic anhydrase (CA, EC 4.2.1.1), the α-, β- and γ-CAsCitation1–3. By catalysing the interconversion between carbon dioxide and bicarbonate, with formation or consumption of a hydronium ion, these widespread enzymes are involved in a multitude of physiologic processes connected with the pH regulation, biosynthetic processes in which CO2/bicarbonate are involved, photosynthesis, acclimation in various environments were the bacteria thrive, colonisation of the host and moreCitation1–5. Since CA inhibition from vertebrates, more exactly humans, in which 15 different α-CA isoforms were describedCitation6, has pharmacologic applications, the idea to exploit bacterial/microbial CA inhibition for obtaining antiinfectives with a new mechanism of action started to be explored in recent yearsCitation1–4,Citation7–11. Indeed, many classes of inhibitors for all three types of bacterial CAs were discovered to date, among which the sulphonamides represent the most investigated chemotypeCitation12–16. CA inhibitors (CAIs) targeting human enzymes (hCAs) are in clinical use for decades for the management of various diseases among which glaucoma, obesity, epilepsy, intracranial hypertension and as diureticsCitation13–16. More recently they started to be used for the treatment of hypoxic tumoursCitation13,Citation14, being also investigated as possible drugs for neuropathic painCitation17, cerebral ischemiaCitation18 and arthritisCitation19.
Brucella suis is one of the bacteria responsible of brucellosis, a disease affecting an increasing number of people and which showed variable degrees of resistance to the clinically used antibiotics [1c,9–11]. Two β-class CAs were discovered in the genome of this pathogen, BsuCA1 and BsuCA2Citation9,Citation10, which have also been investigated for their inhibition with various compounds, such as sulphonamides, sulphamates, anions, phenols, etc.Citation9–12. Furthermore, the growth of the bacterium was also impaired (in cell cultures) by some of these inhibitors, which constitutes the proof-of-concept that BsuCA1/2 inhibition may have a significant antibacterial effectCitation9. Continuing our interest in the discovery of CAIs which effectively target bacterial CAs, we report here an inhibition study of BsuCA1/2 with a class of pyridine-3-sulphonamide derivatives incorporating 4-heterocyclic/heteroaryl moieties, previously designed by our groups for targeting the tumor-associated human isoforms hCA IX and XIICitation12. Some of the investigated sulphonamides from this article are among the most effective and isoform-selective BsuCA1 inhibitors ever reported.
2. Materials and methods
2.1. Chemistry
Sulfonamides 1–18 used in this study were reported earlier by our groupsCitation12 and were used without further purification. Acetazolamide (AAZ), buffers and other inorganic reagents were the highest purity available reagents from Sigma-Aldrich (Milan, Italy).
2.2. Cloning, expression and purification of BsuCA1and BsuCA2
cDNA encoding BsuCA1 and BsuCA2 (a kind gift of Prof J.Y. Winum from University of Montpellier, France) were PCR engineered to be cloned in pETM13 (a kind gift from EMBL, Heidelberg) expression vector. The resulting plasmids, pETM13-bsuca1 and pETM13-bsuca2, were verified by appropriate digestion with restriction enzymes and sequencing. BsCA1 and BsCA2 were expressed in LB culture medium by induction with 1 mM IPTG in Escherichia coli BL21 (DE3) and BL21 (DE3) plusS cells, respectively. After 16hs incubation at 22 °C, cells were harvested by centrifugation, lysed and affinity purified onto a 1 ml His Trap FF column and subsequently on a Superdex 75 10/300 GL column (GE Healthcare). Purity level of BsCA1 and BsCA2 was assessed by LC-MS and SDS-PAGE.
2.3. Carbonic anhydrase inhibition
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activityCitation20. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM TRIS (pH 8.3) as buffer and 20 mM NaClO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters (by Lineweaver–Burk plots) and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (10 mM) were prepared in distilled-deionized water and dilutions up to 0.1 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 6 and the Cheng–Prusoff equation, as reported earlierCitation21–25, and represent the mean from at least three different determinations.
