Abstract
Chalcones are valuable structures for drug discovery due to their broad bioactivity spectrum. In this study, we evaluated 20 synthetic chalcones against estrogen-receptor-positive breast cancer cells (MCF-7 line) and triple-negative breast cancer (TNBC) cells (MDA-MB-231 line). Antiproliferative screening by MTT assay resulted in two most active compounds: 2-fluoro-4’-aminochalcone (11) and 3-pyridyl-4’-aminochalcone (17). Their IC50 values ranged from 13.2 to 34.7 µM against both cell lines. Selected chalcones are weak basic compounds and maintained their antiproliferative activity under acidosis conditions (pH 6.7), indicating their resistance to ion-trapping effect. The mode of breast cancer cells death was investigated and chalcones 11 and 17 were able to induce apoptosis rather than necrosis in both lines. Antiproliferative target investigations with MCF-7 cells suggested 11 and 17 upregulated p53 protein expression and did not affect Sp1 protein expression. Future studies on chalcones 11 and 17 can define their in vivo therapeutic potential.
Introduction
Breast cancer is the most common type of cancer that affects women around the world, corresponding to 25% of cases. It is also the main cause of cancer death among womenCitation1–3. Its classification is based on the presence of cellular receptors: (i) Hormone-Receptor (HR), with Estrogen (ER) and/or Progesterone-Receptors (PR); (ii) Human Epidermal Growth Factor-2 receptor (HER2); and (iii) Triple-Negative Breast Cancer (TNBC), which does not express ER, PR, or HER2 receptorsCitation4–6. Chemotherapy choices for breast cancer are made according to its classification aiming to reach specific targets. ER-positive cancer treatments include ER modulators, such as tamoxifen or aromatase inhibitors. HER2-positive cancer is treated with monoclonal antibodies, such as trastuzumab, which is administered alongside tyrosine-kinase inhibitorsCitation7–9. TNBC cancer treatment is focused on cytotoxic agents, such as taxanes or doxorubicin. This cancer has poor response to chemotherapy, particularly at metastatic sites, with survival rates below 2 years. Altogether, TNBC cancer has been considered the most severe and difficult to treat due to a lack of targeted therapyCitation10–12.
Chalcones are valuable structures for drug discovery due to their broad bioactivity spectrum, as well as their versatile and simple synthesis. Their structures, bearing two benzene rings (A and B), are linked by an enone bridge. They have demonstrated antiproliferative activity against cancer cells, including breast cancerCitation13–15. Mai et al. described chalcone was effective against ER-positive breast cancer cells (MCF-7 line) targeting 20 apoptotic markersCitation16. Iftikhar et al. reported that chalcone bearing chlorine at position 2 was effective against breast cancer cells (CAL-51 line) and induced accumulation of p53 proteinCitation17. Silva and coauthors described unsubstituted chalcone increased p53 protein activity in osteosarcoma cells (U2OS line) through the induction of heat shock protein DNAJB1Citation18.
In our ongoing search for anticancer compounds with structures based on chalcone framework, we evaluated the antiproliferative activity of 20 chalcones against ER-positive cells (MCF-7 line) and TNBC (MDA-MB-231 line). The two most active chalcones (11 and 17) were selected to antiproliferative evaluation under acidosis conditions (pH 6.7) and pro-apoptotic activity in MCF-7 and MDA-MB-231 lines. In addition, we investigated tumour molecular targets of 11 and 17 in MCF-7 line, which were able to upregulate p53 protein expression.
