Abstract
In the present investigation, 48 new tertiary amine derivatives of cinnamic acid, phenylpropionic acid, sorbic acid and hexanoic acid (4d–6g, 10d–12g, 16d–18g and 22d–24g) were designed, synthesized and evaluated for the effect on AChE and BChE in vitro. The results revealed that the alteration of aminoalkyl types and substituted positions markedly influences the effects in inhibiting AChE. Almost of all cinnamic acid derivatives had the most potent inhibitory activity than that of other acid derivatives with the same aminoalkyl side chain. Unsaturated bond and benzene ring in cinnamic acid scaffold seems important for the inhibitory activity against AChE. Among them, compound 6g revealed the most potent AChE inhibitory activity (IC50 value: 3.64 µmol/L) and highest selectivity over BChE (ratio: 28.6). Enzyme kinetic study showed that it present a mixed-type inhibition against AChE. The molecular docking study suggested that it can bind with the catalytic site and peripheral site of AChE.
Introduction
Chalcone, as a special flavone containing an α,β-unsaturated carbonyl group, had demonstrated a wide variety of bioactivities, including anti-cancer, anti-inflammatory, anti-diabetic, cancer chemopreventive, anti-oxidant, anti-microbial activities and enzyme inhibition activitiesCitation1. A few of investigations reported chalcone derivatives as monoamine oxidase inhibitorsCitation2, glucosidase inhibitorsCitation3, xanthine oxidase inhibitorsCitation4 or carbonic anhydrase inhibitorsCitation5, respectively. It suggested that α,β-unsaturated carbonyl group in chalcone scaffold possible contribute its activity markedlyCitation6,Citation7.
In our laboratory, interestingly, several Mannich base derivatives of Flavokawain B with chalcone scaffold were found with AChE inhibitory activity after screening more than one hundred natural productsCitation8. Then a series of other chalcone nitrogen-containing derivatives or the analogs were synthesized and also appeared potent AChE inhibitory activityCitation9–12. Based on these investigations, cinnamic acid, a natural compound also containing an α,β-unsaturated carbonyl group, was selected to study in order to develop potential new AChE inhibitors and elucidate the structure–activity relationship. Cinnamic acid and its derivatives are widely used in foodCitation13, fragrance materialCitation14, cosmeticsCitation15 and drugs. It is applied as the scaffold of some drugs in clinic such as CinepazideCitation16, TranilastCitation17, IlepcimideCitation18, etc. It is also employed to be as lead compound or starting material in a few medicinal chemistry investigations.
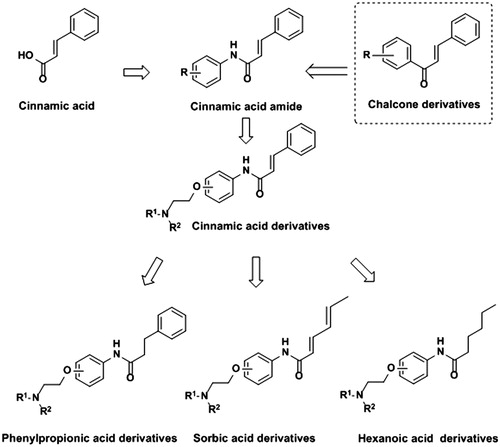
In the present investigation, a series of cinnamic acid derivatives containing aminoalkyl side chains with different substituent position (Ortho, Meta or Para) were designed and synthesized at the beginning. In order to further study the possible influence of unsaturated bond or benzene ring on enzyme inhibition, phenylpropionic acid, sorbic acid and hexanoic acid derivatives were synthesized and evaluated in inhibiting AChE, compared with that of cinnamic acid derivatives (. Then the kinetic experiments and the molecular docking studies were conducted to explore the binding mode of the compound and AChE.
