Abstract
A series of organometallic acylhydrazones was prepared, incorporating Re(CO)3, Mn(CO)3 and ferrocenyl moieties, which were subsequently reacted with amino-sulfonamides in order to obtain carbonic anhydrase (CA, EC 4.2.1.1) inhibitors possessing organometallic moieties in their molecules. The new derivatives were investigated as inhibitors of four human (h) CA isoforms with pharmaceutical applications, such as the cytosolic hCA I, II and VII and the mitochondrial hCA VA. An interesting inhibitory profile against these isoforms was obtained, with some of these metal complexes acting as subnanomolar or low nanomolar inhibitors. They were also thoroughly characterised from the chemical point of view, making them of interest for further developments in the field of metal complexes of sulfonamides with CA inhibitory action.
1. Introduction
Carbonic anhydrase (CA, EC 4.2.1.1) inhibitors (CAIs) are clinically used for several decades as diureticsCitation1, antiglaucoma agentsCitation2, antiobesity drugsCitation3, and more recently, a number of studies showed that CA inhibition has profound antitumor effects by inhibition of hypoxia-inducible isoforms CA IX and XII, overexpressed in many hypoxic tumorsCitation4. Several proof-of-concept studies demonstrated the involvement of some CA isoforms in neuropathic painCitation5 and arthritisCitation6, with inhibitors of the sulfonamide/coumarinCitation7 types demonstrating significant effects in vivo, in animal models of these diseases. This is obviously due to the fact that at least 15 different α-class CA isoforms are present in humans, and many of them are drug targets for the treatment or prevention of this large variety of pathologiesCitation1–7. Thus, the field of drug design, synthesis and in vivo investigations of various types of CAIs is a highly dynamic one, with a large number of interesting new chemotypes acting on these widespread enzymes constantly emergingCitation1–7. Among the clinically used sulfonamide CAIs are acetazolamide (AAZ), methazolamide (MZA), ethoxzolamide (EZA), brinzolamide (BRZ) and dorzolamide (DRZ) – ()Citation1–3. Saccharin (SAC) is a sweetener widely used in beverages and foodCitation1–3.
Figure 1. Clinically used sulfonamides with CA inhibitory activityCitation1–3.
Coordination compounds of sulfonamides with CA inhibitory properties in which the sulfonamides act as ligands to various transition or main group metal ions, leading to sulfonamide metal complexes were also investigated for their interactions with these enzymesCitation8. Originally investigated for obtaining transition metal ion complexes of acetazolamide AAZ, methazolamide MZA, and ethoxzolamide EZA (the main sulfonamide, clinically used drugs belonging to this class of pharmacological agents)Citation8, this approach was subsequently extended to a large set of primary and secondary aromatic/heterocyclic sulfonamides, also including the clinical drugs saccharin (SAC), brinzolamide (BRZ) and dorzolamide (DRZ)Citation9–14. Other sulfonamides possessing a diverse scaffold but effective CA inhibitory properties were also included in such studies together with metal ions which may add a supplementary pharmacological activity, such as Pt(II), Pd(II) and Ru(II) for the antitumor effectsCitation6,Citation14,Citation15, Zn(II) for the antiglaucoma actionCitation11, Al(III) for antacid propertiesCitation10, Co(II), Ag(I) and Cu(II) for antifungal activityCitation10. Imaging tumors overexpressing some CA isoforms (e.g. CA IX and XII) with sulfonamide complexes incorporating isotopes of metal ions which emit positrons (for PET imaging), such as Ga(III), In(III) or Cu(II) were also investigatedCitation14, allowing interesting developments in the field. On the other hand, the organometallic complexes also incorporating sulfonamide CAIs as ligands were less investigated, although some rhenium(I) and ruthenium(II) derivatives were recently reportedCitation14,Citation15.
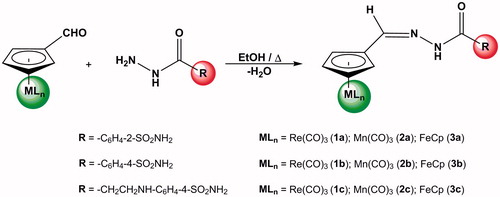
Here we explored the possibility to prepare organometallic-acylhydrazone incorporating Re(CO)3, Mn(CO)3 and ferrocenyl moieties, which were reacted with amino-sulfonamide in order to obtain CAIs possessing organometallic moieties in their molecules.
2. Experimental
2.1. Materials
All manipulations were conducted under an N2 atmosphere using Schlenk techniques. The compounds (η5-C5H4CHO)Re(CO)3Citation16, (η5-C5H4CHO)Mn(CO)3Citation17, 2 or 4-(hydrazinecarbonyl)benzenesulfonamideCitation18 and 4-((3-hydrazinyl-3-oxopropyl)amino)benzenesulfonamideCitation18 were prepared according to published procedures. Ferrocenecarboxaldehyde (98%), sulfanilamide (99%), 4-sulfamoylbenzoic acid (97%), methyl-2-(aminosulfonyl)benzoate (98%) and CF3COOH (99%) were obtained from Sigma-Aldrich and used without additional purification. Solvents such as CH2Cl2, hexane, acetone, EtOH, DMSO, and THF were obtained commercially and purified using standard methods. Infrared spectra were recorded in solid state (KBr pellet) on a Jasco FT-IR 4600 spectrophotometer. 1H NMR spectra were measured on a Bruker spectrometer model ASCEND TM 400 MHz. All NMR spectra are reported in parts per million (ppm, δ) relative to tetramethylsilane (Me4Si), with the residual solvent proton resonances used as internal standards. Coupling constants (J) are reported in Hertz (Hz), and integrations are reported as number of protons. The following abbreviations were used to describe the peak patterns: s = singlet, d = doublet, t = triplet, and m = multiplet. Mass spectra were obtained on a Shimadzu model QP5050A GC-MS at the Laboratorio de Servicios Analíticos, Pontificia Universidad Católica de Valparaíso. Elemental analyses were measured on a Perkin Elmer CHN Analyzer 2400.
