Abstract
The present work describes the design and synthesis of a novel series of 1,3-diaryl-4-sulfonamidoarylpyrazole derivatives 1a–q and 2a–q and their in vitro biological activities. The target compounds were evaluated for antiproliferative activity against NCI-60 cell line panel. Compounds 1c, 1g, 1k–m, 1o, 2g, 2h, 2k–m, 2o, and 2q showed the highest mean inhibition percentages at 10 µM single-dose testing and were selected to be tested at 5-dose mode. The ICs50 of the most potent compounds were determined over the 60 cell lines. Compound 2l exhibited the strongest activity against different cell lines with IC50 0.33 µM against A498 renal cancer cell line. Compound 2l was tested over a panel of 20 kinases to determine its molecular target(s), and its IC50 values over the most sensitive kinases were defined. In vitro stability and in vivo pharmacokinetic profile of compound 2l was also investigated.
Introduction
Cancer is one of the most extensively spreading diseases around the world. In 2015, one in each six global death cases occurred as a result of different cancer typesCitation1. According to American Cancer Society, everyday there are 4750 new cancer cases and 1670 death casesCitation2. The global cancer statistics revealed that cancer is the second fatal condition after cardiovascular diseasesCitation3,Citation4. Despite the rapid development in the diagnostic area, development of new cancer therapy is a quite challenging mission due to the sophisticated biological pathways contributing to cancer progression.
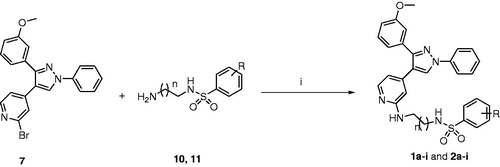
Many compounds possessing sulfonamide moiety have been reported as highly effective antiproliferative agentsCitation5–7. Vemurafenib (Zelboraf®, ) is the first kinase inhibitor drug possessing sulfonamide moiety to be approved by the FDA in 2011 for the treatment of late-stage melanoma. Vemurafenib acts through the inhibition of mutated B-RAFCitation8. Dabrafenib (Tafinlar®, ) is another targeted therapy, which possesses sulfonamide moietyCitation9. Dabrafenib was approved in 2013 for the treatment of mutant B-RAF (V600E-B-RAF)-associated proliferative disordersCitation10. Encorafenib (LGX 818, ) is a drug candidate that carries both sulfonamide and pyrazole backbone which has been recently approved by the FDA to be used in combination with binimetinib for the treatment of unresectable or metastatic melanomaCitation11.
Figure 1. Structures of encorafenib, vemurafenib, dabrafenib, SB203580, and the target compounds 1a–q and 2a–q.
Based on the abovementioned structures and our previous pyrazole ring anticancer investigationsCitation7,Citation12–21, a novel series of 1,3,4-triarylpyrazole was designed and synthesized in which the structure of both rings B and D were fixed and diverse structure modifications were performed in both rings A and C (). The connection length between ring B and sulfonamide terminal moiety ring C was selected from two main chains, ethylene and propylene. The target compounds were tested for in vitro antiproliferative activity against the standard NCI-60 cancer cell line panel. The structure–activity relationships (SAR) are explained in details to show the effects of sulfonamide, linker length, and different substituents on the biological activity. The most promising compounds were further tested against a panel of kinases to study their molecular mechanism of action. The in vitro stability and in vivo pharmacokinetic profile were also investigated for the most potent compound. The synthetic and biological procedures, as well as relevant discussions, are presented in details.
Experimental
Chemistry
General
All solvents and reagents were commercially available and used as such with no further purification. The target compounds and intermediates were purified by column chromatography using silica gel (0.040–0.063 mm, 230–400 mesh) and technical grade solvents. Analytical thin-layer chromatography (TLC) was adopting on silica gel 60 F254 plates from Merck. Purity percentages of the target compounds were confirmed to be more than 96% by LC-MS. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 400 or 300 spectrometer using tetramethylsilane as an internal standard and signals are described as s (singlet), d (doublet), t (triplet), q (quartet), p (pentet), m (multiplet), brs (broad singlet), or dd (doublet of doublets). LC-MS analysis was carried out using the following system: Waters 2998 photodiode array detector, Waters 3100 mass detector, Waters SFO system fluidics organizer, Waters 2545 binary gradient module, Waters reagent manager, Waters 2767 sample manager, Sunfire™ C18 column (4.6 × 50 mm, 5 µm particle size); Solvent gradient = 95% A at 0 min, 1% A at 5 min; solvent A: 0.035% trifluoroacetic acid (TFA) in water; solvent B: 0.035% TFA in CH3OH; flow rate = 3.0 mL/min; the AUC was calculated using Waters MassLynx 4.1 software. Solvents and liquid reagents were transferred using hypodermic syringes. Melting points were obtained on a Walden Precision Apparatus Electro thermal 9300 apparatus and are uncorrected.
Synthesis of N1-(4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)ethane1,2-diamine (8) and N1-(4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)propane-1,3-diamine (9)
They were synthesized utilizing the five-step procedure reported in the literatureCitation19. The detailed procedures are also mentioned in the supplementary file.
General procedure for synthesis of the target compounds N-(2-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)arylsulfonamides (1a-i) and N-(3-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl)arylsulfonamides (2a–i)
To a solution of compound 8 or 9 (0.2 mmol) in anhydrous dichloromethane (5 mL), triethylamine (50.5 mg, 0.5 mmol) was added at 0 °C. A solution appropriate arylsulfonyl chloride (0.21 mmol) in anhydrous dichloromethane (1 mL) was added thereto dropwise. The reaction mixture was stirred at room temperature for 24 h. When the reaction completed, the solvent was removed under vacuo, and the residue was partitioned between ethyl acetate (5 mL) and water (5 mL). The organic layer was separated and the aqueous layer was extracted with ethyl acetate (3 × 10 mL). The combined organic layer was washed with saturated saline (2 × 5 mL) and the organic solvent was evaporated under reduced pressure. The residue was purified by column chromatography (silica gel, hexane-ethyl acetate 4:1 v/v) to give the required product.
N-(2-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl) benzenesulfonamide (1a)
White solid (65%); mp 104–6 °C; 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, Ar-H), 7.86–7.79 (m, 4H, Ar-H), 7.51–7.40 (m, 3H, Ar-H), 7.25–7.21 (m, 5H, Ar-H), 6.92–6.88 (m, 1H, Ar-H), 6.77 (d, J = 9.0 Hz, 1H, Ar-H), 6.70 (s, 1H, Ar-H), 6.42 (d, J = 6.0 Hz, 1H, Ar-H), 6.24 (s, 1H, Ar-H), 4.98 (brs, 1H, NH), 3.65 (s, 3H, OCH3), 3.32 (d, J = 3.0 Hz, 2H, CH2), 3.09 (d, J = 6.0 Hz, 2H, CH2); Citation13C NMR (75 MHz, CDCl3) δ 159.6, 158.6, 147.4, 141.9, 140.1, 140.1, 139.5, 139.4, 132.4, 131.0, 130.0, 129.9, 128.9, 128.8, 127.6, 126.9, 125.1, 122.7, 120.0, 115.8, 114.7, 112.4, 105.9 (Ar-C), 55.3 (OCH3), 43.9 (CH2), 41.6 (CH2).; LC-MS(m/z) calculated for C29H27N5O3S: 525.18, found: 526.0 (M + 1)+.
4-Bromo-N-(2-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl) benzenesulfonamide (1b)
White solid (61%); mp 136–8 °C; 1H NMR (300 MHz, CDCl3) δ 7.96 (s, 1H, Ar-H), 7.89 (d, J = 6.0 Hz, 1H, Ar-H), 7.64 (d, J = 9 Hz, 2H, Ar-H), 7.58–7.55 (m, 2H, Ar-H),7.31–7.28 (m, 6H, Ar-H), 6.93 (d, J = 6.0 Hz, 1H, Ar-H), 6.81 (d, J = 9.0 Hz, 1H, Ar-H), 6.72 (s,1H, Ar-H), 6.48 (d, J = 6.0 Hz, 1H, Ar-H), 6.24 (s, 1H, Ar-H), 4.81 (brs, 1H, NH), 3.69 (s, 3H, OCH3), 3.36 (brs, 2H, CH2), 3.12 (brs, 2H, CH2); Citation13C NMR (75 MHz, CDCl3) δ 159.7, 158.5, 147.2, 142.2, 140.2, 139.5, 139.3, 139.2, 132.6, 132.6, 132.1, 131.0, 130.0, 128.8, 128.5, 127.6, 127.1, 125.1, 122.7, 119.8, 115.8, 114.8, 112.6, 106.1 (Ar-C), 55.3 (OCH3), 44.4 (NH–CH2), 41.6 (CH2–NH); LC-MS(m/z) calculated for C29H26BrN5O3S: 603.09, found: 605.0 (M + 2)+.
4-Chloro-N-(2-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl) benzenesulfonamide (1c)
White solid (60%); mp 132–4 °C; 1H NMR (300 MHz, CDCl3) δ 7.94 (s, 1H, Ar-H), 7.85 (d, J = 6.0 Hz, 1H, Ar-H), 7.72–7.69 (m, 2H, Ar-H), 7.54–7.51 (m, 2H, Ar-H), 7.39–7.22 (m, 6H, Ar-H), 6.91 (d, J = 9.0 Hz, 1H, Ar-H), 6.78 (d, J = 9.0 Hz, 1H, Ar-H), 6.70 (s, 1H, Ar-H), 6.43 (d, J = 3.0 Hz, 1H, Ar-H), 6.24 (s, 1H, Ar-H), 4.95 (brs, 1H, NH), 3.66 (s, 3H, OCH3), 3.33 (d, J = 3.0 Hz, 2H, NH–CH2), 3.09 (d, J = 3.0 Hz, 2H, –CH2–NH); Citation13C NMR (75 MHz, CDCl3) δ 159.6, 158.5, 147.3, 142.0, 140.1, 139.5, 139.3, 138.6, 131.0, 129.9, 129.1, 128.8, 127.6, 125.1, 122.7, 119.9, 115.8, 114.7, 112.5, 106.9 (Ar-C), 55.2 (OCH3), 44.1 (NH-CH2), 41.5 (–CH2-NH).; LC-MS(m/z) calculated for C29H26ClN5O3S: 559.14, found: 560.0 (M + 1)+.