Materials and methods
Chemical procedure for synthesis of chalcones 1–20
Reagents and solvents were purchased from Merck® (Kenilworth, NJ). Series of 20 chalcones was synthesised by Claisen–Schmidt aldol condensation reaction, according to protocol described by Santos and coauthors, with minor modificationsCitation19,Citation20. Reactions were carried out at room temperature using 3.0 mmol of 4'-aminoacetophenone and 3.0 mmol of aldehydes, which were dissolved in ethanol (30 mL). Sodium hydroxide in ethanol (1.0 mol/L) was added as catalyst solution. Reagents conversion was monitored using thin layer chromatography. Crude product was poured onto ice (from distilled and deionised water) and filtered. All compounds were purified over silica gel chromatography column eluted with mixture of hexane and ethyl acetate (3:2). Melting points were determined in TecnoponPFM-II® apparatus (MS Tecnopon Instrumentação, Piracicaba, Brazil) and were uncorrected. Structure of compounds was confirmed by 1H and 13C nuclear magnetic resonance (NMR) spectra analyses. Spectral data were obtained in Bruker Avance III® (14 Tesla, 600 MHz) equipment (Bruker Corporation, Billerica, MA) using deuterated dimethyl sulfoxide (DMSO-d6) as solvent. Chalcones had their UV–vis spectra and chromatograms obtained in High Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD) Agilent Technologies® 1220 Infinity equipment (Agilent Technologies, Palo Alto, CA) coupled with a photodiode array system (1260-Infinity®) and Agilent Zorbax Eclipse Plus C-18® column (250 mm × 4.6 mm, 5 μm), using methanol:water (3:1) as mobile phase (1.0 mL/min).
Antiproliferative activity of chalcones 1–20
Human breast cancer cell lines MCF-7 (HTB-22) and MDA-MB-231 (HTB-26) were purchased from American Type Culture Collection (ATCC). Both cell lines were cultured in DMEM (Gibco®, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS-LGC®), penicillin-streptomycin (100 μg/mL, Merck®, Kenilworth, NJ). Both cell lines were incubated at 37 °C under humidified atmosphere with 5% CO2 (Thermo Fischer Scientific® Incubator, Waltham, MA).
Antiproliferative activity of chalcones 1–20 was evaluated by MTT assayCitation21–23. Cells were seeded in 96-well plate, with an initial cell density of 1 × 104 and 2.5 × 104 cells/well of MCF-7 and MDA-MB-23, respectively. Cells were cultured with DMEM supplemented with 10% FBS and compounds at 20 μM for 48 h at 37 °C under 5% CO2 humidified atmosphere. Cells were incubated with MTT (Sigma Aldrich®, St. Louis, MO) solution (1 mg/mL) for 40 min. Formazan crystals were solubilised in dimethylsulfoxide (DMSO, Sigma Aldrich®, St. Louis, MO), and absorbance rates were measured at 562 nm in ThermoPlate® TP Reader. Chalcones 1, 6, 8, 9, 11, 17, 19, and 20 had the highest antiproliferative activity and were assayed against both cell lines at seven concentrations, ranging from 1.25 to 80 μM for IC50 values determination.
Antiproliferative activity of selected chalcones 11 and 17 under acidosis
Cell viability was evaluated under acidosis conditions by MTT assay, using 4-morpholine-ethanesulfonic acid to produce a pH value of 6.7Citation24,Citation25. Cells were treated with selected chalcones 11 and 17 at their respective IC50 values. All procedures were performed in triplicate and three independent experiments.
Pro-apoptotic activity of selected chalcones 11 and 17
Pro-apoptotic activity of selected chalcones 11 and 17 was evaluated using FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen®, BD Biosciences, Franklin Lakes, NJ) by flow cytometryCitation26. MCF-7 and MDA-MB-231 cells were seeded and cultivated in 6-well plates (6.5 × 105 cells/well), for 24 h, and treated with 40 μM selected chalcones in DMEM containing 10% FBS for 48 h. As negative control 0.1% DMSO was used. Adherent and floating cells were collected, submitted to centrifugation, and washed with PBS (Sigma Aldrich®, St. Louis, MO). Apoptotic cells were determined by flow cytometry in BD FACSCalibur® Flow Cytometer (BD Biosciences® Franklin Lakes, NJ), after double staining (Annexin V/propidium iodide) using FITC Annexin V Apoptosis Detection kit I (BD Pharmigen®, BD Biosciences, Franklin Lakes, NJ) according to instructions of the manufacturer. All procedures were performed in triplicate and in three independent experiments.
