Abstract
To identify anticancer agents with higher potency and lower toxicity, a series of oridonin derivatives with substituted benzene moieties at the C17 position were designed, synthesised, and evaluated for their antiproliferative properties. Most of the derivatives exhibited antiproliferative effects against AGS, MGC803, Bel7402, HCT116, A549, and HeLa cells. Compound 2p (IC50 = 1.05 µM) exhibited the most potent antiproliferative activity against HCT116 cells; it was more potent than oridonin (IC50 = 6.84 µM) and 5-fluorouracil (5-FU) (IC50 = 24.80 µM). The IC50 value of 2p in L02 cells was 6.5-fold higher than that in HCT116 cells. Overall, it exhibited better selective antiproliferative activity and specificity than oridonin and 5-FU. Furthermore, compound 2p arrested HCT116 cells at the G2 phase of the cell cycle and increased the percentage of apoptotic cells to a greater extent than oridonin.
Keywords:
Introduction
Over the last several decades, cancer has been one of the leading causes of death in the worldCitation1. Cytotoxic agents are the mainstay of anticancer therapy. However, due to their inability to differentiate between normal and cancerous cells, they cause severe adverse effects, leading to poor patient complianceCitation2. Therefore, the identification of novel anticancer agents with higher potency and lower toxicity is needed for the treatment of aggressive and refractory cancers. More than 60% of all clinically used anticancer drugs originate from natural sourcesCitation3, highlighting the success of natural products.
Oridonin (1) () is an ent-kaurane diterpenoid isolated from Isodon rubescens (Donglingcao in Chinese). Since the identification of its antitumour potential in 1967, it has been used in traditional Chinese medicineCitation4. It is reported to prevent hepatic fibrosisCitation5–7, Alzheimer’s diseaseCitation8, microbial infectionsCitation9, and inflammationCitation10. It has been shown to exert anticancer effects with limited adverse effectsCitation11–17. However, limited hydrophobic/hydrophilic partitioning and oral bioavailability diminish its therapeutic potential and clinical utilityCitation18. Therefore, it is necessary to develop novel oridonin analogues with potent antitumour efficacy through structural effective modifications.
Figure 1. The structure of oridonin, costunolide, compound 3, 4, 5 and the design of target compound 2a–2y.
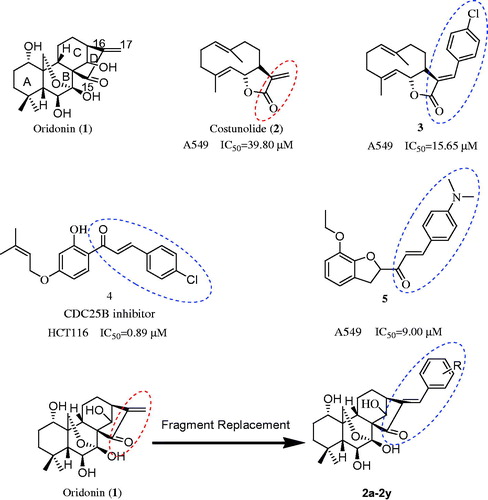
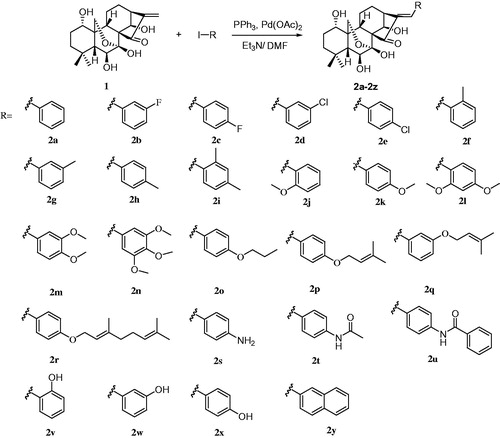
Compounds with substituted benzene rings display a broad spectrum of biological activities; therefore, structural modification by adding substituted benzene rings is widely used in medicinal chemistry. It increases receptor binding by promoting hydrophobic interactions and enhances lipid solubility to facilitate drug permeation. In addition, an arylating α,β-unsaturated ketone system is commonly present in several anticancer agents, such as compounds 3–5Citation19–21 (). In a previous experiment, the arylated α,β-unsaturated ketone was added to the structure of costunolide; the resultant compound 3 was found to have better antiproliferative activity against A549 cells (IC50 = 15.65 µM) than costunolide (IC50 = 39.80 µM)Citation19 (). This substitution in the D ring was also found to be responsible for the antiproliferative activity of oridonin; the reduction or expansion of this ring is expected to significantly reduce efficacy. Inspired by these reports, we synthesised novel oridonin analogues, containing the arylated α,β-unsaturated ketone system in its D ring, to evaluate whether these analogues display better anticancer activity than oridonin. In this study, we designed, synthesised, and evaluated novel oridonin derivatives (2a–2y) containing a substituted benzene at the C-17 position () for their in vitro antitumour efficacy.
Materials and methods
Chemistry
IR spectra were recorded (in KBr) on IR Prestige-21. 1H-NMR and 13C-NMR spectra were measured on an AV-300 (Bruker BioSpin, Switzerland), 13C-NMR spectra were measured on an AV-500 (Bruker BioSpin, Switzerland), and all chemical shifts were given in ppm relative to tetramethylsilane (TMS). High-resolution mass spectra were measured using a matrix-assisted laser desorption/ionisation-time of flight (TOF)/TOF mass spectrometer (Bruker Daltonik, Bremen, Germany). The major chemicals were purchased from Aldrich Chemical Corporation. All other chemicals were of analytical grade.
