Abstract
We report the synthesis and characterisation of a novel series of triazole benzenesulfonamide derivatives, which incorporate the general pharmacophore associated with carbonic anhydrase (CA, EC 4.2.1.1) inhibitors. The synthesised compounds were tested in vitro against four human carbonic anhydrase (hCA, EC 4.2.1.1) isozymes, hCA I, hCA II, hCA IV and hCA IX. The obtained results showed that the tumour-associated hCA IX was the most sensitive to inhibition with the synthesised derivatives, with the triazolo-pyridine benzenesulfonamides 14, 16 and 17 being the most effective inhibitors. Some selected compounds were chosen for a single dose anti-proliferative activity testing against a panel of 57 human tumour cell lines and show some anti-proliferative activity ex vivo.
1. Introduction
Carbonic anhydrases (CA, EC 4.2.1.1) are a large family of zinc-containing metallo-enzymes that catalyse the reversible hydration of carbon dioxide to hydrogen carbonate and H+Citation1,Citation2. In humans (h), 15 CA isoforms are known differing in tissue expression patterns, kinetic properties and subcellular localisationCitation3. Their physiological roles are typically associated with acid–base homeostasis and the transport of CO2 and hydrogen carbonate. Human (h) isoform hCA IX is a transmembrane protein with an extracellular active site, and is poorly expressed in healthy tissues (as GIT, bile duct and gall bladder), being instead over-expressed in many solid tumours as a result of hypoxiaCitation4,Citation5. The function of hCA IX in tumour cell is to maintain acid–base homeostasis under hypoxic conditions and to facilitate the diffusion of H+ through the entire solid tumour leading to low extracellular pH that produces matrix breakdown, invasion, immune suppression and multi-drug resistance leading to more tumour aggression and resistanceCitation6,Citation7. These findings led to great interest for new therapeutics targeting hCA IX. Inhibition of hCA IX with small molecules has emerged as a novel anticancer strategyCitation8–10. The most important and widely studied class of CA inhibitors are the aromatic sulfonamides which are capable to coordinate the catalytic Zn2+ from the enzyme active site, thus blocking the catalytic processCitation11–16. Moreover, several 1,2,3-triazole containing compounds have proved considerable biological activities including antibacterial, antifungal and anticancer activitiesCitation17–22.
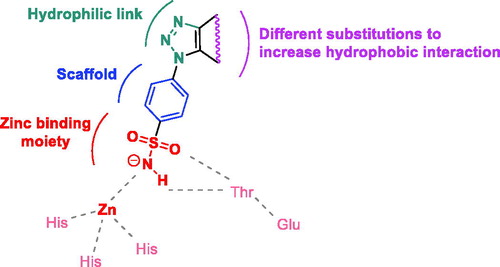
In view of these facts, and in continuation of an ongoing project aiming to develop new biologically active sulfonamide derivativesCitation23–30, we report herein a new set of triazole-benzenesulfonamides designed in agreement with the general pharmacophoric requirements for hCA IX inhibition: an aromatic sulfonamide moiety is used as base unit for the synthesis of the target compounds since necessary to coordinate with the Zn atom and bind to pivotal amino-acids in the active site pocket. An 1,2,3-triazole ring is appended at the aromatic scaffold and used as hydrophilic linker to incorporate several substitution patterns planned to increase the hydrophobic interactions within the active site cavity ().
The synthesised compounds were tested for their inhibitory activity assay against four CA isoforms (hCA I, hCA II, hCA IV and hCA IX). Moreover, they were further evaluated against a panel of 57 human cell lines at National Cancer Institute (NCI, Bethesda, MD).
2. Materials and methods
2.1. Instruments
Melting points were taken in an open capillary tube on a Stuart melting point apparatus (Stuart Scientific, Redhill, UK) and were uncorrected. The IR spectra of the compounds were recorded on FTIR Shimadzu spectrometer (Shimadzu, Tokyo, Japan). 1H NMR and 13C NMR spectra were recorded on a Varian Mercury Plus Oxford (300 MHz for 1H-NMR and 75 MHz for 13C-NMR) spectrometer (Varian Inc., Palo Alto, CA) using TMS as an internal Standard and DMSO-d6 as solvent. Mass spectra were run on HP Model MS-5988 (Hewlett Packard, Palo, Alto, CA). Microanalyses were obtained on a Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany). All values were within ±0.4% of the theoretical values. Purity of the compounds was checked by TLC on pre-coated SiO2 gel (HF254, 200 mesh) aluminium plates (Merk, Darmstadt, Germany). A developing solvent system of chloroform/methanol (8:2) was used and the spots were visualised under UV light. IR, 1H NMR, 13C NMR, Mass and elemental analysis were consistent with the assigned structures. Starting sulfanilamide and all reagents used were of analytical grade and were purchased from Sigma (St. Louis, MO).
2.2. Chemistry
4-(5-amino-4-cyano-1H-1,2,3-triazol-1-yl)benzenesulfonamide 3
A mixture of 2 (1 g, 0.005 mol) and malononitrile (0.33 g, 0.005 mol) was stirred in ethanol containing sodium ethoxide (0.11 g, 0.005 mol) at room temperature overnight and the precipitated solid was filtered off and crystallised from acetic acid to give 3. Yield = 85%; m.p.:100–101 °C. IR(cm−1): 3335, 3205 (NH2), 3096 (CH arom.), 2231 (CN), 1315, 1163 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 7.59 (d, 2H, Ar-H, J = 8.8 Hz), 7.63 (s, 2H, NH2, D2O exch.), 7.89 (d, 2H, Ar-H, J = 8.8 Hz), 8.5 (s, 2H, NH2, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 113.97, 118.1 (2), 128.55(2), 137.48, 138.24, 139.36, 150.62. MS, m/z (%): 264(M+). Anal. Calcd. For C9H8N6O2S (264): C, 40.91; H, 3.05; N, 31.80; Found: C, 40.69; H, 3.35; N, 31.65.
4-(5-amino-4-(4,5-dihydro-1H-imidazol-2-yl)-1H-1,2,3-triazol-1-yl)benzenesulfonamide 4
A mixture of 3 (0.3 g, 0.001 mol) in ethylenediamine (7 ml) and carbon disulfide (7 ml) was heated under reflux for 6 h. After cooling, the reaction mixture was poured onto cold water and the formed solid was filtered off and crystallised from ethanol to give 4. Yield = 80%; m.p.: 278–280 °C. IR(cm−1): 3372, 3183 (NH2), 3002 (CH arom.), 1610 (C = N), 1312, 1116 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 3.33 (d, 2H, CH imidazole, J = 13.0 Hz), 3.61 (d, 2H, CH imidazole, J = 13.2 Hz), 7.49 (d, 2H, Ar-H, J = 8.8 Hz), 7.65 (s, 2H, NH2, D2O exch.) 7.86 (d, 2H, Ar-H, J = 8.8 Hz), 8.2 (s, 2H, NH2, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 49.41 (2), 118.10 (2), 128.55 (2), 137.48, 139.36, 138.24, 150.62, 152.89. MS, m/z (%): 307(M+). Anal. Calcd. For C11H13N7O2S (307): C, 42.99; H, 4.26; N, 31.90; Found: C, 42.75; H, 4.34; N, 31.77.
