?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Glutathione reductase (GR) is a crucial antioxidant enzyme which is responsible for the maintenance of antioxidant GSH molecule. Antimalarial effects of some chemical molecules are attributed to their inhibition of GR, thus inhibitors of this enzyme are expected to be promising candidates for the treatment of malaria. In this work, GR inhibitory properties of N-Methylpyrrole derivatives are reported. It was found that all compounds have better inhibitory activity than the strong GR inhibitor N,N-bis(2-chloroethyl)-N-nitrosourea, especially three molecules, 8 m, 8 n, and 8 q, were determined to be the most powerful among them. Findings of our study indicates that these Schiff base derivatives are strong GR inhibitors which can be used as leads for designation of novel antimalarial candidates.
Introduction
In aerobic organisms, free radicals are produced via normal reactions in the metabolism and can also be generated in the form of reactive oxygen species (ROS), such as superoxide anion radicals (O2•−), hydroxyl radical (•OH), hydrogen peroxide (H2O2), and etc. In the metabolism, equilibrium between the natural antioxidative defence system and ROS exists. If the equilibrium between ROS and antioxidant defence system stops working properly, the reactive oxygen species cause cell damage which then results in several diseases including cancer, cardiovascular diseases, age related degenerative diseases, arthritis, and diabetesCitation1–3.
Glutathione reductase (GR) plays a critical role in gene regulation, maintenance of high rates of GSH/GSSG, intracellular signal transduction, clearing of free radicals and reactive oxygen species, and preservation of redox status of intracellular species and is an important enzyme in the cell. Under normal conditions, glutathione is mostly present in reduced form (GSH), yet it might be rapidly oxidized to GSSG as a response to oxidative stress response in order to protect the cell and cell components. However, glutathione reductase reduces GSSG to GSH with NADPH and the intracellular ratio of GSH/GSSG remains above 99%.
Because of the key function of GSH in numerous cellular processes, GSH level and GSH/GSSG ratio are associated with many human diseases such as cancer, cardiovascular diseases, diabetes, AIDS and Alzheimer. GSH is also used for the detoxification of haem and an increase in the amount of intracellular GSH is responsible for the development of the chloroquine resistance. In addition, glutathione reductase inhibitors have been found to possess antimalarial and anticancer activityCitation4–7.
The reason for investigating Schiff’s base derivatives as GR inhibitors is the fact that simple molecules have been shown to be inhibitors of GR. Grellier et al. have reported the antiplasmodial activity of a number of homologous nitroaromatic compounds with either strong or weak inhibitors of GR. To this end, a new irreversible GR inhibitor 2-acetylamino-3-[4–(2-acetylamino-2-carboxyethyl sulfanyl thiocarbonyl amino) phenyl thiocarbamoyl sulfanyl] propionic acid (2-AAPA) was selected in this study and this study showed that 2-AAPA increased anticancer activity, NADPH/NADP+ and NADH/NAD+ ratios, increased GSSG and decreased GSH and inhibited yeast GRCitation8–11.
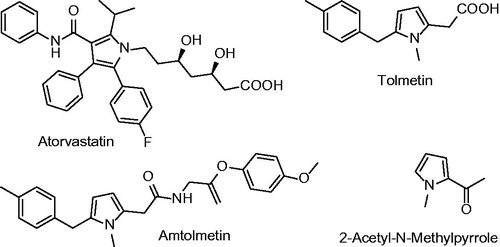
The pyrrole ring, which is found in many natural products and used in many pharmacologically related and other functional syntheses, is one of the most important heterocyclic compounds (). The pyrrole ring is available in a variety of drugs containing antituberculosis agents, analgesics, COX-2 inhibitors, immune system suppressants and antiinflammatory. In addition, 2-acetyl 1-methylpyrrole is the flavouring agent. 1,2,5 tri-substitution pattern pyrrole, displays distinct biological properties as shown by antiinflammatory agents antolmet and tolmetin. As mentioned above, this heterocyclic system is attractive scaffolding that confirms the use of chemical diversity for the purposes of medicinal chemistryCitation12–18.
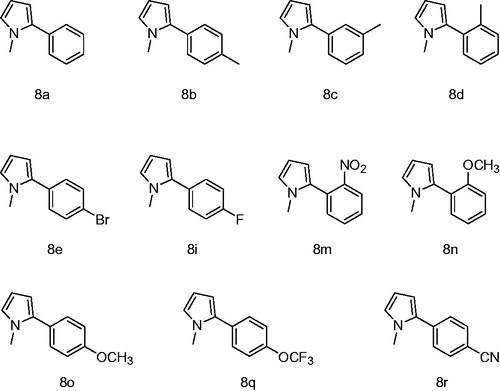
In this study, for the aim of designation of novel GR inhibitors, we have synthesized N-methylpyrrole derivatives and evaluated their ability to inhibit GR (). The inhibition is reported as the IC50 values and the results are averages of at least three independent analyses.
