Abstract
Acidity and hypoxia are crucial phenotypes of tumour microenvironment both contributing to the selection of malignant cells under a micro evolutionistic pressure. During the tumour progression, nanovesicles, called exosomes and the metalloenzyme carbonic anhydrase IX (CA IX) affect the tumour growth and proliferation. Exosomes are released into the tumour microenvironment and spilt all over the body, while CA IX is a tumour-associated protein overexpressed in many different solid tumours. In the present study, to better understand the relationships between exosomes and CA IX, it has been used an in vitro cellular model of cells cultured in different pH conditions. The results showed that the acidic microenvironment induced upregulation of both expression and activity of CA IX in cancer cells and their exosomes, together with increasing the number of released exosomes. These data strongly support the importance of CA IX as a cancer biomarker and as a valuable target of new anticancer therapies.
Introduction
In the last decade, the importance of exosomes has gained attention for their role in intercellular communication. They are cell-derived nanovesicles (40–180 nm) shuttling proteins, DNA, and RNA that could be transferred to recipient cells and modulate their protein expressionCitation1,Citation2. Exosomes membrane is composed of a lipid bilayer enriched in cholesterol and sphingomyelin (typically lipid rafts componentsCitation3), in ceramide (essential for the insertion of microRNAs into exosomesCitation4) and Bis (monoacylglycero) phosphate (BMP) (an exosome-specific markerCitation5). Exosomes are released from cells in physiological and pathological conditions, including cancer. It has been demonstrated that cancer cells produce more exosomes as compared to normal cells, and cancer exosomes differ from normal exosomes in molecular composition and functionCitation6. The tumour microenvironment is acidic, a common feature of virtually all cancersCitation7. The extracellular acidity has a strong influence on the tumour microenvironment fitness, with a critical role in the progressive microevolution of cancers, including the induction of increased exosome releaseCitation7–10. Tumour pH ranges in vivo from 6 to 6.8, with a mean of 6.5 and it is correlated to tumour malignancyCitation11–17. In addition to low pH, tumour microenvironment is characterised by hypoxia and low nutrients supply, and a typical sign of malignancy that is the so-called aerobic glycolysis, also called Warburg Effect, i.e. sugar fermentation in hypoxic conditionCitation7,Citation18–21. These cells produce ATP converting glucose in lactic acid, rather than metabolising it in mitochondria through oxidative phosphorylationCitation20,Citation22–25. However, this process implies an increase of glucose absorption to sustain energy requirement by tumour cells. The most important product of tumour metabolism is lactate leading in turn to H+ accumulation in the extracellular microenvironment with a direct consequence of the low pHCitation26. High levels of carbonic dioxide produced during mitochondrial respiration of oxygenated cancer cells also contribute to a substantial release of H+ into the tumour microenvironmentCitation27–32. Low extracellular pH, lactic and carbonic acid production, uncontrolled growth, low blood and nutrient supply, contribute to generate a tumour microenvironment extremely toxic to normal cells. The toxic microenvironment progressively selects malignant cells, able to survive in this adverse condition thanks to up-regulation of expression and activity of several proton extrusion mechanisms, which release protons and lactate into extracellular microenvironment avoiding cytosol acidificationCitation33,Citation34. In fact, an anti-acidic approach based on either Proton Pump Inhibitors (PPI)Citation9,Citation35 or buffers leads to acidification of cancer cell cytosol followed by quick and non-conventional cell death. Moreover, the anti-acidic treatment sensitises cancer cells to chemotherapeuticsCitation12,Citation15,Citation16, supporting the use of PPI as a new strategy against cancerCitation36–38. Among proton flux regulatorCitation21 there are vacuolar H+-ATPases (V-ATPases), Na+/H+ exchanger (NHE), monocarboxylate transporters (MCTs), carbonic anhydrase IX (CA-IX)Citation10,Citation33,Citation34, and Na+/HCO3 co-transporters (NBC)Citation39. Interference (i.e. inhibition) with one or more of these proton pumps leads to a potent inhibition of cancer growth in a variety of ex vivo and in vivo modelsCitation40–42. In