2-chloro-N-(4-cyano-1-(4-sulfamoylphenyl)-1H-1,2,3-triazol-5-yl)acetamide 5
A mixture of 3 (0.3 g, 0.001 mol) and chloroacetyl chloride (0.15 g, 0.001 mol) was stirred in DMF for 2 h, the reaction mixture was poured onto cold water and the formed solid was filtered off and crystallised from ethanol to give 5. Yield = 91%; m.p.: 165–167 °C. IR(cm−1): 3337, 3187 (NH, NH2), 3099 (CH arom.), 2933, 2858 (CH aliph.), 2231 (CN), 1668 (C = O), 1345, 1112 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 4.26 (s, 2H, CH2Cl), 7.52 (d, 2H, Ar-H, J = 8.6 Hz), 7.69 (s, 2H, NH2, D2O exch.), 7.90 (d, 2H, Ar-H, J = 8.6 Hz), 9.5 (s, 1H, NH, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 42.64, 113.97, 118.10 (2), 128.55 (2), 137.48, 138.24, 139.36, 150.62, 164.79. MS, m/z (%): 340(M+). Anal. Calcd. For C11H9ClN6O3S (340): C, 38.77; H, 2.66; N, 24.66; Found: C, 38.85; H, 2.46; N, 24.45.
N-(4-cyano-1-(4-sulfamoylphenyl)-1H-1,2,3-triazol-5-yl)-3-oxobutanamide 6
A mixture of 3 (0.3 g, 0.001 mol) and ethyl acetoacetate (0.13 g, 0.001 mol) was refluxed in ethanol for 5 h, the reaction mixture was cooled and the formed solid was filtered off and crystallised from ethanol to give 6. Yield = 95%; m.p.: >300 °C. IR(cm−1): 3342, 3270 (NH, NH2), 3097 (CH arom.), 2970, 2890 (CH aliph.), 2205 (CN), 1707 (2C = O), 1342, 1125 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 2.13 (s, 3H, CH3), 3.58 (s, 2H, CH2), 7.69 (s, 2H, NH2, D2O exch.) 7.52 (d, 2H, Ar-H, J = 8.6 Hz), 7.90 (d, 2H, Ar-H), J = 8.6 Hz), 9.1 (s, 1H, NH, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 30.82, 50.25, 113.97, 118.1 (2), 128.55 (2), 137.48, 138.24, 139.36, 150.62, 170.59, 204.29. MS, m/z (%): 348 (M+). Anal. Calcd. For C13H12N6O4S (348): C, 44.83; H, 3.47; N, 24.13; Found: C, 44.90; H, 3.51; N, 24.35.
4-(4-cyano-5-((2-oxo-2-phenylethyl)amino)-1H-1,2,3-triazol-1-yl)benzenesulfonamide 7
A mixture of 3 (0.3 g, 0.001 mol) and phenacyl bromide (0.2 g, 0.001 mol) was refluxed in ethanol for 3 h, the reaction mixture was cooled, poured onto ice water and the formed solid was filtered off and crystallised from acetone to give 7. Yield = 89%; m.p. 216–218 °C. IR(cm−1): 3339, 3206 (NH, NH2), 3088 (CH arom.), 2925, 2889 (CH aliph.), 2224 (CN), 1677 (C = O), 1343, 1119 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 4.03 (s, 2H, CH2), 7.48–7.58 (m, 5H, Ar-H), 7.59 (d, 2H, Ar-H, J = 8.7 Hz), 7.66 (d, 2H, Ar-H, J = 8.8 Hz), 7.88 (s, 2H, NH2, D2O exch.), 10.1 (s, 1H, NH, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 50.35, 113.97, 118.10 (2), 128.47 (2), 128.55 (2), 128.89 (2), 128.92, 133.80, 137.48, 138.24, 139.36, 150.62, 191.85. MS, m/z (%): 382 (M+). Anal. Calcd. For C17H14N6O3S (382): C, 53.40; H, 3.69; N, 21.98; Found: C, 53.72; H, 3.61; N, 21.77.
General procedure for the synthesis of compounds 8–10
(E)-4-(5-((substituted-benzylidene)amino)-4-cyano-1H-1,2,3-triazol-1-yl)-benzenesulfonamide 8–10.
A mixture of 3 (0.3 g, 0.001 mol) and the appropriate aromatic aldehyde (0.001 mol) was refluxed in acetic acid for 5 h and the precipitate formed while hot was filtered off and crystallised from ethanol to give 8–10, respectively.
(E)-4-(5-((2-chlorobenzylidene)amino)-4-cyano-1H-1,2,3-triazol-1-yl)-benzenesulfonamide 8
Yield = 76%; m.p(0).210–211 °C. IR(cm−1): 3342, 3245 (NH2), 3088 (CH arom.), 2188 (CN), 1334, 1120 (SO2), 657 (C-Cl). 1H-NMR (300 MHz, DMSO-d6): δ 7.38–7.60 (m, 4H, Ar-H), 7.72 (s, 2H, NH2, D2O exch.), 7.96 (d, 2H, Ar-H, J = 8.4 Hz), 7.99 (d, 2H, Ar-H, J = 8.5 Hz)), 8.87 (s, 1H, CH). 13C-NMR (75 MHz, DMSO-d6): δ 113.97, 118.1 (2), 127.10, 128.70, 128.55 (2), 130.00, 130.55, 133.39, 136.10, 137.48, 138.24, 139.36, 151.50, 159.86. MS, m/z (%): 386 (M+). Anal. Calcd. For C16H11ClN6O2S (386): C, 49.68; H, 2.87; N, 21.73; Found: C, 49.81; H, 3.01; N, 21.82.