Experimentation
Chemistry
General
All reactions were carried out in air. Anhydrous solvents were distilled prior to use with appropriate drying agents. Thin layer chromatography was performed on Merck silica gel 60 F254. Visualization was performed by means of UV light (254 nm) and by staining with ethanolic phosphomolybdic acid solution. NMR spectra were recorded using a Varian 200 MHz NMR instrument.
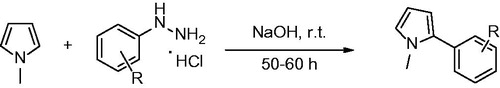
General procedure for arylation of N-methyl pyrrole with phenylhydrazine hydrochloride salts
Six hundred and seventy milligrams pyrrole and 72 mg phenylhydrazine hydrochloride salt were reacted. Then 0.5 M NaOH was added dropwise over a period of 30 min. The resulting mixture was stirred at the room temperature for 50–60 h. Excess of pyrrole and water was evaporated with at room temperature, and the remaining solid was purified by flash column chromatography (EtOAc/hexane %25).
Glutathione reductase inhibition
Activity of the glutathione enzyme was measured by Beutler’s methodCitation22 in which one enzyme unit is defined as the oxidation of 1 mmol NADPH per min under the assay condition at 25 °C, pH 8.0. Different concentrations of the inhibitors were applied to the enzyme solutions and all compounds were tested in triplicate at each concentration usedCitation18–21. Control cuvette activity was assumed as 100% in the absence of inhibitor. A graphic of activity-versus inhibitor concentration was drawn for each compound ().
Results and discussion
Chemistry
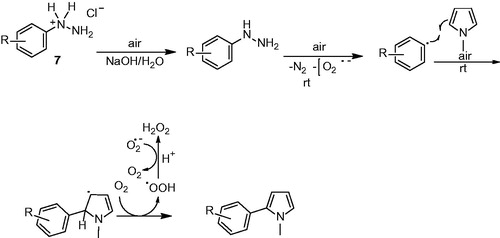
Phenylhydrazine salts have been broadly used for modification of organic molecules with aryl groups. This synthetic procedure achieved transition metal free arylation of pyrrole in a eco-friendly way. The synthetic process started from the reaction of phenylhydrazine hydrochloride salt with NaOH to rapidly forma free phenylhydrazine, a slow oxidation with air to produce aryl radicalCitation15. Aryl radical X reacted with N-methyl pyrrole at room temperature to form allyl radical X which was supported by radical resonance after that losing a single electron loosing with air oxidation and eliminating a proton resulted acrylate pyrroleCitation16.
Arylation mechanism of N-methylpyrrole
To obtain the biologically active target compounds N-methylpyrrole derivatives (8a–r), N-Methylpyrrole was reacted with different arylhydrazine hydrochloride salts in the presence of sodium hyroxyde as a catalyst. These reactions were in moderate yield (70–91%) under room temperature (). The reaction times were from 50 to 60 hoursCitation16.
Biological studies
In this work, we reported GR inhibitory capacity of N-methylpyrrole derivatives (8a-r). As known, glutathione reductase has been purified from various organisms and the influences of drugs, pesticides and various chemicals on GR activity have been investigated. In the current study, GR from baker's yeast (S. cerevisiae) was used and the inhibitory potentials of the synthetized compounds were determined using Beutler method with NADPH and GSSG as substrates and further kinetic studies were performed using the same method.
The data in shows the relation between the compounds 8a–r and glutathione reductase enzyme. The results are also compared with N,N-bis (2-chloroethyl) -N-nitrosourea which is a strong GR inhibitor and anticancer drugCitation23.
Table 1. IC50 values obtained from regression analysis graphs for GR enzyme.
Our results showed that compound 8m behaved as the strongest inhibitor against GR enzyme with the IC50 value of 0.104 µM. Second most powerful inhibition was observed by the structurally similar compound 8n with an IC50 value of 0.678 µM. A very similar compound to 8n is 8o which showed much weaker inhibition (0.678 µM). This result is interesting because the only difference between these two molecules (8n and 8o) is the position of the metoxy group on the aromatic ring. Third most potent inhibitor was 8q with an IC50 value of 0.846 µM which includes electronegative flor atom. Remaining compounds showed similar inhibition values (1.402–1.792 µM) except for 8a which exhibited the weakest inhibition with an IC50 value of 4.942 µM. Nevertheless, all of our compounds showed much more powerful inhibition than N, N-bis(2-chloroethyl)-N-nitrosourea which is a strong GR inhibitor in the literatureCitation23.