this context, a pivotal role has been attributed to the CA IX, which is a metalloenzyme well studied in cancer. CA IX belongs to the α-CA genetic family among the seven CA-families known up to date. It is a membrane protein characterised by an extracellular proteoglycan domain, an extracellular catalytic domain, a transmembrane domain, and a short intracytosolic tail. Its primary function, like the other 14 human isoforms, is to catalyse the reaction of CO2 with H2O to produce H2CO3, which instantly dissociates to H+ and HCO3−. The promoter region of the gene (CA 9) encoding for CA IX contains a hypoxia-responsive element, with CA9 mRNA expression highly upregulated by hypoxia-inducible factor-1 (HIF1)Citation43. Hypoxic tumours express high amounts of CA IX yielding an increase of the intracellular concentration of HCO3− and extracellular acidification. The formed protons (H+) are secreted into the extracellular space through the pump/vacuolar-type ATPase and/or a Na+/H+ exchanger, while the HCO3− is shuttled back to the cytosol mostly via a chloride/bicarbonate exchanger, although other ion exchangers may be involved as well (for both proteins more isoforms are known, some of which present predominantly in tumours)Citation44. Therefore, CA IX activity is one of the main players responsible for the extracellular acidity of hypoxic tumours. One CA IX inhibitor (SLC-0111) actually progressed to Phase Ib clinical trials for the treatment of hypoxic, metastatic tumoursCitation45,Citation46. This study hypothesised that some of the proton exchangers, extremely active in malignant cancer cells, could be expressed on exosomes and actively modulated by the acidic conditions. To this purpose, we explored the expression and activity of CA IX in cancer cells and cancer-released exosomes showing that CA IX expression and related activity are up-regulated under the acidic condition in both cells and exosomes. These results support a key role of CA IX in cancer progression and as both a cancer biomarker and a therapeutic target.
Materials and methods
Cell line
Human prostate carcinoma cell line (LNCaP) is derived from a metastatic site (left supraclavicular lymph node) of a 50-year-old Caucasian male (blood type B+) with confirmed diagnosis of metastatic prostate carcinoma (Istituto dei tumori di Milano). Tumour cells were negative for Mycoplasma contamination as routinely tested by PCR (Venor®GeM, Minerva Biolabs, Germany). The cells were maintained in RPMI culture medium supplemented with antibiotics and 10% foetal calf serum (FCS) (Invitrogen, Milan, Italy). Experiments were performed in buffered medium at pH 7.4 and in RPMI 1640 medium without sodium bicarbonate (pH 6.5) supplemented with antibiotics and 10% foetal calf serum (FCS) (Invitrogen, Milan, Italy). The acid cell culture medium (pH 6.5) was obtained by the addition of 1 M HCl solution. The pH was measured with a pH 123 Microprocessor pH Metre (Hanna Instruments, Milan, Italy).
Confocal microscopy analysis
Cells were fixed 10 min in 4% paraformaldehyde, blocked 30 min in PBS with 1% BSA and labelled overnight with M75 antibody in blocking solutionCitation47. After washing with PBS +0.02% Tween20, cells were labelled with anti-mouse conjugated with AlexaFluor-488 and then with DAPI + ProLong (P36931, ThermoFisher Scientific).
Images were taken by a FV1000 confocal microscope (Olympus, Tokyo, Japan), using a (Olympus) planapo objective 60x oil A.N. 1.42. Excitation light was obtained by a Laser Dapi 408 nm for DAPI, an Argon Ion Laser (488 nm) for Alexa 488, DAPI emission was recorded from 415 to 485 nm, AlexaFluor-488 emission was recorded from 525 to 550 nm.
Exosomes isolation from supernatant cell culture media
After 5 days of cell cultures, supernatants were collected, and exosomes isolated as described in Théry et al. and Kusuzaki et alCitation48. Briefly, after centrifugation of cell at 300 g for 5 min, supernatants were centrifuged at 1200 g for 15 min followed by 12,000 g for 30 min. Supernatants were then filtered using a 0.22 μm filter (Millipore Corp., Bedford, MA) and centrifuged at 110,000 g for 1 h in a Sorvall WX Ultracentrifuge Series (Thermo Fisher Scientific) in order to pellet exosomes. After one wash in a large volume of phosphate-buffered saline (PBS), exosomes were resuspended in PBS (50 μl) for subsequent experimental analysis. To eliminate exosomes of FCS, the FCS was filtered with 0.45 and subsequently 0.22 μm filters (Millipore Corp., Bedford, MA) and then ultracentrifuged at 110,000 g before its addition to the culture media.