(E)-4-(5-((4-chlorobenzylidene)amino)-4-cyano-1H-1,2,3-triazol-1-yl) benzenesulfonamide 9
Yield = 80%; m.p.: 270–272 °C. IR(cm−1): 3335, 3213 (NH2), 3087 (CH arom.), 2197 (CN), 1343, 1123 (SO2), 659 (C-Cl). 1H-NMR (300 MHz, DMSO-d6): δ 7.49 (d, 2H, Ar-H, J = 8.4 Hz), 7.55 (d, 2H, Ar-H, J = 8.4 Hz), 7.97 (d, 2H, Ar-H, J = 8.4 Hz), 7.72 (s, 2H, NH2, D2O exch.), 7.96 (d, 2H, Ar-H, J = 8.4 Hz), 8.86 (s, 1H, CH). 13C-NMR (75 MHz, DMSO-d6): δ 118.1 (2), 113.97, 128.55 (2), 129.26 (2), 129.45 (2), 134.64, 135.68, 137.48, 138.24, 139.36, 151.50, 159.86. MS, m/z (%): 386 (M+). Anal. Calcd. For C16H11ClN6O2S (386): C, 49.68; H, 2.87; N, 21.73; Found: C, 48.77; H, 2.99; N, 21.81.
(E)-4-(4-cyano-5-((4-(dimethylamino)benzylidene)amino)-1H-1,2,3-triazol-1-yl)benzenesulfonamide 10
Yield = 89%; m.p.: 200–201 °C. IR(cm−1): 3336, 3233 (NH2), 3090 (CH arom.), 2207 (CN), 1333, 1115 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 2.92 (s, 6H, 2CH3), 6.72 (d, 2H, Ar-H, J = 8.4 Hz), 7.72 (d, 2H, Ar-H, J = 8.4 Hz), 7.70 (s, 2H, NH2, D2O exch.), 7.91 (d, 2H, Ar-H, J = 8.4 Hz), 7.92 (d, 2H, Ar-H, J = 8.4 Hz), 9.12 (s, 1H, CH). 13C-NMR (75 MHz, DMSO-d6): δ 40.30 (2), 111.54 (2), 113.97, 118.10 (2), 128.55 (2), 130.30 (2), 134.64, 137.48, 138.24, 139.36, 151.43, 151.50, 159.86. MS, m/z (%): 395 (M+). Anal. Calcd. For C18H17N7O2S (395): C, 54.67; H, 4.33; N, 24.79; Found: C, 54.81; H, 4.45; N, 24.59.
General procedure for the synthesis of compounds 11and 10
A mixture of 3 (0.3 g, 0.001 mol) and benzene or toluene sulfonyl chloride (0.001 mol) was refluxed in pyridine for 8 h, the reaction mixture was cooled, poured onto ice water and the formed solid was filtered off and crystallised from ethanol to give 11 and 12, respectively.
N-(4-cyano-1-(4-sulfamoylphenyl)-1H-1,2,3-triazol-5-yl)benzenesulfonamide 11
Yield = 78%; m.p.: >300 °C. IR(cm−1): 3319, 3231 (NH, NH2), 3089 (CH arom.), 2210 (CN), 1321, 1124 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 7.44 (d, 2H, Ar-H, J = 8.5 Hz), 7.49–7.68 (m, 5H, Ar-H), 7.74 (d, 2H, Ar-H, J = 8.5 Hz), 7.90 (s, 2H, NH2, D2O exch.), 8.21 (s, 1H, NH, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 113.97, 118.10 (2), 127.00 (2), 128.55 (2), 129.20 (2), 131.44, 135.14, 137.48, 138.24, 139.36, 150.62. MS, m/z (%): 404 (M+). Anal. Calcd. For C15H12N6O4S2 (404): C, 44.55; H, 2.99; N, 20.78; Found: C, 44.77; H, 2.78; N, 20.58.
N-(4-cyano-1-(4-sulfamoylphenyl)-1H-1,2,3-triazol-5-yl)-4-methylbenzenesulfonamide 12
Yield = 89%; m.p.: >300 °C. IR(cm−1): 3338, 3231 (NH, NH2), 3066 (CH arom.), 2928, 2843 (CH aliph.), 2224 (CN), 1333, 1114 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 2.33 (s, 3H, CH3), 7.32 (d, 2H, Ar-H, J = 8.1 Hz), 7.44 (d, 2H, Ar-H, J = 8.5 Hz), 7.62 (d, 2H, Ar-H, J = 8.1 Hz), 7.90 (d, 2H, Ar-H, J = 8.7 Hz). 7.94 (s, 2H, NH2, D2O exch.), 8.27 (s, 1H, NH, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 21.26, 113.97, 118.10 (2), 127.50 (2), 128.55 (2), 129.69 (2), 135.14, 137.48, 138.24, 139.36, 144.26, 150.62. MS, m/z (%): 418 (M+). Anal. Calcd. For C16H14N6O4S2 (418): C, 45.93; H, 3.37; N, 20.08; Found: C, 45.68; H, 2.91; N, 20.48.
4-(7-amino-6-cyano-5-oxo-4,5-dihydro-3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl) benzenesulfonamide 13
Fusion of 3 (0.3 g, 0.001 mol) with ethyl cyanoacetate (0.1 g, 0.001 mol) was done for 10 min, the reaction mixture was cooled, triturated with diethyl ether and the formed solid was filtered off and crystallised from ethanol to give 13. Yield = 78%; m.p.: >300 °C. IR(cm−1): 3339–3195 (NH, 2NH2), 3080 (CH arom.), 2210 (CN), 1698 (C = O), 1323, 1141 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 7.55 (d, 2H, Ar-H, J = 8.5 Hz), 7.71 (s, 2H, NH2, D2O exch.), 7.89 (d, 2H, Ar-H, J = 8.5 Hz), 8.02 (s, 2H, NH2, D2O exch.), 8.89 (s, 1H, NH, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 91.52, 115.02, 118.10 (2), 128.55 (2), 137.48, 138.24, 139.36, 150.62, 163.08, 163.15. MS, m/z (%): 331 (M+). Anal. Calcd. For C12H9N7O3S (331): C, 43.50; H, 2.74; N, 29.59; Found: C, 43.71; H, 2.55; N, 29.38.
General procedure for the synthesis of compounds 14–17
4-(7-amino-6-cyano-5-(substituted)-3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)-benzenesulfonamide 14–17.
A mixture of 3 (0.3 g, 0.001 mol) and the appropriate benzylidene derivative (0.001 mol) was refluxed in ethanol containing catalytic amount of TEA (0.01 ml) for 5 h, the reaction mixture was left to cool and the precipitate formed was filtered off and crystallised from dioxane to give 14–17, respectively.
4-(7-amino-6-cyano-5-phenyl-3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)benzenesulfonamide 14
Yield = 70%; m.p.: >300 °C. IR(cm−1): 3356–3252 (2NH2), 3077 (CH arom.), 2192 (CN), 1313, 1125 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 7.51 (s, 2H, NH2, D2O exch.), 7.70–7.80 (m, 5H, Ar-H), 7.87 (d, 2H, Ar-H, J = 7.1 Hz), 7.96 (d, 2H, Ar-H, J = 7.1 Hz), 8.35 (s, 2H, NH2, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 116.59, 118.10 (2), 128.55 (2), 128.86 (2), 128.87 (2), 128.92, 130.66, 136.57, 138.24, 139.36, 140.47, 141.02, 156.05, 159.95. MS, m/z (%): 391 (M+). Anal. Calcd. For C18H13N7O2S (391): C, 55.24; H, 3.35; N, 25.05; Found: C, 55.56; H, 3.15; N, 25.29.