4-Fluoro-N-(2-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl) benzenesulfonamide (1d)
White solid (67%); mp155-6 °C; 1H NMR (400 MHz, CDCl3) δ 7.83 (s, 1H, Ar-H), 7.71–7.67 (m, 3H, Ar-H), 7.19–7.15 (m, 5H, Ar-H), 6.99 (t, J = 8.4 Hz, 2H, Ar-H), 6.80 (d, J = 8.4 Hz, 1H, Ar-H), 6.67 (d, J = 8.8 Hz, 1H, ar-H), 6.59 (s, 1H, Ar-H), 6.34 (d, J = 5.2 Hz, 1H, Ar-H), 6.13 (s, 1H, Ar-H), 4.85 (brs, NH), 3.56 (s, 3H, OCH3), 3.23 (s, 2H, NH–CH2), 2.98 (d, J = 4.4 Hz, 2H, –CH2–NH); Citation13C NMR (100 MHz, CDCl3) δ 159.7, 158.6, 147.1, 142.2, 140.1, 139.5, 139.3, 136.1, 130.9, 130.0, 129.7, 129.6, 129.5, 128.9, 128.7, 125.2, 125.0, 122.7, 119.8, 116.0, 115.0, 112.5, 105.9 (Ar-C), 55.3 (OCH3), 44.2 (CH2), 41.6 (CH2); LC-MS(m/z) calculated for C29H26FN5O3S: 543.17, found: 544.0 (M + 1)+.
4-Methoxy-N-(2-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)benzenesulfonamide (1e)
White solid (62%); mp 144–6 °C; 1H NMR (400 MHz, CDCl3) δ 7.94 (s, 1H, Ar-H),7.88 (d, J= 4.0 Hz, 1H, Ar-H), 7.74 (d, J = 8.0 Hz, 2H, Ar-H), 7.33–7.23 (m, 6H, Ar-H), 6.91 (d, J = 8.0 Hz, 3H, Ar-H), 6.79–6.77 (m, 1H, Ar-H), 6.70–6.69 (m, 1H, Ar-H), 6.43 (dd, J= 8.0, J = 4.0 Hz, 1H, Ar-H), 6.23 (s, 1H, Ar-H), 4.85 (s, NH), 3.83 (s, 3H, OCH3), 3.67 (s, 3H, OCH3), 3.34 (t, J = 8.0 Hz, 2H, NH–CH2–), 3.08 (t, J = 8.0 Hz, 2H, –CH2–NH); Citation13C NMR (100 MHz, CDCl3) δ 162.6, 159.6, 158.6, 147.5, 141.9, 140.1, 139.5, 139.4, 131.6, 131.0, 129.9, 129.1, 128.8, 127.6, 125.1, 122.7, 120.0, 115.7, 114.7, 114.1, 112.4, 105.9 (Ar-C), 55.5 (OCH3), 55.3 (OCH3), 43.8 (CH2), 41.5 (CH2); LC-MS(m/z) calculated for C30H29N5O4S: 555.19, found: 556.0 (M + 1)+.
N-(2-((4-(3-(3-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)-4-methylbenzenesulfonamide (1f)
White solid (69%); mp 150–2 °C; 1H NMR (300 MHz, CDCl3) δ 7.97 (s, 1H, Ar-H),7.91 (d, J = 6.0 Hz, 1H), 7.71 (d, J = 9.0 Hz, 2H, Ar-H), 7.32–7.26 (m, 7H), 6.94 (d, J = 9.0 Hz, 1H, Ar-H), 6.81 (d, J= 6.0 Hz, 1H, Ar-H), 6.72 (s, 1H, Ar-H), 6.49 (d, J= 3.0 Hz, 1H, Ar-H), 6.26 (s, 1H Ar-H), 4.97 (brs, 1H, NH), 3.70 (s, 3H, OCH3), 3.36 (brs, 2H, NH–CH2–), 3.12 (t, J = 6.0 Hz, 2H, –CH2–NH), 2.43 (s, 3H, CH3); Citation13C NMR (75 MHz, CDCl3) δ 159.7, 158.5, 147.4, 143.1, 142.1, 140.1, 139.6, 139.3, 137.1, 131.1, 129.9, 129.5, 128.8, 127.6, 127.0, 125.1, 122.7, 120.0, 115.7, 114.7, 112., 105.5 (Ar-C), 55.3 (OCH3), 44.0 (CH2), 41.7 (CH2), 21.4 (CH3); LC-MS(m/z) calculated for C30H29N5O3S: 539.20, found: 540.0 (M + 1)+.
N-(2-((4-(3-(3-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)-4-(trifluoromethyl)benzenesulfonamide (1g)
White solid (74%); mp 132–4 °C; 1H NMR (300 MHz, CDCl3) δ 7.91 (d, J = 9.0 Hz, 4H, Ar-H), 7.71 (s, 1H, Ar-H), 7.30 (brs, 6H, Ar-H), 6.93 (s, 1H, Ar-H), 6.80 (s, 1H, Ar-H), 6.71 (s, 2H, Ar-H), 6.48 (s, 1H, Ar-H), 6.25 (s, 1H, Ar-H), 4.87 (brs, 1H, NH), 3.67 (s, 3H, OCH3), 3.37 (s, 2H, NH–CH2–), 3.14 (s, 2H, –CH2–NHSO2); Citation13C NMR (75 MHz, CDCl3) δ 159.7, 158.5, 147.1, 147.0, 143.8, 142.4, 139.5, 139.4, 139.2, 134.1, 133.7, 130.9, 130.1, 129.9, 128.9, 127.4, 126.0, 125.2, 125.0, 122.7, 119.7, 115.8, 114.7, 112.8, 106.2 (Ar-C), 55.3 (OCH3), 44.7 (CH2), 41.7 (CH2); LC-MS(m/z) calculated for C30H26F3N5O3S: 593.17, found: 594.0 (M + 1)+.
3-Fluoro-N-(2-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)benzenesulfonamide (1h)
White solid (66%); mp 118–20 °C; 1H NMR (300 MHz, CDCl3) δ 7.94 (s, 1H, Ar-H), 7.85 (d, J = 6.0 Hz, 1H, Ar-H), 7.59 (s, 1H, Ar-H), 7.49 (s, 1H, Ar-H), 7.46–7.37 (m, 2H, Ar-H), 7.29–7.21 (m, 6 H), 6.90 (d, J = 9.0 Hz, 1H, Ar-H), 6.78 (d, J = 9.0 Hz, 1H, Ar-H), 6.71 (s, 1H), 6.43 (d, J = 6.0 Hz, 1H, Ar-H), 6.25 (s, 1H, Ar-H), 4.99 (brs, 1H, NH), 3.65 (s, 3H, OCH3), 3.33 (brs, 2H, NH–CH2–), 3.10 (t, J = 6.0 Hz, 2H, –CH2–NH–SO2); Citation13C NMR (75 MHz, CDCl3) δ 159.6, 158.6, 147.3, 142.2, 142.0, 140.2, 139.5, 139.4, 130.9, 130.8, 130.7, 129.9, 128.8, 127.6, 125.1, 122.7, 119.9, 119.6, 119.3, 115.7, 114.7, 114.4, 114.1, 112.5, 106.0 (Ar-C), 55.2 (OCH3), 44.2 (CH2), 41.5 (CH2); LC-MS(m/z) calculated for C29H26FN5O3S: 543.17, found: 544.0 (M + 1)+.
N-(2-((4-(3-(3-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)naphthalene-1-sulfonamide (1i)
White solid (71%); mp 178–80 °C; 1H NMR (400 MHz, CDCl3) δ; 8.38 (s, 1H, Ar-H), 7.91–7.85 (m, 4H, Ar-H), 7.75 (dd, J = 8.8 Hz, J = 1.6 Hz, 1H, Ar-H), 7.64–7.55 (m, 2H, Ar-H), 7.34–7.21 (m, 6H, Ar-H), 7.02 (brs, 1H, NH), 6.89 (dd, J = 8.4 Hz, J = 2.0 Hz, 1H, Ar-H), 6.76 (d, J = 7.6 Hz, 1H, Ar-H), 6.68 (s, 1H, Ar-H), 6.42 (d, J = 4.8 Hz, 1H, Ar-H), 6.17 (s, 1H, Ar-H), 4.87 (brs, 1H, NH), 3.65 (s, 3H, OCH3), 3.34 (d, J = 4.4 Hz, 2H, NH–CH2–), 3.14 (t, J = 5.2 Hz, 2H, –CH2–NH–SO2); Citation13C NMR (100 MHz, CDCl3) δ 159.6, 158.4, 147.1, 142.1, 140.1, 139.5, 139.4, 136.8, 134.6, 132.1, 130.9, 129.8, 129.3, 129.1, 128.8, 128.6, 128.2, 127.8, 127.6, 127.4, 125.1, 122.7, 122.3, 119.9, 115.7, 114.7, 112.4, 105.9 (Ar-C), 55.2 (OCH3), 44.3 (CH2), 41.5 (CH2); LC-MS(m/z) calculated for C33H29N5O3S: 575.20, found: 576.0 (M + 1)+.
N-(3-((4-(3-(3-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl) benzenesulfonamide (2a)
White solid (60%); mp 119–20 °C; 1H NMR (300 MHz, CDCl3) δ 7.98 (d, J = 12.0 Hz, 2H, Ar-H), 7.83 (d, J = 9.0 Hz, 2H, Ar-H), 7.52–7.45 (m, 3H, Ar-H), 7.31–7.24 (m, 6H, Ar-H), 6.93 (d, J = 6.0 Hz, 1H, Ar-H), 6.79 (d, J = 9.0 Hz, 1H, Ar-H), 6.71 (s, 1H, Ar-H), 6.45 (d, J = 3.0 Hz, 1H, Ar-H), 6.22 (s, 1H, Ar-H), 4.73 (brs, 1H, NH), 3.68 (s, 3H, OCH3), 3.31 (brs, 2H, NH–CH2–), 2.97 (brs, 2H, –CH2–NH–SO2), 1.67–1.58 (m, 2H, –CH2–); Citation13C NMR (75 MHz, CDCl3) δ 159.6, 158.7, 147.2, 142.1, 140.4, 140.1, 139.5, 139.3, 132.2, 131.0, 129.9, 128.9, 128.8, 127.6, 126.9, 125.1, 122.7, 120.0, 115.7, 114.8, 112.1, 105.7 (Ar-C), 55.3 (OCH3), 40.1 (CH2), 38.3 (CH2), 29.9 (CH2); LC-MS(m/z) calculated for C30H29N5O3S: 539.20, found: 540.0 (M + 1)+.