Western blot analysis
Lysates were prepared in CHAPS buffer (10 mM Tris-HCl [pH 7.4], MgCl2 1 mM, EGTA 1 mM, CHAPS 0.5%, glycerol 10%, β-mercaptoethanol 5 mM, PMSF 1 mM) containing protease inhibitor cocktail. Cell lysates and exosomes were subjected to electrophoresis on SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Protran Whatman, Dassel, Germany). After blocking in 5% dry milk in PBS1X, membranes were hybridised with primary antibodies: M75Citation47, anti-Tsg101 (4A10, GeneTex, USA), anti-GAPDH (GA1R, Santa Cruz Biotechnology, USA) and anti-actin (C4, Santa Cruz Biotechnology, USA) monoclonal antibodies. After incubation with appropriate peroxidase-conjugated anti-IgG (Amersham Biosciences, Milan, Italy), membranes were revealed using the ECL Chemiluminescent Substrate, (ThermoFisher Scientific).
Enzyme activity
LNCaP cells and their exosomes were obtained from cellular cultures grown at pH 7.4 and 6.5 as described above. Cell and exosome extracts were prepared at 4 °C using the lysis buffer containing 1% Triton X-100, 10 mM Tris-HCl (pH 7.4), MgCl2 1 mM, EGTA 1 mM, CHAPS 0.5%, glycerol 10%, β-mercaptoethanol 5 mM, and supplemented with a cocktail of protease inhibitors. Lysates were collected and clarified by centrifugation at 16,300×g for 15 min at 4 °C. Aliquots of cell or exosomes extracts containing 1 µg of total protein were used to determining the hydratase activity. The enzymatic assay was performed at 0 °C using CO2 as substrate following the pH variation due to the catalysed conversion of CO2 to bicarbonate. Bromothymol blue was used as the indicator of pH variation. The production of hydrogen ions during the CO2 hydration reaction lowers the pH of the solution until the colour transition point of the dye is reached. The time required for the colour change is inversely related to the quantity and activity of CAs present in the sample. Wilbur–Anderson units were calculated according to the following definition: One Wilbur–Anderson unit (WAU) of activity is defined as (T0 − T)/T, where T0 (uncatalysed reaction) and T (catalysed reaction) are recorded as the time (in seconds) required for the pH to drop from 8.3 to the transition point of the dye (pH 6.8) in a control buffer and in the presence of enzyme, respectively. Enzyme activity was expressed as WAU/µg of total protein. Protein concentration was determined using the Bio-Rad protein assay.
Results
CA IX expression is up-regulated in human prostate carcinoma cell line (LNCaP) cultured in acidic condition
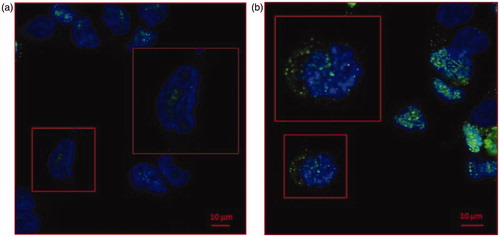
A human prostate cancer cell line (LNCaP) was cultured at two different pH conditions. LNCaP cells were buffered at pH 7.4 and 6.5, with no differences in terms of survival and viability as previously shownCitation6. We checked the cell mortality (Trypan blue assay)(11) by cytofluorimeter analysis with again no differences in terms of mortality (1–2%) in both the cell pH culture conditions (data not shown). To evaluate the CA IX expression in LNCaP cells cultured at different pH, we first performed a Confocal Microscopy analysis. CA IX, predominantly expressed at the plasma membrane, can also be found into the cytoplasm and associated with the nuclear membrane. As shown in , CA IX while expressed in both samples, independently from the culture condition, it was up-regulated in LNCaP cultured at pH 6.5 (). Remarkably, in LNCaP pH 6.5 () CA IX showed an intracellular expression with a granular distribution as compared to the pH 7.4 culture condition ().
Figure 1. Confocal microscopy analysis of CA IX expression in LNCaP cell line cultured at pH 7.4 and pH 6.5. (a)LNCaP cells cultured at pH 7.4 showed low expression of CA IX (green signal) predominately nuclear (blue signal of DAPI). (b) CA IX expression in LNCaP pH 6.5 is higher compared to pH 7.4 and showed a strong cytoplasmic expression.