General procedure for the synthesis of compound (2a–2y)
To a stirred solution of oridonin (0.19 mmol, 70.00 mg), substituted iodobenzene (0.23 mmol) in N, N-dimethylformamide (5.00 ml), triethylamine (0.58 mmol, 58.18 mg), palladium acetate (0.01 mmol, 2.24 mg) and triphenylphosphine (0.020 mmol, 5.24 mg) was added. The reaction mixture was stirred at 90 °0 (oil bath temperature) for 20–24 h monitored by TLC and then concentrated under reduced pressure. The residue was added water (10.0 ml) and extracted with dichloromethane. The organic layer was washed with saturated NaHCO3, saturated NaCl and several portions of 15% hydrochloric acid solution, and dried over anhydrous Na2SO4. Then it can be purified by chromatography on silica eluting with a gradient of methanol/dichloromethane (1:80–1:30) to obtain the compounds (2a–2y) as white solids.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-8-benzylidene-1,5,6,14-tetrahydroxy-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthale-n-7(8H)-one (2a)
Yield 70%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.02 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.16–1.35 (m, 4H), 1.40–1.69 (m, 4H), 1.79–1.85 (m, 1H), 2.10–2.20 (m, 1H), 2.56–2.65 (m, 1H), 3.27–3.30 (m, 1H), 3.52–3.58 (m, 1H), 3.88 (d, 1H, J = 9.9 Hz), 4.15 (d, 1H, J = 9.6 Hz), 4.40 (d, 1H, J = 5.1 Hz), 4.86 (s, 1H), 6.03 (s, 1H), 6.24 (d, 1H, J = 10.5 Hz), 6.90 (s, 1H), 7.42 (s, 1H), 7.46–7.61 (m, 5H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.89, 22.19, 28.44, 29.84, 33.21, 33.85, 38.87, 40.96, 41.37, 53.28, 60.04, 61.46, 63.25, 72.16, 73.47, 73.67, 97.54, 129.54, 130.35, 130.67, 133.19, 134.74, 143.63, 209.50. IR (KBr) cm−1: 3394, 2912, 1693, 1615. HRMS calcd. for C26H32NaO6 ([M + Na]+): 463.2091; found: 463.2085.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-8-(3-fluorobenzylidene)-1,5,6,14-tetrahydroxy-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]n-aphthalen-7(8H)-one (2b)
Yield 43%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.15–1.35 (m, 4H), 1.43–1.67 (m, 4H), 1.79–1.83 (m, 1H), 2.12–2.20 (m, 1H), 2.57–2.67 (m, 1H), 3.27–3.30 (m, 1H), 3.52–3.58 (m, 1H), 3.88 (d, 1H, J = 9.9 Hz), 4.15 (d, 1H, J = 9.6 Hz), 4.41 (d, 1H, J = 5.1 Hz), 4.86 (s, 1H), 6.03 (s, 1H), 6.15 (d, 1H, J = 10.5 Hz), 6.91 (s, 1H), 7.29–7.59 (m, 5H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.85, 22.17, 28.39, 29.81, 33.19, 33.84, 40.97, 41.33, 53.34, 60.01, 61.53, 63.27, 72.17, 73.39, 73.67, 97.50, 117.05, 126.56, 131.56, 137.15, 144.98, 161.77, 163.71, 209.43. IR (KBr) cm−1: 3404, 2902, 1695, 1618. HRMS calcd. for C26H31FNaO6 ([M + Na]+): 481.1997; found: 481.1989.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-8-(4-fluorobenzylidene)-1,5,6,14-tetrahydroxy-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]n-aphthalen-7(8H)-one (2c)
Yield 46%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.14–1.34 (m, 4H), 1.43–1.66 (m, 4H), 1.80–1.82 (m, 1H), 2.12–2.20 (m, 1H), 2.57–2.67 (m, 1H), 3.27–3.30 (m, 1H), 3.50–3.56 (m, 1H), 3.88 (d, 1H, J = 9.9 Hz), 4.14 (d, 1H, J = 9.6 Hz), 4.41 (d, 1H, J = 5.1 Hz), 4.85 (s, 1H), 6.03 (s, 1H), 6.23 (d, 1H, J = 10.5 Hz), 6.91 (s, 1H), 7.31–7.37 (m, 2H), 7.42 (s, 1H), 7.64–7.70 (m, 2H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.84, 22.15, 28.29, 29.79, 33.18, 33.83, 38.84, 40.95, 41.20, 53.26, 55.31, 56.52, 60.03, 61.42, 63.25, 72.18, 73.45, 73.65, 97.51, 116.71, 132.08, 133.06, 143.20, 162.23, 164.21, 209.44. IR (KBr) cm−1: 3395, 2900, 1693, 1625. HRMS calcd. for C26H31FNaO6 ([M + Na]+): 481.1997; found: 481.1987.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-8-(3-chlorobenzylidene)-1,5,6,14-tetrahydrox-y-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]-naphthalen-7(8H)-one (2d)
Yield 51%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.14–1.34 (m, 4H), 1.51–1.67 (m, 4H), 1.79–1.85 (m, 1H), 2.12–2.20 (m, 1H), 2.51–2.65 (m, 1H), 3.27–3.30 (m, 1H), 3.51–3.57 (m, 1H), 3.88 (d, 1H, J = 9.9 Hz), 4.15 (d, 1H, J = 9.6 Hz), 4.41 (d, 1H, J = 5.1 Hz), 4.86 (s, 1H), 6.03 (s, 1H), 6.14 (d, 1H, J = 10.5 Hz), 6.89 (s, 1H), 7.40 (s, 1H), 7.52–7.54 (m, 2H), 7.63–7.64 (m, 2H) 13C-NMR (DMSO-d6, 125 MHz): δ 19.85, 22.17, 28.43, 29.81, 33.20, 33.84, 38.85, 40.96, 41.35, 53.34, 60.01, 61.54, 63.27, 72.16, 73.37, 73.66, 97.49, 128.73, 129.96, 130.24, 131.38, 131.55, 134.15, 136.93, 145.16, 209.38. IR (KBr) cm−1: 3450, 2925, 1689, 1621. HRMS calcd. for C26H31ClNaO4 ([M + Na]+): 497.1701; found: 497.1692.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-8-(4-chlorobenzylidene)-1,5,6,14-tetrahydroxy-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]-naphthalen-7(8H)-one (2e)
Yield 53%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.14–1.39 (m, 4H), 1.43–1.66 (m, 4H), 1.78–1.82 (m, 1H), 2.12–2.20 (m, 1H), 2.57–2.67 (m, 1H), 3.27–3.30 (m, 1H), 3.51–3.57 (m, 1H), 3.87 (d, 1H, J = 9.9 Hz), 4.14 (d, 1H, J = 9.9 Hz), 4.41 (d, 1H, J = 5.1 Hz), 4.86 (s, 1H), 6.03 (s, 1H), 6.18 (d, 1H, J = 10.5 Hz), 6.90 (s, 1H), 7.40 (s, 1H), 7.54–7.63 (m, 4H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.85, 22.16, 28.36, 29.80, 33.19, 33.83, 38.85, 40.96, 41.30, 53.31, 60.02, 61.48, 63.26, 72.17, 73.41, 73.66, 97.51, 129.60, 131.83, 132.32, 133.62, 134.94, 144.28, 209.41. IR (KBr) cm−1: 3441, 2932, 1685, 1615. HRMS calcd. for C26H31ClNaO4 ([M + Na]+): 497.1701; found: 497.1693.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-4,4-dimethyl-8-(2-methylbenzylidene)decahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]-naphthalen-7(8H)-one (2f)