4-Bromo-N-(3-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl) benzenesulfonamide (2b)
White solid (62%); mp 117–19 °C; 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H, Ar-H), 7.90 (d, J = 5.6 Hz, 1H, Ar-H), 7.69 (d, J = 8.8 Hz, 2H, Ar-H), 7.60 (d, J = 8.8 Hz, 2H, Ar-H), 7.35–7.20 (m, 6H, Ar-H), 6.95 (dd, J = 8.0, J = 2.0 Hz, 1H, Ar-H), 6.72 (t, J = 2.0 Hz, 1H, Ar-H), 6.49 (dd, J = 6.0 Hz, J = 1.6 Hz, 1H, Ar-H), 6.28 (s, 1H, Ar-H), 5.21 (brs, 1H, NH), 3.70 (s, 3H, OCH3), 3.36 (q, J = 6.4 Hz, 2H, NH-CH2-), 2.99 (d, J = 5.6 Hz, 2H, –CH2NHSO2), 1.68 (p, J = 6.4 Hz, 2H, CH2–CH2–CH2); Citation13C NMR (75 MHz, CDCl3) δ 159.7, 158.9, 147.3, 142.1, 140.2, 139.6, 139.4, 132.2, 131.0, 130.0, 128.8, 128.5, 127.6, 127.1, 125.1, 122.8, 120.0, 115.8, 114.8, 112.1, 105.9 (Ar-C), 55.3 (OCH3), 40.2 (CH2), 38.3(CH2), 29.9(CH2); LC-MS(m/z) calculated for C30H28BrN5O3S: 617.11, found: 618.00 (M + 1)+.
4-Chloro-N-(3-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl) benzenesulfonamide (2c)
White solid (62%); mp 98–100 °C; 1H NMR (400 MHz, CDCl3) δ 7.95 (s, 1H, Ar-H), 7.93 (d, J = 5.2 Hz, 1H, Ar-H), 7.75 (d, J = 8.4 Hz, 2H, Ar-H), 7.42 (d, J = 8.4 Hz, 2H, Ar-H), 7.32–7.25 (m, 6H, Ar-H), 6.93 (dd, J = 8.0 Hz, J = 2.0 Hz, 1H, Ar-H), 6.79 (d, J = 7.6 Hz, 1H, Ar-H), 6.71 (s, 1H, Ar-H), 6.45 (d, J = 5.6 Hz, 1H, Ar-H), 6.25 (s, 1H, Ar-H), 4.84 (brs, 1H, NH), 3.68 (s, 3H, OCH3), 3.34 (d, J = 5.6 Hz, 2H, NH–CH2–), 2.90 (brs, 2H, –CH2NHSO2), 1.66 (t, J = 6.0 Hz, 2H, -CH2-); Citation13C NMR (100 MHz, CDCl3) δ 159.4, 158.4, 146.8, 142.8, 140.2, 139.3, 139.3, 138.9, 138.6, 130.9, 130.0, 129.5, 129.2, 128.8, 128.4, 127.6, 125.1, 122.7, 119.8, 115.7, 114.8, 112.0, 105.9 (Ar-C), 55.2 (OCH3), 40.0 (CH2), 38.2 (CH2), 29.9 (CH2); LC-MS(m/z) calculated for C30H28ClN5O3S: 573.16, found: 574.0 (M + 1)+.
4-Fluoro-N-(3-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl) benzenesulfonamide (2d)
White solid (75%); mp 92–94 °C; 1H NMR (300 MHz, CDCl3) δ 7.96 (s, 2H, Ar-H), 7.82 (d, J = 3.0 Hz, 2H, Ar-H), 7.32–7.28 (m, 6H, Ar-H), 7.14 (t, J = 9.0 Hz, 2H, Ar-H), 6.94 (d, J = 6.0 Hz, 1H, Ar-H), 6.80 (d, J = 6.0 Hz, 1H, Ar-H), 6.72 (s, 1H, ar-H), 6.46 (d, J = 6.0 Hz, 1H, Ar-H), 6.24 (s, 1H, Ar-H), 4.60 (brs, 1H, NH), 3.69 (s, 3H, OCH3), 3.36 (s, 2H, NH–CH2–), 2.98 (brs, 2H, –CH2NHSO2), 1.66 (d, J = 6.0 Hz, 2H, –CH2–); Citation13C NMR (75 MHz, CDCl3) δ 159.6, 158.7, 147.2, 140.1, 139.3, 131.0, 129.9, 129.6, 129.5, 128.8, 127.6, 125.1, 122.7, 119.9, 116.2, 115.9, 115.7, 114.8, 112.2, 106.0 (Ar-C), 55.2 (OCH3), 40.0 (CH2), 38.2 (CH2), 30.0 (CH2); LC-MS(m/z) calculated for C30H28FN5O3S: 557.19, found: 558.0 (M + 1)+.
4-Methoxy-N-(3-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino) propyl)benzenesulfonamide (2e)
White solid (71%); mp 124–6 °C; 1H NMR (300 MHz, CDCl3) δ 7.96 (s, 1H, Ar-H), 7.94 (d, J = 6.0 Hz, 1H, Ar-H), 7.76 (d, J = 9.0 Hz, 2H, Ar-H),7.33–7.24 (m, 6H, Ar-H), 6.93 (d, J = 9.0 Hz, 3H, Ar-H), 6.79 (d, J = 9.0 Hz, 1H, Ar-H), 6.71 (s, 1H, Ar-H), 6.54 (brs, 1H, NH), 6.45 (d, J = 3.0 Hz, 1H, Ar-H), 6.22 (s, 1H, Ar-H), 4.61 (brs, 1H, NH), 3.86 (s, 3H, OCH3), 3.68 (s, 3H, OCH3), 3.32 (t, J = 6.0 Hz, 2H, NH–CH2–), 2.96 (t, J = 6.0 Hz, 2H, –CH2NHSO2), 1.65 (p, J = 6.0 Hz, 2H, –CH2–); Citation13C NMR (75 MHz, CDCl3) δ 159.7, 158.8, 147.5, 142.0, 140.1, 139.6, 139.4, 132.0, 131.1, 130.0, 128.8, 127.6, 125.1, 122.8, 120.1, 115.7, 114.8, 114.1, 112.2, 105.7(Ar-C), 55.6 (OCH3), 55.3 (OCH3), 40.2 (CH2), 38.4(CH2), 30.0 (CH2); LC-MS(m/z) calculated for C31H31N5O3S: 569.21, found: 570.0 (M + 1)+.
N-(3-((4-(3-(3-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl)-4-methylbenzenesulfonamide (2f)
Buff solid (72%); mp 94–6 °C; 1H NMR (300 MHz, CDCl3) δ 7.95 (s, 1H, Ar-H), 7.91 (d, J = 6.0 Hz, 1H, Ar-H), 7.71 (d, J = 9.0 Hz, 2H, Ar-H),7.30–7.24 (m, 7H, Ar-H), 6.92 (d, J = 6.0 Hz, 1H, Ar-H), 6.79 (d, J = 6.0 Hz, 1H, Ar-H), 6.71 (s, 1H, Ar-H), 6.44 (d, J = 6.0 Hz, 1H, Ar-H), 6.23 (s, 1H, Ar-H), 4.78 (brs, 1H, NH), 3.67 (s, 3H, OCH3), 3.26 (brs, 2H, NH–CH2–), 2.94 (d, J = 3.0 Hz, 2H, –CH2NHSO2), 2.60 (s, 3H, CH3), 1.62 (d, J = 6.0 Hz, 2H, -CH2-); Citation13C NMR (75 MHz, CDCl3) δ 160.2, 159.3, 147.9, 143.5, 142.5, 140.6, 140.1, 139.9, 137.8, 131.5, 130.4, 130.1, 129.3, 128.1, 127.5, 125.6, 123.2, 120.6, 116.2, 115.3, 112.5, 106.1 (Ar-C), 55.8 (OCH3), 40.7 (CH2), 38.9 (CH2), 30.2 (CH2), 22.0 (CH3); LC-MS(m/z) calculated for C31H31N5O4S: 553.21, found: 554.0 (M + 1)+.
N-(3-((4-(3-(3-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl)-4-(trifluoromethyl)benzenesulfonamide (2g)
White solid (32%); mp 150–2 °C; 1H NMR (300 MHz, CDCl3) δ 7.84–7.81 (m, 4H, Ar-H), 7.61 (d, J = 6.0 Hz, 2H, Ar-H), 7.49 (brs, 1H, NH), 7.21–7.16 (m, 6H, Ar-H), 6.82 (d, J = 6.0 Hz, 1H, Ar-H), 6.68 (d, J = 5.4, 1H, Ar-H), 6.60 (s, 1H, Ar-H), 6.33 (d, J = 3.9 Hz, Ar-H), 6.14 (s, 1H, Ar-H), 4.52 (brs, 1H, NH), 3.56 (s, 3H, OCH3), 3.26 (brs, 2H, NH–CH2–), 2.89 (brs, 2H, CH2NHSO2), 1.95 (brs, 2H, -CH2-); Citation13C NMR (75 MHz, CDCl3) δ 159.7, 158.8, 147.1, 142.2, 140.1, 139.5, 139.3, 131.0, 129.9, 128.8, 127.6, 127.4, 126.1, 126.0, 125.1, 122.7, 119.9, 115.7, 114.8, 112.2, 106.1 (Ar-C), 55.2 (OCH3), 40.1 (CH2), 38.3 (CH2), 30.1 (CH2); LC-MS(m/z) calculated for C31H28 F3N5O3S: 607.19, found: 608.0 (M + 1)+.
3-Fluoro-N-(3-((4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl)benzenesulfonamide (2h)
White solid (36%); mp 120–2 °C; 1H NMR (300 MHz, CDCl3) δ 7.94 (s, 1H, Ar-H), 7.91 (d, J = 6.0 Hz, 1H, Ar-H), 7.30–7.22 (m, 10H, Ar-H), 6.93 (dd, J = 2.0 Hz, J = 6.0 Hz, 1H, Ar-H), 6.89 (d, J = 6.0 Hz, 1H, Ar-H), 6.70 (s, 1H, Ar-H), 6.44 (d, J = 6.0 Hz, 1H, Ar-H), 6.24 (s, 1H, Ar-H), 4.74 (s, 1H, NH) 3.67 (s, 3H, OCH3), 3.31 (brs, 2H, NH–CH2–), 2.98 (t, J = 6.0 Hz, 2H, CH2NHSO2), 1.65 (brs, 2H, –CH2–); Citation13C NMR (75 MHz, CDCl3) δ 159.6, 158.8, 147.2, 142.5, 140.1, 139.5, 139.3, 131.0, 130.8, 130.7, 129.9, 128.8, 127.6, 125.1, 122.7, 122.7, 120.0, 119.5, 119.2, 115.7, 114.8, 114.4, 114.1, 112.1, 105.9 (Ar-C), 55.2 (OCH3), 40.1 (CH2), 38.2(CH2), 29.9 (CH2); LC-MS(m/z) calculated for C30H28 FN5O3S: 557.19, found: 558.0 (M + 1)+.