3. Results and discussion
Aromatic and heterocyclic sulphonamides were showed earlier by some of us to act as inhibitors of the two β-CAs from B. suis, with various degrees of efficacyCitation9,Citation10. Usually, the heterocyclic derivatives, such as acetazolamide (5-acetamido-1,3,4-thiadiazole-2-sulfonamide, AAZ) were among the best bacterial CA inhibitors, but their efficacy was much better for the human isoforms hCA I and II (highly abundant proteins found in the blood, GI tract and many other tissuesCitation6,Citation13) which may lead to a series of side effects if such inhibitors should be used as anti-bacterials. Thus, exploration of structurally different sulphonamides may lead to the discovery of compounds with a better inhibitory profile and better selectivity for the bacterial over the human isoforms. Thus, in the present study, we investigated a series of pyridine-3-sulfonamide derivatives incorporating 4- heterocyclic/heteroaryl moieties 1–18, reported earlier by our groups as effective tumor-associated human isoforms hCA IX and XII inhibitorsCitation12.
Inhibition data of hCA I and II (offtargets) as well as BsuCA1/2 with sulfonamides 1–18 and acetazolamide as standard inhibitor are shown in and .
Table 1. Inhibition of human (h) CA isoforms hCA I and II and bacterial (Brucella suis) enzymes BsuCA1 and BsuCA2 with sulfonamides 1–12, by a stopped-flow CO2 hydrase assayCitation20. Acetazolamide (AAZ) was used as standard inhibitor.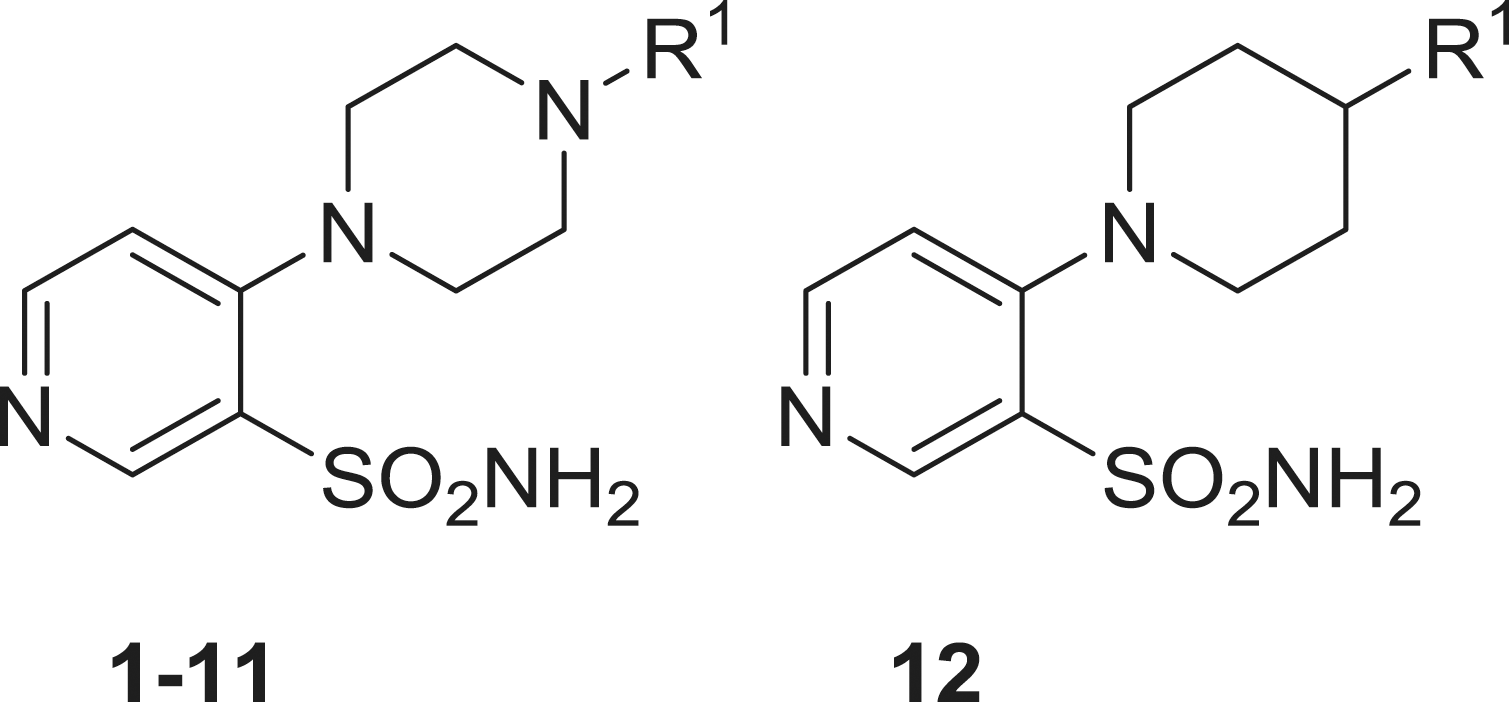
Table 2. Inhibition of human (h) CA isoforms hCA I and II and bacterial (Brucella suis) enzymes BsuCA1 and BsuCA2 with sulfonamides 13–18, by a stopped-flow CO2 hydrase assayCitation20. Acetazolamide (AAZ) was used as standard inhibitor.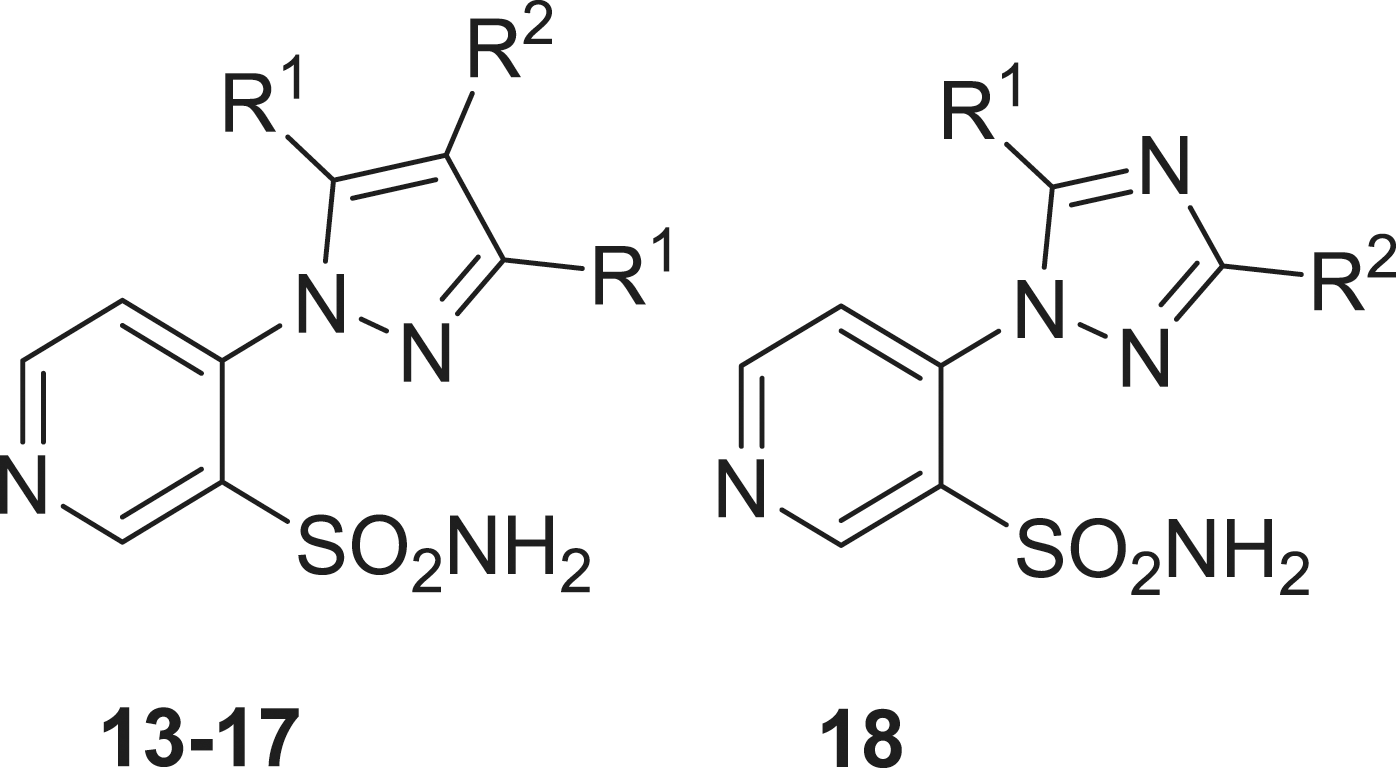
The following structure–activity relationship for the inhibition of the two bacterial CAs with sulphonamides 1–18 can be drawn from data of and :