Conclusions
Here we synthesized and evaluated the inhibition potential of a new class of GR inhibitors. Our compounds showed higher inhibition capacity than reference GR inhibitor and also than those of many drugs, metal ions, and other chemical compounds which have been tested for GR inhibition so far. Kinetic measurements allowed us to define N-methylpyrrole derivatives besides N,N-bis (2-chloroethyl) -N-nitrosourea as submicromolar-low micromolar inhibitors. This novel class of inhibitors might bind differently than any other known GR inhibitors and might be located between the glutathione binding sites in the enzyme cavity. As the inhibitors of GR are very important for both designation of antimalaria agents and other drugs, our findings provide useful data for further investigations in medicinal chemistry and pharmacology with the possibility to design novel molecules with higher inhibition potentials as compared to clinically used inhibitors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lee JY, Hwang WI, Lim ST. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J Ethnopharmacol 2004;93:409–15.
- Kannan RRR, Arumugam R, Anantharaman P. In vitro antioxidant activities of ethanol extract from Enhalus acoroides (LF) Royle. Asian Pac J Trop Med 2010;3:898–901.
- Liu J, Wang C, Wang Z, et al. The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem 2011;126:261–9.
- Krauth-Siegel RL, Bauer H, Schirmer RH. Dithiol proteins as guardians of the intracellular redox milieu in parasites: old and new drug targets in trypanosomes and malaria-causing plasmodia. Angew Chem Int Ed Engl 2005;44:690–715.
- Pastore A, Federici G, Bertini E, et al. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 2003;333:19–39.
- Perricone C, De Carolis C, Perricone R. Glutathione: a key player in autoimmunity. Autoimmun Rev 2009;8:697–701.
- Biot C, Bauer H, Schirmer RH, et al. 5-Substituted tetrazoles as bioisosteres of carboxylic acids. Bioisosterism and mechanistic studies on glutathione reductase inhibitors as antimalarials. J Med Chem 2004;47:5972–83.
- Şentürk M, Talaz O, Ekinci D, et al. In vitro inhibition of human erythrocyte glutathione reductase by some new organic nitrates. Bioorg Med Chem Lett 2009;19:3661–3.
- Senturk M, Kufrevioglu OI, Ciftci M. Effects of some antibiotics on human erythrocyte glutathione reductase: an in vitro study. J Enzym Inhib Med Chem 2008;23:144–8.
- Grellier P, Sarlauskas J, Anusevicius Z, et al. Antiplasmodial activity of nitroaromatic and quinoidal compounds: Redox potential vs inhibition of erythrocyte glutathione reductase. Arch Biochem Biophys 2001;393:199–206.
- Zhao Y, Seefeldt T, Chen W, et al. Effects of glutathione reductase inhibition on cellular thiol redox state and related systems. Archiv Biochem Biophys 2009;485:56–62.
- Bellina F, Rossi R. Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl groups on adjacent positions. Tetrahedron 2006; 62:7213–56.
- Sawant P, Maier ME. A novel strategy towards the atorvastatin lactone. Tetrahedron 2010;66:9738–44.
- Sheu M-T, Wu J, Chen C-J, et al. Photolysis of NSAIDs. Part 3: Structural elucidation of photoproducts of tolmetin in methanol. Tetrahedron Lett 2004;45:8107–9.
- Chauhan P, Ravi M, Singh S, et al. Regioselective alpha-arylation of coumarins and 2-pyridones with phenylhydrazines under transition-metal-free conditions. RSC Adv 2016;6:109–18.
- Kocaoğlu E, Karaman MA, Tokgöz H, et al. Transition-metal catalyst free oxidative radical arylation of n-methylpyrrole. ACS Omega 2017;2:5000–4.
- Guler M, Eraslan E, Tanyeli A, et al. Determination of glutathione reductase enzyme activity in liver of ischemia/reperfusion damaged rats. Acta Physiologica 2017;221:91.
- Senturk M, Kufrevioglu OI, Ciftci M. Effects of some analgesic anaesthetic drugs on human erythrocyte glutathione reductase: an in vitro study. J Enzym Inhib Med Chem 2009;24:420–424.
- Akkemik E, Senturk M, Ozgeris FB, et al. In vitro effects of some drugs on human erythrocyte glutathione reductase. Turk J Med Sci 2011;41:235–241.
- Cakmak R, Durdagi S, Ekinci D, et al. Design, synthesis and biological evaluation of novel nitroaromatic compounds as potent glutathione reductase inhibitors. Bioorg Med Chem Lett 2011;21:5398–402.
- Sahin A, Senturk M, Akkemik E, et al. The effects of chemical and radioactive properties of Tl-201 on human erythrocyte glutathione reductase activity. Nuc Med Biol 2012;39:161–5.
- Beutler Red Cell Metabolism. A manual of biochemical methods. Orlando: Grune and Stratton Inc; 1984. p. 134.
- Seefeldt T, Zhao Y, Chen W, et al. Characterization of a Novel Dithiocarbamate Glutathione Reductase Inhibitor and Its Use as a Tool to Modulate Intracellular Glutathione. J Biol Chem 2009;284:2729–37.