N-(3-((4-(3-(3-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl) naphthalene-1-sulfonamide (2i)
White solid (66%); mp 134–6 °C; 1H NMR (300 MHz, CDCl3) δ 8.40 (s, 1H, Ar-H), 7.97–7.78 (m, 5H, Ar-H), 7.60 (d, J = 6.0 Hz, 2H, Ar-H), 730–7.22 (m, 6H, Ar-H), 6.91 (d, J = 9.0 Hz, 1H, Ar-H), 6.77 (d, J = 6.0 Hz, 1H, Ar-H), 6.70 (s, 1H, Ar-H), 6.45 (d, J = 6.0 Hz, 1H, Ar-H), 6.20 (s, 1H, Ar-H), 4.61 (s, 1H, NH) 3.65 (s, 3H, OCH3), 3.31 (brs, 2H, NH–CH2–), 3.00 (s, 2H, CH2NHSO2), 1.64 (brs, 2H, –CH2–); Citation13C NMR (75 MHz, CDCl3) δ 159.6, 158.8, 147.5, 142.0, 140.1, 139.6, 139.4, 137.2, 134.6, 132.1, 131.0, 129.9, 129.3, 129.1, 128.8, 128.5, 128.1, 127.8, 127.6, 127.4, 125.1, 122.7, 122.4, 120.0, 115.7, 114.8, 112.1, 105.8 (Ar-C), 55.2 (OCH3), 40.1 (CH2), 38.2 (CH2), 29.9 (CH2); LC-MS(m/z) calculated for C30H28 FN5O3S: 589.21, found: 590.0 (M + 1)+.
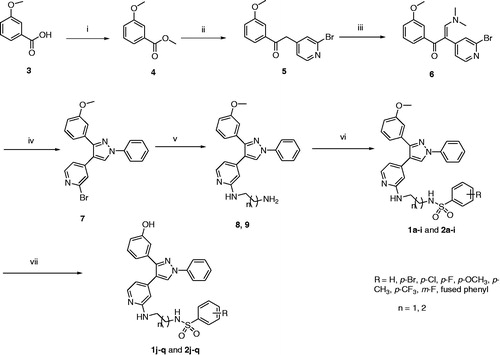
General procedure for synthesis of N-(2-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)benzenesulfonamide (1j), N-(2-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)benzenesulfonamide (1k-q), N-(3-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl)benzenesulfonamide (2j) and N-(3-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl) (substituted)benzenesulfonamide (2k-q)
To a mixture of compound (1a–i) or (2a–i) (0.1 mmol) in methylene chloride (5 mL), BBr3 (0.13 g, 1.0 mmol) was added dropwise at –78 °C under nitrogen, and the reaction mixture was stirred at 0 °C for 24 h. The mixture was quenched with saturated aqueous NaHCO3. Ethyl acetate (10 mL) was added and the organic layer was separated. The aqueous layer was extracted with ethyl acetate (3 × 10 mL). The combined organic layer extracts were washed with brine and dried over anhydrous Na2SO4. The organic solvent was evaporated under reduced pressure, and the residue was purified by column chromatography.
N-(2-((4-(3-(3-Hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl) benzenesulfonamide (1j)
Light brown solid (36%); mp 100–2 °C; 1H NMR (400 MHz,CD3OD) δ 8.05 (s, 1H, Ar-H), 7.83 (d, J = 7.2 Hz, 2H, Ar-H), 7.76 (d, J = 5.6 Hz, 1H, Ar-H), 7.54–7.50 (m, 3H, Ar-H), 7.37–7.34 (m, 3H, Ar-H), 7.29–7.27 (m, 2H, Ar-H), 7.18 (t, J = 8.0 Hz, 1H, Ar-H), 6.84–6.81 (m, 1H, Ar-H), 6.67–6.64 (m, 2H, Ar-H), 6.46 (dd, J = 5.2 Hz, J = 1.2 Hz, 1H, Ar-H), 6.38 (s, 1H, Ar-H), 3.26 (t, J = 6.0 Hz, 2H, NH–CH2–), 2.99 (t, J = 6.0 Hz, 2H, –CH2NHSO2), Citation13C NMR (100 MHz, CD3OD) δ 158.6, 157.6, 149.5, 141.9, 140.3, 139.3, 138.9, 132.1, 130.7, 129.7, 128.7, 128.5, 127.7, 126.5, 121.2, 119.7, 116.8, 115.9, 112.3, 105.7 (Ar-C), 42.2 (CH2), 40.9 (CH2); LC-MS(m/z) calculated for C28H25N5O3S: 511.17, found: 512.0 (M + 1)+.
4-Bromo-N-(2-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl) benzenesulfonamide (1k)
Buff solid (30%); mp 178–80 °C; 1H NMR (400 MHz,CDCl3) δ 7.88 (s, 1H, Ar-H), 7.56 (d, J = 6.0 Hz, 3H, Ar-H), 7.42 (d, J = 8.1 Hz, 2H, Ar-H), 7.17–7.08 (m, 6H, Ar-H), 6.80 (d, J = 7.6 Hz, 1H, Ar-H), 6.64 (s, 1H, Ar-H), 6.58 (d, J = 7.1 Hz, 1H, Ar-H), 6.37 (d, J = 4.7 Hz, 1H, Ar-H), 6.17 (s, 1H, Ar-H), 5.08 (brs, 1H, NH), 3.13 (brs, 2H, NH–CH2–), 2.93 (brs, 2H, –CH2NHSO2); Citation13C NMR (100 MHz, CD3OD) δ 158.0, 157.3, 146.6, 146.4, 142.4, 140.6, 139.1, 138.7, 132.3, 132.1, 130.7, 129.0, 128.5, 128.3, 127.4, 125.2, 124.9, 119.6, 117.4, 117.2, 116.2, 112.2, 105.4 (Ar-C), 42.9 (CH2), 41.5 (CH2); LC-MS(m/z) calculated for C28H24BrN5O3S: 589.17, found: 590.0 (M + 1)+.
4-Chloro-N-(2-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl) benzenesulfonamide (1l)
Light brown solid (41%); mp 100–2 °C; 1H NMR (400 MHz, CD3OD) δ 8.05 (s, 1H, Ar-H), 7.78–7.74 (m, 3H, Ar-H), 7.47 (d, J = 8.8 Hz, 2H, Ar-H), 7.35–7.32 (m 3H, Ar-H), 7.28–7.27 (m, 2H, Ar-H), 7.18 (t, J = 8.0 Hz, 1H, Ar-H), 6.84–6.81 (m, 1H, Ar-H), 6.67 (s, 1H, Ar-H), 6.66 (t, J = 2.0 Hz, 1H, Ar-H), 6.47 (dd, J = 5.2 Hz, J = 1.2 Hz, 1H, Ar-H), 6.34 (s, 1H, Ar-H), 3.25 (t, J = 6.0 Hz, 2H, NH–CH2–), 3.01 (t, J = 6.0 Hz, 2H, –CH2NHSO2), Citation13C NMR (100 MHz, CD3OD) δ 158.5, 157.6, 146.5, 1141.9, 14.0.9, 139.3, 139.1, 138.9, 138.2, 130.7, 129.8, 129.2, 129.0, 128.9, 128.5, 128.2, 127.7, 126.2, 121.2, 119.7, 116.9, 115.9, 111.3, 105.6 (Ar-C), 42.2 (CH2), 40.8(CH2); LC-MS(m/z) calculated for C28H24ClN5O3S: 545.13, found: 546.0 (M + 1)+.
4-Fluoro-N-(2-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl) benzenesulfonamide (1m)
Light yellow solid (40.5%); mp 106–8 °C; 1H NMR (400 MHz, CDCl3) δ 7.90 (s, 1H, Ar-H), 7.78–7.75 (m, 2H, Ar-H), 7.60 (d, J = 8.0 Hz, 1H, Ar-H), 7.20 (s, 5H, Ar-H), 7.12 (t, J = 8.0 Hz, 1H, Ar-H), 7.03 (t, J = 8.0 Hz, 2H, Ar-H), 6.83 (d, J = 8.0 Hz, 1H, Ar-H), 6.67 (s, 1H, Ar-H), 6.61 (d, J = 8.0 Hz, 1H, Ar-H), 6.40 (d, J = 8.0 Hz, 1H, Ar-H), 6.22 (s, 1H, Ar-H) 3.18 (s, 2H, NH–CH2–), 2.97 (s, 2H, –CH2NHSO2), Citation13C NMR (100 MHz, CDCl3) δ 166.1, 163.6, 158.1, 157.3, 146.6, 142.4, 140.6, 139.2, 135.7, 130.7, 130.2, 129.7, 129.6, 128.8, 127.8, 125.1, 121.7, 119.6, 116.7, 116.3, 116.1, 112.3, 105.5 (Ar-C), 42.9 (CH2), 41.6 (CH2); LC-MS(m/z) calculated for C28H24FN5O3S: 529.59, found: 530.0 (M + 1)+.
N-(2-((4-(3-(3-Hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)-4-methylbenzenesulfonamide (1n)
Yellow solid (38%); mp 114–6 °C; 1H NMR (400 MHz, CDCl3) δ 7.91 (s, 1H, Ar-H), 7.69 (d, J = 8.0 Hz, 3H, Ar-H), 7.29–7.21 (m, 6H, Ar-H), 7.15 (t, J = 8.0 Hz, 1H, Ar-H), 6.87 (d, J = 8.0 Hz, 1H, Ar-H), 6.72 (s, 1H, Ar-H), 6.61 (d, J = 8.0 Hz, 1H, Ar-H), 6.44 (s, 1H, Ar-H), 6.22 (s, 1H, Ar-H), 5.32 (brs, 1H, NH), 3.20 (brs, 2H, NH–CH2–), 3.00–2.98 (m, 2H, –CH2NHSO2), 2.78 (s, 3H, CH3); Citation13C NMR (100 MHz, CDCl3) δ 158.2, 157.1, 146.7, 143.4, 142.4, 140.4, 139.3, 139.2, 136.7, 130.8, 130.2, 129.7, 128.8, 127.6, 127.0, 125.0, 121.8, 119.7, 116.8, 112.3, 105.6 (Ar-C), 42.9 (CH2), 41.6 (CH2), 21.0(CH3); LC-MS(m/z) calculated for C29H29N5O3S: 525.59, found: 526.0 (M + 1)+.