Yield 74%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.16–1.34 (m, 4H), 1.45–1.66 (m, 4H), 1.81–1.85 (m, 1H), 2.10–2.17 (m, 1H), 2.37 (s, 3H, Ar–CH3), 2.53–2.58 (m, 1H), 3.18 (d, 1H, J = 9.9 Hz), 3.52–3.55 (m, 1H), 3.87 (d, 1H, J = 9.9 Hz), 4.14 (d, 1H, J = 9.9 Hz), 4.38 (d, 1H, J = 5.1 Hz), 4.85 (s, 1H), 6.02 (s, 1H), 6.20 (d, 1H, J = 10.5 Hz), 6.85 (s, 1H), 7.32–7.34 (m, 3H), 7.39 (d, 1H, J = 7.0 Hz), 7.58 (s, 1H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.93, 20.06, 22.15, 28.99, 29.83, 33.18, 33.86, 38.88, 40.94, 40.99, 53.42, 60.13, 61.64, 63.27, 72.15, 73.41, 73.63, 97.54, 126.80, 128.53, 130.09, 130.21, 131.20, 133.54, 138.87, 144.18, 209.55. IR (KBr) cm−1: 3405, 2925, 1674, 1632. HRMS calcd. for C27H34NaO6 ([M + Na]+): 477.2248; found: 477.2239.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-4,4-dimethyl-8-(3-methylbenzylidene)decahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one (2g)
Yield 67%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.17–1.31 (m, 4H), 1.44–1.67 (m, 4H), 1.78–1.83 (m, 1H), 2.12–2.21 (m, 1H), 2.37 (s, 3H), 2.59–2.65 (m, 1H), 3.27–3.30 (m, 1H), 3.52–3.57 (m, 1H), 3.87 (d, 1H, J = 9.9 Hz), 4.14 (d, 1H, J = 9.6 Hz), 4.40 (d, 1H, J = 5.1 Hz), 4.86 (s, 1H), 6.02 (s, 1H), 6.18 (d, 1H, J = 10.5 Hz), 6.90 (s, 1H), 7.29 (s, 1H), 7.39 (s, 4H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.88, 21.45, 22.18, 28.46, 29.83, 33.20, 33.84, 38.86, 40.95, 41.39, 53.28, 55.28, 60.03, 63.25, 72.17, 73.46, 73.67, 97.54, 127.65, 129.43, 131.08, 131.34, 133.32, 134.69, 138.76, 143.46, 209.49. IR (KBr) cm−1: 3434, 2933, 1675, 1627. IR (KBr) cm−1: 3429, 2921, 1684, 1620. HRMS calcd. for C27H34NaO6 ([M + Na]+): 477.2248; found: 477.2236.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-4,4-dimethyl-8-(4-methylbenzylidene)decahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one (2h)
Yield 78%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.14–1.33 (m, 4H), 1.44–1.65 (m, 4H), 1.78–1.80 (m, 1H), 2.13–2.17 (m, 1H), 2.36 (s, 3H, Ar–CH3), 2.55–2.61 (m, 1H), 3.29–3.30 (m, 1H), 3.52–3.55 (m, 1H), 3.87 (d, 1H, J = 9.9 Hz), 4.14 (d, 1H, J = 9.9 Hz), 4.37 (d, 1H, J = 5.1 Hz), 4.84 (s, 1H), 6.00 (s, 1H), 6.27 (d, 1H, J = 10.5 Hz), 6.88 (s, 1H), 7.31 (d, 2H, J = 7.0 Hz), 7.37 (s, 1H), 7.49 (d, 2H, J = 7.0 Hz). 13C-NMR (DMSO-d6, 125 MHz): δ 19.88, 21.52, 22.19, 28.31, 29.84, 33.21, 33.85, 38.87, 40.95, 41.38, 53.23, 60.04, 61.39, 63.24, 72.17, 73.49, 73.67, 97.55, 130.18, 130.74, 131.97, 133.32, 140.43, 142.55, 209.44. IR (KBr) cm−1: 3425, 2925, 1678, 1615. HRMS calcd. for C27H34NaO6 ([M + Na]+): 477.2248; found: 477.2238.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-8-(3,4-dimethylbenzylidene)-1,5,6,14-tetrahydroxy-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohept-a-[a]naphthalen-7(8H)-one (2i)
Yield 72%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.14–1.34 (m, 4H), 1.43–1.64 (m, 4H), 1.76–1.80 (m, 1H), 2.12–2.19 (m, 1H), 2.27 (s, 6H), 2.57–2.65 (m, 1H), 3.27–3.30 (m, 1H), 3.51–3.57 (m, 1H), 3.87 (d, 1H, J = 9.9 Hz), 4.14 (d, 1H, J = 9.6 Hz), 4.39 (d, 1H, J = 5.1 Hz), 4.84 (s, 1H), 6.00 (s, 1H), 6.30 (d, 1H, J = 10.5 Hz), 6.90 (s, 1H), 7.25–7.36 (m, 4H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.88, 19.91, 22.19, 28.35, 29.84, 33.22, 33.85, 33.88, 40.95, 41.40, 53.23, 55.38, 60.04, 61.38, 63.23, 72.16, 73.48, 73.67, 97.56, 128.14, 130.67, 131.92, 132.34, 133.48, 137.47, 139.34, 142.41, 209.42. IR (KBr) cm−1: 3452, 2935, 1669, 1612. HRMS calcd. for C28H36NaO6 ([M + Na]+): 491.2404; found: 491.2396.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-8-(2-methoxybenzylidene)-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta-[a]naphthalen-7(8H)-one (2j)
Yield 78%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.15–1.34 (m, 4H), 1.43–1.65 (m, 4H), 1.79–1.85 (m, 1H), 2.11–2.19 (m, 1H), 2.57–2.63 (m, 1H), 3.19–3.22 (m, 1H), 3.48–3.55 (m, 1H), 3.81–3.92 (m, 4H), 4.14 (d, 1H, J = 9.6 Hz), 4.40 (d, 1H, J = 5.1 Hz), 4.84 (s, 1H), 6.01 (s, 1H), 6.27 (d, 1H, J = 10.5 Hz), 6.87 (s, 1H), 7.06–7.13 (m, 2H), 7.40–7.45 (m, 2H), 7.72 (s, 1H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.84, 22.15, 28.29, 29.79, 33.18, 33.83, 38.84, 40.95, 41.20, 53.26, 56.53, 60.03, 61.42, 72.18, 73.45, 73.65, 97.51, 116.71, 131.34, 132.99, 133.06, 143.20, 162.23, 164.21, 209.44. IR (KBr) cm−1: 3437, 2928, 1673, 1622. HRMS calcd. for C27H34NaO7 ([M + Na]+): 493.2197; found: 493.2190.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-8-(4-methoxybenzylidene)-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta-[a]naphthalen-7(8H)-one (2k)
Yield 83%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.00 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.14–1.34 (m, 4H), 1.44–1.65 (m, 4H), 1.76–1.81 (m, 1H), 2.13–2.22 (m, 1H), 2.55–2.63 (m, 1H), 3.26–3.29 (m, 1H), 3.50–3.55 (m, 1H), 3.82 (s, 3H), 3.87 (d, 1H, J = 9.9 Hz), 4.13 (d, 1H, J = 9.6 Hz), 4.40 (d, 1H, J = 5.1 Hz), 4.84 (s, 1H), 6.02 (s, 1H), 6.38 (d, 1H, J = 10.5 Hz), 6.90 (s, 1H), 7.07 (d, 2H, J = 8.4 Hz), 7.37 (s, 1H), 7.57 (d, 2H, J = 8.4 Hz). 13C-NMR (DMSO-d6, 125 MHz): 19.86, 22.13, 28.13, 29.78, 33.17, 33.82, 38.84, 40.94, 41.31, 53.15, 56.54, 60.07, 61.28, 63.21, 72.19, 73.57, 73.64, 97.55, 115.13, 127.21, 132.67, 133.37, 140.77, 161.16, 209.34. IR (KBr) cm−1: 3430, 2924, 1670, 1626. HRMS calcd. for C27H34NaO7 ([M + Na]+): 493.2197; found: 493.2189.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-8-(2,4-dimethoxybenzylidene)-1,5,6,14-tetrahydroxy-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohe-pta[a]naphthalen-7(8H)-one (2l)