Effect of selected chalcones 11 and 17 on Sp1 and p53 proteins expressions
Protein expression was assessed using western blot assay. Breast cancer cells (MCF-7) were seeded in 6-well plates at 7.5 × 105 cells/well, treated with selected chalcones 11 and 17 at 10 and 20 μM or with negative control (0.1% DMSO) for 24 hCitation26. Cells were suspended in PBS solution, collected in Radioimmunoprecipitation Assay buffer – (RIPA buffer at 4 °C), supplemented with proteinase inhibitors, sonicated for lysis, and submitted to centrifugation (14,000 rpm, 4 °C, 20 min, Eppendorf®, Hamburg, Germany). Protein total concentration was obtained using Pierce™ BCA Protein Assay reagent (Thermo Scientific®, Waltham, MA). Total proteins (30 μg) in sodium dodecyl sulphate (SDS) buffer were warmed at 95 °C, cooled at 4 °C and submitted to sodium dodecyl sulphate–polyacrylamide gel electrophoresis SDS-PAGE (12%, 100 V, 360 mA) for 90 min at room temperature, and were transferred to nitrocellulose membrane (PALL Corporation®, Port Washington, NY). Membranes were blocked using TBST buffer (25 mM Tris, 3 mM KCl, 0.14 M NaCl, 0.05% Tween 20) containing 5% nonfat milk at room temperature for 1 h, followed by overnight incubation at 4 °C, with primary antibodies to Sp1 (MilliPore®, Burlington, MA), p53, and β-actin (Santa Cruz Biotechnology®, Dallas, TX). Subsequently, membranes were washed with TBST (3×) and incubated with peroxidase-conjugated secondary antibodies diluted in TBST buffer-5% nonfat milk (1:5000) at room temperature and washed several times with TBST buffer. Proteins were detected through chemiluminescence using Enhanced Chemiluminescent (ECL®) western blotting detection reagent (Amersham Biosciences®, Little Chalfont, UK) in LAS 500 luminescence analyser (GE Healthcare®, Chicago, IL)Citation26. Bands intensity in image was quantified using ImageJ® software (NIH Image J system, Bethesda, MD).
Statistical analysis
Statistical analyses were carried out using one-way ANOVA multiple comparison tests followed by Tukey’s HSD testCitation19,Citation27. Statistical significance difference was considered if p< 0.05.
Results and discussion
Chemistry
Chalcones 1–20 were synthesised by Claisen–Schmidt reaction with yields of 30–93% after chromatography purification. Series of compounds has chalcones with amino at position 4’ (ring A) and benzene ring B substituted by electron-withdrawing (EWG) and electron-donating groups (EDG). Also, aryl analogues with benzene ring B replaced for furan, thiophene, pyridine, and naphthyl rings were synthesised (Scheme 1).
All NMR parameters, including hydrogen and carbon chemical shifts (δH and δC in ppm), integrations, multiplicities, and coupling constants (J in Hz), corresponded to proposed structures of chalcones 1–20. Two main signals in 1H NMR spectra were diagnosed: (i) a pair of doublets with J ranging from 15 to 16 Hz (7.43–8.10 ppm), attributed to methyne hydrogens of trans carbon–carbon double bonds and (ii) broad singlets between 6.18 and 6.27 ppm related to geminal hydrogens of amino group. In 13C NMR spectra, signals in 185.9–186.1 confirmed ketone carbonyls, which are shifted to downfield due to conjugation with carbon–carbon double bonds. UV–Vis had λmax at 298–378 nm, which were related to a pivotal chromophore of chalcone framework (conjugation between ring B and enone bridge). HPLC-DAD analyses of peak areas integrations indicated chalcones purity of 94.0–99.9%. All chromatograms and spectra are presented in supplemental data (Figures S1–S62).