4-(7-amino-5-(2-chlorophenyl)-6-cyano-3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)benzenesulfonamide 15
Yield = 87%; m.p.: 115–117 °C. IR (cm−1): 3330–3223 (2NH2), 3087 (CH arom.), 2195 (CN), 1333, 1121 (SO2), 678 (C-Cl). 1H-NMR (300 MHz, DMSO-d6): δ 7.51 (s, 2H, NH2, D2O exch.), 7.59–7.77 (m, 4H, Ar-H), 7.89 (d, 2H, Ar-H, J = 8.4 Hz), 8.05 (d, 2H, Ar-H, J = 8.4 Hz), 8.08 (s, 2H, NH2, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 116.59, 118.10 (2), 127.58, 127.82, 128.55 (2), 128.71, 130.55, 130.66 (2), 133.16, 138.24, 139.36, 140.47, 141.02, 156.05, 159.95. MS, m/z (%): 425 (M+). Anal. Calcd. For C18H12ClN7O2S (425): C, 50.77; H, 2.84; N, 23.02; Found: C, 50.56; H, 3.02; N, 23.22.
4-(7-amino-5-(4-chlorophenyl)-6-cyano-3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)benzenesulfonamide 16
Yield = 87%; m.p.: >300 °C. IR(cm−1): 3358–3254 (2NH2), 3011 (CH arom.), 2191 (CN), 1333, 1121 (SO2), 688 (C-Cl). 1H-NMR (300 MHz, DMSO-d6): δ 7.12 (s, 2H, NH2, D2O exch.), 7.49 (d, 2H, Ar-H, J = 8.6 Hz), 7.89 (d, 2H, Ar-H, J = 8.4 Hz), 8.11 (d, 2H, Ar-H, J = 8.4 Hz), 8.21 (d, 2H, Ar-H, J = 8.6 Hz), 8.55 (s, 2H, NH2, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 116.59, 118.10 (2), 128.20 (2), 128.55 (2), 130.66, 130.70 (2), 135.68, 136.57, 138.24, 139.36. 140.47, 141.02, 156.05, 159.95. MS, m/z (%): 425 (M+). Anal. Calcd. For C18H12ClN7O2S (425): C, 50.77; H, 2.84; N, 23.02; Found: C, 50.58; H, 3.11; N, 23.29.
4-(7-amino-6-cyano-5-(4-(dimethylamino)phenyl)-3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)benzenesulfonamide 17
Yield = 89%; m.p.: 130–132 °C. IR(cm−1): 3343–3211 (2NH2), 3081 (CH arom.), 2199 (CN), 1333, 1126 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 3.02 (s, 6H, 2CH3), 6.88 (s, 2H, NH2, D2O exch.), 7.37 (d, 2H, Ar-H, J = 8.4 Hz), 7.79 (d, 2H, Ar-H, J = 8.4 Hz), 7.82 (d, 2H, Ar-H, J = 8.8 Hz), 8.06 (d, 2H, Ar-H, J = 8.8 Hz), 8.50 (s, 2H, NH2, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 40.30 (2), 113.84 (2), 116.59, 118.10 (2), 127.82 (2), 128.55 (2), 130.66, 136.57, 138.24, 139.36, 140.47, 141.02, 151.43, 156.05, 159.95. MS, m/z (%): 434 (M+). Anal. Calcd. For C20H18N8O2S (434): C, 55.29; H, 4.18; N, 25.79; Found: C, 55.52; H, 3.89; N, 25.55.
4-(7-amino-5-thioxo-4,5-dihydro-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)benzenesulfonamide 18
Fusion of 3 (0.3 g, 0.001 mol) with thiourea (0.1 g, 0.001 mol) was done for 10 min, the reaction mixture was cooled, triturated with diethyl ether and the formed solid was filtered off and crystallised from ethanol to give 18.
Yield = 91%; m.p. 285–287 °C. IR(cm−1): 3360–3198 (NH, 2NH2), 3089 (CH arom.), 1623 (C = S), 1323, 1121 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 7.18 (s, 2H, NH2, D2O exch.), 7.52 (d, 2H, Ar-H, J = 8.5 Hz), 7.90 (d, 2H, Ar-H, J = 8.5 Hz), 8.01 (s, 2H, NH2, D2O exch.), 8.55 (s, 1H, NH, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 118.10 (2), 128.55 (2), 137.48, 138.24, 139.36, 150.62, 152.89, 179.08. MS, m/z (%): 323 (M+). Anal. Calcd. For C10H9N7O2S2 (323): C, 37.15; H, 2.81; N, 30.32; Found: C, 37.43; H, 3.03; N, 30.10.
4-(7-imino-5-oxo-6-phenyl-4,5,6,7-tetrahydro-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)benzenesulfonamide 19
A mixture of 3 (0.3 g, 0.001 mol) and phenyl isocyanate (0.13 g, 0.001 mol) was refluxed in DMF containing catalytic amount of TEA (0.01 ml) for 18 h, the reaction mixture was cooled, poured onto ice water and the formed solid was filtered off and crystallised from ethanol to give 19. Yield = 89%; m.p.: 180–182 °C. IR(cm−1): 3320, 3292, 3198, 3133 (2NH, NH2), 3064 (CH arom.), 1675 (C = O), 1343, 1123 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 7.47–7.55 (m, 5H, Ar-H), 7.52 (d, 2H, Ar-H, J = 8.8 Hz), 7.62 (s, 2H, NH2, D2O exch.), 7.89 (d, 2H, Ar-H, J = 8.8 Hz), 8.01, 8.11 (2 s, 2H, 2NH, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 118.10 (2), 122.11 (2), 124.72, 128.55 (2), 129.25 (2), 137.48, 138.24, 139.36, 144.73, 149.00, 150.62, 151.73. MS, m/z (%): 383 (M+). Anal. Calcd. For C16H13N7O3S (383): C, 50.13; H, 3.42; N, 25.57; Found: C, 50.37; H, 3.21; N, 25.38.