2.2. Synthesis of organometallic-acylhydrazones. General procedure
The new organometallic-acylhydrazones were prepared following the same procedure as for their organic analoguesCitation19. In each case, the respective acylhydrazide (1 eq.) was charged in a two neck round bottomed flask with dried ethanol (10 mL) and a magnetic stir bar. The solution was stirred under nitrogen atmosphere to obtain a clear solution. To the reaction mixture, formyl organometallic precursor (1 eq.) and four drops of CF3COOH were added at room temperature and under stirring condition and the reaction was continued for 4 h. After this time, the reaction mixture was filtered and the precipitate was washed with cold hexane (3 × 10 mL) and dried under vacuum for 2 h. The solid obtained was purified using slow diffusion crystallization from THF/hexane (1:5) at −18 °C.
2.2.1. [{(η5-C5H4)–CH=N–N(H)C(O)–C6H4-2–SO2NH2}]Re(CO)3 (1a)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)Re(CO)3 (121 mg, 0.33 mmol) and 2-(hydrazinecarbonyl)benzenesulfonamide (72 mg, 0.33 mmol). Brown solid, yield 78% (146 mg, 0.26 mmol). IR (KBr, cm−1): 3300–3198 (νNH/NH2); 2027 (νRe–CO); 1913 (νRe–CO); 1658 (νCO); 1558 (νC = N); 1338 (νS–O). 1H NMR (DMSO-d6): δ 5.63 (t, 0.5H, J = 2.2 Hz, C5H4); 5.77 (t, 1.5H, J = 2.2 Hz, C5H4); 5.87 (t, 0.5H, J = 2.2 Hz, C5H4); 6.24 (t, 1.5H, J = 2.2 Hz, C5H4); 7.09 (s, 0.5H, NH2); 7.12 (s, 1.5H, NH2); 7.69 (m, 3H, Ar–H); 7.95 (m, 1H, Ar–H); 8.02 (s, 0.8H, CH = N); 8.32 (s, 0.2H, CH = N); 11.99 (s, 1H, NH). Mass spectrum (based on 187Re) (m/z): 560 [M+]; 532 [M+ - CO]; 504 [M+ - 2CO]; 476 [M+ - 3CO]. Anal. (%) Calc. for C16H12N3O6SRe: C, 34.28; H, 2.16 and N, 7.50; found: C, 34.34; H, 2.17 and N, 7.49.
2.2.2. [{(η5-C5H4)–CH=N–N(H)C(O)–C6H4-4–SO2NH2}]Re(CO)3 (1b)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)Re(CO)3 (121 mg, 0.33 mmol) and 4-(hydrazinecarbonyl)benzenesulfonamide (72 mg, 0.33 mmol). Yellow solid, yield 83% (155 mg, 0.28 mmol). IR (KBr, cm−1): 3243–3112 (νNH/NH2); 2027 (νRe–CO); 1950 (νRe–CO); 1664 (νCO); 1556 (νC=N); 1341 (νS–O). 1H NMR (DMSO-d6): δ 5.78 (t, 2H, J = 2.2 Hz, C5H4); 6.26 (t, 2H, J = 2.2 Hz, C5H4); 7.52 (s, 2H, NH2); 7.94 (d, 2H, J = 8.2 Hz, Ar–H); 8.04 (d, 2H, J = 8.2 Hz, Ar–H); 8.20 (s, 1H, CH=N); 11.96 (s, 1H, NH). Mass spectrum (based on 187Re) (m/z): 560 [M+]; 476 [M+ - 3CO]. Anal. (%) Calc. for C16H12N3O6SRe: C, 34.28; H, 2.16 and N, 7.50; found: C, 34.33; H, 2.16 and N, 7.52.
2.2.3. [{(η5-C5H4)–CH=N–N(H)C(O)–CH2CH2–NH–C6H4-4–SO2NH2}]Re(CO)3 (1c)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)Re(CO)3 (121 mg, 0.33 mmol) and 4-((3-hydrazinyl-3-oxopropyl)amino)benzenesulfonamide (86 mg, 0.33 mmol). Pale yellow solid, yield 77% (155 mg, 0.26 mmol). IR (KBr, cm−1): 3368-3115 (νNH/NH2); 2958 (νCsp3–H); 2025 (νRe–CO); 1912 (νRe–CO); 1641 (νCO); 1605 (νC = N); 1321 (νS–O). 1H NMR (DMSO-d6): δ 2.45 (t, 0.9H, J= 6.8 Hz, CH2CO); 2.80 (t, 1.1H, J= 6.8 Hz, CH2CO); 3.36 (m, 2H, CH2NH); 5.73 (m, 2H, C5H4); 6.11 (t, 1.1H, J = 2.2 Hz, C5H4); 6.16 (t, 0.9H, J = 2.2 Hz, C5H4); 6.43 (m, 1H, NH); 6.63 (m, 2H, Ar–H); 6.91 (s, 2H, NH2); 7.51 (d, 2H, J = 8.2 Hz, Ar–H); 7.68 (s, 0.6H, CH=N); 7.91 (s, 0.4H, CH = N); 11.32 (s, 0.6H, NH); 11.34 (s, 0.4H, NH). Mass spectrum (based on 187Re) (m/z): 603 [M+]; 606 [M+ - 3CO]. Anal. (%) Calc. for C18H17N4O6SRe: C, 35.82; H, 2.84 and N, 9.28; found: C, 35.85; H, 2.83 and N, 9.30.
2.2.4. [{(η5-C5H4)–CH=N–N(H)C(O)–C6H4-2–SO2NH2}]Mn(CO)3 (2a)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)Mn(CO)3 (77 mg, 0.33 mmol) and 2-(hydrazinecarbonyl)benzenesulfonamide (72 mg, 0.33 mmol). Yellow solid, yield 76% (108 mg, 0.25 mmol). IR (KBr, cm−1): 3360–3096 (νNH/NH2); 2020 (νMn–CO); 1940 (νMn–CO); 1683 (νCO); 1537 (νC = N); 1343 (νS–O). 1H NMR (DMSO-d6): δ 5.01 (s, 0.5H, C5H4); 5.16 (s, 1.5H, C5H4); 5.25 (s, 0.5H, C5H4); 5.61 (s, 1.5H, C5H4); 7.11 (s, 2H, NH2); 7.62 (m, 4H, Ar–H); 7.96 (s, 0.8H, CH = N); 8.25 (s, 0.2H, CH = N); 12.03 (s, 1H, NH). Mass spectrum (m/z): 429 [M+]; 401 [M+ - CO]; 373 [M+ - 2CO]; 345 [M+ - 3CO]. Anal. (%) Calc. for C16H12N3O6SMn: C, 44.77; H, 2.82 and N, 9.79; found: C, 44.76; H, 2.81 and N, 9.78.