CA IX expression is up-regulated in human prostate carcinoma cell line (LNCaP) cultured in acidic condition and in exosomes isolated from cell culture supernatant
To validate the different CA IX expression, we performed a Western Blot Analysis; as shown in , CA IX is characterised by the presence of two bands (58/54 kDa) with an up-regulated expression in cells cultured under acidic condition compared to pH 7.4 (1.5-fold higher calculated with densitometry software ImageJ) (data not shown). This result was consistent with previous reports showing that CA IX higher expression was associated with acidic microenvironmentCitation49–51.
Figure 2. Western Blot of lysates from LNCaP pH 7.4 and LNCaP pH 6.5 cell and exosomes. CA IX expression was analysed in LNCaP lysates from cells and exosomes cultured at different condition. CA IX expression in increased both in cells and exosomes cultured at pH 6.5 compared to samples at pH 7.4. In particular, there is an increase of CA IX 54KDa band in samples at pH 6.5. Membranes were also incubated with anti-GAPDH, a housekeeping protein, and anti-Tsg101, a typical exosomal marker.
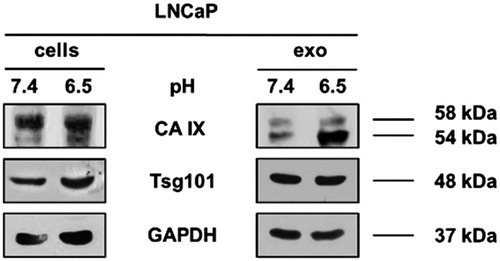
Horie et al.Citation52, demonstrated the presence of CA IX associated with exosomes fractions after OptiPrep density gradient centrifugation, so we analysed the whole exosomal purification lysates by Western Blot Analysis. Exosomes were characterised and quantified by Nanoparticle Tracking Analysis (NTA) (data not shown) and the expression of Tsg101, a typical exosomal marker. Consistent with the results obtained in cellular lysates, we showed that the CA IX expression in exosomes was up-regulated in acidic condition as compared to the 7.4 buffered condition (2.3-fold higher calculated with densitometry software) (data not shown). We also displayed that there was a different expression of the two CA IX bands (58/54 kDa) between cells and exosomes. In fact, cells extracts showed higher expression of 58 kDa CA IX while exosome preparation showed higher expression of the 54 kDa band. Interestingly, 54 kDa-form is always up-regulated in pH 6.5 condition both in cells and exosomes, suggesting to be the results of posttranscriptional changes of CA IX expression induced by the acidic state.
CA enzymatic activity analysis of LNCaP cells and exosomes
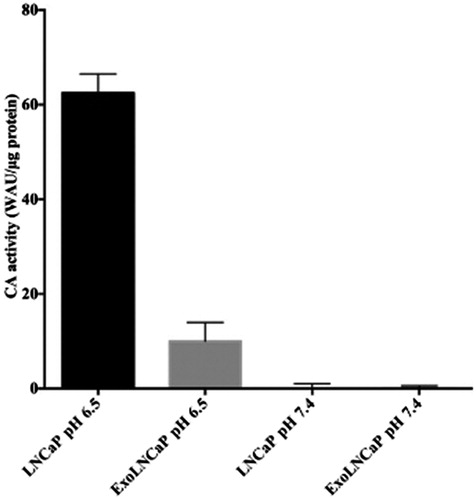
As shown in , the CA-activity has been found only in LNCaP cells and in the exosomes isolated from LNCaP cells grown at pH 6.5, while it was undetectable in LNCaP cells and in exosomes isolated from LNCaP cells grown at pH 7.4. These results are supported by the fact that the hypoxia condition, a state of all solid tumours, stimulates the hypoxia-inducible factor 1 (HIF-1), which induces the expression of the CA IX. The enzyme activity is responsible for the intracellular acidification and the acidity of the extracellular tumour microenvironment.
Acidic microenvironment influences cellular and exosomal CA IX expression.