N-(2-((4-(3-(3-Hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)-4-(trifluoromethyl)benzenesulfonamide (1o)
White solid (40%); mp 138–40 °C; 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 9.0 Hz, 3H, Ar-H), 7.62–7.57 (m, 3H, Ar-H), 7.17 (s, 5H, Ar-H), 7.10 (t, J = 7.6 Hz, 1H, Ar-H), 6.80 (d, J = 7.1 Hz, 1H, Ar-H), 6.64 (s, 1H, Ar-H), 6.58 (d, J = 7.0 Hz, 1H, Ar-H), 6.38 (s, 1H, Ar-H), 5.32 (brs, 1H, NH), 3.20 (brs, 2H, NH–CH2–), 3.00–2.98 (m, 2H, –CH2NHSO2), 2.78 (s, 3H, CH3); LC-MS(m/z) calculated for C29H24F3N5O3S: 579.59, found: 580.0 (M + 1)+.
3-Fluoro-N-(2-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino) ethyl)benzenesulfonamide (1p)
White solid (32%); mp 146–8 °C; 1H NMR (400 MHz, CD3OD) δ 8.05 (s, 1H, Ar-H), 7.87 (d, J = 5.2 Hz, 1H, Ar-H), 7.64 (d, J = 7.6 Hz, 1H, Ar-H), 7.56–7.51 (m, 2H, Ar-H), 7.37–7.28 (m, 6H, Ar-H), 7.19 (t, J = 8.0 Hz, 1H, Ar-H), 6.82 (d, J = 8.0 Hz, 1H, Ar-H), 6.68–6.65 (m, 2H, Ar-H), 6.47 (d, J = 5.6 Hz, 1H, Ar-H), 6.37 (s, 1H, Ar-H), 3.27 (t, J = 5.2 Hz, 2H, NH–CH2–), 3.02 (t, J = 6.0 Hz, 2H, -CH2NHSO2); Citation13C NMR (75 MHz, CD3OD) δ 163.6, 161.1, 158.6, 157.6, 146.6, 142.6, 141.9, 139.3, 138.9, 130.8, 130.0, 130.7, 129.7, 128.5, 127.7, 125.2, 122.5, 121.1, 119.7, 119.1, 118.8, 116.8, 113.7, 113.5, 111.3, 105.7 (Ar-C), 42.2 (CH2), 40.8 (CH2); LC-MS(m/z) calculated for C28H24 FN5O3S: 529.16, found: 530.0 (M + 1)+.
N-(2-((4-(3-(3-Hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl)naphthalene-1-sulfonamide (1q)
White solid (33%); mp 146–8 °C; 1H NMR (400 MHz, CDCl3) δ 8.37 (s, 1H, Ar-H), 7.87–7.73 (m, 5H, Ar-H), 7.61–7.51 (m, 3H, Ar-H), 7.22 (d, J = 4.0 Hz, 5H, Ar-H), 7.11 (t, J = 8.0 Hz, 1H, Ar-H), 6.84 (dd, J = 8.0 Hz, J = 4.0 Hz, 1H, Ar-H), 6.69 (t, J = 4.0 Hz, 1H, Ar-H), 6.58 (d, J = 8.0 Hz, 1H, Ar-H), 6.38 (dd, J = 8.0 Hz, J = 4.0 Hz, 1H, Ar-H), 6.15 (s, 1H, Ar-H), 5.03 (brs, 1H, NH), 3.19 (brs, 2H, NH–CH2–), 3.02 (t, J = 8.0 Hz, 2H, –CH2NHSO2); Citation13C NMR (75 MHz, CDCl3) δ 158.0, 157.2, 146.5, 142.4, 140.5, 139.2, 136.4, 134.7, 132.0, 130.7, 130.2, 129.4, 129.2, 128.8, 128.3, 127.8, 127.7, 127.4, 125.0, 122.1, 121.7, 119.6, 117.3, 116.8, 112.3, 105.5 (Ar-C), 43.0 (CH2), 41.6 (CH2); LC-MS(m/z) calculated for C32H27N5O3S: 561.66, found: 562.0 (M + 1)+.
N-(3-((4-(3-(3-Hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl) benzenesulfonamide (2j)
White solid (37%); mp 154–6 °C; 1H NMR (400 MHz, CDCl3) δ 7.92 (s, 1H, Ar-H), 7.81–7.74 (m, 3H, Ar-H), 7.51 (d, J = 7.2 Hz, 1H, Ar-H), 7.44 (t, J = 7.6 Hz, 2H, Ar-H), 7.26 (s, 5H, Ar-H), 7.14 (t, J = 7.9 Hz, 1H, Ar-H), 6.87 (d, J = 7.88 Hz, 1H, Ar-H), 6.74 (s, 1H, Ar-H), 6.60 (d, J = 7.4 Hz, 1H, Ar-H), 6.51 (d, J = 5.3 Hz, 1H, Ar-H), 6.11(s, 1H, Ar-H), 4.83 (brs, 1H, NH), 3.06 (brs, 2H, NH–CH2–), 2.88 (d, J = 5.8 Hz, 2H, –CH2NHSO2), 1.53 (p, J = 5.8 Hz, 2H, CH2CH2CH2); LC-MS(m/z) calculated for C29H27N5O3S: 525.63, found: 526.0 (M + 1)+.
4-Bromo-N-(3-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl)benzenesulfonamide (2k)
Light yellow solid (42%); mp 184–6 °C; 1H NMR (400 MHz, CD3OD) δ 8.06 (s, 1H, Ar-H), 7.79 (d, J = 5.2 Hz, 1H, Ar-H), 7.80–7.67 (m, 4H, Ar-H), 7.37–7.34 (m, 3H, Ar-H), 7.29–7.27 (m, 2H, Ar-H), 7.19 (t, J = 8.4 Hz, 1H, Ar-H), 6.84–6.81 (m, 1H, Ar-H), 6.68 (s, 1H, Ar-H), 6.66 (s, 1H, Ar-H), 6.51 (dd, J = 5.6 Hz, J = 1.2 Hz, 1H, Ar-H), 6.34 (s, 1H, Ar-H), 3.14 (t, J = 8.0 Hz, 2H, NH–CH2–), 2.93 (t, J = 8.0 Hz, 2H, –CH2NHSO2); 1.61 (d, J = 6.0 Hz, 2H, CH2CH2CH2); Citation13C NMR (100 MHz, CD3OD) δ 158.9, 157.6, 146.6, 141.9, 140.9, 139.7, 139.3, 138.9, 132.0, 130.8, 129.8, 128.5, 128.3, 127.7, 126.6, 125.2, 121.2, 119.8, 116.8, 115.9, 110.9, 105.4 (Ar-C), 40.2 (CH2), 38.1 (CH2), 28.8 (CH2); LC-MS(m/z) calculated for C29H26BrN5O3S: 604.10, found: 605.0 (M + 1)+.
4-Chloro-N-(3-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl) benzenesulfonamide (2l)
White solid (52%); mp 166–8 °C; 1H NMR (400 MHz, CD3OD) δ 8.06 (s, 1H, Ar-H), 7.87–7.75 (m, 3H, Ar-H), 7.60–7.46 (m, 2H, Ar-H), 7.36 (t, J = 5.8 Hz, 3H, Ar-H), 7.32–7.26 (m, 2H, Ar-H), 7.19 (t, J = 7.9 Hz, 1H, Ar-H), 6.83 (ddd, J = 8.3 Hz, 2.3 Hz, 0.8 Hz, 1H, Ar-H), 6.67 (t, J = 5.0 Hz, 2H, Ar-H), 6.51 (dd, J = 5.5 Hz, 1.4 Hz, 1H, Ar-H), 6.34 (s, 1H, Ar-H), 3.14 (t, J = 6.6 Hz, 2H, NH–CH2-), 2.93 (t, J = 6.8 Hz, 2H, –CH2NHSO2), 1.61 (p, J = 6.7 Hz, 2H, CH2CH2CH2); Citation13C NMR (100 MHz, CD3OD) δ 158.9, 157.6, 146.7, 141.9, 140.9, 139.3, 139.2, 138.9, 138.3, 130.8, 129.8, 129.0, 128.5, 128.2, 127.7, 125.2, 121.2, 119.8, 116.8, 115.9, 110.9, 105.4 (Ar-C), 40.2 (CH2), 38.1 (CH2), 28.8 (CH2); LC-MS(m/z) calculated for C29H26ClN5O3S: 560.7, found: 561.0 (M + 1)+.
4-Fluoro-N-(3-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl)benzenesulfonamide (2m)
White solid (45%); mp 144–6 °C; 1H NMR (400 MHz, CD3OD) δ 8.06 (s, 1H, Ar-H), 7.98–7.86 (m, 2H, Ar-H), 7.80 (d, J = 5.5 Hz, 1H, Ar-H), 7.37 (d, J = 6.6 Hz, 3H, Ar-H), 7.33–7.24 (m, 4H, Ar-H), 7.20 (t, J = 7.8 Hz, 1H, Ar-H), 6.83 (d, J = 8.2 Hz, 1H, Ar-H), 6.67 (d, J = 8.4 Hz, 2H, Ar-H), 6.51 (d, J = 5.5 Hz, 1H, Ar-H), 6.35 (s, 1H, Ar-H), 3.15 (t, J = 6.6 Hz, 2H, NH–CH2–), 2.92 (t, J = 6.7 Hz, 2H, –CH2NHSO2), 1.74–1.53 (m, 2H, CH2CH2CH2); Citation13C NMR (100 MHz, CD3OD) δ 158.9, 157.6, 146.7, 141.9, 139.3, 138.8, 136.7, 130.8, 129.7, 129.5, 129.4, 128.5, 127.7, 125.2, 121.2, 119.8, 116.8, 115.9, 115.8, 115.6, 110.9, 105.4 (Ar-C), 40.2 (CH2), 38.2 (CH2), 28.8 (CH2); LC-MS(m/z) calculated for C29H26FN5O3S: 543.17, found: 544.0 (M + 1)+.