4-(6-ethyl-7-imino-5-thioxo-4,5,6,7-tetrahydro-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)benzenesulfonamide 20
A mixture of 3 (0.3 g, 0.001 mol) and ethyl isothiocyanate (0.13 g, 0.001 mol) was refluxed in pyridine for 10 h, the reaction mixture was cooled, poured onto ice water, acidified with diluted HCl and the formed solid was filtered off and crystallised from ethanol to give 20. Yield = 90%; m.p. 278–279 °C. IR(cm−1): 3340–3214 (2NH, NH2), 3069 (CH arom.), 2929, 2843 (CH aliph.), 1601 (C = S), 1333, 1120 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 1.25 (t, 3H, CH3, J = 7.1 Hz), 3.85 (q, 2H, CH2, J = 7.1 Hz), 7.51 (d, 2H, Ar-H, J = 8.8 Hz), 7.89 (d, 2H, Ar-H, J = 8.8 Hz), 7.92 (s, 2H, NH2, D2O exch.), 8.00, 8.10 (2 s, 2H, 2NH, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 12.98, 44.37, 118.10 (2), 128.55 (2), 137.48, 138.24, 139.36, 149.00, 150.62, 177.16. MS, m/z (%): 351 (M+). Anal. Calcd. For C12H13N7O2S2 (351): C, 41.02; H, 3.73; N, 27.90; Found: C, 41.31; H, 3.51; N, 27.73.
7-imino-N-(quinoxalin-2-yl)-3-(4-sulfamoylphenyl)-5-thioxo-3,4,5,7-tetrahydro-6H-[1,2,3]triazolo[4,5-d]pyrimidine-6-sulfonamide 21
A mixture of 3 (0.3 g, 0.001 mol) and 4-isothiocyanato-N-(quinoxalin-2-yl) benzenesulfonamide29,30 (0.4 g, 0.001 mol) was refluxed in DMF containing catalytic amount of TEA (0.01 ml) for 6 h, the reaction mixture was cooled, poured onto ice water and the formed solid was filtered off and crystallised from ethanol to give 21. Yield = 89%; m.p.: 212–215 °C. IR(cm−1): 3435–3238 (3NH, NH2), 3074 (CH arom.), 2924, 2846 (CH aliph.), 1599 (C = S), 1320, 1146 (SO2). 1H-NMR (300 MHz, DMSO-d6): δ 7.51 (d, 2H, Ar-H, J = 8.7 Hz), 7.68–7.81 (m, 4H, Ar-H), 7.73 (d, 2H, Ar-H, J = 8.8 Hz), 7.91 (d, 2H, Ar-H, J = 7.9 Hz), 8.09 (s, 1H, CH quinoxaline), 8.10 (d, 2H, Ar-H, J = 8.0 Hz), 8.22 (s, 2H, NH2, D2O exch.), 8.32, 8.36, 8.55 (3 s, 3H, 3NH, D2O exch.). 13C-NMR (75 MHz, DMSO-d6): δ 117.29 (2), 118.10 (2), 126.14 (2), 128.08 (2), 128.55 (3), 128.71, 129.18, 133.28, 136.75, 137.48, 138.24, 139.36, 143.64, 144.73, 149.00, 150.62, 153.92, 177.16. MS, m/z (%): 530 (M+). Anal. Calcd. For C24H18N10O4S2 (606): C, 47.52; H, 2.99; N, 23.09; Found: C, 47.41; H, 2.72; N, 23.25.
2.3. Carbonic anhydrase inhibition
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalysed CO2 hydration activityCitation31. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionised water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were pre-incubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3 and the Cheng–Prusoff equation, as reported earlierCitation32,Citation33 and represent the mean from at least three different determinations. All CA isoforms were recombinant ones obtained in-house as reported earlierCitation34–38.
2.4. In vitro anti-proliferative activity
Ten of the newly synthesised 1,2,3-triazolo benzenesulfonamide derivatives were selected by NCI (Bethesda, MD) for evaluating their anti-proliferative activity. The selected compounds were subjected to a primary in vitro one-dose (10 mM) anti-proliferative assay against 57 human tumour cell lines following the previously reported methodCitation30 and their obtained growth inhibition percent (GI%) are presented in .
Table 2. Percentage growth inhibition (GI%) of in vitro human tumour cell lines at 10 µM concentration for ten compounds.
3. Results and discussion
3.1. Chemistry
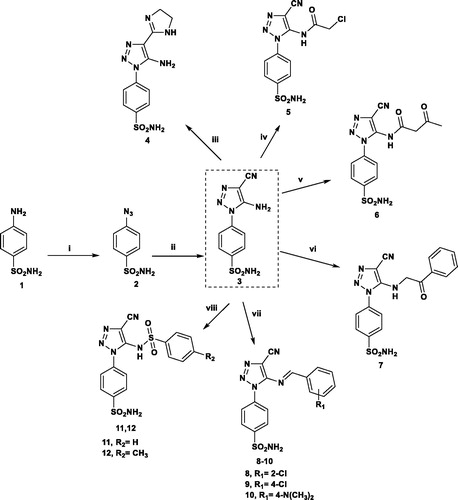
Synthesis of the series of 1,2,3-triazolo-benzensulfonamide derivatives 3–21 begins with 4-azido benzenesulfonamideCitation32 a which was subjected to reaction with malononitrile under stirring at room temperature to give 4-(5-amino-4-cyano-1H-1,2,3-triazol-1-yl)benzenesulfonamide 3, which represents the key intermediate to produce the target compounds 4–21. Reaction of 3 with ethylene diamine and carbon disulfide as catalystCitation32b afforded the imidazoline derivative 4 whose structure was confirmed by disappearance of the carbonotrile band in IR and the presence of the corresponding protons and carbons of imidazoline ring in NMR spectra. Substitution on the amino group of 3 proceeded successfully via simple reactions with chloroacetyl chloride, ethyl acetoacetate and phenacyl bromide affording the corresponding compounds 5–7 in good yields. The Schiff’s bases 8–10 were obtained by reaction of 3 with substituted aromatic aldehydes, and in compounds 11 and 12, a new sulfonamide moiety is introduced to the amino group of 3 through reaction with benzene/toluene sulfonyl chloride (Scheme 1). In compounds 5–12 the 1H-NMR spectra showed the disappearance of NH2 signals and the presence of the corresponding signals for the introduced groups as listed in the experimental section.
Scheme 1. Reagents and conditions: (i) NaN3/H2SO4/NaNO2/r.t.; (ii) CH2(CN)2/EtONa/EtOH/r.t.; (iii) NH2CH2CH2NH2/CS2/reflux 6 h; (iv) ClCH2COCl/DMF/r.t.; (v) CH3COCH2COOC2H5/reflux 3 h; (vi) PhCOCH2Br/EtOH/reflux 3 h; (vii) Ar-CHO/AcOH/reflux 5 h; (viii) Ar-SO2Cl/pyridine/8 h.