2.2.5. [{(η5-C5H4)–CH = N–N(H)C(O)–C6H4-4–SO2NH2}]Mn(CO)3 (2b)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)Mn(CO)3 (77 mg, 0.33 mmol) and 4-(hydrazinecarbonyl)benzenesulfonamide (72 mg, 0.33 mmol). Yellow solid, yield 83% (118 mg, 0.28 mmol). IR (KBr, cm−1): 3233–3008 (νNH/NH2); 2025 (νMn–CO); 1933 (νMn–CO); 1663 (νCO); 1557 (νC=N); 1343 (νS–O). 1H NMR (DMSO-d6): δ 4.92 (s, 0.5H, C5H4); 5.16 (s, 1.5H, C5H4); 5.28 (s, 0.5H, C5H4); 5.63 (s, 1.5H, C5H4); 7.52 (s, 2H, NH2); 7.94 (d, 2H, J = 8.2 Hz, Ar–H); 8.04 (d, 2H, J = 8.2 Hz, Ar–H); 8.10 (s, 0.25H, CH=N); 8.13 (s, 0.75H, CH=N); 11.90 (s, 0.25H, NH); 11.99 (s, 0.75H, NH). Mass spectrum (m/z): 429 [M+]; 345 [M+ - 3CO]. Anal. (%) Calc. for C16H12N3O6SMn: C, 44.77; H, 2.82 and N, 9.79; found: C, 44.76; H, 2.83 and N, 9.78.
2.2.6. [{(η5-C5H4)–CH=N–N(H)C(O)–CH2CH2–NH–C6H4-4–SO2NH2}]Mn(CO)3 (2c)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)Mn(CO)3 (77 mg, 0.33 mmol) and 4-((3-hydrazinyl-3-oxopropyl)amino)benzenesulfonamide (86 mg, 0.33 mmol). Brown solid, yield 77% (121 mg, 0.26 mmol). IR (KBr, cm−1): 3369–3080 (νNH/NH2); 2960 (νCsp3–H); 2025 (νMn–CO); 1929 (νMn–CO); 1646 (νCO); 1602 (νC=N); 1327 (νS–O). 1H NMR (DMSO-d6): δ 2.45 (t, 0.9H, J= 6.8 Hz, CH2CO); 2.81 (t, 1.1H, J= 6.8 Hz, CH2CO); 3.36 (m, 2H, CH2NH); 5.11 (s, 2H, C5H4); 5.50 (s, 1.1H, C5H4); 5.54 (s, 0.9H, C5H4); 6.44 (m, 1H, NH); 6.64 (m, 2H, Ar–H); 6.92 (s, 2H, NH2); 7.51 (d, 2H, J = 7.9 Hz, Ar–H); 7.62 (s, 0.6H, CH=N); 7.83 (s, 0.4H, CH = N); 11.34 (s, 0.6H, NH); 11.36 (s, 0.4H, NH). Mass spectrum (m/z): 472 [M+]; 388 [M+ - 3CO]. Anal. (%) Calc. for C18H17N4O6SMn: C, 45.77; H, 3.63 and N, 11.86; found: C, 45.79; H, 3.62 and N, 11.89.
2.2.7. [{(η5-C5H4)–CH=N–N(H)C(O)–C6H4-2–SO2NH2}]FeCp (3a)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)FeCp (107 mg, 0.50 mmol) and 2-(hydrazinecarbonyl)benzenesulfonamide (107 mg, 0.50 mmol). Red solid, yield 71% (146 mg, 0.36 mmol). IR (KBr, cm−1): 3308–3088 (νNH/NH2); 1640 (νCO); 1598 (νC = N); 1334 (νS–O). 1H NMR (DMSO-d6): δ 4.18 (s, 1H, C5H5); 4.26 (s, 4H, C5H5); 4.32 (s, 0.4H, C5H4); 4.37 (s, 0.4H, C5H4); 4.48 (s, 1.6H, C5H4); 4.67 (s, 1.6H, C5H4); 7.01 (s, 0.5H, NH2); 7.15 (s, 1.5H, NH2); 7.72 (m, 3H, Ar–H); 7.96 (m, 1H, Ar–H); 8.15 (s, 0.9H, CH=N); 8.43 (s, 0.1H, CH=N); 11.71 (s, 0.2H, NH); 11.77 (s, 0.8H, NH). Mass spectrum (m/z): 411 [M+]. Anal. (%) Calc. for C18H17N3O3SFe: C, 52.57; H, 4.17 and N, 10.22; found: C, 52.59; H, 4.18 and N, 10.23.
2.2.8. [{(η5-C5H4)–CH=N–N(H)C(O)–C6H4-4–SO2NH2}]FeCp (3b)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)FeCp (107 mg, 0.50 mmol) and 4-(hydrazinecarbonyl)benzenesulfonamide (107 mg, 0.50 mmol). Red solid, yield 78% (160 mg, 0.39 mmol). IR (KBr, cm−1): 3226–3209 (νNH/NH2); 1635 (νCO); 1567 (νC = N); 1336 (νS–O). 1H NMR (DMSO-d6): δ 4.26 (s, 5H, C5H5); 4.48 (s, 2H, C5H4); 4.69 (s, 2H, C5H4); 7.52 (s, 2H, NH2); 7.95 (d, 2H, J = 8.2 Hz, Ar–H); 8.06 (d, 2H, J = 8.2 Hz, Ar–H); 8.31 (s, 1H, CH=N); 11.68 (s, 1H, NH). Mass spectrum (m/z): 411 [M+]. Anal. (%) Calc. for C18H17N3O3SFe: C, 52.57; H, 4.17 and N, 10.22; found: C, 52.55; H, 4.19 and N, 10.24.