N-(3-((4-(3-(3-Hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl)-4-methylbenzenesulfonamide (2n)
White solid (33%); mp 158–60 °C; 1H NMR (400 MHz, CD3OD) δ 8.06 (s, 1H, Ar-H), 7.79 (d, J = 5.5 Hz, 1H, Ar-H), 7.72 (d, J = 8.3 Hz, 2H, Ar-H), 7.41–7.25 (m, 7H, Ar-H), 7.19 (t, J = 7.9 Hz, 1H, Ar-H), 6.83 (ddd, J = 8.3 Hz, 2.3 Hz, 1.0 Hz, 1H, Ar-H), 6.71–6.61 (m, 2H, Ar-H), 6.51 (dd, J = 5.5, 1.5 Hz, 1H, Ar-H), 6.34 (s, 1H, Ar-H), 3.13 (t, J = 6.7 Hz, 2H, NH–CH2–), 2.89 (t, J = 6.8 Hz, 2H,–CH2NHSO2), 2.41 (s, 3H, CH3), 1.60 (p, J = 6.7 Hz, 2H, CH2CH2CH2); Citation13C NMR (100 MHz, CD3OD) δ 158.9, 157.6, 146.7, 143.1, 141.9, 140.9, 139.3, 138.9, 137.4, 130.8, 129.8, 129.3, 128.5, 127.7, 126.6, 125.2, 121.2, 119.8, 116.8, 115.9, 110.9, 105.4 (Ar-C), 40.2 (CH2), 38.2 (CH2), 28.9 (CH2), 20.1 (CH3); LC-MS(m/z) calculated for C30H29N5O3S: 539.20, found: 540.0 (M + 1)+.
N-(3-((4-(3-(3-Hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl)-4-(trifluoromethyl)benzenesulfonamide (2o)
White solid (39%); mp 150–2 °C; 1H NMR (400 MHz, CD3OD) δ 8.09–7.99 (m, 3H, Ar-H), 7.87 (d, J = 8.3 Hz, 2H, Ar-H), 7.80 (d, J = 5.5 Hz, 1H, Ar-H), 7.41–7.34 (m, 3H, Ar-H), 7.34–7.25 (m, 2H, Ar-H), 7.20 (t, J = 7.9 Hz, 1H, Ar-H), 6.83 (ddd, J = 8.3 Hz, 2.4 Hz, 0.9 Hz, 1H, Ar-H), 6.71–6.63 (m, 2H, Ar-H), 6.52 (dd, J = 5.6 Hz, 1.5 Hz, 1H, Ar-H), 6.36 (d, J = 6.0 Hz, 1H, Ar-H), 3.16 (t, J = 6.7 Hz, 2H, NH–CH2–), 2.96 (t, J = 6.8 Hz, 2H, –CH2NHSO2), 1.64 (p, J = 6.7 Hz, 2H, CH2CH2CH2); Citation13C NMR (100 MHz, CD3OD) δ 158.8, 157.6, 146.5, 142.0, 140.9, 139.3, 138.8, 130.7, 129.7, 128.5, 127.7, 127.3, 125.9, 125.2, 121.2, 119.7, 116.8, 115.9, 110.9, 105.5, (Ar-H), 40.2 (CH2), 38.1 (CH2), 28.9 (CH2); LC-MS(m/z) calculated for C30H26F3N5O3S: 593.20 found: 594.0 (M + 1)+.
3-Fluoro-N-(3-((4-(3-(3-hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino) propyl) benzenesulfonamide (2p)
White solid (41%); mp 120–2 °C; 1H NMR (400 MHz, CD3OD) δ 8.05 (s, 1H, Ar-H), 7.79 (d, J = 5.5 Hz, 1H, Ar-H), 7.67 (d, J = 7.9 Hz, 1H, Ar-H), 7.63–7.51 (m, 2H, Ar-H), 7.41–7.25 (m, 6H, Ar-H), 7.19 (t, J = 7.9 Hz, 1H, Ar-H), 6.87–6.79 (m, 1H, Ar-H), 6.67 (t, J = 4.2 Hz, 2H, Ar-H), 6.50 (dd, J = 5.5 Hz, 1.3 Hz, 1H, Ar-H), 6.36 (s, 1H, Ar-H), 3.15 (t, J = 6.7 Hz, 2H, NH–CH2–), 2.93 (t, J = 6.8 Hz, 2H, –CH2NHSO2) , 1.63 (p, J = 6.7 Hz, 2H, CH2CH2CH2), Citation13C NMR (100 MHz, CD3OD) δ 163.7, 161.2, 158.8, 157.6, 146.5, 142.7, 141.9, 140.9, 139.3, 138.9, 130.9, 130.8, 130.8, 129.8, 128.5, 127.7, 125.2, 122.5, 122.5, 121.2, 119.8, 119.1, 118.9, 116.8, 115.9, 113.7, 113.5, 110.9, 105.5 (Ar-C), 40.2 (CH2), 38.2 (CH2), 28.9 (CH2); LC-MS(m/z) calculated for C29H26FN5O3S: 543.17, found: 544.0 (M + 1)+.
N-(3-((4-(3-(3-Hydroxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)propyl) naphthalene-1-sulfonamide (2q)
White solid (40%); mp 168–70 °C; 1H NMR (400 MHz, CD3OD) δ 8.40 (s, 1H, Ar-H), 8.01 (s, 1H, Ar-H), 7.97 (d, J = 8.4 Hz, 2H, Ar-H), 7.92 (d, J = 8.0 Hz, 1H, Ar-H), 7.81 (dd, J = 8.8 Hz, 1.6 Hz, 1H, Ar-H), 7.73 (d, J = 5.2 Hz, 1H, Ar-H), 7.65–7.57 (m, 2H, Ar-H), 7.36–7.25 (m, 5H, Ar-H), 7.13 (t, J = 8.0 Hz, 1H, Ar-H), 6.79 (dd, J = 8.4 Hz, 2.0 Hz, 1H, Ar-H), 6.62 (s, 1H, Ar-H), 6.59 (d, J = 6.4 Hz, 1H, Ar-H), 6.46 (d, J = 4.8 Hz, 1H, Ar-H), 6.27 (s, 1H, Ar-H), 3.11 (t, J = 6.8 Hz, 2H), 2.94 (t, J = 6.8 Hz, 2H, Ar-H), 1.60 (t, J = 6.8 Hz, 2H, Ar-H); Citation13C NMR (100 MHz, CD3OD) δ 158.7, 157.6, 146.4, 141.9, 140.9, 139.3, 138.9, 137.2, 134.7, 132.1, 130.7, 129.7, 129.1, 128.8, 128.5, 128.3, 127.7, 127.6, 127.5, 127.2, 125.2, 122.0, 121.2, 119.7, 116.8, 115.9, 110.9, 105.3 (Ar-C), 40.2 (CH2), 38.2 (CH2), 28.8 (CH2); LC-MS(m/z) calculated for C33H29N5O3S: 575.20 found: 576.0 (M + 1)+.
Antiproliferative screening of the target compounds against NCI-55 cancer cell line panel
Screening against the cancer cell lines was carried out at the National Cancer Institute (NCI, Bethesda, Maryland, USA) applying the standard protocol of the NCICitation22.
In vitro kinase screening
To investigate the biological target of compound, Reaction Biology Corp. Kinase HotSpotSM service was used adopting standard assay protocolCitation23.
Plasma stability
About 10 µM of compound 2l was mixed with human plasma and rat plasma and the mixture was shaked at 37 °C for 30 min and 120 min. After each time interval, acetonitrile was added and the mixture was centrifugated (14000 rpm, 4 °C). The supernatant was injected into the LC-MS to detect the remaining amount of compound 2l.
hERG binding assay
hERG binding assay was performed using hERG Fluorescence Polarization Assay (Invitrogen: PV5365) assay kit and Synergy Neo (Biotek) and standard procedures were applied.
In vivo pharmacokinetic assay
Adult male rats (N = 3/group) were administered with compound 2l dissolved in distilled water (tween 80) at a single dose of 10 mg/kg by oral administration and 10 mg/mL by injection. Serum samples were collected each 30 min. The blood concentration of the test compounds was determined by LC-MS/MS (Agilent 1290 infinity II series equipped with on-line degasser, binary pump, thermostatted well-plate autosampler and column compartment, Waters Atlantis® HSS T3 (2.1 × 100 mm, 1.9 µm) column, and mobile phase linear gradient from 95% A (0.1% formic acid in water) /5% B (0.1% formic acid in acetonitrile) to 5% A/95% B, 6500+ QTRAP LC-MS/MS/MS system, Turbo Spray Ion Drive as ion source, and Carbamazepine as internal standard. Pharmacokinetic parameters were obtained by non-compartmental analysis of the plasma concentration–time profiles using KineticaTM 4.4.1 (Thermo Fisher Scientific, Inc., Woburn, MA, USA).
Result and discussion
Chemistry
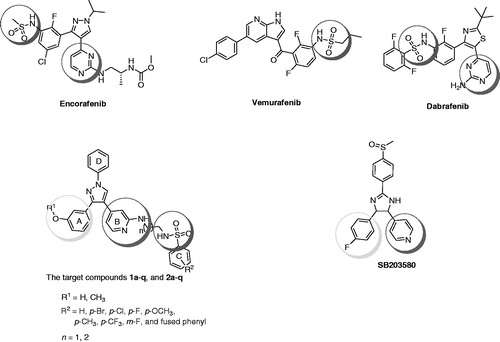
Synthesis of the final compounds was achieved using the pathway illustrated in Scheme 1. Esterification of 3-methoxybenzoic acid (3) using methanol and Conc. sulphuric acid produced methyl 3-methoxybenzoate (4). Reaction of 4 with 2-bromo-4-methylpyridine in the presence of LiHMDS led to formation of 2-(2-bromopyridin-4-yl)-1-(3-methoxyphenyl)ethan-1-one (5). Compound 5 was refluxed with DMF-DMA to give (Z)-2-(2-bromopyridin-4-yl)-3-(dimethylamino)-1-(3-methoxyphenyl)prop-2-en-1-one (6), which further reacts with phenylhydrazine produced 2-bromo-4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridine (7). Compound 7 reacted with ethylenediamine or 1,3-propylenediamine to produce N1-(4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)ethane-1,2-diamine (8) and N1-(4-(3-(3-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)propane-1,3-diamine (9). Reaction of compound 8 or 9 with the appropriate arylsulfonyl chloride in the presence of Et3N afforded the first group of final compounds 1a–i and 2a–i which bears the m-methoxyphenyl group at position 3 of the pyrazole ring. Demethylation of compounds 1a–i and 2a–i using boron tribromide produced corresponding hydroxyl derivatives 1j–q and 2j–q. An alternative pathway was investigated to synthesize the final compounds starting from compound 7. Arylation of N-(2-aminoethyl)benzenesulfonamide (10) or N-(3-aminopropyl)benzenesulfonamide (11) with compound 7 in the presence of pyridine at 110 °C led to formation of compounds 1a–i and 2a–i (Scheme 2).