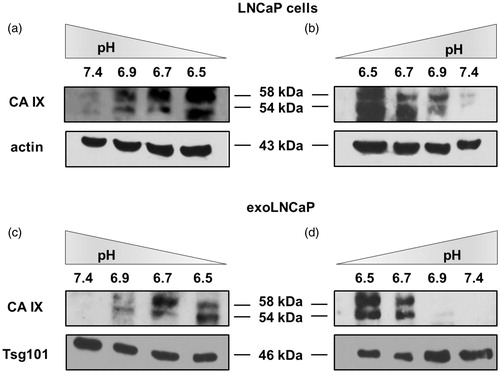
Considering that CA IX expression is related to microenvironmental pH, we cultured LNCaP cell line at different pH conditionsCitation6 ranging from 7.4 to 6.5 and vice-versa. After five days, cells were lysed to perform Western Blot Analysis and cell culture media were collected to obtain exosome purifications and perform WB analysis. CA IX expression in LNCaP cells progressively increased with the decrease of pH (), while decreasing with the increase of microenvironmental pH (). The same for the exosomes release at the different pH conditions from pH 7.4 to 6.5 () and vice-versa (). NTA showed that pH-related change in CA IX expression was directly related to the number and size of exosomes released by the cells (i.e. higher number in acidic conditions lower at pH 7.4) (Supplementary Figure 1 and Figure 2, Supplementary Table 1 and Table 2).
Figure 4. CA IX expression is related to microenvironmental pH. (a) Cells gradually cultured from pH 7.4 to 6.5 showed an increased CA IX expression. (b) CA IX decreased expression in LNCAP cells from pH 6.5 to 7.4. (c) Exosomes isolated from cells cultured from pH 7.4 to 6.5 showed an increased CA IX expression. (d) Exosomes isolated from cells cultured from pH 6.5 to 7.4 showed a decreased CA IX expression.
Discussion and conclusions
Tumour microenvironment (TME) exerts a key role in cancer pathogenesis, including initiation, progression, and response to therapy. As described in the literature, low pH, hypoxia and low blood and nutrients supply all contribute to setting a very hostile and selective microenvironment, that is common to virtually all cancersCitation11. The adverse microenvironmental induces a Darwinian selection allowing the survival and proliferation of the cells most suited to live in those conditions. For example, malignant cells up-regulate the expression of many proton-efflux regulators to avoid the acidification of the cytosol, which leads to a quick cell deathCitation33,Citation53. Among these proton-efflux regulators, there are several CAs, zinc metalloenzymes catalysing the conversion of carbon dioxide to bicarbonate and protons. CAs are expressed in almost all living organisms and they are involved in many physiological processes based on transport and pH balance (respiration, digestion, renal acidification, bone resorption etcCitation45,Citation54. Several isoforms are known and CA IX is markedly associated with solid tumours and by the way to the acidification of the tumour microenvironment pHCitation49–51; thus representing a suitable target in cancer therapyCitation45,Citation51,Citation55–58.
Recently, exosomes acquired a crucial role in the oncologic scenario since they are involved in cell-cell communicationCitation59, angiogenesisCitation52, metastasisCitation60 and drug resistanceCitation61. Moreover, exosomes contain a cell-type specific signature, thus representing a source of biomarkers in a variety of diseases, including cancerCitation2,Citation6,Citation62,Citation63. Furthermore, exosomes number and distribution could be included between the phenotypes common to virtually all cancers. We found, in fact, a higher number of exosomes in the plasma of cancer patients as compared to healthy donorsCitation6,Citation62, correlating with the results obtained in vitro modelsCitation6.
Since CA IX is a suitable marker of prostate cancerCitation64 and its expression is up-regulated in hypoxia conditionCitation64,Citation65, in the present paper we analysed how the acidic microenvironment of tumours could affect the CA IX expression and activity in human prostatic cancer cell line and exosomes released from them. The results showed different expression of CA IX in cells cultured in the acidic media (pH 6.5) respect to those at pH 7.4. The obtained results were consistent with the previous data showing that the low-pH is the most suitable condition for CA IX catalysisCitation49–51. Moreover, the Confocal Analysis showed a remarkable expression of the protein in the cytoplasm of the cells cultured in the acidic microenvironment, with different internal distribution, suggesting that acidity influences CA IX expression and localisation within tumour cells. Notably, the CA IX expression in exosomes lysates reflected the CA IX expression at the cellular level in both acidic and physiological conditions. In addition, WB Analysis evidenced that the expression of the CA IX 54 kDa band increased in acidic condition at both cellular and exosome levels. All these experiments support the CA IX role in the in vivo tumour condition, since CA IX expression increased with progressive acidification and decreased with alkalinisation at both cellular and exosome levels. Thus, the tumour acidic microenvironment is the key factor in influencing the CA IX expression and activity in cancer cells and cancer-released exosomes. Previous reports have shown that CA IX is related to cancer development and progressionCitation66 and some malignant cancer activities such as cell spreading and migrationCitation67. Therefore, the possibility to obtain exosomes from plasma samples of tumour patients may offer the opportunity to consider CA IX a key tumour biomarker. Considering that CA IX is involved in the metastatic activity of cancer cellsCitation52,Citation68, measuring its expression and activity in the exosomes may be helpful in monitoring cancer patients, as we have shown here for prostate cancer. Lastly, having exosomes a pivotal role in the metastatic process, both in preparing a metastatic niche and in inducing a tumour-like transformation in mesenchymal stem cells in target organsCitation60, it is highly conceivable that the expression and activity of CA IX in tumour exosomes may be the key factor in the spreading of cancer all over the body. In conclusion, the future of anti-tumour strategies in primary cancers should be focussed in inhibiting the exosomes release and CA IX expression in malignant cancers. All strategies aimed at inhibiting microenvironmental acidity of tumours might represent a possible way to do it.