2.2.6. [{(η5-C5H4)–CH = N–N(H)C(O)–CH2CH2–NH–C6H4-4–SO2NH2}]FeCp (3c)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)FeCp (107 mg, 0.50 mmol) and 4-((3-hydrazinyl-3-oxopropyl)amino)benzenesulfonamide (129 mg, 0.50 mmol). Brown solid, yield 74% (168 mg, 0.37 mmol). IR (KBr, cm−1): 3365-3090 (νNH/NH2); 2949 (νCsp3–H); 1667 (νCO); 1600 (νC=N); 1316 (νS–O). 1H NMR (DMSO-d6): δ 2.42 (t, 0.92H, J= 6.8 Hz, CH2CO); 2.80 (t, 1.08H, J= 6.8 Hz, CH2CO); 3.36 (m, 2H, CH2NH); 5.19 (s, 2.7H, C5H5); 5.21 (s, 2.3H, C5H5); 4.41 (m, 2H, C5H4); 4.59 (m, 2H, C5H4); 6.46 (m, 1H, NH); 6.65 (m, 2H, Ar–H); 6.91 (s, 2H, NH2); 7.52 (m, 2H, Ar–H); 7.83 (s, 0.54H, CH = N); 7.98 (s, 0.46H, CH=N); 11.05 (s, 0.54H, NH); 11.08 (s, 0.46H, NH). Mass spectrum (m/z): 454 [M+]. Anal. (%) Calc. for C20H22N4O3SFe: C, 52.87; H, 4.88 and N, 12.33; found: C, 52.86; H, 4.89 and N, 12.37.
2.3. CA inhibition studies
An Sx.18Mv-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used to assay the catalytic activity of various CA isozymes for CO2 hydration reactionCitation10,Citation11,Citation20. Phenol red (at a concentration of 0.2 mM) was used as indicator, working at the absorbance maximum of 557 nm, with 10 mM Hepes (pH 7.5) as buffer, 0.1 M Na2SO4 (for maintaining constant ionic strength), following the CA-catalyzed CO2 hydration reaction for a period of 10 s at 25 ◦C. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitors (10 mM) were prepared in distilled-deionised water and dilutions up to 1 nM were done thereafter with the assay buffer. Enzyme and inhibitor solutions were pre-incubated together for 15 min (standard assay at room temperature) prior to assay, in order to allow for the formation of the enzyme-inhibitor complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3 and the Cheng–Prusoff equation, as reported earlierCitation10–12. All CAs were recombinant proteins produced as reported earlier by our groupCitation10–12,Citation20.
3. Results and discussion
3.1. Synthesis and characterisation of organometallic-acylhydrazones containing sulfonamide fragments
The preparation of these new family of organometallic-acylhydrazones described in the experimental section involved the synthesis of the appropriated organic acylhydrazide precursor 2-(hydrazinecarbonyl)benzenesulfonamide, 4 (hydrazinecarbonyl)benzenesulfonamide and 4-((3-hydrazinyl-3-oxopropyl)amino)benzenesulfonamide, which were prepared according to published proceduresCitation18,Citation19. The organometallic-acylhydrazones containing sulfonamide moieties were obtained as described in Scheme 1, following the same procedure reported for some organic analoguesCitation19, that is, by the condensation reaction of the appropriate acylhydrazide and the corresponding formyl organometallic complex in anhydrous EtOH. All compounds were isolated in good yields (71–83%) as solids, after crystallisation from THF/hexane mixture. These products are air-stable and slightly soluble in most polar organic solvents (e.g. CH2Cl2, acetone, CH3CN).
In all cases, the infrared spectral analysis of these compounds showed the characteristic absorption corresponding stretching vibration of the ν(C = N) bond in the range of 1605–1537 cm−1 in KBr disk. Similar ν(C = N) frequency values have been previously reported for other organometallic-acylhydrazones derived from ferrocenylCitation21 and cymantrenyl groupsCitation22. The absence of the band assigned to the aldehyde carbonyl group of organometallic complexes confirmed the formation of the organometallic Schiff bases. Moreover, all compounds showed the expected absorption bands for the νN–H, νCO, and νSO2 stretches in the ranges of 3369–3080 cm−1, 1683–1635 cm−1 and 1343–1321 cm−1, respectively. In addition, the spectra for 1c, 2c, and 3c exhibited an absorption band for νCsp3–H at ∼2950 cm−1. Furthermore, the IR spectra of cyrhetrenyl (1a–c) and cymantrenyl (2a–c) acylhydrazones revealed the presence of terminal metal carbonyl groups in the region of 2027–1912 cm−1Citation23–25. A strong molecular ion was shown in the mass spectrum of each organometallic-acylhydrazones, in addition to the detection of notable successive losses of CO ligands for the cyrhetrenyl (1a–c) and cymantrenyl (2a–c) derivatives. The elemental analysis data determined for all compounds are in agreement with their proposed formulas.
For all complexes, the 1H NMR spectra showed the presence of a sharp singlet in the range of 8.43–7.62 ppm, and it was assigned to the iminic proton. These results are in agreement with the values reported for organometallic Schiff basesCitation26–28. Moreover, 1H NMR spectra for 1a–c and 2a–c showed sets of resonances in the region of 6.26–4.92 ppm, which are ascribed to the protons of the cyrhetrenyl and cymantrenyl moietiesCitation29. On this regard, the ferrocenyl derivatives 3a–c exhibited resonances around δ 4.69–4.32 due to the non-equivalent alpha and beta protons containing in the substituted Cp ring and a singlet in the region of 4.26–4.18 ppm, which was assigned to the proton resonances of the unsubstituted cyclopentadienyl group. For all compounds, the multiplets observed between 8.06 and 6.63 ppm were assigned to the hydrogen atoms of the C6H4 ring. As per literature reports, the broad singlet observed at 7.52–6.91 ppm was assigned to the hydrogen nuclei of the SO2NH2 groupCitation18,Citation30,Citation31.
The presence of the –NH–CO– group registered as a broad singlet in the range of 12.03–11.05 ppm. Similar δ have been reported for other organometallic hydrazonesCitation21,Citation22,Citation32. On this regard, it is an important remark that the chemical shifts of the –NH– resonance showed a dependence on the presence of the organometallic moiety bound to the iminic entity. In fact, the downfield shift observed for the cyrhetrenyl (1a–c) and cymantrenyl (2a–c) derivatives (Δδ∼0.5) compared with ferrocenyl analogues (3a–c) can be related to the electron-withdrawing properties of the (η5-C5H4)M(CO)3 moietiesCitation33,Citation34, which produce a deshielding of the NH resonance, thus suggesting that the nature of the organometallic framework modifies the degree of electronic delocalization on the –C(H)=N–NH– unit. We have found similar results for ferrocenyl and cyrhetrenyl hydrazonesCitation19,Citation35 and 1,3,4-thiadiazolesCitation36. In the case of the acylhydrazones 1c, 2c and 3c, additional signals were observed at 6.44 ppm, 3.36 ppm and 2.80–2.42 ppm, respectively. These resonances were attributed to the presence of the –CH2CH2NH– groupCitation37.