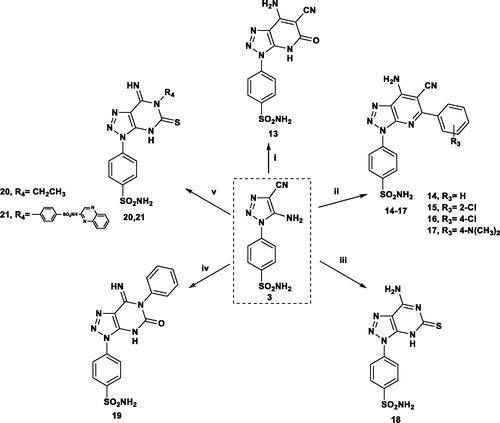
The triazole derivative 3, bearing two active functional groups, was further cyclised by reaction with 3-ethylcyanoacetate and different 4-substituted benzylidene derivatives affording the corresponding triazolo-pyridine derivatives 13–17. On the other hand, reaction of 3 with thiourea and phenyl thiocyanate afforded the triazolo-pyrimidines 18 and 19. Similarly, the isothiocyanate derivatives reacted with 3 to give the triazolo-pyrimidine derivatives 20 and 21 bearing ethyl or sulfaquinoxaline moiety, respectively (Scheme 2).
Scheme 2. Reagents and conditions: (i) CNCH2COOC2H5/3 h; (ii) Ar-CHC(CN)2/EtOH/TEA/5 h; (iii) NH2CSNH2/15 min; (iv) PhNCO/DMF/TEA/reflux 18 h; (v) CH3CH2 or Ar-NCS/DMF/TEA/reflux 5 h.
The structures of the target compounds were proved by elemental and spectral data and were in consistency with assigned structures as presented in details in the experimental section.
3.2. Carbonic anhydrase inhibition
The synthesised compounds were tested against the cytosolic hCA I, II the transmembrane IV and the tumour-associated membrane bound hCAIX by a stopped-flow CO2 hydrase assay in comparison to acetazolamide (AAZ) as standard CAICitation31. The results presented in allowed to depict the following SAR.
Table 1. Inhibition data of human CA isoforms hCA I, II, IV and IX with compounds 2–21 reported here and the standard sulfonamide inhibitor acetazolamide (AAZ) by a stopped flow CO2 hydrase assay.
The cytosolic hCA I was moderately inhibited by all the tested compounds with inhibition constant (KI) ranging from 604.8 to 9938.3 nM. The most active compounds were the triazolo-pyridine derivatives 13, 16 and 17 (789.7, 604.8 and 682.6 nM, respectively).
The physiologically dominant isoform hCA II was moderately inhibited by all the synthesised compounds with different extents having inhibitory constants in the range of 16.7–6836.6 nM The 4-(5-amino-4-cyano-1H-1,2,3-triazol-1-yl)benzenesulfonamide 3 showed the best activity (16.7 nM), very close to that of acetazolamide (12 nM). Moreover, a good activity was observed for the triazolo-pyridine 17 (56.8 nM).
A relatively poor affinity for the tested compounds was observed against hCA IV, with their inhibitory constant spanning between 116.8 and 5030.30 nM, whereas compounds 2 and 8 showed no activity.
The tumour-associated hCA IX was the most significantly inhibited by the tested compounds which showed inhibitory constants ranging from 35.1 to 3809.6 nM. The most active compounds were the triazolo-pyridine derivatives 14, 16 and 17 (46.4, 42.6 and 35.1 nM, respectively).
Hence, p-substitution on benzenesulfonamide with triazolo-pyridine moiety was the most successful towards CA inhibition. Introduction of aryl group to position 5 of triazolo-pyridine led to high affinity towards hCA IX, whereas p-substitution on 5-aryl group increased activity especially for the N-dimethyl derivative 17, which is the most potent candidate in this study.
3.3. In vitro anti-proliferative activity
A subset of ten triazolo-benzenesulfonamides (3, 5, 6, 7, 9, 11, 16–18, and 20) were selected and subjected to an in vitro anti-proliferative screening against a panel of 57 cancer cell lines at NCI at an initial high dose (10 mM). The human cell lines used were derived from nine cancer types: leukaemia, lung, colon, CNS, melanoma, ovarian, renal, prostate and breast cancers. The mean percentages growth inhibition (GI%) of the tested compounds are shown in .
The tested compounds showed fair anti-proliferative activity over the different cell lines. They inhibited cell growth by different extents and their sensitivity varies as presented in the table. The tested compounds were active mostly against leukaemia and lung cancer cell lines. Compound 16 was sensitive against 9 cell lines showing the highest GI% on EKVX (39.5%) and HOP-92 (34.4%) lung cancer cell lines. Compound 17 was active against 12 cell lines with the highest GI% on SNB-75 CNS cancer cell line (21.6%). Compound 18 was mostly active on HL-60(TB) Leukaemia cell line with GI% = 26.1%. While, compound 20 showed highest activity (20.9%) on CCRF-CEM leukaemia cell line. An exceptional activity was observed for compound 5 on HS 578 T breast cancer cell line showing GI% of 99.8%.
4. Conclusions
The present work describes the design and synthesis of novel series of 1.2.3-triazolo benzensulfonamide derivatives according to the general pharmacophoric requirements of the tumour-associated hCA IX. The synthesised compounds were tested in vitro against four human carbonic anhydrase isozymes (hCA I, hCA II, hCA IV and hCA IX). hCA IX was the most sensitive isozyme towards the tested compounds, with the most active inhibitors being the triazolo-pyridine benzenesulfonamides 14, 16, and 17. In addition, they showed fair anti-proliferative activity against a panel of 57 human tumour cell lines. These results present a novel group of selective hCA IX inhibitors having triazolo-pyridine benzensulfonamide moiety. Future work will focus on varying hydrophobic substitutions on the pyridine ring to enhance selectivity.
Acknowledgments
The authors are grateful to National Cancer Institute (NCI) Developmental Therapeutic Program (www.dtp.nci.nih.gov) for screening the anticancer activity of the newly synthesised compounds in vitro.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74.
- (a) Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72; (b) Boztaş M, Çetinkaya Y, Topal M, et al. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxybromophenol derivatives incorporating cyclopropane moieties. J Med Chem 2015;58:640–50.
- Swietach P, Patiar S, Supuran CT, et al. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J Biol Chem 2009;284:20299–310.
- (a) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88; (b) Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32; (c) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77; (d) Supuran CT, Vullo D, Manole G, Casini A, Scozzafava A. Designing of novel carbonic anhydrase inhibitors and activators. Curr Med Chem Cardiovasc Hematol Agents 2004;2:49–68.
- Supuran CT, Alterio V, Di Fiore A, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev 2018; in press.
- Ledaki I, McIntyre A, Wigfield S, et al. Carbonic anhydrase IX induction defines a heterogeneous cancer cell response to hypoxia and mediates stem cell-like properties and sensitivity to HDAC inhibition. Oncotarget 2015;6:19413–27.