Fais_Supplemental_Material.docx
Download MS Word (159.3 KB)Acknowledgements
We would like to thank Dr Silvia Pastoreková (Biomedical Research Centre, Slovak Academy of Sciences) for kindly providing us with the antibody M75 used in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Yáñez-Mó M, Siljander PR-M, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015;4:27066.
- Fais S, O’Driscoll L, Borras FE, et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano 2016;10:3886–99.
- Parolini I, Federici C, Raggi C, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 2009;284:34211–22.
- Kosaka N, Iguchi H, Yoshioka Y, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010;285:17442–52.
- Gallala HD, Sandhoff K. Biological function of the cellular lipid BMP-BMP as a key activator for cholesterol sorting and membrane digestion. Neurochem Res 2011;36:1594–600.
- Logozzi M, Angelini DF, Iessi E, et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett 2017;403:318–29.
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891–9.
- Lugini L, Matarrese P, Tinari A, et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res 2006;66:3629–38.
- De Milito A, Iessi E, Logozzi M, et al. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res 2007;67:5408–17.
- Spugnini E, Fais S. Proton pump inhibition and cancer therapeutics: a specific tumor targeting or it is a phenomenon secondary to a systemic buffering? Semin Cancer Biol 2017;43:111–8.
- De Milito A, Canese R, Marino ML, et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer 2010;127:207–19.
- Luciani F, Spada M, De Milito A, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst 2004;96:1702–13.
- Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res 2012;72:2746–56.
- Fais S, Venturi G, Gatenby B. Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy. Cancer Metastasis Rev 2014;33:1095–108.
- Azzarito T, Venturi G, Cesolini A, Fais S. Lansoprazole induces sensitivity to suboptimal doses of paclitaxel in human melanoma. Cancer Lett 2015;356:697–703.
- Azzarito T, Lugini L, Spugnini EP, et al. Effect of modified alkaline supplementation on syngenic melanoma growth in CB57/BL mice. PLoS ONE 2016;11:e0159763.
- Canitano A, Iessi E, Spugnini EP, et al. Proton pump inhibitors induce a caspase-independent antitumor effect against human multiple myeloma. Cancer Lett 2016;376:278–83.
- Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. MRI of the tumor microenvironment. J Magn Reson Imaging 2002;16:430–50.
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029–33.
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011;11:85–95.
- Iessi E, Logozzi M, Mizzoni D, et al. Rethinking the combination of proton exchanger inhibitors in cancer therapy. Metabolites 2017;8:2.
- Warburg O. On the origin of cancer cells. Science 1956;123:309–14.
- Semenza GL, Artemov D, Bedi A, et al. The metabolism of tumours: 70 years later. Novartis Found Symp 2001;240:251–60.
- Xu XD, Shao SX, Jiang HP, et al. Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat 2015;38:117–22.
- Icard P, Shulman S, Farhat D, et al. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat 2018;38:1–11.
- Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 2007;99:1441–54.
- Newell K, Franchi A, Pouysségur J, Tannock I. Studies with glycolysis-deficient cells suggest that production of lactic acid is not the only cause of tumor acidity. Proc Natl Acad Sci USA 1993;90:1127–31.
- Yamagata M, Hasuda K, Stamato T, Tannock IF. The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br J Cancer 1998;77:1726–31.
- Helmlinger G, Sckell A, Dellian M, et al. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res 2002;8:1284–91.