BsuCA1 was rather sensitive to be inhibited by sulphonamides 1–18 investigated here, showing KIs ranging between 34 and 624 nM (). The nature of the ring appended in position 4 of the pyridine sulphonamide and the moieties substituting it were the most important factors influencing enzyme inhibitory properties of these compounds. Thus, the derivatives incorporating the five-membered heterocyclic rings present in 13–18 were generally more effective than sulfonamides incorporating substituted piperazines/piperidines 1–12. For the six-membered ring substituted derivatives, the substitution patterns leading to the most effective inhibitors were 4-chloro/fluorophenyl (2 and 3); benzyl (10 and 12) and piperonyl (11), all these compounds being more effective as BsuCA1 inhibitors compared to the standard inhibitor acetazolamide. For the second subset, only the triazole derivative 18 was slightly less effective as BsuCA1 inhibitor (KI of 112 nM) whereas all pyrazoles 13–17 had KIs < 100 nM, in the range of 38–96 nM. The substitution patterns connected with the most effective inhibition were those present in 13–16 (R1 a methyl group, and R2 being a H, Me, Bu or ethoxycarbonylethyl moiety). For the first subset the least effective inhibitors (4–9) incorporated diverse substituents at the 4-phenyl-piperazine moiety, of the type o-fluoro-phenyl; 3,4-dichlorophenyl; o-methoxyphenyl, 2,5-dimethylphenyl). It is obvious that small modifications in the substitution pattern and nature of the moieties present on the phenyl ring in the 4-arylsubstituted piperazines strongly influence the biological activity.
BsuCA2 was slightly less sensitive to inhibition with sulphonamides 1–18 compared to BsuCA1, but this behaviour was already reported in previous inhibition studies of these two enzymesCitation9–11. Thus, 5–7 did not substantially inhibit this enzyme up to 10 µM concentration of inhibitor within the assay system. Weak BsuCA2 inhibitors were also sulfonamides 1, 3, 4, 9, 11, 12 and 13–18, with KIs in the range of 237 – 4650 nM ( and ). Thus, for this enzyme, the 5-membered ring-substituted derivatives 13–18 were only medium potency – weak