Materials and methods
General procedure for the synthesis of amide derivatives (3a–3c, 9a–9c, 15a–15c and 21a–21c)
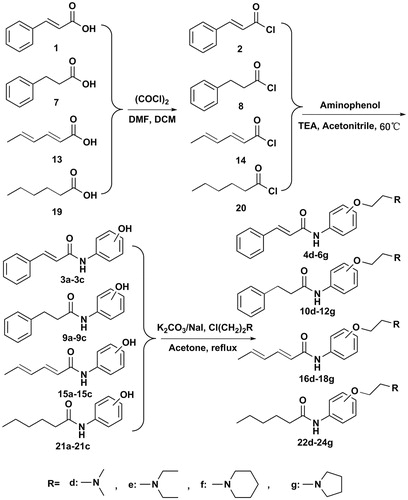
Cinnamic acid, phenylpropionic acid, sorbic acid or hexanoic acid (10 mmol) were added into CH2Cl2 (40 ml), followed by the addition of oxalyl chloride (5 ml, 60 mmol) and a little of dimethylformamide (DMF) as catalyst. The mixture was stirred for 30 min at 40 °C. After the solvent and excess oxalyl chloride was removed by the evaporation under reduced pressure, the acyl chlorides were gained. These chloride derivatives were used as the initial materials to conduct subsequent reactions without further purification.
Aminophenol (2-aminophenol, 3-aminophenol, or 4-aminophenol, 11 mmol) was added to the acetonitrile solution (20 ml) containing acyl chlorides (10 mmol), then the mixture was refluxed and stirred for 7–10 h until the reaction was completed. After the solvent was removed under vacuum, the crude product was added into 20% sodium hydroxide solution. The solution was cooled to 5–6 °C and filtered. The filtrate was slowly added into 10% cold hydrochloric acid solution, followed by adjusting the solution to pH = 3–4. Then gray or white precipitates were gained with the yield of 85–95%. The intermediates mentioned above are known compounds but with no reports about bioactivity in inhibiting AchE and BChECitation19–22.
General procedure for the synthesis of compounds 4d–6g, 10d–12g, 16d–18g and 22d–24g
The aminoethyl chloride (4.5 mmol) were added into a solution containing compounds 3a–3c, 9a–9c, 15a–15c or 21a–21c (1.5 mmol), K2CO3 (1.055 g, 7.5 mmol) and NaI (0.012 g, 0.05 mmol) in acetone (20 ml), respectively. The reaction mixture was refluxed and stirred for 10–15 h, then cooled to room temperature. After the mixture was filtered, the filtrate was concentrated under reduced pressure and partitioned between brine and CH2Cl2. The separated CH2Cl2 layer was washed with 10% NaOH, dried over by anhydrous Na2SO4. The solvent was removed in vacuum and the desired compounds were gained with 50–70% yield through silica gel column chromatography.
Compounds 4d and 6d are known compounds but without reports about bioactivityCitation23. Experimental details for the characterization of compounds can be found in the Supplemental materials.
AChE and BChE inhibition assay
The effects of the newly synthesized compounds on AChE/BChE were assayed by Ellman method with slight modificationCitation24. The brain and serum of Sprague–Dawley rat was used as the resource of AChE and BChE, respectively. The individual compound was dissolved in Tween 80 (final concentration was 0.06% in each reaction) and diluted with water to various concentrations immediately before use. Five different concentrations were tested for each compound in triplicate. The reaction mixture contained 2.70 ml Na2HPO4/NaH2PO4 buffer (pH = 7.4), 100 μL of the different concentrations of test compounds, 100 μL AChE or BChE (0.04 U/100 μL) and 100 μL 15 mmol/L acetylthiocholine iodide/S-butyrylthiocholine iodide, the mixture was incubated for 30 min. Then, the reaction was terminated by the addition of 100 μL 20% sodium dodecylsulfate (SDS), then 100 μL 10 mmol/L 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) was added to generate the yellow anion 5-thio-2-nitrobenzoic acid. The absorbance of each assay mixture was measured at 412 nm by UV–Vis spectroscopy. The IC50 values were calculated by the Bliss method and expressed as Mean ± SD. All assays were conducted in triplicate. Rivastigmine was used as the control drug.
Kinetic study
Kinetic characterization of AChE was performed by a reported methodCitation25. Compound 6g was added into the assay solution and preincubated with the enzyme at 30 °C, followed by the addition of 100 μL acetylthiocholine iodide including five concentrations. The assay solution contained 100 μL compound 6g, 100 μL DTNB, 2.79 ml 0.1 mol/l Na2HPO4/NaH2PO4 buffer (pH 7.4). Kinetic profile of the hydrolysis of acetylthiocholine iodide catalyzed by AChE was determined by the UV–Vis spectrometry at 412 nm. The parallel control experiment was carried out without compound 6g in the mixture.