Yield 82%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.14–1.34 (m, 4H), 1.44–1.65 (m, 4H), 1.75–1.79 (m, 1H), 2.08–2.20 (m, 1H), 2.55–2.61 (m, 1H), 3.26–3.29 (m, 1H), 3.49–3.55 (m, 1H), 3.84 (s, 3H), 3.87 (s, 3H), 4.10–4.14 (m, 1H), 4.37 (d, 1H, J = 5.1 Hz), 4.83 (s, 1H), 5.98 (s, 1H), 6.40 (d, 1H, J = 10.5 Hz), 6.66 (s, 2H), 66.86 (s, 1H), 7.40 (d, 2H, J = 8.4 Hz), 7.69 (s, 1H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.92, 22.16, 28.29, 29.85, 33.19, 33.85, 38.89, 40.92, 41.23, 53.15, 56.00, 56.35, 60.14, 61.30, 63.22, 72.16, 73.62, 97.60, 106.62, 116.09, 127.30, 130.60, 140.29, 160.58, 163.00, 209.32. IR (KBr) cm−1: 3445, 2913, 1682, 1628. HRMS calcd. for C28H36NaO8 ([M + Na]+): 523.2302; found: 493.2295.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-8-(3,4-dimethoxybenzylidene)-1,5,6,14-tetrahydroxy-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one (2m)
Yield 85%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.14–1.34 (m, 4H), 1.43–1.67 (m, 4H), 1.76–1.80 (m, 1H), 2.08–2.21 (m, 1H), 2.51–2.59 (m, 1H), 3.18 (d, J = 5.4 Hz, 1H), 3.26–3.29 (m, 1H), 3.51–3.57 (m, 1H), 3.81 (s, 3H, -OCH3), 3.82 (s, 3H, -OCH3), 3.87 (d, 1H, J = 9.9 Hz), 4.10–4.16 (m, 1H), 4.38 (d, 1H, J = 5.1 Hz), 4.85 (s, 1H), 6.00 (s, 1H), 6.91 (d, 1H, J = 10.5 Hz), 7.08–7.21 (m, 3H), 7.38 (s, 1H).13C-NMR (DMSO-d6, 125 MHz): δ 19.89, 22.19, 28.11, 29.84, 33.21, 33.84, 38.87, 40.94, 41.40, 53.17, 55.35, 55.91, 56.09, 60.04, 63.22, 72.18, 73.54, 73.67, 97.58, 112.44, 113.72, 124.48, 127.45, 133.75, 140.95, 149.24, 150.96, 209.21. IR (KBr) cm−1: 3440, 2923, 1673, 1632. HRMS calcd. for C28H36NaO8 ([M + Na]+): 523.2302; found: 493.2296.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-4,4-dimethyl-8-(3,4,5-trimethoxybenzylidene)decahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclo-hepta[a]naphthalen-7(8H)-one (2n)
Yield 87%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.02 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.15–1.35 (m, 4H), 1.47–1.64 (m, 4H), 1.78–1.82 (m, 1H), 2.08–2.19 (m, 1H), 2.55–2.63 (m, 1H), 3.35–3.37 (m, 1H), 3.52–3.57 (m, 1H), 3.72 (s, 3H, -OCH3), 3.83 (s, 6H, -OCH3), 3.89 (d, 1H, J = 9.9 Hz), 4.14 (d, 1H, J = 9.6 Hz), 4.39 (d, 1H, J = 5.1 Hz), 4.85 (s, 1H), 5.98 (s, 1H), 6.28 (d, 1H, J = 10.5 Hz), 6.90 (s, 2H), 6.93 (s, 1H), 7.38 (s, 1H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.88, 22.21, 28.21, 29.84, 33.23, 33.85, 38.86, 40.95, 41.45, 53.24, 56.38, 59.97, 60.04, 61.42, 63.25, 72.17, 73.46, 73.68, 97.56, 108.10, 130.23, 133.62, 139.57, 142.60, 153.46, 209.29. IR (KBr) cm−1: 3390, 2923, 1675, 1616. HRMS calcd. for C29H38NaO9 ([M + Na]+): 553.2408; found: 553.2398.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-4,4-dimethyl-8-(4-propoxybenzylidene)decahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta-[a]naphthalen-7(8H)-one (2o)
Yield 85%. 1H-NMR (DMSO-d6, 300 MHz): δ 0.98–1.03 (m, 9H, –CH3), 1.15–1.34 (m, 6H), 1.47–1.64 (m, 4H), 1.73–1.78 (m, 2H), 2.08–2.20 (m, 1H), 2.57–2.65 (m, 1H), 3.25–3.28 (m, 1H), 3.50–3.55 (m, 1H), 3.87 (d, 1H, J = 9.9 Hz), 4.00 (t, 2H, J = 6.0 Hz), 4.13 (d, 1H, J = 9.6 Hz), 4.40 (d, 1H, J = 5.1 Hz), 4.83 (s, 1H), 6.02 (s, 1H), 6.37 (d, 1H, J = 10.5 Hz), 6.90 (s, 1H), 7.05 (d, 2H, J = 8.1 Hz), 7.55 (d, 2H, J = 8.1 Hz). 13C-NMR (DMSO-d6, 125 MHz): δ 10.80, 19.01, 19.88, 22.18, 22.40, 28.14, 29.84, 33.21, 33.84, 40.95, 41.31, 53.16, 56.51, 60.09, 61.30, 63.22, 69.66, 72.19, 73.66, 97.58, 115.56, 127.12, 132.66, 133.34, 140.74, 160.62, 209.28. IR (KBr) cm−1: 3341, 2936, 1678, 1626. HRMS calcd. for C29H38NaO7 ([M + Na]+): 521.2510; found: 521.2501.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-4,4-dimethyl-8-(4-((3-methylbut-2-en1yl)oxy)benzylidene)decahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one (2p)
Yield 72%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.00 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.13–1.29 (m, 4H), 1.43–1.63 (m, 4H), 1.72 (s, 3H, –CH3), 1.75 (s, 3H, –CH3), 1.79–1.83 (m, 1H), 2.07–2.25 (m, 1H), 2.55–2.66 (m, 1H), 3.25–3.28 (m, 1H), 3.50–3.55 (m, 1H), 3.86 (d, 1H, J = 9.9 Hz), 4.13 (d, 1H, J = 9.6 Hz), 4.40 (d, 1H, J = 5.1 Hz), 4.59 (d, 2H, J = 6.3 Hz, –OCH2–) 4.83 (s, 1H), 5.44 (t, 1H, J = 6.0 Hz), 6.02 (s, 1H), 6.37 (d, 1H, J = 10.5 Hz), 6.90 (s, 1H), 7.05 (d, 2H, J = 8.4 Hz), 7.37 (s, 1H), 7.54 (d, 2H, J = 8.4 Hz). 13C-NMR (DMSO-d6, 125 MHz): δ 18.51, 19.01, 19.89, 22.18, 25.89, 28.14, 29.84, 33.21, 33.84, 38.89, 40.94, 41.30, 53.16, 56.51, 60.09, 61.30, 63.22, 65.02, 72.19, 73.66, 97.58, 115.74, 116.99, 127.14, 132.61, 138.11, 140.75, 160.40, 209.29. IR (KBr) cm−1: 3343, 2927, 1674, 1628. HRMS calcd. for C31H40NaO7 ([M + Na]+): 547.2666; found: 547.2655.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-4,4-dimethyl-8-(3-((3-methylbut-2-en1yl)oxy)benzylidene)decahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one (2q)
Yield 65%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.02 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.15–1.30 (m, 4H), 1.44–1.61 (m, 5H), 1.72 (s, 3H, –CH3), 1.76 (s, 3H, –CH3), 1.81–1.84 (m, 1H), 2.08–2.20 (m, 1H), 2.55–2.67 (m, 1H), 3.26–3.29 (m, 1H), 3.52–3.58 (m, 1H), 3.88 (d, 1H, J = 9.9 Hz), 4.14 (d, 1H, J = 9.6 Hz), 4.40 (d, 1H, J = 5.1 Hz), 4.58 (d, 2H, J = 6.3 Hz, –OCH2–) 4.86 (s, 1H), 5.46 (t, 1H, J = 6.0 Hz), 6.02 (s, 1H), 6.23 (d, 1H, J = 10.5 Hz), 6.90 (s, 1H), 7.02–7.05 (m, 1H), 7.13–7.17 (m, 2H), 7.39–7.40 (m, 1H). 13C-NMR (DMSO-d6, 125 MHz): δ 18.54, 19.89, 22.19, 25.89, 28.44, 29.83, 33.21, 33.85, 38.87, 40.96, 41.44, 53.27, 60.02, 61.47, 63.25, 64.90, 72.16, 73.45, 73.67, 97.53, 116.54, 116.93, 120.26, 122.70, 130.55, 133.25, 136.05, 137.74, 143.78, 159.16, 209.47. IR (KBr) cm−1: 3333, 2924, 1680, 1625. HRMS calcd. for C31H40NaO7 ([M + Na]+): 547.2666; found: 547.2657.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-8-(4-(((Z)-3,6-dimethylhepta-2,5-dien1yl)oxy)benzylidene)-1,5,6,14-tetrahydroxy-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one (2r)