It is important to mention that acylhydrazones can form four isomers owing to the presence of amide (–CONH–) and azomethine groups (–CH = N–) in their structureCitation38,Citation39. The geometrical isomers (E/Z) originate from the azomethine group and rotamer (cis/trans) formation is due to the restricted rotation of the amide group (see ). However, the survey of the literature reveals that the N-acyl hydrazones synthesised from aromatic carbaldehyde are essentially planar and exist completely in the form of geometric (E)-configuration about the C = N bond due to steric hindrance on the imine bondCitation38–41. Therefore, we discarded the formation of Z, cis and Z, trans isomers.
Figure 2. General structure of possible Z/E geometrical isomers and cis/trans amide rotamers of organometallic-acylhydrazones.
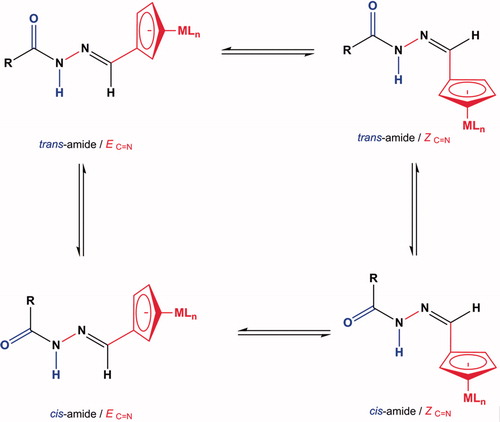
Based on 1H NMR data, the organometallic-acylhydrazones (1a–c), (2a–c) and (3a–c) reported in this work exist as a mixture of cis/trans isomers in DMSO-d6 solutions. On this regard, 1H NMR spectra for all compounds show resonances for the CO–NH and CH = N group protons are present in double sets and the signal intensity ratio is ∼0.6 cis: 0.4 trans. This splitting signals pattern was also observed for cyclopentadienyl (C5H5, C5H4) and ethyl (–CH2CH2–) protons. The cis isomer predominates because of a hindered rotation around the CO–NH bondCitation19. Similar results have been reported for organic-acylhydrazones derived from other benzenesulfonamide derivativesCitation37.
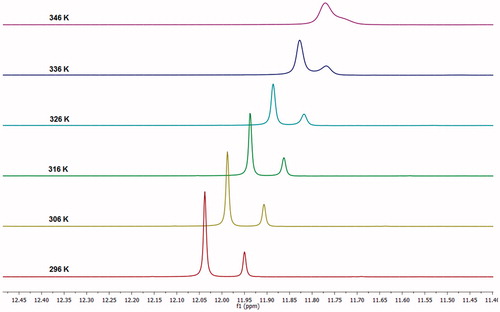
In order to confirm the existence of cis-/trans-amide rotamers, we carried out 1H NMR spectra of 1a measurements at several temperatures (). Variable temperature (VT) 1H NMR spectra showed that increasing the temperature within the range of 296-346 K led to coalescence of the –CONH– resonance of cis- and trans-amide rotamers. Similar results have been published previously by Barhoumi-Slimi and co-workers in organic-acylhydrazones derived from cinnamaldehyde and β-chloro-α,β-unsaturated aldehydesCitation42.
Unfortunately, the low solubility of all the compounds in deuterated solvents precluded us to measure their 13C NMR and bidimensional NMR spectra to complement their characterisation.
3.2. CA inhibition studies
The sulfonamide containing organometallic-acylhydrazones have been evaluated in vitro as CAIs. Three cytosolic human (h) isoforms (hCA I, II, and VII) and one mitochondrial (hCA VA) have been included for the screening, and the results revealed interesting selectivity profiles for some of the evaluated compounds. Inhibition data obtained with the standard stopped-flow CO2 hydrase assay are compared to those of the standard sulfonamide inhibitor acetazolamide (AAZ)Citation43–47 (). Structure-activity relationships have been delineated dividing the compounds into three classes, depending on the organic portion responsible for the CA inhibition.
Table 1. Inhibition of human (h) CA isoforms hCA I, II, VA, and VII with acetazolamide (AAZ) and the organometallic derivatives reported here, by a stopped-flow, CO2 hydrase assayCitation47.
The 2-(hydrazinecarbonyl)benzenesulfonamide derivatives (series a) revealed to be ineffective in inhibiting hCA I (Ki > 10,000 nM) and showed poor active against hCA II, with Ki values in the micromolar range (2595.6 < Ki < 10,000 nM), regardless of the different metal substituted Cp ring contained in them. On the other hand, the insertion of the sulfonamide group in ortho position of the aromatic ring turned out to be favourable for the inhibition of hCA VA and VII, which were strongly inhibited by all the compounds investigated here, with Ki values in the nanomolar range for the cyrhetrenyl 1a, cymantrenyl 2a and ferrocenyl 3a derivatives. Therefore, compounds 1a, 2a, and 3a were potent and selective hCA VA and VII inhibitors (over the cytosolic enzymes hCA I and II).
The insertion of 4 (hydrazinecarbonyl)benzenesulfonamide fragment on the molecular scaffolds (series b) led to a dramatic enhancement of inhibition potency against hCA II, particularly for compounds 1b and 2b, which were 8-fold more potent than the ferrocenyl derivative 3b. A slight enhancement of potency against hCA I was also observed for the cyrhetrenyl 1b and cymantrenyl 2b derivatives, which were effective in the high nanomolar range (Ki values of 595.4 nM and 373.6 nM respectively). The same isosteric substitution on the organic scaffold did not affect the inhibition potency against hCA VA and VII, with the best Ki values showed by the cymantrenyl 2b (30.3 nM) and ferrocenyl 3b (34.2 nM) derivatives. For hCA VII, the ferrocenyl derivative 3b turned out to be 3-fold more potent (Ki of 27.2 nM) than the other organometallic analogues investigated here.
