?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
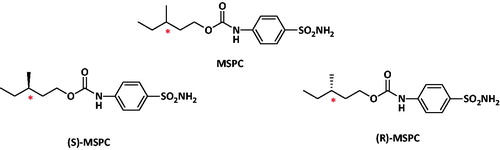
3-Methylpentyl(4-sulphamoylphenyl)carbamate (MSPC) came as the most potent compound out of a new series of carbamates composed of phenyl-ethanol or branched aliphatic alcohols, and 4-benzenesulphonamide-carbamic acid. In this study, the anticonvulsant activity and pharmacokinetics (PKs) of MSPC-two individual enantiomers were comparatively analysed in rats as well as their carbonic anhydrase (CA) inhibition. The anticonvulsant activity of MSPC enantiomers was evaluated at the rat-maximal electroshock (MES) test, and their CA inhibition evaluated. (R)-MSPC had a 29% higher clearance and consequently, a lower plasma exposure area under the curve (AUC) than (S)-MSPC and racemic-MSPC. Nevertheless, (R)-MSPC had a better brain permeability than its (S)-enantiomer with brain-to-plasma-(AUC)-ratio (BPR) of 2.07 ((R)-enantiomer), 1.85 (racemate), and 0.79 ((S)-enantiomer). As a whole body (in vivo) pharmacodynamic (PD) measure, MSPC-anticonvulsant maximal electroshock seizure (MES) activity was less enantioselective than MSPC-CA inhibition. The lack of significant differences between racemic-MSPC and its individual enantiomers suggest that their anticonvulsant activity might be due to multiple mechanisms of action.
1. Introduction
Despite the availability of more than 25 old and new antiepileptic drugs (AEDs), about 30% of the patients with epilepsy are not seizure-free with their current treatment. In addition, adverse events of existing AEDs restrict their use in certain segments of patients like women of child-bearing age. Therefore, there is an unmet clinical need to discover and develop novel chemical entities with a potential to become new effective and safer AEDsCitation1–3.
As part of the attempts to develop new AEDs, a novel class of carbamates composed of phenyl-ethanol or branched aliphatic alcohols with 6–9 carbons and 4-benzenesulphonamide-carbamic acid was recently designed and evaluated for their anticonvulsant activity and carbonic anhydrase (CA) inhibitionCitation4.
Out of the ten synthesised new carbamates the compound 3-methylpentyl(4-sulphamoylphenyl)carbamate (MSPC) came as the most potent compound with rat-MES-ED50 values of 13 mg/kg (i.p.) and 28 mg/kg (po)Citation4. MSPC is a chiral compound with one stereogenic centre and thus, racemic-MSPC is composed of two individual enantiomers depicted in . Enantiomers possess the same chemical formula, but differ in their three-dimensional arrangement around the stereogenic carbon and exist as two non-super imposable fromsCitation5,Citation6.
The issue of drug chirality is a major theme in the design, discovery, and development of new active pharmaceutical entities due to the understanding of the role of stereospecificity and molecular recognition in drugs activity. Thus, most of the new chiral drugs reaching the market are single enantiomers, rather than racemic mixturesCitation7. Enantiomers are often readily distinguished by biological systems and may have different pharmacokinetic (PK) or pharmacodynamic (PD) properties in their wanted (designated) indication or unwanted effectsCitation5,Citation6.
In this study, we assessed the effect of enantioselectivity and stereospecificity of the two enantiomers of MSPC, a new molecule containing carbamate and sulphonamide, two moieties incorporated in the olds AEDs (e.g. acetazolamide felbamate and zonisamide) as well as new AEDs in the pipeline (e.g. cenobamate and padsevonil)Citation2,Citation3. The experience learnt from felbamate and carisbamate coupled with the results of the currently ongoing clinical studies with cenobamate and padsevonil emphasises the desire to develop new AEDs containing sulphamoylphenyl and/or carbamate moieties in their chemical structure. Thus, in this study, we synthesised and comparatively analysed the anticonvulsant activity of MSPC two individual enantiomers as well as their CA inhibition and PK in rats.