Scheme 1. Synthetic pathway for final target compounds 1a–q and 2a–q. Reagents and conditions: (i) H2SO4, CH3OH, reflux, 8 h; (ii) 2-bromo-4-methylpyridine, LiHMDS, THF, –25 °C to rt, overnight; (iii) DMF-DMA, reflux, 18 h; (iv) phenylhydrazine, C2H5OH, rt, overnight; (v) 1,2-ethylenediamine or 1,3-propylenediamine, reflux, 8 h.; (vi) appropriate sulfonyl chloride, Et3N, CH2Cl2, 0 °C, overnight; (vii) BBr3, CH2Cl2, –78 °C; 0 °C, overnight.
Biology
Antiproliferative activity of the target compounds
Single-dose testing
The final target compounds were submitted to the National Cancer Institute (NCI), Maryland, USACitation23. Thirty-one compounds were selected to be tested over 60 cell lines at single dose 10 µM. The mean per cent inhibition of the tested compounds are presented in and .
Table 1. Structures of compounds 1a–i and 2a–i and their mean inhibition percentages in single-dose (10 μM) 60-cancer cell line screening.
Table 2. Structures of compounds 1j–q and 2j–q and their mean inhibition percentages in single-dose (10 μM) 60-cancer cell line screening.
The results in represent the activity of methoxy compounds 1a–i and 2a–i. In general, the methoxy compounds have moderate activity over the cell line panel with the highest activity for compound bearing p-trifluoromethyl group and ethylene bridge 1g with per cent inhibition 92.80% followed by compound 2h with m-fluorobenzensulfonamide moiety and propylene linker and compound 2g having p-trifluoromethyl group and propylene spacer and mean inhibition percentages 76.81% and 76.53%, respectively. Compounds with ethylene bridge and electron-withdrawing group and moderate size such as the chloro derivative 1c possess mean per cent inhibition higher compared to one with electron-donating group (1e and 1f) and large electron-withdrawing group such as bromo derivative 1b. Regarding compounds with propylene bridge, compound without any substituents 2a was more active than corresponding one with both elctron withdrawing groups and electron donating one with exception of p-trifluoromethyl and m-fluoro (compounds 2g and 2h).
On the other side, all demethylated compounds 1j–p and 2j–p showed more than 50% per cent inhibition (). Compounds containing electron-withdrawing group at para position such as bromo (1k and 2k), chloro (1l and 2l), and fluoro (1 m and 2m) showed the highest per cent inhibition. Compounds with propylene bridge are more potent compared to compounds having ethylene bridge. The propylene bridge might be suitable for optimum fitting at the receptor site. The most potent compounds in this series were 2 l with mean per cent inhibition 97.80%, and 2k with mean per cent inhibition 90.64%. Target compounds with ethylene bridge and electron-withdrawing groups (1k–m) were equipotent to 2m with propylene bridge and p-fluoro electron-withdrawing group. The meta substitution at sulfonamide moiety was less active than para substitution, but more active than methoxy series (1a–i and 2a–1).
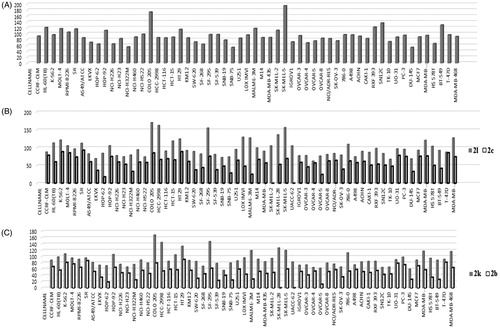
The detailed inhibitory effects of the most potent hydroxyl compounds 2k and 2l and their corresponding methoxy analogues 2b and 2c, and the most potent methoxy compound 2g against NCI-60 cell line panel are depicted in . Compound 2g exhibited more than 100% inhibition over 13 cell lines and showed lethal effect on two cell lines belonging to colon cancer (Colo 205 and HT29) and melanoma (SK-MEL-5). Regarding compounds 2 b and 2k, the cellular activity of compound 2k is much greater than 2b. The maximum inhibition percentage of compound 2b was 83.30% over T-47D cell line, while compound 2k showed per cent inhibition over 100% against 13 cell lines and maximum inhibitions against Colo 205 (168%), HCC-116 (140%) [colon cancer cell lines], and SF-295 (147%) [CNS cancer cell line]. Compound 2c showed maximum activity 92.74% against SR leukaemia cell line and 88.66% against HT29 colon cancer cell line. Finally, compound 2l exhibited more than 100% against 24 cell lines and maximum activity against Colo 205, HCC-2998 [colon cancer cell lines], SF-295 [CNS cancer cell line], LOX IMVI, SK-MEL-28, and SK-MEL-5 [melanoma cell lines] with per cent inhibition 170.28%, 162.39%, 155.19%, 129.50%, 136.19%, and 156.35, respectively.
Figure 2. (A) Per cent inhibition of compound 2g over all cancer cell lines of the NCI panel; (B) per cent inhibition of compound 2c and 2l over all cancer cell lines of the NCI panel; (C) mean per cent inhibition of compound 2b and 2k over all cancer cell lines of the NCI panel. The compounds were tested at 10 µM concentration.
Five-dose results
Compounds 1c, 1g, 1k–m, 1o, 2g, 2 h, 2k–m, 2o, and 2q with the highest activity in single-dose tests were selected to be tested in a five-dose testing mode to determine their IC50, TGI, and LD50. The mean IC50 of the selected compounds over different cancer subtypes are shown in . Compounds 1k, 2k, 2l, 2o, and 2q were more potent against leukaemia cell lines than sorafenib with mean IC50 ranging from 1.44 µM to 2.11 µM. Regarding non-small cell lung cancer, compound 2k was the most potent compound with IC50 1.96 µM. Compounds 1k, 1m, 2k, 2l and 2o were more potent against colon cancer and CNS cancer cell line with IC50 1.48 µM for 2k against colon cancer and IC50 1.65 µM against CNS cell lines for 2l. All tested compounds were more active than sorafenib with IC50 1.62 µM for compound 2k. Compound 2l was the most potent compound against ovarian cancer with mean IC50 of 2.09 µM. Compound 2k was the most potent compound against renal cancer cell lines, prostate cancer cell lines, and breast cancer cell lines with mean IC50 1.89, 2.03, and 1.11 µM, respectively.
Table 3. Mean ICs50 of the most potent compounds and sorafenib over different cancer subtypes.
The activity of the most potent compounds 1c, 1g, 1k–m, 1o, 2g, 2h, 2k–m, 2o, and 2q against the most sensitive cell lines is represented in . Compounds 2k, 2l, 2o, and 2q were the most potent compounds against K-562 leukaemia cell line with IC50 0.44, 0.43, 0.61, and 0.92 µM, respectively. Compound 2o was the most potent compound against HOP-92 non-small cell lung cancer cell line with IC50 1.41 µM followed by compound 2l with IC50 1.52 µM and compound 2k with IC50 1.83μM. Compounds 2l, 2o, and 2k were most potent against HT-29 cell line. Compound 2k was the most potent compound against U251 CNS cancer cell line and OVCAR-4 ovarian cancer cell line with IC50 1.21 µM and 1.66 µM, respectively. On the other hand, compound 2l was the most potent compound against SK-Mel-5 [melanoma], A498 [renal], PC-3 [prostate], and MDA-MB-468 [breast] with an IC50 values of 1.23, 0.33, 1.74, and 1.16 µM, respectively. In general, compounds having 3-hydroxyphenyl and propylene bridge were more potent than the corresponding derivatives with 3-methoxyphenyl and ethylene bridge. Also, compounds with electron-withdrawing group showed high potency than compounds with electron-donating group. Both chloro and bromo substituents were more potent than fluoro compounds.
Table 4. IC50 values of the most potent compounds over the most sensitive cell lines from each cancer subpanel.
In addition to IC50, total growth inhibition (TGI) concentration and lethal dose 50 (LD50) for the most potent compounds were determined (). The total growth inhibition in case of K-562 leukaemia cell line was ranging from 2.5 µM for compound 2l to 19 µM for compound 1g. For non-small cell lung cancer cell line HOP-92, compound 2o exerted the lowest TGI (3.6 µM) and LD50 (9.7 µM). Compounds 2l and 2k exhibited TGI 4.7 and 7.5 µM, respectively. Compounds 2q and 2l were the most efficacious among this new series against HT-29 colon cancer cell line with TGI 1.7 µM for each of them and LD50 4.5 and 4.7 µM, respectively. Compounds 2o and 2l showed the highest efficacy against U251 CNS cancer cell line with TGI 3.2 and 3.4 µM, respectively, and LD50 8.1 and 8.8 µM, respectively. All the tested compounds except 2g and 2m exhibited significant one-digit micromolar TGI and LD50 values against SK-MEL-5 melanoma cell line. Both compounds 2k and 2 l showed lethal dose 50 5.3 µM. Compound 2k had TGI of 2.6 µM, while compound 2l had TGI equal to 2.5 µM. Compound 2l was the most potent against OVCAR-4, A498, PC-3 and MDA-MB-468 with TGI 5.9, 2.2, 6.1, and 4.6 µM and LD50 6.9 µM for A498 cell line. Compound 2k was the second most potent compound against the same cell lines with TGI 8.6, 2.8, 7.8, and 5.6 µM and LD50 11.9 µM against A498 cell line.
Table 5. TGI and LD50 values of the most potent compounds over the most sensitive cell lines from each cancer subpanel.