- (a) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60; (b) Alterio V, Di Fiore A, D'Ambrosio K, Supuran CT, De Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68; (c) Abbate F, Winum JY, Potter BV, Casini A, Montero JL, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: X-ray crystallographic structure of the adduct of human isozyme II with EMATE, a dual inhibitor of carbonic anhydrases and steroid sulfatase. Bioorg Med Chem Lett 2004;14:231–4; (d) Capasso C, Supuran CT. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32
- (a) Wichert M, Krall N. Targeting carbonic anhydrase IX with small organic ligands. Curr Opin Chem Biol 2015;26:48–54; (b) Supuran CT. Carbon- versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors? J Enzyme Inhib Med Chem 2018;33:485–95; (c) Di Fiore A, Maresca A, Supuran CT, De Simone G. Hydroxamate represents a versatile zinc binding group for the development of new carbonic anhydrase inhibitors. Chem Commun (Camb) 2012;48:8838–40; (d) Marques SM, Nuti E, Rossello A, Supuran CT, Tuccinardi T, Martinelli A, Santos MA. Dual inhibitors of matrix metalloproteinases and carbonic anhydrases: iminodiacetyl-based hydroxamate-benzenesulfonamide conjugates. J Med Chem 2008;51:7968–79
- (a) Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:E48; (b) Ward C, Langdon SP, Mullen P, Harris AL, Harrison DJ, Supuran CT, Kunkler IH. New strategies for targeting the hypoxic tumour microenvironment in breast cancer. Cancer Treat Rev. 2013;39:171–9; (c) Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005;15:3102–08; (d) Casey JR, Morgan PE, Vullo D, Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors. Design of selective, membrane-impermeant inhibitors targeting the human tumor-associated isozyme IX. J Med Chem 2004;47:2337–47.
- Gul HI, Yamali C, Sakagami H, et al. New anticancer drug candidates sulfonamides as selective hCA IX or hCA XII inhibitors. Bioorg Chem 2018;77:411–9.
- Havrankova E, Csollei J, Vullo D, et al. Novel sulfonamide incorporating piperazine, aminoalcohol and 1,3,5-triazine structural motifs with carbonic anhydrase I, II and IX inhibitory action. Bioorg Chem 2018;77:25–37.
- Lolak N, Akocak S, Bua S, et al. Design and synthesis of novel 1,3-diaryltriazene-substituted sulfonamides as potent and selective carbonic anhydrase II inhibitors. Bioorg Chem 2018;77:542–7.
- (a) Monti SM, Meccariello A, Ceruso M, Szafranski K, Slawinski J, Supuran CT. Inhibition studies of Brucella suis beta-carbonic anhydrases with a series of 4-substituted pyridine-3-sulphonamides. J Enzyme Inhib Med Chem 2018;33:255–9; (b) Modak JK, Liu YC, Supuran CT, Roujeinikova A. Structure-Activity relationship for sulfonamide inhibition of helicobacter pylori α-carbonic anhydrase. J Med Chem 2016;59:11098–109; (c) Buzás GM, Supuran CT. The history and rationale of using carbonic anhydrase inhibitors in the treatment of peptic ulcers. In memoriam Ioan Puşcaş (1932–2015). J Enzyme Inhib Med Chem 2016;31:527–33; (d) Supuran CT. Bacterial carbonic anhydrases as drug targets: toward novel antibiotics? Front Pharmacol. 2011;2:34; (e) Nishimori I, Onishi S, Takeuchi H, Supuran CT. The alpha and beta classes carbonic anhydrases from Helicobacter pylori as novel drug targets. Curr Pharm Des 2008;14:622–30.
- Nocentini A, Bua S, Lomelino CL, et al. Discovery of new sulfonamide carbonic anhydrase IX inhibitors incorporating nitrogenous bases. ACS Med Chem Lett 2017;8:1314–9.
- Vullo D, Lehneck R, Poggeler S, Supuran CT. Sulfonamide inhibition studies of two beta-carbonic anhydrases from the ascomycete fungus Sordaria macrospora, CAS1 and CAS2. J Enzyme Inhib Med Chem 2018;33:390–6.
- Chipoline IC, Alves E, Branco P, et al. Synthesis and cytotoxic evaluation of 1H-1,2,3-triazol-1-ylmethyl-2,3-dihydronaphtho[1,2-b]furan-4,5-diones. An Acad Bras Cienc 2018;90:1027–1033.
- El-Sherief HAM, Youssif BGM, Bukhari SNA, et al. Novel 1,2,4-triazole derivatives as potential anticancer agents: Design, synthesis, molecular docking and mechanistic studies. Bioorg Chem 2018;76:314–25.
- Lal K, Yadav P, Kumar A, et al. Design, synthesis, characterization, antimicrobial evaluation and molecular modeling studies of some dehydroacetic acid-chalcone-1,2,3-triazole hybrids. Bioorg Chem 2018;77:236–44.
- Lopez-Rojas P, Janeczko M, Kubinski K, et al. Synthesis and antimicrobial activity of 4-substituted 1,2,3-triazole-coumarin derivatives. Molecules 2018;23:199.
- Tsai YH, Borini Etichetti CM, Di Benedetto C, et al. Synthesis of triazole derivatives of levoglucosenone as promising anticancer agents. Effective exploration of the chemical space through retro-aza-Michael//aza-Michael isomerizations. J Org Chem 2018;83:3516–3528.
- Paprocka R, Modzelewska-Banachiewicz B, Kutkowska J, et al. Antibacterial and central nervous system activity of (4,5-diaryl-4H-1,2,4-triazol-3-YL)methacrylic acid derivatives. Acta Pol Pharm 2017;74:289–92.
- Ghorab MM, Alsaid MS, Ceruso M, et al. Carbonic anhydrase inhibitors: synthesis, molecular docking, cytotoxic and inhibition of the human carbonic anhydrase isoforms I, II, IX, XII with novel benzenesulfonamides incorporating pyrrole, pyrrolopyrimidine and fused pyrrolopyrimidine moieties. Bioorg Med Chem 2014;22:3684–95.
- Ghorab MM, Ceruso M, Alsaid MS, et al. Novel sulfonamides bearing pyrrole and pyrrolopyrimidine moieties as carbonic anhydrase inhibitors: synthesis, cytotoxic activity and molecular modeling. Eur J Med Chem 2014;87:186–96.
- Ghorab MM, Ragab FA, Heiba HI, et al. In vitro anticancer screening and radiosensitizing evaluation of some new quinolines and pyrimido[4,5-b]quinolines bearing a sulfonamide moiety. Eur J Med Chem 2010;45:3677–84.
- Ghorab MM, Ragab FA, Heiba HI, et al. Synthesis of novel pyrrole and pyrrolo[2,3-d]pyrimidine derivatives bearing sulfonamide moiety for evaluation as anticancer and radiosensitizing agents. Bioorg Med Chem Lett 2010;20:6316–20.