- Svastová E, Hulíková A, Rafajová M, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 2004;577:439–45.
- Mookerjee SA, Goncalves RLS, Gerencser AA, et al. The contributions of respiration and glycolysis to extracellular acid production. Biochim Biophys Acta 2015;1847:171–81.
- Corbet C, Feron O. Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer 2017;17:577–93.
- Izumi H, Torigoe T, Ishiguchi H, et al. Cellular pH regulators: potentially promising molecular targets for cancer chemotherapy. Cancer Treat Rev 2003;29:541–9.
- Spugnini EP, Sonveaux P, Stock C, et al. Proton channels and exchangers in cancer. Biochim Biophys Acta 2015;1848:2715–26.
- Marino ML, Fais S, Djavaheri-Mergny M, et al. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis 2010;1:e87.
- Fais S, De Milito A, You H, Qin W. Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res 2007;67:10627–30.
- Lugini L, Federici C, Borghi M, et al. Proton pump inhibitors while belonging to the same family of generic drugs show different anti-tumor effect. J Enzyme Inhib Med Chem 2016;31:538–45.
- Lu Z-N, Tian B, Guo X-L. Repositioning of proton pump inhibitors in cancer therapy. Cancer Chemother Pharmacol 2017;80:925–37.
- Gorbatenko A, Olesen CW, Boedtkjer E, Pedersen SF. Regulation and roles of bicarbonate transporters in cancer. Front Physiol 2014;5:130.
- Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 2011;71:3364–76.
- a) Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 2011;54:1896–902; b) Krall N, Pretto F, Decurtins W, et al. A small‐molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew Chem Int Ed Engl 2014;53:4231–5; c) Bozdag M, Carta F, Ceruso M, et al. Discovery of 4-Hydroxy-3-(3-(phenylureido)benzenesulfonamides as SLC-0111 analogues for the treatment of hypoxic tumors overexpressing carbonic anhydrase IX. J Med Chem 2018;61:6328–38; d) Borras J, Scozzafava A, Menabuoni L, et al. Carbonic anhydrase inhibitors: synthesis of water-soluble, topically effective intraocular pressure lowering aromatic/heterocyclic sulfonamides containing 8-quinoline-sulfonyl moieties: is the tail more important than the ring? Bioorg Med Chem 1999;7:2397–406.
- a) McDonald PC, Swayampakula M, Dedhar S. Coordinated regulation of metabolic transporters and migration/invasion by carbonic anhydrase IX. Metabolites 2018;8:E2; b) Kuchuk O, Tuccitto A, Citterio D, et al. pH regulators to target the tumor immune microenvironment in human hepatocellular carcinoma. OncoImmunology 2018;7:e1445452.
- Kaluz S, Kaluzová M, Liao S-Y, et al. Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: a one transcription factor (HIF-1) show? Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2009;1795:162–72.
- a) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767–77; b) Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Pat 2013;23:737–49.
- a) Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:E48; b) Supuran CT. Carbonic anhydrases and metabolism. Metabolites 2018;8:E25.
- Boyd NH, Walker K, Fried J, et al. Addition of carbonic anhydrase 9 inhibitor SLC-0111 to temozolomide treatment delays glioblastoma growth in vivo. JCI Insight 2017;2:92928. Available at: https://insight.jci.org/articles/view/92928
- Pastoreková S, Závadová Z, Kostál M, et al. A novel quasi-viral agent, MaTu, is a two-component system. Virology 1992;187:620–6.
- a) Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002;2:569–79; b) Kusuzaki K, Matsubara T, Murata H, et al. Natural extracellular nanovesicles and photodynamic molecules: is there a future for drug delivery? J Enzyme Inhib Med Chem 2017;32:908–16.
- Chen LQ, Howison CM, Spier C, et al. Assessment of carbonic anhydrase IX expression and extracellular pH in B-cell lymphoma cell line models. Leuk Lymphoma 2015;56:1432–9.
- a) Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68; b) Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- a) Andreucci E, Peppicelli S, Carta F, et al. Carbonic anhydrase IX inhibition affects viability of cancer cells adapted to extracellular acidosis. J Mol Med 2017;95:1341–53; b) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60; c) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88.
- Horie K, Kawakami K, Fujita Y, et al. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem Biophys Res Commun 2017;492:356–61.