2. Materials and methods
2.1. Chemistry
All the solvents were of analytical grade or high-performance liquid chromatography (HPLC) grade and were purchased from Sigma-Aldrich, St. Louis, MO. The synthesis and chemical structures of racemic-MSPC was previously describedCitation4.
2.2. General procedure for the synthesis of (S)- or (R)-MSPC
A solution of (S)-methylvaleric acid (for the synthesis of (S)-MSPC) or (R)-methylvaleric acid (for the synthesis of (R)-MSPC) (1 equiv) in dry tetrahydrofuran (THF) (10 ml) was added dropwise to a stirred solution of lithium aluminium hydride (1.2 equiv) in dry THF (20 ml). The reaction mixture was allowed to stir for 2 h and then quenched with water and extracted with ethyl ether (3 × 30 ml). The organic layer was dried over sodium sulphate, filtered, and evaporated to yield 80% oily product. The coupling product was conjugated with sulfanilamide according to a previously published methodCitation4. MSPC enantiomers and their purity were assessed by 1H NMR, HPLC and elemental analysis and the results obtained were as follows.
2.2.1 (R)- or (S)-3-methylpentyl(4-sulphamoylphenyl)carbamate (MSPC)
1H NMR (300 MHz, DMSO-d6) δ 10.00 (s, 1H), 7.70 (d, J = 8.8 Hz, 2H), 7.59 (d, J = 8.7 Hz, 2H), 7.20 (s, 1H), 4.18–4.07 (m, 2H), 1.73–1.56 (m, 1H), 1.11–1.21 (m, 4H), and 0.85 (m, 6H). Calculated for C13H20N2O4S: C, 51.98; H, 6.71; N, 9.33; S, 10.68. Found: C, 52.12; H, 6.54; N, 9.35; S, 10.77.
2.3. Pharmacokinetics studies
The pharmacokinetics (PK) of racemic-MSPC and its two individual enantiomers were studied following i.p. (80 mg/kg) to rats and their major PK parameters were estimated. MSPC has low water solubility, and consequently, it was administered to rats in multisol of propylene glycol, alcohol, and water for injection at a ratio of 8:1:1.
The study details were as described previouslyCitation8 and plasma and brain levels of racemic-MSPC and it individual enantiomers were monitored at: 20, 40, 60, 90, 120, 160, 180, 200, 220, 260, 320, and 360 min after dosing.
2.4. Analysis of MSPC and its two individual enantiomers in plasma and brain
Plasma and brain concentrations of each compound were quantified by an HPLC assay. The HPLC analysis was performed on a system (2695 Separation Module; Waters, Milford, MA) with a photodiode array UV detector (2996 PDA Detector; Waters, Milford, MA). Conditioned as follows: Kinetex, 5 u EVO C18 100 A, 150*4.6 mm column (Phenomenex®, Torrance, CA). Linear gradients (5–95% acetonitrile content) with H2O (0.1% formic acid) and acetonitrile were used as the eluents with flow rate of 1 ml/min at 20 °C. The compounds and the internal standard were detected at 250 nm. Plasma and brain concentrations of MSPC and its two individual enantiomers were quantified by assay that its extraction method was previously describedCitation8.
2.5. Analysis of MSPC in urine
MSPC urine concentrations were quantified by the same HPLC assay as its plasma assay.
2.6. Calculation of pharmacokinetic (PK) parameters
The PK parameters of each compound were calculated by non-compartmental analysis based on statistical moment theory using the PK software Phoenix Winnonlin Tripos L.P. (Pharsight Co., Mountain View, CA) as previously describedCitation9. MSPC amount excreted unchanged in the urine was calculated by multiplying its urine concertino by the excreted urine volume for up to 6 h after dosing.
2.7. Anticonvulsant activity of MSPC and its individual enantiomers
The experiments at the MES model were done in male Sprague-Dawley rats (8 rats per dose) weighing 100−120 g (Charles River Laboratories, Wilmington, MA) as previously describedCitation9–11.
2.8. Carbonic anhydrase inhibition of MSPC and its individual enantiomers
The CA inhibition of MSPC and its individual enantiomers were assessed by a stopped flow CO2 hydrase assay as previously describedCitation12–15.