Molecular docking
Molecular docking studies were carried out with Molecular Operating Environment (MOE) software package (Chemical Computing Group, Montreal, Canada), and structure models of AChE/BChE X-ray crystal structures (PDB ID: 1EVE/1P0I)Citation26 were gained from protein data bank. The 3D structures of compounds were built with virtue of the builder interface of MOE program, and docked into the active site of the protein after energy being minimized. The dock scoring in MOE software was done by ASE scoring function.
Log P and pKa assay
Log P is defined as the logarithm of octanol–water partition coefficient. In the present investigation, log P of compounds 4d∼24g was measured by the shake flask method, and PBS (pH = 7.4) was used as the water phaseCitation27. Ultraviolet spectrophotometry was applied to determine the concentration of compounds. Log p values were calculated based on the data in triplicate.
pKa is determined by pH-titration method according to the reportCitation28. The titration curve E vs. VT was recorded and pKa value was calculated.
Results and discussion
Chemistry
The amide compounds were synthesized as followed. Briefly, the amide compounds were prepared by the condensation reaction of compounds 1, 7, 13 or 19 with oxalyl chloride using DMF as the catalyst. Then, the acyl chloride was reacted with the aminophenol to synthesize amide derivatives 3a–3c, 9a–9c, 15a–15c and 21a–21c, respectively, which were subsequently reacted with four commercially available aminoethyl chlorides (chloroethyldimethylamine hydrochloride, chloroethyldiethylamine hydrochloride, chloroethylpiperidinehydrochloride and chloroethylpyrrolidine hydrochloride) in the presence of K2CO3 and NaI to gain compound 4d–6g, 10d–12g, 16d–18g and 22d–24g (Scheme 1). The structures of these compounds were characterized by proton nuclear magnetic resonance spectroscopy (1H NMR), infrared spectrum (IR) and mass spectrometry (MS). The purity of all synthesized compounds was determined by HPLC.
AChE and BChE inhibition effects
As shown in , it seemed that the variation of aminoalkyl position significantly influences the inhibitory potency against AChE. Except for compounds 18e and 24e, almost all compounds with para-substituted aminoalkyl had more potent inhibitory activity than the ones with meta- or ortho-substituted aminoalkyl, while most compounds with ortho-substituted aminoalkyl but not compounds 16d and 22e had poorer inhibitory activity against AChE than those compounds with meta- or para-substituted aminoalkyl. In addition, most compounds with para-substituted aminoalkyl but not compounds 18e and 24e revealed the higher selectivity in inhibiting AChE over BChE than the ones with meta- or ortho-substituted aminoalkyl.
Table 1. The effects in inhibiting AChE and BChE, and logp, pKa of new synthesized tertiary amine derivatives.
The alteration of aminoalkyl side chains also had significant impact on inhibitory activity. In general, the derivatives with diethylamino ethyl side chain had the weaker inhibitory activity than the others. Among all compounds with meta- or para-substituted aminoalkyl, most derivatives with pyrrolidine ethyl side chain but not compound 18g had more potent activity than the others in inhibiting AChE, while the derivatives with ortho-substituted aminoalkyl did not accord with this manner.
The alteration of compound scaffold also influenced the effect in inhibiting AChE. In general, all cinnamic acid derivatives but not compound 4d had the most potent inhibitory activity than that of sorbic acid, phenylpropionic acid or hexanoic acid derivatives with the same aminoalkyl side chain. Most sorbic acid derivatives but not compound 16e are more potent than that of hexanoic acid derivatives, which indicated that double bond was possible important for these compounds to possessing AChE inhibitory activity. Most of cinnamic acid derivatives but not compound 4d showed more potent effects in inhibiting AChE than that of sorbic acid derivatives. It suggested that benzene ring also had significant influence on the inhibitory activity against AChE.