inhibitors (in contrast to what observed for the first isoform, BsuCA1, as mentioned above). The most effective BsuCA2 inhibitors were 2, 8 and 10, with KIs in the range 62–97 nM. It may be observed that these three sulfonamides are 3–5 times better BsuCA2 inhibitors compared to acetazolamide, and these are indeed relevant data. As for BsuCA1, small changes in the scaffold of the inhibitor lead to drastic differences of activity. For example, introduction of Cl in the para position of the phenyl moiety of 1 led to an increase in the inhibitory power of 13.9 times for the sulphonamide 2, the best BsuCA2 inhibitor detected so far ().
Most of the investigated sulphonamides were weak hCA I and II inhibitorsCitation12 () making them of great interest for more detailed inhibition of growth studies of the pathogen, ex vivo and possible also in vivo.
4. Conclusions
We have investigated in this article the inhibition of the two β-CAs present in the pathogenic bacterium Brucella suis, BsuCA1 and BsuCA2, for their inhibition profile with a series of pyridine-3-sulfonamide derivatives incorporating 4-heterocyclic/heteroaryl moieties, originally reported as inhibitors of the tumour-associated human isoforms hCA IX and XII. BsuCA1 was effectively inhibited by these sulphonamides with inhibition constants ranging between 34 and 624 nM. BsuCA2 was less sensitive to these inhibitors, with KIs in the range of 62 nM - > 10 µM. The nature of the 4-substituent present on the pyridine ring was the main factor influencing the inhibitory profile against both isoforms, with 4-halogenophenylpiperazin-1-yl and 3,4,5-trisubstituted-pyrazol-1-yl derivatives showing the most effective inhibition. Some of these sulphonamides were most effective bacterial CA than human (h) CA I and II inhibitors, making them selective for the prokaryotic enzymes. Investigation of bacterial CA inhibitors may be relevant for finding antibiotics with a new mechanism of action compared to the clinically used agents for which substantial drug resistance emerged.