Yield 79%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.14–1.30 (m, 5H), 1.43–1.53 (m, 3H), 1.57 (s, 3H), 1.63 (s, 3H), 1.67 (s, 1H), 1.72 (s, 3H, –CH3), 1.76–1.81 (m, 1H), 2.03–2.20 (m, 5H), 2.26–2.29 (m, 1H), 2.51–1.62 (m, 1H), 3.51–3.56 (m, 1H), 3.87 (d, 1H, J = 9.9 Hz), 4.14 (d, 1H, J = 9.6 Hz), 4.38 (d, 1H, J = 5.1 Hz), 4.62 (d, 2H, J = 6.3 Hz, –OCH2–) 4.84 (s, 1H), 5.07 (t, 1H, J = 6.0 Hz), 5.43 (t, 1H, J = 6.0 Hz), 6.01 (s, 1H), 6.37 (d, 1H, J = 10.5 Hz), 6.89 (s, 1H), 7.05 (d, 2H, J = 9.0 Hz), 7.37 (s, 1H), 7.55 (d, 2H, J = 9.0 Hz). 13C-NMR (DMSO-d6, 125 MHz): δ 16.84, 18.03, 19.58, 22.18, 22.63, 25.94, 26.25, 28.14, 29.84, 33.21, 33.84, 38.88, 40.94, 41.30, 53.16, 60.08, 61.30, 63.22, 65.07, 72.18, 73.66, 97.58, 110.69, 115.77, 119.80, 124.21, 127.41, 131.52, 132.60, 133.35, 140.74, 141.18, 160.39, 209.29. IR (KBr) cm−1: 3349, 2934, 1670, 1623. HRMS calcd. for C36H49NaO7([M + Na]+): 615.3292; found: 615.3286.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-8-(4-aminobenzylidene)-1,5,6,14-tetrahydroxy-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]-naphthalen-7(8H)-one (2s)
Yield 70%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.02 (s, 3H, –CH3), 1.12–1.33 (m, 4H), 1.42–1.62 (m, 4H), 1.74–1.80 (m, 1H), 2.07–2.22 (m, 1H), 3.17 (s, 2H), 3.22–3.27 (m, 1H), 3.48–3.55 (m, 1H), 3.85 (d, 1H, J = 9.9 Hz), 4.12 (d, 1H, J = 9.6 Hz), 4.38 (d, 1H, J = 5.1 Hz), 4.81 (s, 1H), 5.98 (s, 1H), 6.43 (d, 1H, J = 9.9 Hz), 6.80 (d, 1H, J = 8.4 Hz), 6.90 (s, 1H), 7.20 (d, 1H, J = 8.7 Hz), 7.30 (d, 1H, J = 8.7 Hz), 7.37–7.48 (m, 2H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.90, 22.16, 27.89, 29.85, 33.84, 38.90, 40.92, 41.42, 53.02, 55.35, 56.51, 60.16, 61.11, 63.18, 72.19, 73.64, 97.65, 113.56, 122.72, 132.79, 134.78, 137.32, 149.84, 208.86. IR (KBr) cm−1: 3345, 2934, 1667, 1621. HRMS calcd. for C26H33NNaO6 ([M + Na]+): 478.2200; found: 478.2192.
N-(4-((E)-((1S,4aR,5S,6S,6aR,9S,11aS,14R)-1,5,6,14-tetrahydroxy-4,4-dimethyl-7-oxodecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-8(7H)-ylidene)methyl)phenyl)acetamide (2t)
Yield 63%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.00 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.15–1.29 (m, 4H), 1.43–1.67 (m, 4H), 1.78–1.82 (m, 1H), 1.99 (s, 1H), 2.08 (s, 3H), 2.57–2.66 (m, 1H), 3.53–3.55 (m, 1H), 3.87 (d, 1H, J = 9.9 Hz), 4.04 (q, 1H), 4.14 (d, 1H, J = 9.6 Hz), 4.40 (d, 1H, J = 5.1 Hz), 4.83 (s, 1H), 6.02 (s, 1H), 6.30 (d, 1H, J = 10.5 Hz), 6.91 (s, 1H), 7.33 (s, 1H), 7.55 (d, 2H, J = 8.4 Hz), 7.70 (d, 2H, J = 8.4 Hz), 10.23 (s, 1H, –CONH–). 13C-NMR (DMSO-d6, 125 MHz): δ 19.00, 19.91, 22.14, 28.63, 29.82, 33.16, 33.86, 38.86, 40.93, 41.16, 53.29, 56.18, 60.11, 63.26, 72.16, 73.62, 97.54, 112.04, 121.15, 123.25, 127.27, 129.37, 132.13, 143.10, 158.88, 209.57. IR (KBr) cm−1: 3351, 2940, 1668, 1619. HRMS calcd. for C28H35NNaO7 ([M + Na]+): 520.2306; found: 520.2297.
N-(4-((E)-((1S,4aR,5S,6S,6aR,9S,11aS,14R)-1,5,6,14-tetrahydroxy-4,4-dimethyl-7-oxodecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-8(7H)-ylidene)methyl)phenyl)benzamide (2u)
Yield 65%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.02 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.15–1.35 (m, 4H), 1.44–1.66 (m, 4H), 1.78–1.82 (m, 1H), 2.11–2.21 (m, 1H), 2.57–2.68 (m, 1H), 3.26–3.30 (m, 1H), 3.52–3.58 (m, 1H), 3.88 (d, 1H, J = 9.9 Hz), 4.15 (d, 1H, J = 9.6 Hz), 4.40 (d, 1H, J = 5.1 Hz), 4.86 (s, 1H), 6.04 (s, 1H), 6.33 (d, 1H, J = 10.5 Hz), 6.92 (s, 1H), 7.39 (s, 1H), 7.53–7.63 (m, 5H), 7.96 (t, 4H, J = 8.7 Hz), .10.51 (s, 1H, –CONH–). 13C-NMR (DMSO-d6, 125 MHz): δ 19.89, 22.19, 28.25, 29.85, 33.21, 33.85, 38.88, 40.96, 41.39, 53.25, 60.07, 61.38, 63.24, 72.18, 73.54, 73.68, 97.58, 120.79, 128.23, 128.92, 129.88, 131.57, 131.85, 132.28, 133.07, 141.27, 141.99, 166.34, 209.40. IR (KBr) cm−1: 3343, 2949, 1666, 1621. HRMS calcd. for C33H37NNaO7 ([M + Na]+): 582.2462; found: 582.2458.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-8-(2-hydroxybenzylidene)-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one (2v)
Yield 79%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.14–1.35 (m, 4H), 1.44–1.63 (m, 4H), 1.77–1.87 (m, 1H), 2.06–2.21 (m, 1H), 3.20–3.26 (m, 1H), 3.49–3.58 (m, 1H), 3.86 (d, 1H, J = 9.9 Hz), 4.13 (d, 1H, J = 9.6 Hz), 4.41 (d, 1H, J = 5.1 Hz), 4.84 (s, 1H), 6.02 (s, 1H), 6.34 (d, 1H, J = 10.5 Hz), 6.88–6.95 (m, 3H), 7.27–7.38 (m, 2H), 7.76 (s, 2H), 10.23 (s, 1H, –ArOH). 13C-NMR (DMSO-d6, 125 MHz): δ 19.02, 22.16, 28.52, 29.84, 33.19, 33.85, 38.89, 40.94, 41.19, 53.24, 56.50, 60.13, 61.37, 63.24, 72.17, 73.64, 97.58, 116.38, 119.88, 121.74, 127.95, 129.33, 131.94, 141.85, 157.95, 209.55. IR (KBr) cm−1: 3356, 2943, 1672, 1617. HRMS calcd. for C26H32NaO7 ([M + Na]+): 479.2040; found: 479.2031.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-8-(3-hydroxybenzylidene)-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one (2w)