The expansion of the organic scaffold due to the insertion of 4-((3-hydrazinyl-3-oxopropyl)amino)benzenesulfonamide moiety in the derivatives of series c revealed to be detrimental for the inhibition potency against hCA I for the cyrhetrenyl 1c and ferrocenyl 3c derivatives, that possessed Ki-s in the micromolar range. On the other hand, the cymantrenyl 2c inhibition potency was not affected by this structural modification. The potency against hCA II was retained with a slight worsening of the Ki values, mostly for the ferrocenyl derivative 3c (4.5 fold potency decrease when compared to 3b). hCA VA was strongly inhibited by all derivatives investigated here. Noteworthy, the same modification in the organometallic-acylhydrazones scaffold led to a clear enhancement of the potency against hCA VII, with low nanomolar Ki values showed by all derivatives, (ranging between 9.2 nM and 9.6 nM).
In conclusion, we report a new class of organometallic CAIs possessing an interesting inhibitory profile against isoforms with important pharmacologic applications, such as CA II, VA, and VII. Some of these metal complexes were subnanomolar or low nanomolar inhibitors of many such enzymes. They were also thoroughly characterised from the chemical point of view, making them of interest for further developments in the field of metal complexes of sulfonamides with CA inhibitory action.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- (a) Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005 - 2013). Expert Opin Ther Pat 2013;23:681–91; (b) Temperini C, Cecchi A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Sulfonamide diuretics revisited-old leads for new applications?. Org Biomol Chem 2008;6:2499–506; (c) Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709–12; (d) Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin Ther Pat 2018;28:713–21; (e) Nocentini A, Supuran CT. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: a patent review (2008-2018). Expert Opin Ther Pat 2018;28:729–40; (f) Supuran CT, Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin Ther Pat 2018;28:745–54.
- (a) Masini E, Carta F, Scozzafava A, Supuran CT. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Pat 2013;23:705–16; (b) Borras J, Scozzafava A, Menabuoni L, et al. Carbonic anhydrase inhibitors: synthesis of water-soluble, topically effective intraocular pressure lowering aromatic/heterocyclic sulfonamides containing 8-quinoline-sulfonyl moieties: is the tail more important than the ring?. Bioorg Med Chem 1999;7:2397–06; (c) Capasso C, Supuran CT. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria?. J Enzyme Inhib Med Chem 2015;30:325–32.
- (a) Scozzafava A, Supuran CT, Carta F. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat 2013;23:725–35; (b) Supuran CT. Carbonic anhydrases and metabolism. Metabolites 2018;8:E25; (c) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov. 2017;12:61–88.
- (a) Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008 - 2013). Expert Opin Ther Pat 2013;23:737–49; (b) Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:E48; (c) Ward C, Langdon SP, Mullen P, et al. New strategies for targeting the hypoxic tumour microenvironment in breast cancer. Cancer Treat Rev 2013;39:171–79; (d) Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005;15:3102–08; (e) Casey JR, Morgan PE, Vullo D, et al. Carbonic anhydrase inhibitors. Design of selective, membrane-impermeant inhibitors targeting the human tumor-associated isozyme IX. J Med Chem 2004;47:2337–47.
- (a) Supuran CT. Carbonic anhydrase inhibition and the management of neuropathic pain. Expert Rev Neurother 2016;16:961–68; (b) Di Cesare Mannelli L, Micheli L, Carta F, et al. Carbonic anhydrase inhibition for the management of cerebral ischemia: in vivo evaluation of sulfonamide and coumarin inhibitors. J Enzyme Inhib Med Chem 2016;31:894–99.
- (a) Margheri F, Ceruso M, Carta F, et al. Overexpression of the transmembrane carbonic anhydrase isoforms IX and XII in the inflamed synovium. J Enzyme Inhib Med Chem 2016;31:60–3; (b) Bua S, Di Cesare Mannelli L, Vullo D, et al. Design and synthesis of novel nonsteroidal anti-inflammatory drugs and carbonic anhydrase inhibitors hybrids (NSAIDs-CAIs) for the treatment of rheumatoid arthritis. J Med Chem 2017;60:1159–70.
- (a) Supuran CT. Carbon- versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors?. J Enzyme Inhib Med Chem 2018;33:485–95; (b) Di Fiore A, Maresca A, Supuran CT, et al. Hydroxamate represents a versatile zinc binding group for the development of new carbonic anhydrase inhibitors. Chem Commun (Camb) 2012;48:8838–40; (c) Marques SM, Nuti E, Rossello A, et al. Dual inhibitors of matrix metalloproteinases and carbonic anhydrases: iminodiacetyl-based hydroxamate-benzenesulfonamide conjugates. J Med Chem 2008;51:7968–79; (d) Bozdag M, Carta F, Angeli A, et al. Synthesis of N'-phenyl-N-hydroxyureas and investigation of their inhibitory activities on human carbonic anhydrases. Bioorg Chem 2018;78:1–6.
- (a) Andruh M, Cristurean E, Stefan R, Supuran CT. Carbonic anhydrase inhibitors. Part 6. Novel coordination compounds of Pd(II), Pt(II) and Ni(II) with 6-ethoxybenzothiazole-2-sulfonamide. Rev Roum Chim 1991;36:727–32; (b) Luca C, Barboiu M, Supuran CT. Carbonic anhydrase inhibitors. Part 7. Stability constants of complex inhibitors and their mechanism of action. Rev Roum Chim 1991;36:1169–73; (c) Supuran CT, Manole G, Andruh M. Carbonic anhydrase inhibitors. Part 11. Coordination compounds of heterocyclic sulfonamides with lanthanides are potent inhibitors of isozymes I and II. J Inorg Biochem 1993;49:97–103; (d) Borras J, Alzuet G, Ferrer S, Supuran CT. Metal complexes of heterocyclic sulfonamides as carbonic anhydrases inhibitors. In Supuran CT, Scozzafava A, Conway J, eds. Carbonic anhydrase – its inhibitors and activators. Boca Raton (FL), USA: CRC Press; 2004:183–207.