Kinetic study
Compound 6g was selected for further kinetic study since it had the strongest inhibiting activity against AChE among new synthesized compounds. The graphical analysis of the steady-state inhibition data of compound 6g is shown in Figure 2 Supplement. According to the analysis, the increase of Km and the decline of Vmax with the increasing concentration of compound 6g presents the characteristics of mixed-type inhibition. The competitive inhibit constant (Ki) of compounds 6g is 7.27 µmol/L, and the non-competitive constant (Ki′) is 2.14 µmol/L, respectively. This result suggested that compound 6g could simultaneously bind the active center and other sites of AChE.
Log P and pKa assay
Log P was thought as an important physical chemistry parameter to predict the ability to cross blood brain barrier (BBB). According to report, optimum log P for central nervous system (CNS) penetration was around 2.0 ± 0.7Citation29. Log p values of synthesized compounds are presented in . It was ranged 0.16–3.22. The results suggested that most of these compounds were able to pass BBB with sufficiently lipophilicity.
In another hand, as tertiary amine derivatives, new synthesized compounds present weakly alkaline with pKa ranged 6.45–7.73. It is known that weak basic drugs or compounds are easy to absorb from small intestine and pass the BBBCitation30. The results indicated that all new synthesized compounds in present investigation exhibit excellent potency in absorb ability.
Molecular docking
To explore the molecular mechanism of compound 6g to inhibit AChE and BChE, molecular docking was performed with software MOE2008. As shown in Figure 3 Supplement, compound 6g exhibited multiple points binding modes with AChE (−31.15 kJ/mol) (Figure 3A, Supplement). In the top of the gorge, the aromatic moiety adopted an appropriate orientation for its binding to peripheral anionic site (PAS), via the π–π stacking interaction with Trp279 (4.36 Å) and Tyr334 (3.66 Å).In the bottom of the gorge, the charged nitrogen of pyrrolidine ring was observed to bind to catalytic active sites (CAS) via a π–cation interaction with Trp84 (4.34 Å) and Phe330 (2.82 Å). However, compound 6g could only binds to BChE (−13.19 kJ/mol) (Figure 3B, Supplement) via Trp82 (3.76 Å) with a π–π interaction. These results may provide partial explain for its potent and high selective inhibition against AChE over BChE.
Conclusions
The present investigation on the effects of cinnamic acid, phenylpropionic acid, sorbic acid or hexanoic acid derivatives in inhibiting AChE revealed that the alteration of aminoalkyl types and the substituted position markedly influenced the inhibitory activity. The unsaturated bond and aromatic ring of cinnamic acid derivatives are seemed important for AChE inhibitory activity. These results provide valuable information for the development of potent and/or selective AChE inhibitors in the future.
Disclosure statement
The authors have declared no conflict of interest.
IENZ_1436053_Supplementary_Material.pdf
Download PDF (3.8 MB)Additional information
Funding
References
- Zhou B, Xing CG. Diverse molecular targets for chalcones with varied bioactivities. Med Chem (Los Angeles) 2015;5:388–404.
- Mathew B, Mathew GE, Ucar G, et al. Monoamine oxidase inhibitory activity of methoxy-substituted chalcones. Int J Biol Macromol 2017;104:1321–9.
- Cai CY, Rao L, Rao Y, et al. Analogues of xanthones–chalcones and bis-chalcones as α-glucosidase inhibitors and anti-diabetes candidates. Eur J Med Chem 2017;130:51–9.
- Hofmann E, Webster J, Do T, et al. Hydroxylated chalcones with dual properties: xanthine oxidase inhibitors and radical scavengers. Bioorg Med Chem 2016;24:578–87.
- Kocyigit UM, Budak Y, Eligüzel F, et al. Synthesis and carbonic anhydrase inhibition of tetrabromo chalcone derivatives. Arch Pharm 2017;350:e1700198.
- Singh P, Anand A, Kumar V. Recent developments in biological activities of chalcones: a mini review. Eur J Med Chem 2014;85:758–77.
- Mathew B, Suresh J, Anbazghagan S, et al. Heteroaryl chalcones: mini review about their therapeutic voyage. Biomed Prev Nutr 2014;4:451–8.
- Liu HR, Huang XQ, Lou DH, et al. Synthesis and acetylcholinesterase inhibitory activity of Mannich base derivatives flavokawain B. Bioorg. Med Chem Lett 2014;24:4749–53.