Yield 66%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.14–1.34 (m, 4H), 1.43–1.66 (m, 4H), 1.78–1.82 (m, 1H), 2.09–2.26 (m, 1H), 2.57–2.64 (m, 1H), 3.26–3.29 (m, 1H), 3.50–3.56 (m, 1H), 3.86 (d, 1H, J = 9.9 Hz), 4.13 (d, 1H, J = 9.6 Hz), 4.41 (d, 1H, J = 5.1 Hz), 4.85 (s, 1H), 6.04 (s, 1H), 6.25 (d, 1H, J = 10.5 Hz), 6.84–6.88 (m, 2H), 6.99–7.03 (m, 2H), 7.26–7.31 (m, 2H), 9.72 (s, 1H, –ArOH). 13C-NMR (DMSO-d6, 125 MHz): δ 19.88, 22.17, 28.50, 29.83, 33.20, 33.84, 38.87, 40.95, 41.45, 53.26, 60.04, 61.44, 63.25, 72.17, 73.47, 73.65, 97.53, 116.69, 117.63, 122.02, 130.52, 133.42, 135.90, 143.35, 158.13, 209.50. IR (KBr) cm−1: 3339, 2945, 1668, 1620. HRMS calcd. for C26H32NaO7 ([M + Na]+): 479.2040; found: 479.2032.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-8-(4-hydroxybenzylidene)-4,4-dimethyldecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one (2x)
Yield 80%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.01 (s, 3H, –CH3), 1.03 (s, 3H, –CH3), 1.14–1.39 (m, 4H), 1.43–1.65 (m, 4H), 1.75–1.79 (m, 1H), 2.04–2.26 (m, 1H), 2.56–2.62 (m, 1H), 3.24–3.27 (m, 1H), 3.50–3.55 (m, 1H), 3.86 (d, 1H, J = 9.9 Hz), 4.13 (d, 1H, J = 9.6 Hz), 4.39 (d, 1H, J = 5.1 Hz), 4.83 (s, 1H), 6.01 (s, 1H), 6.42 (d, 1H, J = 10.5 Hz), 6.88 (m, 3H), 7.33 (s, 1H), 7.47 (d, 2H, J = 7.5 Hz), 10.15 (s, 1H, –ArOH). 13C-NMR (DMSO-d6, 125 MHz): δ 18.97, 19.84, 22.21, 27.99, 29.79, 33.21, 33.77, 40.89, 41.26, 53.06, 56.48, 60.05, 61.22, 63.17, 72.12, 73.67, 97.58, 116.52, 125.71, 132.93, 133.81, 139.72, 159.92, 209.26. IR (KBr) cm−1: 3339, 2945, 1668, 1620. HRMS calcd. for C26H32NaO7 ([M + Na]+): 479.2040; found: 479.2031.
(1S,4aR,5S,6S,6aR,9S,11aS,14R,E)-1,5,6,14-tetrahydroxy-4,4-dimethyl-8-(napht-halen-2-ylmethylene)decahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one (2y)
Yield 67%. 1H-NMR (DMSO-d6, 300 MHz): δ 1.03 (s, 3H, –CH3), 1.04 (s, 3H, –CH3), 1.19–1.35 (m, 4H), 1.44–1.66 (m, 4H), 1.87–1.91 (m, 1H), 2.08–2.23 (m, 1H), 2.55–2.63 (m, 1H), 3.19–3.23 (m, 1H), 3.53–3.58 (m, 1H), 3.88 (d, 1H, J = 9.9 Hz), 4.15 (d, 1H, J = 9.6 Hz), 4.43 (d, 1H, J = 5.1 Hz), 4.88 (s, 1H), 6.07 (s, 1H), 6.26 (d, 1H, J = 10.5 Hz), 6.90 (s, 1H), 7.62–7.64 (m, 4H), 8.01–8.05 (m, 2H), 8.14 (s, 2H). 13C-NMR (DMSO-d6, 125 MHz): δ 19.00, 19.95, 22.13, 29.18, 29.82, 33.16, 33.87, 38.87, 40.97, 41.29, 53.53, 56.51, 60.17, 61.89, 63.30, 72.17, 74.65, 97.56, 123.97, 126.09, 126.95, 127.26, 127.63, 129.28, 130.49, 131.38, 131.98, 133.74, 145.85, 209.36. IR (KBr) cm−1: 3332, 2937, 1668, 1625. HRMS calcd. for C30H34NaO6 ([M + Na]+): 513.2248; found: 513.2240.
Biological evaluation
Materials
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) was purchased from Sigma Chemical Co. (St. Louis, MO). The propidium iodide (PI) and Annexin V-FITC apoptosis detection kit was purchased from BD Pharmingen (SanDiego, CA).
Cell lines and cell culture
Human gastric cancer (AGS), human differentiation of advanced gastric cancer (MGC803), human colorectal cancer (HCT116), human lung cancer (A549), human hepatocellular carcinoma (Bel7402) and human cervical cancer (HeLa) cell lines were obtained from the State Key Laboratory of Natural Resources and Functional Molecules of the Changbai Mountain (Yanbian University) and maintained in Dulbecco’s modified Eagle’s medium and RPMI Media 1640 (RPMI1640), supplemented with 10% foetal bovine serum 100 IU/ml penicillin, 100 mg/ml streptomycin and 2 mmol/l L-glutamine (Sigma) at 37 °7 in a humidified atmosphere containing 5% CO2.
Determination of anticancer activity in vitro
All six human cancer cell lines and normal L02 cells were seeded in 96-well plates at the appropriate densities to ensure exponential growth throughout the experimental period (9 × 103 cells per well) and then allowed to adhere for 24 h. The cells were then treated for 48 h with four serial dilutions (100, 50, 10, and 1 µM) of each compound. 5-Fluorouracil (5-FU) was used as the positive control. After incubation for 48 h, 10 µl MTT solution was added to each well to give a final concentration of 2 mg ml−1. The plates were then incubated for further 4 h. After incubation, the MTT solution was removed and 150 µl DMSO was added to each well to solubilise the formed product. To ensure complete solubilisation, the plates were shaken vigorously for 10 min at room temperature. The optometric density (OD) was read on a microplate reader (ELx800, BioTek, Highland Park, Winooski, VT) at a wavelength of 492 nm and the data were subsequently analysed. The percentage of cell growth inhibition was calculated from the following equation: inhibitory rate (%) = [1–(ODtreated–ODblank)/(ODcontrol–ODblank)] × 100.
Analysis of the cell cycle distribution and apoptosis by flow cytometry
HCT116 cells were plated in six-well plates (1.0 × 106 cells per well) and incubated at 37 °7 for 12 h. Exponentially growing cells were then incubated with compound 2p at different concentrations (0 , 5.0 µM) and oridonin at 5.0 µM. After 48 h, the cells were centrifuged at 1000 rpm for 10 min and then fixed in 70% ethanol at −20 °0 for at least 24 h. The cells were subsequently resuspended in phosphate-buffered saline (PBS) containing 50 µg ml−1 RNase A and 50 µg ml−1 PI. The cellular DNA content for the cell cycle distribution analysis was measured by flow cytometry using a FACSCalibur flow cytometer with Cell Quest software (Becton-Dickinson, Franklin Lakes, NJ), plotting at least 30,000 events per sample. The percentage of cells in the G1, S and G2 phases of the cell cycle were determined using the ModFit LT V4.0 software package (Verity Software, Topsham, ME).