3. Results
The synthesis of (R)- and (S)-MSPC enantiomers was as follows:
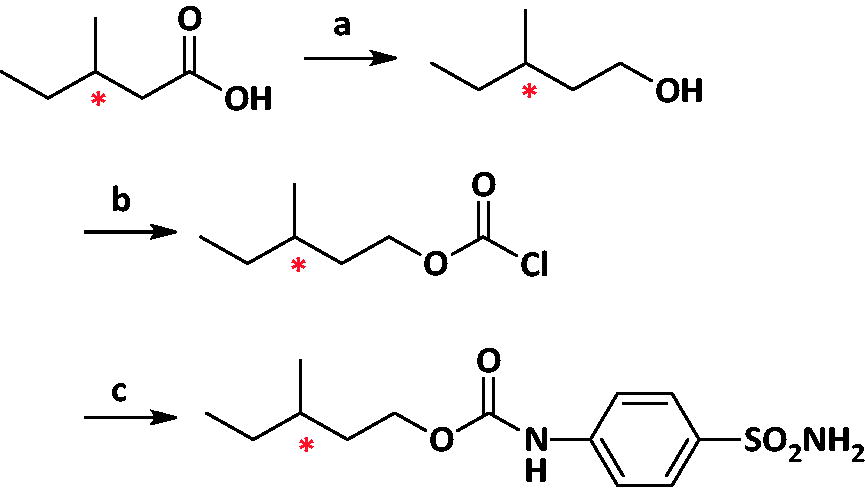
Conditions and reagents: (a) Lithium aluminium hydride, Ethyl ether, 0 °C 4 h (b) pyridine, triphosgene, DCM, RT, 2 h; (c) 4-aminobenzenesulphonamide, THF, RT 12 h.
The major PK parameters of MSPC (racemate) and its individual enantiomers are depicted in and . The mean MSPC plasma and brain concentration versus time plots are presented in and , respectively. Less than 0.1% of MSCP dose was excreted unchanged in urine.
Figure 2. MSPC, (S)-MSPC, and (R)-MSPC plasma concentrations after i.p. (80 mg/kg) administration to rats.
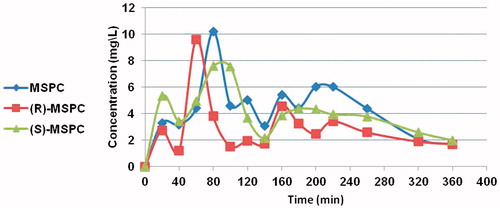
Figure 3. MSPC, (S)-MSPC, and (R)-MSPC brain concentrations after i.p. (80 mg/kg) administration to rats.
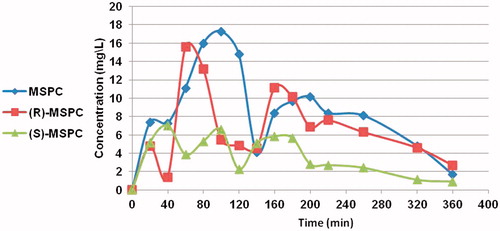
Table 1. Mean PK parameters of the MPSC (racemate) and its two individual enantiomers calculated from plasma levels following i.p. (80 mg/kg dose) administration to rats.
Table 2. Mean PK parameters of the MPSC (racemate) and its two individual enantiomers calculated from brain levels following i.p. (80 mg/kg dose) administration to rats.
The brain-to-plasma-(AUC)-ratio (BPR) of MSPC and its two individual enantiomers were as follows:
The rat-MES (po)-ED50 values (and their 95% confidence interval - 95% CI) of racemic-, (S)- and (R)-MSPC were: 28 mg/kg (18–25 mg/kg), 24 mg/kg (17–29 mg/kg), and 30 mg/kg (22–37 mg/kg), respectively.
shows the inhibition constant (Ki) of racemic-MSPC and its two individual enantiomers against four human carbonic anhydrase (hCA) isoforms. The compounds’ potency varied from nanomolar to hundreds of nanomolars, depending on the hCA isoform. MSPC was not a potent inhibitor of CAs, CAs IV, and VII, but inhibited of CAs I and II. (R)-MSPC was 3.8 and 6.1 a more effective inhibitor than (S)-MSPC, against hCAs I and II, respectively.