Disclosure statement
The authors do not declare any conflict of interest.
References
- (a) Supuran CT, Capasso C. An overview of the bacterial carbonic anhydrases. Metabolites 2017;7:E56.(b) Capasso C, Supuran CT. An overview of the carbonic anhydrases from two pathogens of the oral cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr Top Med Chem 2016;16:2359–68.(c) Köhler S, Ouahrani-Bettache S, Winum JY. Brucella suis carbonic anhydrases and their inhibitors: towards alternative antibiotics? J Enzyme Inhib Med Chem 2017;32:683–7.(d) Supuran CT. Bacterial carbonic anhydrases as drug targets: toward novel antibiotics? Front Pharmacol 2011;2:34.
- (a) Supuran CT, Capasso C. Carbonic anhydrase from Porphyromonas Gingivalis as a drug target. Pathogens 2017;6:E30. (b) Capasso C, Supuran CT. Inhibition of bacterial carbonic anhydrases as a novel approach to escape drug resistance. Curr Top Med Chem 2017;17:1237–48. (c) Supuran CT, Capasso C. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. Enzyme Inhib Med Chem 2016;31:1254–60. (d) Supuran CT. Legionella pneumophila carbonic anhydrases: underexplored antibacterial drug targets. Pathogens 2016;5:E44.
- (a) De Vita D, Angeli A, Pandolfi F, et al. Inhibition of the α-carbonic anhydrase from Vibrio cholerae with amides and sulfonamides incorporating imidazole moieties. J Enzyme Inhib Med Chem 2017;32:798–804. (b) Del Prete S, Vullo D, Osman SM, et al. Sulfonamide inhibition profiles of the β-carbonic anhydrase from the pathogenic bacterium Francisella tularensis responsible of the febrile illness tularemia. Bioorg Med Chem 2017;25:3555–61. (c) Vullo D, Del Prete S, Di Fonzo P, et al. Comparison of the sulfonamide inhibition profiles of the β- and γ-carbonic anhydrases from the pathogenic bacterium Burkholderia pseudomallei. Molecules 2017;22:E421.
- (a) Vullo D, Kumar RSS, Scozzafava A, et al. Sulphonamide inhibition studies of the β-carbonic anhydrase from the bacterial pathogen Clostridium perfringens. Enzyme Inhib Med Chem 2018;33:31–6. (b) Angeli A, Abbas G, Del Prete S, et al. Acyl selenoureido benzensulfonamides show potent inhibitory activity against carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg Chem 2017;75:170–2. (c) Aspatwar A, Hammarén M, Koskinen S, et al. β-CA-specific inhibitor dithiocarbamate Fc14-584B: a novel antimycobacterial agent with potential to treat drug-resistant tuberculosis. J Enzyme Inhib Med Chem 2017;32:832–40.
- (a) Mohamed MA, Abdel-Aziz AA, Sakr HM, et al. Synthesis and human/bacterial carbonic anhydrase inhibition with a series of sulfonamides incorporating phthalimido moieties. Bioorg Med Chem 2017;25:2524–9. (b) Modak JK, Liu YC, Supuran CT, Roujeinikova A. Structure–activity relationship for sulphonamide inhibition of Helicobacter pylori α-carbonic anhydrase. J Med Chem 2016;59:11098–109. (c) Cau Y, Mori M, Supuran CT, Botta M. Mycobacterial carbonic anhydrase inhibition with phenolic acids and esters: kinetic and computational investigations. Org Biomol Chem 2016;14:8322–30.