- Reshkin SJ, Greco MR, Cardone RA. Role of pHi, and proton transporters in oncogene-driven neoplastic transformation. Philos Trans R Soc Lond, B, Biol Sci 2014;369:20130100.
- Pastorekova S, Ratcliffe PJ, Pastorek J. Molecular mechanisms of carbonic anhydrase IX-mediated pH regulation under hypoxia. BJU Int 2008;101:8–15.
- Cianchi F, Vinci MC, Supuran CT, et al. Selective inhibition of carbonic anhydrase IX decreases cell proliferation and induces ceramide-mediated apoptosis in human cancer cells. J Pharmacol Exp Ther 2010;334:710–9.
- Federici C, Lugini L, Marino ML, et al. Lansoprazole and carbonic anhydrase IX inhibitors sinergize against human melanoma cells. J Enzyme Inhib Med Chem 2016;31:119–25.
- a) Mahmood S-U, Saeed A, Bua S, et al. Synthesis, biological evaluation and computational studies of novel iminothiazolidinone benzenesulfonamides as potent carbonic anhydrase II and IX inhibitors. Bioorg Chem 2018;77:381–6; b) Menchise V, De Simone G, Alterio V, et al. Carbonic anhydrase inhibitors: stacking with Phe131 determines active site binding region of inhibitors as exemplified by the X-ray crystal structure of a membrane-impermeant antitumor sulfonamide complexed with isozyme II. J Med Chem 2005;48:5721–7; c) Supuran CT, Mincione F, Scozzafava A, et al. Carbonic anhydrase inhibitors—part 52. Metal complexes of heterocyclic sulfonamides: a new class of strong topical intraocular pressure-lowering agents in rabbits. Eur J Med Chem 1998;33:247–54; d) Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005;15:3102–8; e) Şentürk M, Gülçin İ, Beydemir Ş, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9.
- a) Métayer B, Angeli A, Mingot A, et al. Fluoroenesulphonamides: N-sulphonylurea isosteres showing nanomolar selective cancer-related transmembrane human carbonic anhydrase inhibition. J Enzyme Inhib Med Chem 2018;33:804–8; b) Rehman SU, Chohan ZH, Gulnaz F, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. J Enzyme Inhib Med Chem 2005;20:333–40; c) Clare BW, Supuran CT. Carbonic anhydrase activators. 3: Structure‐activity correlations for a series of isozyme II activators. J Pharm Sci 1994;83:768–73; d) Dubois L, Peeters S, Lieuwes NG, et al. Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol 2011;99:424–31; e) Ozensoy Guler O, Capasso C, Supuran CT. A magnificent enzyme superfamily: carbonic anhydrases, their purification and characterization. J Enzyme Inhib Med Chem 2016;31:689–94; f) De Simone G, Langella E, Esposito D, et al. Insights into the binding mode of sulphamates and sulphamides to hCA II: crystallographic studies and binding free energy calculations. J Enzyme Inhib Med Chem 2017;32:1002–11.
- Khalyfa A, Almendros I, Gileles-Hillel A, et al. Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget 2016;7:54676–90.
- Zhao H, Achreja A, Iessi E, et al. The key role of extracellular vesicles in the metastatic process. Biochim Biophys Acta 2018;1869:64–77.
- Federici C, Petrucci F, Caimi S, et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE 2014;9:e88193.
- Logozzi M, De Milito A, Lugini L, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE 2009;4:e5219.
- Cappello F, Logozzi M, Campanella C, et al. Reprint of exosome levels in human body fluids: a tumor marker by themselves? Eur J Pharm Sci 2017;98:64–9.
- Ambrosio MR, Di Serio C, Danza G, et al. Carbonic anhydrase IX is a marker of hypoxia and correlates with higher Gleason scores and ISUP grading in prostate cancer. Diagn Pathol 2016;11:45.
- Li Y, Wang H, Oosterwijk E, et al. Antibody-specific detection of CAIX in breast and prostate cancers. Biochem Biophys Res Commun 2009;386:488–92.
- Juhász M, Chen J, Lendeckel U, et al. Expression of carbonic anhydrase IX in human pancreatic cancer. Aliment Pharmacol Ther 2003;18:837–46.
- Csaderova L, Debreova M, Radvak P, et al. The effect of carbonic anhydrase IX on focal contacts during cell spreading and migration. Frontier Physiol 2013;4:271.
- Ramteke A, Ting H, Agarwal C, et al. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog 2015;54:554–65.