Antiproliferative activity of chalcones 1–20
Preliminary antiproliferative activity of chalcones 1–20 was evaluated by MTT assay for 48 h against MCF-7 (ER) and MDA-MB-231 (TNBC) lines. Percentages of metabolically viable cells (%MVC) are summarised in . Series of compounds demonstrated active (e.g. 20) and inactive (e.g. 3) chalcones, suggesting molecular variations were relevant for antiproliferative screening against both cell lines. Experiments at 20 μM allowed us to derive preliminary structural features related to antiproliferative activityCitation28–30.
Table 1. Percentage of metabolically viable cells (%MVC) treated with chalcones 1–20 (at 20 μM) and IC50 values (in μM) of selected chalcones.
In previous studies conducted by our group, chalcone with unsubstituted rings A and B had potent antiproliferative and pro-apoptotic activitiesCitation18. However, its low solubility in water enables limited additional pharmacological assays, mainly in vivo experiments. Therefore, we designed a series of analogues with amino group at position 4’ on ring A, which had higher solubility in water compared to unsubstituted chalconeCitation16. We evaluated chalcone with amino at position 4’ and unsubstituted ring B (1), which was inactive against MCF-7 (%MVC = 97.3 ± 6.0) and active against MDA-MB-231 (%MVC = 80.6 ± 4.0). Such result encouraged us to prepare further analogues with substitutions on ring B. Mai et al. reported EWG on ring B of chalcones improved antiproliferative activity against a broad panel of cancer cellsCitation16. We assayed analogues substituted by nitro (2), trifluoromethyl (3), cyano (4), and halogens (5–12). 3-Chloro-4’-aminochalcone (9) and 2-fluoro-4’-aminochalcone (11) were the two most active compounds against MCF-7 line, with %MVC rates of 52.5 ± 6.7 and 50.3 ± 7.8, respectively. 3-Fluoro-4’-aminochalcone (8) was the most potent against MDA-MB-231 line, with %MVC of 73.3 ± 5.4. In summary, comparison among %MVC values of chalcones with EWG on ring B and 1 suggested more electronegative halogens corroborated to antiproliferative activity. 4-Methyl-4’-aminochalcone (13) and 4-methoxy-4’-aminochalcone (14) were less active than halogenated-chalcones (5–15), corroborating negative effect of EDG towards bioactivity. We hypothesised rings containing six π electrons, similarly to phenyl ring of 1, could conduce to bioactive analoguesCitation31. 2-Furyl-4’-aminochalcone (15) and 2-thiophenyl-4’-aminochalcone (16) were inactive compounds. Pyridylchalcones 17 and 18 were strongly active against MCF-7 line, with %MVC rates of 51.8 ± 2.5 and 61.3 ± 3.4, respectively. We also investigated the effect of additional benzene ring towards bioactivity, evaluating naphthylchalcone 19, and biphenyl chalcone 20, which have ten and twelve π electrons on ring B, respectively. Both chalcones presented strong antiproliferative activity against MCF-7 line, with %MVC rates of 53.4 ± 4.3 and 44.2 ± 3.3, respectively. Despite effect against both lines, MCF-7 line (ER) was more sensitive to chalcones than MDA-MB-231 line (TNBC) ().
The five most antiproliferative compounds against MCF-7 were 9, 11, 17, 19, and 20 (%MVC values lower than 60). Against MDA-MB-231, the five most potent ones were 1, 6, 8, 11, and 17 (%MVC values lower than 85). These seven compounds were selected to determine IC50 values for both cell lines (). Chalcones 1, 6, and 8 were inactive against MCF-7 (IC50 > 100 µM) and active against MDA-MB-231, with IC50 values of 60.3, 69.0, and 74.1 µM, respectively. Chalcones 19 and 20 were active against MCF-7 with IC50 values of 14.3 and 22.7 µM, respectively, and inactive against MDA-MB-231 (IC50 > 100 µM). Chalcones 11 and 17 were active against both cell lines, displaying IC50 values of 13.2–34.2 µM and were selected for additional bioassays.