- (a) Sumalan SL, Casanova J, Alzuet G, et al. Synthesis and characterization of metal(II)-8-quinolinsulfonamidato (sa) complexes (M = Co, Ni, Cu, and Zn). Crystal structure of [Zn(sa)2(NH3)]NH3 complex. Carbonic anhydrase inhibitory properties. J Inorg Biochem 1996;62:31–9; (b) Borràs J, Casanova J, Cristea T, et al. Complexes with biologically active ligands. Part 6 Ni(II) coordination compounds of hydrazine and heterocyclic sulfonamides as inhibitors of the zinc enzyme carbonic anhydrase. Met Based Drugs 1996;3:143–48.
- (a) Diaz JR, Fernández Baldo M, Echeverría G, et al. A substituted sulfonamide and its Co (II), Cu (II), and Zn (II) complexes as potential antifungal agents. J Enzyme Inhib Med Chem 2016;31:51–62; (b) Ilies MA, Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors. Part 91. Metal complexes of heterocyclic sulfonamides as potential pharmacological agents in the treatment of gastric acid secretion imbalances. Met Based Drugs 2000;7:57–62; (c) Mastrolorenzo A, Supuran CT. Antifungal activity of Ag(I) and Zn(II) complexes of sulfacetamide derivatives. Met Based Drugs 2000;7:49–54; (d) Nakai M, Pan J, Lin KS, et al. Evaluation of 99mTc-sulfonamide and sulfocoumarin derivatives for imaging carbonic anhydrase IX expression. J Inorg Biochem 2018;185:63–70.
- (a) Supuran CT, Scozzafava A, Menabuoni L, et al. Carbonic anhydrase inhibitors part 72 synthesis and antiglaucoma properties of metal complexes of p-fluorobenzolamide. Met Based Drugs 1999;6:67–73; (b) Supuran CT, Scozzafava A, Briganti F, et al. Carbonic anhydrase inhibitors. Part 55 metal complexes of 1,3,4-thiadiazole-2-sulfonamide derivatives: in vitro inhibition studies with carbonic anhydrase isozymes I, II and IV. Met Based Drugs 1998;5:103–14; (c) Supuran CT, Scozzafava A, Jitianu A. Carbonic anhydrase inhibitors. Part 54: metal complexes of heterocyclic sulfonamides: a new class of antiglaucoma agents. Met Based Drugs 1997;4:307–15.
- (a) Mincione G, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Part 46 inhibition of carbonic anhydrase isozymes i, ii and iv with trifluoromethylsulfonamide derivatives and their zinc(ii) and copper(ii) complexes. Met Based Drugs 1997;4:27–34; (b) Scozzafava A, Supuran CT. Complexes with biologically active ligands. part 10 inhibition of carbonic anhydrase isozymes i and ii with metal complexes of imidazo[2,1-b ]-1,3,4-thiadiazole-2-sulfonamide. Met Based Drugs 1997;4:19–26; (c) Jitianu A, Ilies MA, Briganti F, et al. Complexes with biologically active ligands. Part 9 metal complexes of 5-benzoylamino- and 5-(3-nitrobenzoyl-amino)-1,3,4-thiadiazole-2-sulfonamide as carbonic anhydrase inhibitors. Met Based Drugs 1997;4:1–7.
- (a) Supuran CT. Complexes with biologically active ligands. Part 4. Coordination compounds of chlorothiazide with transition metal ions behave as strong carbonic anhydrase inhibitors. Met Based Drugs 1996;3:79–83; (b) Supuran CT. Metal complexes of 1,3,4-thiadiazole-2,5-disulfonamide are strong dual carbonic anhydrase inhibitors, although the ligand possesses very weak such properties. Met Based Drugs 1995;2:331–36; (c) Supuran CT. Thienothiopyransulfonamides as complexing agents for the preparation of dual carbonic anhydrase inhibitors. Met Based Drugs 1995;2:327–30.
- (a) Can D, Spingler B, Schmutz P, et al. [(Cp-R)M(CO)3] (M = Re or 99mTc) Arylsulfonamide, arylsulfamide, and arylsulfamate conjugates for selective targeting of human carbonic anhydrase IX. Angew Chem Int Ed Engl 2012;51:3354–57; (b) Dilworth JR, Pascu SI, Waghorn PA, et al. Synthesis of sulfonamide conjugates of Cu(II), Ga(III), In(III), Re(V) and Zn(II) complexes: carbonic anhydrase inhibition studies and cellular imaging investigations. Dalton Trans 2015;44:4859–73.
- Biancalana L, Batchelor LK, Ciancaleoni G, et al. Versatile coordination of acetazolamide to ruthenium(II) p-cymene complexes and preliminary cytotoxicity studies. Dalton Trans 2018;47:9367–84.
- Heldt J-M, Fischer-Durand N, Salmain M, et al. Preparation and characterization of poly(amidoamine) dendrimers functionalized with a rhenium carbonyl complex and PEG as new IR probes for carbonyl metallo immunoassay. J Organomet Chem 2004;689:4775–82.
- Hromadová M, Salmain M, Sokolová R, et al. Novel redox label for proteins: electron transfer properties of (η5-cyclopentadienyl) tricarbonyl manganese bound to bovine serum albumin. J Organomet Chem 2003;668:17–24.
- Winum JY, Dogné JM, Casini A, et al. Carbonic anhydrase inhibitors: synthesis and inhibition of cytosolic/membrane-associated carbonic anhydrase isozymes i, ii, and ix with sulfonamides incorporating hydrazino moieties. J Med Chem 2005;48:2121–5.
- (a) Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J Enzyme Inhib Med Chem 2013;28:229–30; (b) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist?. J Enzyme Inhib Med Chem 2016;31:345–60; (c) Alterio V, Di Fiore A, D'Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms?. Chem Rev 2012;112:4421–28.
- Soydan E, Güler A, Bıyık S, et al. Carbonic anhydrase from Apis mellifera: purification and inhibition by pesticides. J Enzyme Inhib Med Chem 2017;32:47–50.
- Maguene GM, Jakhlal J, Ladyman M, et al. Synthesis and antimycobacterial activity of a series of ferrocenyl derivatives. Eur J Med Chem 2011;46:31–8.
- Tirkey V, Mishra S, Dash HR, et al. Synthesis, characterization and antibacterial studies of ferrocenyl and cymantrenyl hydrazone compounds. J Organomet Chem 2013;732:122–9.