Table 3. Inhibition data of human CA isoforms hCA I, II, IV, and VII with MPSC as racemate and single enantiomers and the standard sulphonamide inhibitor acetazolamide (AAZ).
4. Discussion
Among our current antiepileptic armamentarium as well as among the AEDs in development there are drugs containing carbamate or sulphonamide moieties in their chemical structure. Felbamate is a carbamate that exhibits a broad anticonvulsant activity and has been on the market since 1993. However, currently, it is seldom used due to the fatal aplastic anaemia and hepatotoxicity that is associated with its therapyCitation16,Citation17. Two additional carbamates, carisbamate, and cenobamate completed phase III clinical trials and their new drug application (NDA) were submitted to the Food and Drug Administration (FDA). Carisbamate regulatory application was withdrawn in 2010, due to lack of consistent efficacy across a clinically-relevant dose rangeCitation18–19. Two cenobamate well-controlled studies demonstrated statistically significant reduction in seizure frequency as well as high responder rates, including seizure freedom, in patients with uncontrolled partial seizures treated with cenobamate for up to 18 weeksCitation18–22. Due to three reported cases of drug reaction with eosinophilia and systemic symptoms (DRESS) in patients exposed to cenobamate, a large Phase III open-label study took place to evaluate the long-term safety of cenobamate when using a lower starting dose (<100 mg) and slower titration rate in order to mitigate the serious coetaneous reactions (e.g. DRESS)Citation22. Subsequently, cenobamate-NDA submission was accepted by the FDA on 4/2/2019 and its Prescription Drug User Fee Act (PDUFA) date is set for 21/11/2019Citation23.
The human central nervous system (CNS) is among the tissues/organs having the highest number of CA isoforms, among which CA I, II, III, IV, VA, VII, XII, and XIVCitation24. Given the multitude of CA isoforms in the CNS, their inhibition was exploited already in the 1970s as a source for new AEDs. The mechanisms by which CA isoforms possess antiepileptic activity is rather complex and there is no definitive consensus among researchers about this issueCitation24.
Thus, CA inhibitors (e.g. acetazolamide and zonisamide) are among the AEDs in our current therapeutic arsenal. Acetazolamide (1953) and zonisamide (Japan-1989; USA-2000) are marketed AEDs containing sulphonamide in their chemical structureCitation25,Citation26. Padsevonil is a new AED in development designed by UCB Pharma that contains benzenesulphonamide in its chemical structure and inhibits seizure activity via presynaptic modulation of the SV2 isoforms as well as postsynaptic enhancement of GABA-mediated inhibitionCitation3,Citation27.
Researchers at Sumimoto Dainippon Pharma are also currently developing new AEDs based on the scaffold of benzenesulphonamide such as 2'-fluoro-N-methyl-[1,1'-biphenyl]-2-sulphonamide and other N-alkyl-[1,1'-biphenyl]-2-sulphonamide derivativesCitation28,Citation29. In addition, a few groups designed and evaluated substituted 4-aminobenzene sulphonamides, relying on Lipinski rule or hCA I inhibitors as potential novels AEDsCitation30,Citation31.
MSPC was the most potent compound of a series of sulphamoylphenyl or alkyl carbamate deraivtivesCitation4. As a chiral molecule, MSPC has to be developed as a single individual enantiomerCitation7,Citation32,Citation33. The current stereoselective PK and PD analysis showed that in spite of its lower (78%) plasma exposure (compared to (S)-MSPC), (R)-MSPC had a 2.6 higher BPR than its enantiomer. Nevertheless, the rat-MES-ED50 values of MSPC and its individual enantiomers were similar, ranging from 24 to 30 mg/kg. Thus, both MSPC-two individual enantiomers might be candidates for further evaluation as potential new AEDs.
This research also demonstrated that a whole body (in vivo) PD measure such as MSPC-anticonvulsant activity (MES) was less enantioselective than MSPC-specific PD measures CA inhibition or its primary PK parameter clearance that was mainly metabolic. (R)-MSPC better BPR might be also due to the fact that it is a more potent inhibitor (compared to the (S)-enantiomer) of the brain-abundant CA isoforms CA I and CA II. The lack of significant differences between MSPC and its individual stereoisomers suggest that their anticonvulsant (MES) activity might be due to multiple mechanisms of action.