Antiproliferative activity of selected chalcones 11 and 17 under acidosis
In normal cells, intracellular and extracellular pH values are 7.2 and 7.4, respectively. On the other hand, in breast tumour cells, intracellular and extracellular pH values are 7.1–7.4 and 6.5–7.1, respectivelyCitation24,Citation32. This extracellular acidosis has been related to Warburg effect, in which tumour cells are in intense anaerobic metabolism, producing and exporting acid compounds through transporters. The biological central interest in acidosis is due to clear relationship with invasiveness and metastasis potentialCitation24,Citation33. Tumour cells in acidic extracellular microenvironment can block membrane permeation of weak basic compounds, leading to ion-trapping effect. This phenomenon is caused by protonation of basic functionalities, converting neutral into cationic compounds. Cationic form exhibits reduced passive permeation through membrane phospholipids when compared to neutral form. Doxorubicin is antineoplastic and weak basic drug and has demonstrated low efficacy in acidosis microenvironment due to ion-trapping effectCitation34.
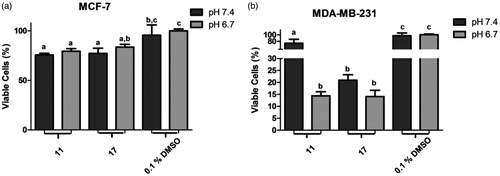
Chalcones 11 and 17 were submitted to antiproliferative assays under acid microenvironment (at pH 6.7) against both breast cancer cell lines (). In experiments with MCF-7 line, both chalcones maintained their antiproliferative effect, showing ion-trapping resistance. In MDA-MB-231 line, chalcone 17 maintained similar %MVC rate at both pH values. Interestingly, chalcone 11 was five times more active at pH 6.7 (acidosis) than at 7.4, with %MVC of 14.4 and 73.7, respectively.
Figure 1. Percentage of metabolically viable cells (%MVC) under acidosis. (a) treatments with selected chalcones 11 and 17 against MCF-7 line; (b) treatments with selected chalcones 11 and 17 against MDA-MB-231 line. Different letters in the graph indicate statistical difference with significance p< 0.05 in Tukey’s multiple comparisons test. DMSO 0.1% was used as negative control.
Pro-apoptotic activity of selected chalcones 11 and 17
Antiproliferative compounds can induce tumour cell death by several mechanismsCitation35. Among these, apoptosis and necrosis are the most common and studied ones. Apoptosis events have been induced by chalcones towards different cancer cell linesCitation36,Citation37. In order to investigate antiproliferative effect mechanism of selected chalcones 11 and 17 towards MCF-7 and MDA-MB-231 lines, Annexin V/PI staining and flow cytometry were used to detect whether cells were dying due to apoptosis or necrosis events ().
Figure 2. Pro-apoptotic activity of selected chalcones 11 and 17 towards MCF-7 (a) and MDA-MB-231 (b) cells. Different letters indicate statistical difference with significance p< 0.05 in Tukey’s multiple comparisons test. DMSO 0.1% was used as a vehicle control.
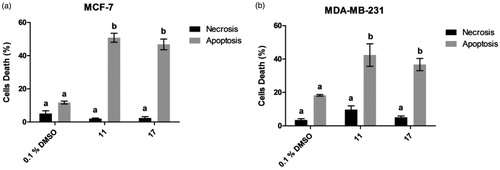
Chalcones 11 and 17 induced 50.8% and 46.1% apoptotic events in MCF-7 line, 5- and 4-fold increases compared to cell treated with 0.1% DMSO, respectively. In MDA-MB-231 line, chalcones 11 and 17 caused death through apoptosis in 42.4 and 36.3% of cells, respectively. Additionally, selected chalcones induced apoptosis rather than necrosis.