- (a) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88. (b) Supuran Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. (c) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- (a) Supuran CT. Bortezomib inhibits bacterial and fungal β-carbonic anhydrases. Bioorg Med Chem 2016;24:4406–9. (b) Lomelino CL, Supuran CT, McKenna R. Non-classical inhibition of carbonic anhydrase. Int J Mol Sci 2016;17:E1150. (c) Annunziato G, Angeli A, D'Alba F, et al. Discovery of new potential anti-infective compounds based on carbonic anhydrase inhibitors by rational target-focused repurposing approaches. ChemMedChem 2016;11:1904–14.
- (a) Ferraroni M, Del Prete S, Vullo D, et al. Crystal structure and kinetic studies of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Acta Crystallogr D Biol Crystallogr 2015;71:2449–56.(b) Orhan F, Şentürk M, Supuran CT. Interaction of anions with a newly characterized alpha carbonic anhydrase from Halomonas sp. J Enzyme Inhib Med Chem 2016;31:1119–23. (c) Eminoğlu A, Vullo D, Aşık A, et al. Cloning, expression and biochemical characterization of a β-carbonic anhydrase from the soil bacterium Enterobacter sp. B13. J Enzyme Inhib Med Chem 2016;31:1111–8.
- (a) Joseph P, Turtaut F, Ouahrani-Bettache S, et al. Cloning, characterization, and inhibition studies of a beta-carbonic anhydrase from Brucella suis. J Med Chem 2010;53:2277–85. (b) Joseph P, Ouahrani-Bettache S, Montero JL, et al. A new β-carbonic anhydrase from Brucella suis, its cloning, characterization, and inhibition with sulfonamides and sulfamates, leading to impaired pathogen growth. Bioorg Med Chem 2011;19:1172–8.
- (a) Winum JY, Köhler S, Supuran CT. Brucella carbonic anhydrases: new targets for designing anti-infective agents. Curr Pharm Des 2010;16:3310–6. (b) Vullo D, Nishimori I, Scozzafava A, et al. Inhibition studies of a beta-carbonic anhydrase from Brucella suis with a series of water soluble glycosyl sulfanilamides. Bioorg Med Chem Lett 2010;20:2178–82. (c) Supuran CT. Inhibition of bacterial carbonic anhydrases and zinc proteases: from orphan targets to innovative new antibiotic drugs. Curr Med Chem 2012;19:831–44.
- (a) Riafrecha LE, Vullo D, Supuran CT, Colinas PA. C-glycosides incorporating the 6-methoxy-2-naphthyl moiety are selective inhibitors of fungal and bacterial carbonic anhydrases. J Enzyme Inhib Med Chem 2015;30:857–61. (b) Riafrecha LE, Vullo D, Ouahrani-Bettache S, et al. Inhibition of β-carbonic anhydrases from Brucella suis with C-cinnamoyl glycosides incorporating the phenol moiety. J Enzyme Inhib Med Chem 2015;30:1017–20. (c) Ombouma J, Vullo D, Köhler S, et al. N-glycosyl-N-hydroxysulfamides as potent inhibitors of Brucella suis carbonic anhydrases. J Enzyme Inhib Med Chem 2015;30:1010–2. (d) Ceruso M, Carta F, Osman SM, et al. Inhibition studies of bacterial, fungal and protozoan β-class carbonic anhydrases with Schiff bases incorporating sulfonamide moieties. Bioorg Med Chem 2015;23:4181–7.
- Sławiński J, Szafrański K, Vullo D, Supuran CT. Carbonic anhydrase inhibitors. Synthesis of heterocyclic 4-substituted pyridine-3-sulfonamide derivatives and their inhibition of the human cytosolic isozymes I and II and transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 2013;69:701–10.
- (a) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. (b) Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72. (c) Alterio V, Di Fiore A, D'Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68.
- (a) Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J Enzyme Inhib Med Chem 2013;28:229–30. (b) Capasso Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin Ther Targets 2015;19:1689–704. (c) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov 2011;10:767–77. (d) Monti SM, Supuran CT, Simone GD. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Patents 2013;23:737–49.
- (a) Masini E, Carta F, Scozzafava A, Supuran CT. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Patents 2013;23:705–16. (b) Supuran CT. Acetazolamide for the treatment of idiopathic intracranial hypertension. Expert Rev Neurother 2015;15:851–6. (c) Scozzafava A. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Patents 2013;23:725–35.