Kinase profiling
In order to determine the molecular target(s) of the newly synthesized compounds, a panel of 20 kinases of different families was used. The inhibitory effects of compounds 1l, 2c, and 2l, which showed the strongest potencies against the NCI-60 cancer cell line panel, on different kinases are depicted in . It can be concluded that compound 2l had a strong activity against JNK1, JNK2, JNK3, P38a/MAPK14, and BRAF (V600E) with mean per cent inhibitions 99.02%, 98.47%, 89.50%, 86.54%, and 93.67%, respectively. Also, compound 2l showed a moderate activity against GSK3b and BRAF with mean per cent inhibitions 75.26% and 72.56%, respectively. On the other hand, compounds 1l and 2c showed moderate activity against JNK1, JNK2, and BRAF(V600E). The stronger kinase inhibitory effects of the hydroxyl compound 2l compared to the corresponding methoxy derivative 2c can be rationalized that the presence of hydrogen bond donor and the lower bulkiness of OH group may contribute to stronger affinity with the enzymes. Furthermore, the propylene linker of 2l seems to be more appropriate for activity than ethylene.
Table 6. Inhibitory effect of compounds 1l, 2c, and 2 l on different kinases activity at a single dose of 10 µM.
ICs50 of compound 2l against its target enzymes are tabulated in . Compound 2l exhibited an IC50 0.35 µM over JNK1 and 0.36 µM over JNK2. JNK kinases are hyperactivated in different types of cancer such as leukaemia, lung cancer, skin cancer, glioblastoma and other brain tumours, and breast cancer. JNK kinases induce tumorigenesis through induction of cell proliferation and survival, and their inhibition is a potential avenue for cancer therapyCitation24. So inhibition of JNK1 and JNK2 kinases could be, at least partially, a possible mechanism of antiproliferative activity of compound 2l.
Table 7. IC50 of compound 2l against BRAF, BRAF(V600E), JNK1, JNK2, P38a/MAPK14 and RAF1.
Plasma stability
The plasma stability test for compound 2l revealed that compound 2l has high stability profile in both human and rat plasma. After 30 min, 100% of compound 2 l remained unchanged which decreased to 95.3% after 2 h, while that of procaine was 1.2% after 30 min and 0.2% after 2 h and the percentage for diltiazem was 91.5% after 30 min and 89.3% after 2 h ().
Table 8. Plasma stability of compound 2l.
One of the most important features to be investigated during drug development is the ability of the compound to bind to the human ether-a-go-go related gene (hERG). The inhibition of hERG can lead to sudden death and should be avoided during drug discoveryCitation25. Compound 2l was tested for its ability to inhibit hERG and the IC50 was 8.28 µM which is fifteen times greater than its IC50 against the most sensitive cell line. So the compound has high safety and selectivity index, and low risk of sudden death induction.
In vivo pharmacokinetic
Compound 2 l was the most potent compound against both in vitro cell line assay and enzyme activity. The human plasma stability is excellent compared to standard reference compounds. The in vivo pharmacokinetic profile following oral or intravenous administration, and oral bioavilability of 2 l are represented in and .
Figure 3. Plasma concentration-time curves of 2l in rats following iv (10 mg/kg) or oral (10 mg/kg) administration.
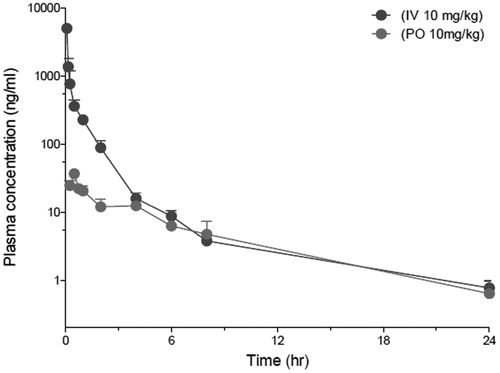
Table 9. PK Parameters of 2l in SD Rats (N = 3).
Conclusion
In the current work, design and synthesis of a new N-(2-((4-(3-(3-methoxy and/or hydroxyl)phenyl)-1-phenyl-1H-pyrazol-4-yl)pyridin-2-yl)amino)ethyl and/or propyl) substituted benzene sulfonamides 1a–q and 2a–q were accomplished. The antiprolifertave activity of the new synthesized compounds revealed that compounds having propyl bridge between the pyridine ring and sulfonamide moiety 2a–q were more potent compared to compounds having ethylene bridge 1a–q. In addition, compounds possessing m-hydroxyl group at ring A were more potent than their methoxy analogues with an exception of compounds 2g and 2h which carry out terminal p-triflouromethyl sulfonamide and m-fluoro sulfonamide moieties which showed excellent activity compared to other methoxy compounds. Compounds with electron-withdrawing groups such as chloro 2l and bromo 2k presented the highest per cent inhibition among the newly synthesized compounds and lowest IC50 0.33 µM against A498 renal cancer cell line for compound 2l. Compound 2l significantly inhibited the activity of JNK1, JNK2, JNK3, V600E BRAF, and P38 alpha with IC50 0.35 and 0.36 µM against JNK1 and JNK2. Moreover, compound 2l exhibited high plasma stability in both human and rat. Compound 2l had 9.2% oral bioavailability with t1/2 ranging from 5.8 h for intravenously administrated to 6.1 h for orally administrated doses.
IENZ_1530225_Supplementary Material
Download MS Word (24.2 MB)Acknowledgements
The authors are thankful to Korea Institute of Science and Technology (KIST), Seoul, Republic of Korea, for financially supporting this work (KIST Project 2E26900). We would like to thank the National Cancer Institute (NCI), Bethesda, Maryland, USA, for performing the in vitro anticancer testing over the cell lines.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- World Health Organization, Media Centre, Cancer. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/ [last accessed 30 Jun 2018].
- Cancer statistic center. Available from: https://cancerstatisticscenter.cancer.org.
- Frankish H. 15 million new cancer cases per year by 2020, says WHO. Lancet 2003;361:1278.
- Belpomme D, Irigaray P, Sasco AJ, et al. The growing incidence of cancer: role of lifestyle and screening detection. Int. J. Oncol 2007;30:1037–49.
- Abdel-Maksoud MS, Kim MR, El-Gamal MI, et al. Design, synthesis, in vitro antiproliferative evaluation, and kinase inhibitory effects of a new series of imidazo[2,1- b] thiazole derivatives. Eur J Med Chem 2015;95:453–63.
- Gamal El-Din MM, El-Gamal MI, Abdel-Maksoud MS, et al. Synthesis and in vitro antiproliferative activity of new 1,3,4-oxadiazole derivatives possessing sulfonamide moiety. Eur J Med Chem 2015;90:45–52.
- Gamal El-Din MM, El-Gamal MI, Abdel-Maksoud MS, et al. Design, synthesis, and in vitro antiproliferative and kinase inhibitory effects of pyrimidinylpyrazole derivatives terminating with arylsulfonamido or cyclic sulfamide substituents. J Enz Inhibit Med Chem 2016;31:111–22.
- Sharma A, Shah SR, Illum H, Dowell J. Vemurafenib: targeted inhibition of mutated BRAF for treatment of advanced melanoma and its potential in other malignancies. Drugs 2012;72:2207–22.
- Rheault TR, Stellwagen JC, Adjabeng GM, et al. Discovery of Dabrafenib: a selective inhibitor of Raf kinases with antitumor activity against B-Raf-driven tumors. ACS Med Chem Lett 2013;4:358–62.
- Karoulia Z, Gavathiotis E, Poulikakos PI. New perspectives for targeting RAF kinase in human cancer. Nature Rev Cancer 2017;17:676–91.
- BRAFTOVI and MEKTOVI, Array BioPharma Inc. FDA approves encorafenib and binimetinib in combination for unresectable or metastatic melanoma with BRAF mutations. Available from: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm611981.htm [last accessed 24 Sep 2018].
- El-Gamal MI, Sim TB, Hong JH, et al. Synthesis of 1H-pyrazole-1-carboxamide derivatives and their antiproliferative activity against melanoma cell line. Arch Pharm Chem Life Sci 2011;344:197–204.
- El-Gamal MI, Oh CH. Design and synthesis of 3-(3-chloro-4-substituted phenyl)-4-(pyridin-4-yl)-1H-pyrazole-1-carboxamide derivatives and their antiproliferative activity against melanoma cell line. Bull Korean Chem Soc 2011;32:821–8.
- El-Gamal MI, Choi HS, Cho HG, et al. Design, synthesis and antiproliferative activity of 3,4-diarylpyrazole-1-carboxamide derivatives against melanoma cell line. Arch Pharm Chem Life Sci 2011;344:745–54.
- Choi WK, El-Gamal MI, Choi HS, et al. New diarylureas and diarylamides containing 1,3,4-triarylpyrazole scaffold: Synthesis, antiproliferative evaluation against melanoma cell lines, ERK kinase inhibition and molecular docking studies. Eur J Med Chem 2011;46:5754–62.
- Choi WK, El-Gamal MI, Choi HS, et al. Synthesis and antiproliferative activity of new aminoisoquinolinylurea derivatives against melanoma cell line. Bull Korean Chem Soc 2012;33:2991–8.
- El-Gamal MI, Park YS, Chi DY, et al. New triarylpyrazoles as broad-spectrum anticancer agents: Design, synthesis and biological evaluation. Eur J Med Chem 2013;65:315–22.
- El-Gamal MI, Choi HS, Yoo KH, et al. Antiproliferative diarylpyrazole derivatives as dual inhibitors of the ERK pathway and COX-2. Chem Biol Drug Des 2013;82:336–47.
- Gamal El-Din MM, El-Gamal MI, Abdel-Maksoud MS, et al. Design, synthesis, broad-spectrum antiproliferative activity and kinase inhibitory effect of triarylpyrazole derivatives possessing arylamides or arylureas moieties. Eur J Med Chem 2016;119:122–31.
- Khan MA, El-Gamal MI, Tarazi H, et al. Design and synthesis of a new series of highly potent RAF kinase-inhibiting triarylpyrazole derivatives possessing antiproliferative activity against melanoma cells. Future Med Chem 2016;8:2197–211.
- El-Gamal MI, Abdel-Maksoud MS, Gamal El-Din MM, et al. Synthesis, in vitro antiproliferative and antiinflammatory activities and kinase inhibitory effects of new 1,3,4-triarylpyrazole derivatives. Anti-Cancer Agent Med Chem 2017;17:75–84.
- National Cancer Institute, USA. NCI-60 screening methodology. Available from: https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm [last accessed 24 Sep 2018].
- Kinase Assays (Reaction Biology Corp.) accessed on 24 Sep 2018. Available from: http://www.reactionbiology.com/ [last accessed 24 Sep 2018].
- Bubici C, Papa S. JNK signalling in cancer: in need of new, smarter therapeutic targets. Br J Pharmacol 2014;171:24–37.
- Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature 2006;440:463–9.