Apoptosis was detected using an Apoptosis Detection Kit (Invitrogen, Eugene, OR). Briefly, cells were plated in six-well plates (1.0 × 106 cells per well) and incubated at 37 °C for 12 h. Exponentially growing cells were then incubated with compound 2p at different concentrations (0 , 5.0 µM) and oridonin at 5.0 µM. Following 48 h of incubation, the cells were collected and washed twice with PBS, once with 1 × binding buffer, and then stained with 5 µM annexin V-FITC and 2.5 µM PI in 1 × binding buffer for 30 min at room temperature in the dark. Apoptotic cells were quantified using a FACSCalibur flow cytometer with the Cell Quest software (Becton-Dickinson).
Results and discussion
Chemistry
As shown in Scheme 1, oridonin (1) was coupled with different aryl halides under standard Heck reaction conditions [5 mol% Pd(OAc)2, 10 mol% PPh3, NEt3, DMF, 90 °0]. The reaction proceeded smoothly at the α,β-unsaturated ketone system of oridonin (1) to specifically yield E-olefin products (2a–2y)Citation22. Before biological evaluation, the compounds were characterised via IR, 1H-NMR, and 13C-NMR spectrometry as well as high-resolution mass spectrometry.
Biological evaluation
MTT assay and structure-activity relationship (SAR) studies
Antiproliferative activities of the synthesised compounds were evaluated against six human cancer cell lines (AGS, MGC803, Bel7402, HCT116, A549, and HeLa) and compared with those of oridonin and 5-FU. The MTT assay was performed using a standard protocolCitation23.
The results of this screening are summarised in . Most of the tested compounds exhibited similar or better bioactivities in vitro than 5-FU. Among them, five alkoxyphenyl-substituted compounds (2j, 2k, 2l, 2p, and 2q) and two methylphenyl substituted compounds (2h and 2i) displayed lower IC50 values (2.87–5.26 µM) than oridonin (8.35 µM) in human gastric cancer cells (AGS). The order of antitumour activity was o-OCH3 > p-OCH3 > m-isopentenyloxy > p-isopentenyloxy > 2,4–CH3 > p–CH3. Analogously, chlorophenyl-substituted compounds (2d and 2e) and alkoxyphenyl-substituted compounds (2k, 2l, 2p, 2q, and 2r) exhibited better antiproliferative activities (IC50 = 2.13, 7.27, 2.79, 3.49, 4.54, 9.10, and 6.86 µM, respectively) against human advanced gastric cancer cells (MGC803) than oridonin (IC50 = 9.18 µM). Among the tested compounds, the order of antitumour activities against hepatocellular carcinoma cells (Bel7402) was 2,4-OCH3 > 3,4-OCH3 > p-isopentenyloxy > o-OCH3 > p-OCH3 > p-propoxy > p-NH2 > p-phenylacetamide > p-geranyloxy > 2,4–CH3 = m-isopentenyloxy; these compounds exhibited IC50 values of 2.41–8.62 µM, whereas the IC50 value of oridonin was 9.59 µM. Analysis using human colorectal cancer cells (HCT116) revealed that compound 2p showed the highest activity (IC50 = 1.08 µM), followed by compounds 2l, 2r, 2k, 2o, 2u, and 2j with IC50 values of 2.28, 2.39, 2.99, 3.09, 4.02, and 5.08 µM, respectively. Therefore, the order of antiproliferative activities was p-isopentenyloxy > 2,4-OCH3 > p-geranyloxy > p-OCH3 > p-propoxy > p-phenylacetamide > o-OCH3. Overall, 14 compounds (2d, 2e, 2h, 2j–2r, 2u, and 2y) showed more potent antiproliferative activities than oridonin (IC50 = 24.66 µM) in human lung cancer cells (A549); compound 2r was the most potent with an IC50 value of 4.16 µM. Compounds 2i, 2n–2r, and 2u against human cervical cancer cells (HeLa) exhibited better antiproliferative activities (IC50 = 14.33, 8.89, 8.21, 6.33, 5.43, and 9.85 µM, respectively) than oridonin (IC50 = 15.03 µM). Overall, compounds 2l (2,4-OCH3) and 2p (p-isopentenyloxy) showed more potent antiproliferative activities in vitro than oridonin against all the six cancer cell lines. Remarkably, compound 2p was 6.8-fold more active than oridonin, inhibiting HCT116 cell proliferation with an IC50 value of 1.05 µM. However, three phenol-substituted compounds (2v, 2w, and 2x) and two aminophenyl-substituted compounds (2s and 2t) displayed poor antiproliferative activities against the six tumour cell lines.
Table 1. Antiproliferative efficacy of oridonin derivatives of 2a–2y in six human cancer cell linesTable Footnoteb.
Based on these preliminary results, we identified the following structure-activity relationships: (1) the introduction of benzene with hydrophobic groups, such as alkoxy, methyl, and halogen, at the C17 position of oridonin, significantly improves antitumour activity with the order of positive potency as alkoxy > methyl > halogen; and (2) the introduction of benzene with hydrophilic groups, such as –OH and –NH2, reduces the antitumour activity of oridonin. Based on this interpretation, we suspected that the lipid partition coefficient crucially affects the antitumour activity of oridonin derivatives.
Selective inhibition of cancer cell growth by compounds 2l and 2q
Lack of selective cytotoxicity is the main factor that restricts the dose of most conventional chemotherapeutic agentsCitation24. We compared the toxicity of compounds 2l and 2p with oridonin or 5-FU on human normal liver cells (L-02). Selectivity indexes between cancer cells and L-02 cells were calculated. As shown in , compound 2p exhibited 6.5-fold higher selectivity for HCT116 cells than for normal L-02 cells; this selectivity displayed by 2p was significantly higher than that displayed by oridonin. Therefore, compound 2p was further analysed to identify its mechanism of selective cytotoxicity.
Table 2. In vitro antiproliferative activities of compounds 2l and 2p against normal cell line (L02).
Cell cycle regulation by compound 2p
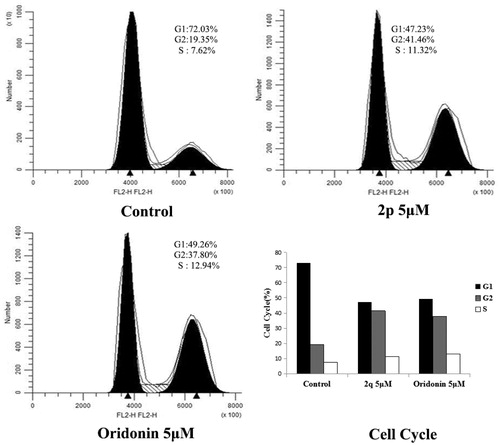
Numerous cytotoxic compounds exert their antiproliferative effect by inducing cell cycle arrest (at a particular cell cycle checkpoint), apoptosis, or bothCitation25. These mechanisms are considered to be effective anticancer strategiesCitation26. We used fluorescence-activated cell sorting analysis to explore the mechanism by which compound 2p reduced the viability of HCT116 cells. Compound 2p and oridonin were selected and tested against HCT116 cell lines at a concentration of 5 µM. As shown in , compound 2p significantly increased the percentage of G2 cell population from 19.35 to 41.46% after 48 h of incubation, whereas oridonin increased it to 37.80%. This finding suggests that compound 2p induces cell cycle arrest at the G2 phase.