- Toro P, Suazo C, Acuña A, et al. Cyrhetrenylaniline and new organometallic phenylimines derived from 4- and 5-nitrothiophene: synthesis, characterization, X-Ray structures, electrochemistry and in vitro anti-T. brucei activity. J Organomet Chem 2018;862:13–21.
- Muñoz-Osses M, Godoy F, Fierro A, et al. New organometallic imines of rhenium(i) as potential ligands of GSK-3β: synthesis, characterization and biological studies. Dalt Trans 2018;47:1233–42.
- Oyarzo J, Acuña A, Klahn H, et al. Isomeric and hybrid ferrocenyl/cyrhetrenyl aldimines: a new family of multifunctional compounds. Dalt Trans 2018;47:1635–49.
- Arancibia R, Dubar F, Pradines B, et al. Synthesis and antimalarial activities of rhenium bioorganometallics based on the 4-aminoquinoline structure. Bioorg Med Chem 2010;18:8085–91.
- Arancibia R, Klahn AH, Buono-Core GE, et al. Synthesis, characterization and anti-Trypanosoma cruzi evaluation of ferrocenyl and cyrhetrenyl imines derived from 5-nitrofurane. J Organomet Chem 2011;696:3238–44.
- Arancibia R, Hugo Klahn A, Buono-Core GE, et al. Organometallic Schiff bases derived from 5-nitrothiophene and 5-nitrofurane: synthesis, crystallographic, electrochemical, ESR and antiTrypanosoma cruzi studies. J Organomet Chem 2013;743:49–54.
- Concha C, Quintana C, Klahn AH, et al. Organometallic tosyl hydrazones: synthesis, characterization, crystal structures and in vitro evaluation for anti-Mycobacterium tuberculosis and antiproliferative activities. Polyhedron 2017;131:40–5.
- Casini A, Scozzafava A, Mincione F, et al. Carbonic anhydrase inhibitors: synthesis of water soluble sulfonamides incorporating a 4-sulfamoylphenylmethylthiourea scaffold, with potent intraocular pressure lowering properties. J Enzyme Inhib Med Chem 2002;17:333–3.
- Karalı N, Akdemir A, Göktaş F, et al. Novel sulfonamide-containing 2-indolinones that selectively inhibit tumor-associated alpha carbonic anhydrases. Bioorg Med Chem 2017;25:3714–8.
- Chohan ZH, Pervez H, Khan KM, Supuran CT. Organometallic-based antibacterial and antifungal compounds: transition metal complexes of 1,1′-diacetylferrocene-derived thiocarbohydrazone, carbohydrazone, thiosemicarbazone and semicarbazone. J Enzyme Inhib Med Chem 2005;20:81–9.
- Bolm C, Xiao L, Hintermann L, et al. Synthesis of chiral cyrhetrenes and their application in asymmetric catalysis. Organometallics 2004;23:2362–9.
- Laws DR, Chong D, Nash K, et al. Cymantrene radical cation family: spectral and structural characterization of the half-sandwich analogues of ferrocenium ion. J Am Chem Soc 2008;130:9859–70.
- Gómez J, Leiva N, Arancibia R, et al. Synthesis, characterization, crystal structures and computational studies on novel cyrhetrenyl hydrazones. J Organomet Chem 2016;819:129–37.
- Quintana C, Klahn AH, Artigas V, et al. Cyrhetrenyl and ferrocenyl 1,3,4-thiadiazole derivatives: synthesis, characterization, crystal structures and in vitro antitubercular activity. Inorg Chem Commun 2015;55:48–50.
- (a) Durgun M, Turkmen H, Ceruso M, Supuran CT. Synthesis of 4-sulfamoylphenyl-benzylamine derivatives with inhibitory activity against human carbonic anhydrase isoforms I, II, IX and XII. Bioorg Med Chem 2016;24:982–8;
- (b) Durgun M, Turkmen H, Ceruso M, Supuran CT. Synthesis of Schiff base derivatives of 4-(2-aminoethyl)-benzenesulfonamide with inhibitory activity against carbonic anhydrase isoforms I, II, IX and XII. Bioorg Med Chem Lett 2015;25:2377–81;
- (c) Sarikaya B, Ceruso M, Carta F, Supuran CT. Inhibition of carbonic anhydrase isoforms I, II, IX and XII with novel Schiff bases: identification of selective inhibitors for the tumor-associated isoforms over the cytosolic ones. Bioorg Med Chem 2014;22:5883–90.
- Syakaev VV, Podyachev SN, Buzykin BI, et al. NMR study of conformation and isomerization of aryl- and heteroarylaldehyde 4-tert-butylphenoxyacetylhydrazones. J Mol Struct 2006;788:55–62.
- Ershov AY, Lagoda I V, Yakimovich SI, et al. Tautomerism and conformational isomerism of mercaptoacetylhydrazones of aliphatic and aromatic aldehydes. Russ J Org Chem 2009;45:660–6.
- Patorski P, Wyrzykiewicz E, Bartkowiak G. Synthesis and conformational assignment of N-(E)-stilbenyloxymethylenecarbonyl-substituted hydrazones of acetone and o-(m- and p-) chloro- (nitro-) benzaldehydes by means of 1H and 13C NMR spectroscopy. J Spectrosc 2013;2013:12.
- Gonzaga DTG, Silva FC, da Ferreira VF, et al. Crystal structures of 1-aryl-1H- and 2-aryl-2H-1,2,3-triazolyl hydrazones. Conformational consequences of different classical hydrogen bonds. J Braz Chem Soc 2016;27:2322–33.
- Hamzi I, Barhoumi-Slimi TM, Abidi R. Synthesis, characterization, and conformational study of acylhydrazones of α,β-unsaturated aldehydes. Heteroat Chem 2016;27:139–48.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- Supuran CT, De Simone G, (eds). Carbonic anhydrases as biocatalysts: from theory to medical and industrial applications. Amsterdam: Elsevier B.V.; 2015.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- (a) Scozzafava A, Briganti F, Mincione G, et al. Carbonic anhydrase inhibitors: synthesis of water-soluble, aminoacyl/dipeptidyl sulfonamides possessing long-lasting intraocular pressure-lowering properties via the topical route. J Med Chem 1999;42:3690–3700; (b) Supuran CT, Scozzafava A, Mastrolorenzo A. Bacterial proteases: current therapeutic use and future prospects for the development of new antibiotics. Expert Opin Ther Pat 2001;11:221–59.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.