Acknowledgements
This work is abstracted from the Ph.D. thesis of David Bibi in partial fulfillment of the Ph.D. degree requirements for The Hebrew University of Jerusalem. The authors thank Drs. John Keane, Brian Klein, and Shamsi Raeissi, of the NIH-NINDS Epilepsy Therapy Screening Program (ETSP) for testing the compounds in the ETSP.
Disclosure statement
David Bibi, Bella Shusterman, Alessio Nocentini and Claudiu T. Supuran have no conflict of interest to disclose. Meir Bialer has received in the last three years speakers or consultancy fees from Alkaloid, Bial, Boehringer Inglheim, Upsher-Smith US WorldMeds and Vidac Pharma. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov 2010;9:68–83.
- Bialer M, Johannessen SI, Koepp MJ, et al. Progress report on new antiepileptic drugs: a summary of the Fourteenth Eilat Conference on new antiepileptic drugs and devices (EILAT XIV). I. Drugs in preclinical and early clinical development. Epilepsia 2018;59:1811–41.
- Bialer M, Johannessen SI, Koepp MJ, et al. Progress report on new antiepileptic drugs: a summary of the fourteenth Eilat conference on new antiepileptic drugs and devices (EILAT XIV). II. Drugs in more advanced clinical development. Epilepsia 2018;59:1842–66.
- Bibi D, Mawasi H, Nocentini A, et al. Design and comparative evaluation of the anticonvulsant profile, carbonic-anhydrate inhibition and teratogenicity of novel carbamate derivatives of branched aliphatic carboxylic acids with 4-aminobenzensulfonamide. Neurochem Res 2017;42:1972–82.
- Brocks DR. Drug disposition in three dimensions: an update on stereoselectivity in pharmacokinetics. Biopharm Drug Disposit 2006;27:387–406.
- Levy RH, Boddy AV. Stereoselectivity in pharmacokinetics: a general theory. Pharm Res 1991;8:551–6.
- Agranat I, Caner H, Caldwell J. Putting chirality to work: the strategy of chiral switches. Nat Rev Drug Discov 2002;1:753–68.
- Mawasi H, Bibi D, Shekh-Ahmad T, et al. Pharmacokinetic-pharmacodynamic correlation and brain penetration of sec-butylpropylacetamide, a new CNS drug possessing unique activity against status epilepticus. Mol Pharm 2016;13:2492–6.
- Hen N, Shekh-Ahmad T, Yagen B, et al. Stereoselective pharmacodynamic and pharmacokinetic analysis of sec-butylpropylacetamide (SPD), a new CNS-active derivative of valproic acid with unique activity against status epilepticus. J Med Chem 2013;56:6467–77.
- Shekh-Ahmad T, Hen N, Yagen B, et al. Stereoselective anticonvulsant and pharmacokinetic analysis of valnoctamide, a CNS-active derivative of valproic acid with low teratogenic potential. Epilepsia 2014;55:353–61.
- Shekh-Ahmad T, Mawasi H, McDonough JH, et al. The potential of valnoctamide and sec-butylpropylacetamide (SPD) for acute seizures and status epilepticus (SPD) and valnoctamide and their individual stereoisomers in status epilepticus. Epilepsy Behav 2015;49:298–302.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- (a) Casini A, Antel J, Abbate F, et al. Carbonic anhydrase inhibitors: SAR and X-ray crystallographic study for the interaction of sugar sulfamates/sulfamides with isozymes I, II and IV. Bioorg Med Chem Lett 2003;13:841–5. (b) Supuran CT. Carbonic anhydrase inhibitors in the treatment and prophylaxis of obesity. Expert Opin Ther Pat 2003;13:1545–50. (c) Briganti F, Pierattelli R, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Part 37. Novel classes of isozyme I and II inhibitors and their mechanism of action. Kinetic and spectroscopic investigations on native and cobalt-substituted enzymes. Eur J Med Chem 1996;31:1001–10.