- Liu HR, Liu XJ, Fan HQ, et al. Design, synthesis and pharmacological evaluation of chalcone derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem 2014;22:6124–33.
- Liu HR, Fan HQ, Gao XH, et al. Design, synthesis and preliminary structure activity relationship investigation of nitrogen-containing chalcone derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors: a further study based on Flavokawain B Mannich base derivatives. J Enzyme Inhib Med Chem 2016;31:580–9.
- Gao XH, Zhou C, Liu HR, et al. Tertiary amine derivatives of chlorochalcone as acetylcholinesterase (AChE) and buthylcholinesterase (BChE) inhibitors: the influence of chlorine, alkyl amine side chain and α,β-unsaturated ketone group. J Enzyme Inhib Med Chem 2017;32:146–52.
- Liu HR, Liu LB, Gao XH, et al. Novel ferulic amide derivatives with tertiary amine side chain as acetylcholinesterase and butyrylcholinesterase inhibitors: the influence of carbon spacer length, alkylamine and aromatic group. Eur J Med Chem 2017;126:810–22.
- Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A 2004;1054:95–111.
- Letizia CS, Cocchiara J, Lapczynski A, et al. Fragrance material review on cinnamic acid. Food Chem Toxicol 2005;43:925–43.
- Khan NR, Rathod VK. Enzyme catalyzed synthesis of cosmetic esters and its intensification: a review. Process Biochem 2015;50:1793–806.
- Zhao JY, Song Y, Wang HJ, et al. High performance liquid chromatographic method for the determination of cinepazide maleate and its application to a pharmacokinetic study in rats. J Chromatogr B 2014;957:105–9.
- Darakhshan S, Pour AB. Tranilast: a review of its therapeutic applications. Pharmacol Res 2015;91:15–28.
- Xiao FL, Yan B, Chen L, et al. Review of the use of botanicals for epilepsy in complementary medical systems – traditional Chinese medicine. Epilepsy Behav 2015;52:281–9.
- Shi ZH, Li NG, Shi QP, et al. Synthesis and structure–activity relationship analysis of caffeic acid amides as selective matrix metalloproteinase inhibitors. Bioorg Med Chem Lett 2013;23:1206–11.
- Xu T, Sha F, Alper H. Highly ligand-controlled regioselective Pd-catalyzed aminocarbonylation of styrenes with aminophenols. J Am Chem Soc 2016;138:6629–35.
- Narasimhan B, Judge V, Narang R, et al. Quantitative structure–activity relationship studies for prediction of antimicrobial activity of synthesized 2,4-hexadienoic acid derivatives. Bioorg Med Chem Lett 2007;17:5836–45.
- Froyen P. Phosphorus in organic synthesis. Acyloxyphospho-nium salts as chemoselective acylating reagents. Tetrahedron Lett 1997;38:5359–62.
- Krapcho J, Schwartz J. 6-(or 8)-[[(Substituted amino)alkyl] oxy(or thio)]-3,4-dihydro-4-phenyl-2(1H)-quinolinones US. Pat 1976; 3994900.
- Ellman GL, Courtney KD, Andres VJ, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Luo ZH, Sheng J, Sun Y, et al. Synthesis and evaluation of multi-target-directed ligands against Alzheimer’s disease based on the fusion of donepezil and ebselen. J Med Chem 2013;56:9089–99.
- Alpan AS, Parlar S, Carlino L, et al. Synthesis, biological activity and molecular modeling studies on 1H-benzimidazole derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem 2013;21:4928–37.
- Yu H, Li WM, Kan KK, et al. The physicochemical properties and the in vivo AChE inhibition of two potential anti-Alzheimer agents, bis(12)-hupyridone and bis(7)-tacrine. J Pharm Biomed Anal 2008;46:75–81.
- González AG, Herrador MA. Ionization constants of water insoluble arylpropionic acids in aqueous N, N -dimethylformamide mixtures from potentiometric pH-titrations. Analytica Chimica Acta 1997;356:253–8.
- Glave WR, Hansch CJ. Relationship between lipophilic character and anesthetic activity. J Pharm Sci 1972;61:589–91.
- Manallack DT. The pKa distribution of drugs: Application to drug discovery. Perspect Med Chem 2007;1:25–38.