- Ghorab MM, Ragab FA, Heiba HI, et al. Synthesis, anticancer and radiosensitizing evaluation of some novel sulfonamide derivatives. Eur J Med Chem 2015;92:682–92.
- Ghorab MM, Ragab FA, Heiba HI, Soliman AM. Design and synthesis of some novel 4-Chloro-N-(4-(1-(2-(2-cyanoacetyl)hydrazono)ethyl)phenyl) benzenesulfonamide derivatives as anticancer and radiosensitizing agents. Eur J Med Chem 2016;117:8–18.
- Ghorab M, Ragab F, Heiba H, et al. Synthesis, in vitro anticancer screening and radiosensitizing evaluation of some new 4-[3-(substituted)thioureido]-N-(quinoxalin-2-yl)-benzenesulfonamide derivatives. Acta Pharm 2011;61:415–25.
- (a) Ghorab MM, Ragab FA, Heiba HI, El-Gazzar MG. Synthesis, in-vitro anticancer screening and radiosensitizing evaluation of some new N-(quinoxalin-2-yl)benzenesulfonamide derivatives. Arzneimittelforschung 2012;62:46–52; (b) Abou-Seri SM, Eldehna WM, Ali MM, Abou El Ella DA. 1-Piperazinylphthalazines as potential VEGFR-2 inhibitors and anticancer agents: Synthesis and in vitro biological evaluation. Eur J Med Chem 2016;107:165–79.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase: I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- (a) Singer M, Lopez M, Bornaghi LF, Innocenti A, Vullo D, Supuran CT, Poulsen SA. Inhibition of carbonic anhydrase isozymes with benzene sulfonamides incorporating thio, sulfinyl and sulfonyl glycoside moieties. Bioorg Med Chem Lett 2009;19:2273–6; (b) Radwan SM, El-Kashef HS. Synthesis and anti-microbial activity of some imidazo [1′,2′:5,6]pyrimido [4,5-c] pyridazines and related heterocycles. Il Farmaco 1998;53:113–7.
- (a) Angeli A, Del Prete S, Osman SM, et al. Activation studies of the α- and β-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae with amines and amino acids. J Enzyme Inhib Med Chem 2018;33:227–33; (b) Bua S, Bozdag M, Del Prete S, et al. Mono- and di-thiocarbamate inhibition studies of the δ-carbonic anhydrase TweCAδ from the marine diatom Thalassiosira weissflogii. J Enzyme Inhib Med Chem 2018;33:707–13.
- (a) Diaz JR, Fernández Baldo M, Echeverría G, et al. A substituted sulfonamide and its Co (II), Cu (II), and Zn (II) complexes as potential antifungal agents. J Enzyme Inhib Med Chem 2016;31(suppl. 2):51–62; (b) Menchise V, De Simone G, Alterio V, et al. Carbonic anhydrase inhibitors: stacking with Phe131 determines active site binding region of inhibitors as exemplified by the X-ray crystal structure of a membrane-impermeant antitumor sulfonamide complexed with isozyme II. J Med Chem 2005;48:5721–7; (c) Supuran CT, Mincione F, Scozzafava A, et al. Carbonic anhydrase inhibitors—part 52. Metal complexes of heterocyclic sulfonamides: a new class of strong topical intraocular pressure-lowering agents in rabbits. Eur J Med Chem 1998;33:247–54; (d) Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett. 2005;15:3102–8; (e) Şentürk M, Gülçin İ, Beydemir Ş, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des. 2011;77:494–9; (f) Fabrizi F, Mincione F, Somma T, et al. A new approach to antiglaucoma drugs: carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J Enzyme Inhib Med Chem 2012;27:138–47.
- (a) Krall N, Pretto F, Decurtins W, et al. A small‐molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew Chem Int Ed Engl 2014;53:4231–35; (b) Rehman SU, Chohan ZH, Gulnaz F, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. J Enzyme Inhib Med Chem 2005;20:333–40; (c) Clare BW, Supuran CT. Carbonic anhydrase activators. 3: structure‐activity correlations for a series of isozyme II activators. J Pharm Sci 1994;83:768–73; (d) Dubois L, Peeters S, Lieuwes NG, et al. Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol 2011;99:424–31; (e) Chohan ZH, Munawar A, Supuran CT. Transition metal ion complexes of Schiff-bases. Synthesis, characterization and antibacterial properties. Met Based Drugs 2001;8:137–43; (f) Zimmerman SA, Ferry JG, Supuran CT. Inhibition of the archaeal β-class (Cab) and γ-class (Cam) carbonic anhydrases. Curr Top Med Chem 2007;7:901–8.
- (a) Supuran CT, Nicolae A, Popescu A. Carbonic anhydrase inhibitors: part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;31:431–8; (b) Pacchiano F, Aggarwal M, Avvaru BS, et al. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb) 2010;46:8371–3; (c) Ozensoy Guler O, Capasso C, Supuran CT. A magnificent enzyme superfamily: carbonic anhydrases, their purification and characterization. J Enzyme Inhib Med Chem 2016;31:689–94; (d) De Simone G, Langella E, Esposito D, et al. Insights into the binding mode of sulphamates and sulphamides to hCA II: crystallographic studies and binding free energy calculations. J Enzyme Inhib Med Chem 2017;32:1002–11.
- (a) Supuran CT. Carbonic anhydrase inhibition and the management of neuropathic pain. Expert Rev Neurother 2016;16:961–8; (b) Di Cesare Mannelli L, Micheli L, Carta F, Cozzi A, Ghelardini C, Supuran CT. Carbonic anhydrase inhibition for the management of cerebral ischemia: in vivo evaluation of sulfonamide and coumarin inhibitors. J Enzyme Inhib Med Chem 2016;31:894–9; (c) Margheri F, Ceruso M, Carta F, et al. Overexpression of the transmembrane carbonic anhydrase isoforms IX and XII in the inflamed synovium. J Enzyme Inhib Med Chem 2016;31(suppl. 4):60–3; (d) Bua S, Di Cesare Mannelli L, Vullo D, et al. Design and synthesis of novel nonsteroidal anti-inflammatory drugs and carbonic anhydrase inhibitors hybrids (NSAIDs-CAIs) for the treatment of rheumatoid arthritis. J Med Chem 2017;60:1159–70.
- (a) Alper Türkoğlu E, Şentürk M, Supuran CT, Ekinci D. Carbonic anhydrase inhibitory properties of some uracil derivatives. J Enzyme Inhib Med Chem 2017;32:74–7; (b) Soydan E, Güler A, Bıyık S, et al. Carbonic anhydrase from Apis mellifera: purification and inhibition by pesticides. J Enzyme Inhib Med Chem 2017;32:47–50.