- De Simone G, Di Fiore A, Menchise V, et al. Carbonic anhydrase inhibitors. Zonisamide is an effective inhibitor of the cytosolic isozyme II and mitochondrial isozyme V: solution and X-ray crystallographic studies. Bioorg Med Chem Lett 2005;15:2315–20.
- (a) Temperini C, Innocenti A, Scozzafava A, et al. The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example. J Med Chem 2010;53:850–4. (b) Clare BW, Supuran CT. Carbonic anhydrase activators. Part 3. Structure-activity correlations for a series of isozyme II activators. J Pharm Sci 1994;83:768–73.
- Pellock JM, Perhach JM, Sofia RD. Felbamate. In: Levy RH, Mattson RH, Meldrum BS, et al. eds. Antiepileptic drugs. 5nd ed. Philadelphia (PA): Lipincott Williams & Wilkins Co.; 2002.
- Thakkar K, Billa G, Rane J, et al. The rise and fall of felbamate as a treatment for partial epilepsy - aplastic anemia and hepatic failure to blame? Expert Rev Neurother 2015;15:1373–5.
- Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT IX). Epilepsy Res 2009;83:1–43.
- Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the tenth Eilat conference (EILAT X). Epilepsy Res 2010;92:89–124.
- Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the eleventh Eilat conference (EILAT XI). Epilepsy Res 2013;103:2–30.
- Krauss G, French J, Kamin J, et al. Seizure freedom with YKP3089 as adjunctive therapy for refractory partial-onset seizures in double-blind placebo-controlled trials. Neurology 2016;86:2–19.
- Sperling MR, Klein P, Kamin M. Safety of cenobamate (YKP3089) as adjunctive treatment for uncontrolled partial (focal) seizures: results from a large, phase 3, multicenter, open-label study. AES Meeting; 2018 Nov 30–Dec 4; New Orleans (LA).
- SK Life Sciences Press Release on February, 4th, 2019.
- (a) Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin Ther Patent 2018;28:713–21. (b) Winum JY, Temperini C, El Cheikh K, et al. Carbonic anhydrase inhibitors: clash with Ala65 as a means for designing inhibitors with low affinity for the ubiquitous isozyme II, exemplified by the crystal structure of the topiramate sulfamide analogue. J Med Chem 2006;49:7024–31.
- Resor SR, Resor LD, Woodbury DM, Kemp JW. Acetazolamide. In: Levy RH, Mattson RH, Meldrum B, et al., eds.. Antiepileptic drugs. 4th ed. Winnipeg, Canada: Raven Press Co.; 1995.
- Kwan SY, Chuang YC, Huang CW, et al. Zonisamide: review of recent clinical evidence for treatment of epilepsy. CNS Neurosci Ther 2015;21:683–91.
- Mula M. Emerging drugs for focal epilepsy. Expert Opin Emerg Drugs 2018;23:243–9.
- Tanaka T, Yajima N, Kiyoshi T, et al. N-alkyl-[1,1'-biphenyl]-2-sulfonamide derivatives as novel broad spectrum anti-epileptic drugs with efficacy equivalent to that of sodium valproate. Biorg Med Chem Lett 2017;27:4118–21.
- Tanaka T, Yajima N, Kiyoshi T, et al. Identification of 2-(2'-fluoro-[1,1'-biphenyl]-2-yl)acetamide as a sodium valproate-like broad spectrum anti-epileptic drug candidate. Bioorg Med Chem Lett 2019;29:138–42.
- Sethl KK, Nayak PN, Sarker H, et al. A rational approach toward the development of human carbonic anhydrase inhibitors as antiepileptic agents. Med Chem 2016;6:405–10.
- Ajeet A, Kumar A, Mishra AK. Design, synthesis and pharmacological evaluation of sulfonamide derivatives screened against maximal electroshock seizure test. Mol Biol 2018;7:206–15.
- Agranat I, Wainschtein SR. The strategy of enantiomer patents of drugs. Drug Discov Today 2010;15:163–70.
- Caner H, Groner E, Levy L, Agranat I. Trends in the drug development of chiral drugs. Drug Discov Today 2004;9:105–10.