Apoptotic effects of compound 2p
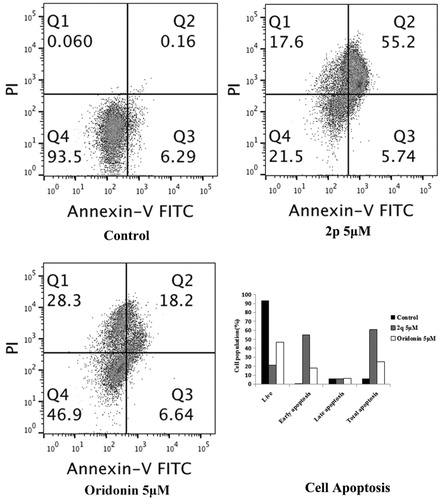
Because compound 2p arrested the cells in the G2 phase, we investigated whether it also causes apoptosis. HCT116 cells were treated with the vehicle, compound 2p (5 µM), or oridonin (5 µM) for 48 h, and then stained with annexin V-FITC and PI. As shown in , the percentage of total apoptotic cells (right quadrants, Q2 + Q3) increased to 60.94% after treatment with compound 2p, whereas it was 24.84% after oridonin treatment; vehicle treatment induced apoptosis in 6.20% of the cells. This result indicated that compound 2p is a more potent inducer of apoptosis than oridonin.
Conclusion
A series of oridonin derivatives with substituted benzene analogues at the C17 position were designed, synthesised, and evaluated for their antiproliferative properties against six human cancer cell lines (AGS, MGC803, Bel7402, HCT116, A549, and HeLa), and the noncancerous human L02 cells. Most of the synthesised oridonin derivatives displayed significant antiproliferative effects in these cancer cell lines. SAR analysis indicated that the alkoxyphenyl ring at the C17 position of oridonin effectively improves its antitumour efficacy. Compound 2p possessed the highest antiproliferative activity against HCT116 cells; it was 6.8-fold more potent than oridonin. The IC50 value of 2p in L02 cells was 6.5-fold higher than that in HCT116 cells, indicating that it exhibits selective antiproliferative effects.
In addition, cell cycle analysis revealed that compound 2p arrested HCT116 cells at the G2 phase. It increased the percentage of apoptotic cells to a greater extent than oridonin. Therefore, compound 2p could serve as a promising lead candidate for further studies.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Mahapatra DK, Bharti SK, Asati V. Anti-cancer chalcones: structural and molecular target perspectives. Eur J Med Chem 2015;98:69–114.
- Bray F, Møller B. Predicting the future burden of cancer. Nat Rev Cancer 2006;6:63–74.
- Cragg GM, Newman DJ. Nature: a vital source of leads for anticancer drug development. Phytochem Rev 2009;8:313–31.
- Fujita E, Nagao Y, Kaneko K, et al. The antitumor and antibacterial activity of the Isodon diterpenoids. Chem Pharm Bull (Tokyo) 1976; 24:2118–27.
- Bohanon FJ, Wang XF, Ding CY, et al. Oridonin inhibits hepatic stellate cell proliferation and fibrogenesis. J Surg Res 2014;190:55–63.
- Bohanon FJ, Wang XF, Graham BM, et al. Enhanced effects of novel oridonin analog CYD0682 for hepatic fibrosis. J Surg Res 2015;199:441–9.
- Bohanon FJ, Wang XF, Graham BM, et al. Enhanced anti-fibrogenic effects of novel oridonin derivative CYD0692 for hepatic stellate cells. Mol Cell Biochem 2015;410:293–300.
- Wang SL, Yang H, Yu LJ, et al. Oridonin attenuates Aβ1–42-induced neuroinflammation and inhibits NF-κB pathway. PLoS One 2014;9:e104745.
- Xu ST, Pei LL, Li DH, et al. Synthesis and anti-mycobacterial evaluation of natural oridonin and its enmein-type derivatives. Fitoterapia 2014;99:300–6.
- Ku CM, Lin JY. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem 2013;141:1104–13.
- Xu ST, Yao H, Luo SS, et al. A novel potent anticancer compound optimized from a natural oridonin scaffold induces apoptosis and cell cycle arrest through the mitochondrial pathway. J Med Chem 2017;60:1449–68.
- Ding CY, Zhang YS, Chen HJ, et al. Oridonin ring A-based diverse constructions of enone functionality: identification of novel dienone analogues effective for highly aggressive breast cancer by inducing apoptosis. J Med Chem 2013;56:8814–25.
- Xu ST, Pei LL, Wang CQ, et al. Novel hybrids of natural oridonin-bearing nitrogen mustards as potential anticancer drug candidates. ACS Med Chem Lett 2014;5:797–802.
- Li DH, Han T, Tian HT, et al. Novel nitric oxide-releasing spirolactone-type diterpenoid derivatives with in vitro synergistic anticancer activity as apoptosis inducer. Bioorg Med Chem Lett 2016;26:4191–6.
- Li DH, Xu ST, Cai H, et al. Enmein-type diterpenoid analogs from natural kaurene-type oridonin: synthesis and their antitumor biological evaluation. Eur J Med Chem 2013;64:215–21.
- Ding CY, Wang LL, Chen HJ, et al. ent-Kaurane-based regio-and stereoselective inverse electron demand hetero-Diels-Alder reactions: synthesis of dihydropyran-fused diterpenoids. Org Biomol Chem 2014;12:8442.
- Li DH, Xu ST, Cai H, et al. Library construction and biological evaluation of enmein-type diterpenoid analogues as potential anticancer agents. ChemMedChem 2013;8:812–18.
- Xu W, Sun J, Zhang TT, et al. Pharmacokinetic behaviors and oral bioavailability of oridonin in rat plasma. Acta Pharmacol Sin 2006;27:1642–6.
- Rao Vadaparthi PR, Pavan Kumar C, Kumar K, et al. Synthesis of costunolide derivatives by Pd-catalyzed Heck arylation and evaluation of their cytotoxic activities. Med Chem Res 2015;24:2871–8.
- Zhang J, Ji F, Gu Y, et al. Chalcones derivatives as potent cell division cycle 25B phosphatase inhibitors. Pharmacol Rep 2014;66:515–19.
- Coskun D, Erkisa M, Ulukaya E, et al. Novel 1-(7-ethoxy-1-benzofuran-2-yl) substituted chalcone derivatives: synthesis, characterization and anticancer activity. Eur J Med Chem 2017;136:212–22.
- Patrick TB, Agboka TY, Gorrell K. Heck reaction with 3-fluoro-3-buten-2-one. J Fluorine Chem 2008;129:983–5.
- Liu CF, Shen QK, Li JJ, et al. Synthesis and biological evaluation of novel 7-hydroxy-4-phenylchromen-2-one-linked to triazole moieties as potent cytotoxic agents. J Enzyme Inhib Med Chem 2017;32:1111–19.
- Zhou HY, Dong FQ, Du XL, et al. Antitumor activities of biscoumarin and dihydropyran derivatives. Bioorg Med Chem Lett 2016;26:3876–80.
- Hileman EO, Liu J, Albitar M, et al. Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol 2004;53:209–19.
- Luo GS, Muyaba M, Lyu W, et al. Design, synthesis and biological evaluation of novel 3-substituted 4-anilino-coumarin derivatives as antitumor agents. Bioorg Med Chem Lett 2017;27:867–74.