- (a) Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat 2013;23:681–91. (b) Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74. (c) Supuran CT. Drug interaction considerations in the therapeutic use of carbonic anhydrase inhibitors. Expert Opin Drug Metab Toxicol 2016;12:423–31. (d) Karioti A, Carta F, Supuran CT. An Update on Natural Products with Carbonic Anhydrase Inhibitory Activity. Curr Pharm Des 2016;22:1570–91. (e) Winum JY, Supuran CT. Recent advances in the discovery of zinc-binding motifs for the development of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2015;30:321–4.
- (a) Carta F, Di Cesare Mannelli L, Pinard M, et al. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg Med Chem 2015;23:1828–40. (b) Supuran CT. Carbonic anhydrase inhibition and the management of neuropathic pain. Expert Rev Neurother 2016;16:961–8.
- Di Cesare Mannelli L, Micheli L, Carta F, et al. Carbonic anhydrase inhibition for the management of cerebral ischemia: in vivo evaluation of sulfonamide and coumarin inhibitors. J Enzyme Inhib Med Chem 2016;31:894–9.
- (a) Margheri F, Ceruso M, Carta F, et al. Overexpression of the transmembrane carbonic anhydrase isoforms IX and XII in the inflamed synovium. J Enzyme Inhib Med Chem 2016;31(sup4):60–3. (b) Bua S, Di Cesare Mannelli L, Vullo D, et al. Design and synthesis of novel nonsteroidal anti-inflammatory drugs and carbonic anhydrase inhibitors hybrids (NSAIDs-CAIs) for the treatment of rheumatoid arthritis. J Med Chem 2017;60:1159–70.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- (a) Scozzafava A, Briganti F, Mincione G, et al. Carbonic anhydrase inhibitors: synthesis of water-soluble, aminoacyl/dipeptidyl sulfonamides possessing long-lasting intraocular pressure-lowering properties via the topical route. J Med Chem 1999;42:3690–700. (b) Puccetti L, Fasolis G, Vullo D, et al. Carbonic anhydrase inhibitors. Inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with Schiff's bases incorporating chromone and aromatic sulfonamide moieties, and their zinc complexes. Bioorg Med Chem Lett 2005;15:3096–101.
- (a) Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1, 3, 5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005;1:3102–8. (b) Scozzafava A, Menabuoni L, Mincione F, Supuran CT. Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyamino − polycarboxylate tails and of their metal complexes possessing long-lasting, topical intraocular pressure-lowering properties. J Med Chem 2002;45:1466–76.
- (a) Supuran CT, Nicolae A, Popescu A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;31:431–8. (b) Şentürk M, Gülçin I, Beydemir Ş, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9. (c) Carta F, Aggarwal M, Maresca A, et al. Dithiocarbamates: a new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chem Commun (Camb) 2012;48:1868–70.
- (a) Supuran CT, Barboiu M, Luca C, et al. Carbonic anhydrase activators. Part 14. Syntheses of mono and bis pyridinium salt derivatives of 2-amino-5-(2-aminoethyl)- and 2-amino-5-(3-aminopropyl)-1,3,4-thiadiazole and their interaction with isozyme II. Eur J Med Chem 1996;31:597–606. (b) Carta F, Aggarwal M, Maresca A, et al. Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. J Med Chem 2012;55:1721–30. (c) Maresca A, Carta F, Vullo D, Supuran CT. Dithiocarbamates strongly inhibit the β-class carbonic anhydrases from Mycobacterium tuberculosis. J Enzyme Inhib Med Chem 2013;28:407–11.
- (a) Fabrizi F, Mincione F, Somma T, et al. A new approach to antiglaucoma drugs: carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J Enzyme Inhib Med Chem 2012;27:138–47. (b) Zimmerman SA, Ferry JG, Supuran CT. Inhibition of the archaeal beta-class (Cab) and gamma-class (Cam) carbonic anhydrases. Curr Top Med Chem 2007;7:901–8. (c) Oztürk Sarikaya SB, Topal F, Sentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62.