Chalcones are known to induce apoptosis in several cancer cells, being able to up regulate more than 15 pro-apoptotic markers expression, such as Bad, Bax, Bid, Bim, CD40, Fas, IGFBP-5, IGFBP-6, p21, and sTNF-R116. Hsu and Bortolloto and their respective collaborators have demonstrated pro-apoptotic activity of unsubstituted chalcone against MCF-7 breast cancer cellsCitation38,Citation39. These authors have described intrinsic apoptotic pathway induced by unsubstituted chalcone, with inhibition of Bcl-2 and induction of Bax apoptotic markers.
Effect of selected chalcones 11 and 17 on Sp1 and p53 proteins expression
MCF-7 and MDA-MB-231 lines have demonstrated wild and mutant p53 protein, respectivelyCitation40. Thus, we selected MCF-7 to conduct molecular target experiments. Western blot assay was performed to evaluate effect of chalcones 11 and 17 on p53 and Sp1 proteins expression in MCF-7 line for 24 h at 10 and 20 µM ().
Figure 3. Effect of selected chalcones 11 and 17 on Sp1 and p53 proteins expression in MCF-7 cell line.
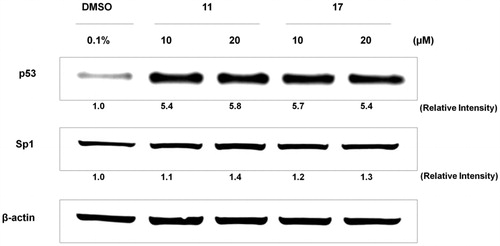
Sp1 protein is transcription factor involved in cell proliferation and differentiation. In breast cancers, it acts on invasion and metastasis processesCitation41,Citation42, and has been classified as marker for poor prognosisCitation43. Chalcones 11 and 17 were not able to modulate Sp1 protein expression, at either concentration, presenting similar effect to DMSO 0.1% (negative control).
p53 protein is tumour suppressor and its mutated status has been related to several types of cancers. Expression of p53 in breast cancer cells varies according to its classificationCitation44,Citation45. ER-positive and ER-negative cancer types have wild-type p53 and mutated protein forms, respectivelyCitation46. Activation and stabilisation of wild-type p53 induce cell-cycle arrest and cell death through apoptosis, reducing cancer progression. This cell pathway has been recognised as attractive target to simple and low-weight-molecular compounds with promising antineoplastic potential. Chalcones 11 and 17 induced 5-fold upregulation of p53 expression in MCF-7 cells (), indicating these compounds are able to activate and stabilise p53 protein expression. This result is the first evaluation of low-molecular-weight compounds against breast cancer cells. In this context, methoxychalcones and naphthylchalcones have been described as agents of p53 activation and stabilisation in prostate cancer and osteosarcoma cell lines, respectivelyCitation26,Citation47.
Conclusions
In summary, we reported activity of series of 20 chalcones against two types of breast cancer cells, ER-positive (MCF-7 line) and TNBC (MDA-MB-231 line). Preliminary investigations suggested halogens on ring B and additional benzene rings play central role in antiproliferative activity. Basic chalcones 11 and 17 were antiproliferative agents under acidosis (at pH 6.7), displaying resistance to ion-trapping effect. These compounds induced apoptosis rather than necrosis in both cells, upregulating p53 expression in ER-positive cells (MCF-7 line).
Supplemental Material
Download PDF (2.7 MB)Acknowledgements
M. B. S. thanks FAPESP for her research fellowship # 2016/00580–5. Authors acknowledge Centre of Biomolecular Innovation (FAPESP Grant # 2009/53989–4) and National Institute of Science and Technology – Biodiversity and Natural Products INCT-BioNat (Grants FAPESP # 2014/50926–0 and CNPq # 465637/2014–0).
Disclosure statement
No potential conflict of interest was reported by authors.
Additional information
Funding
References
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidem Biomar 2016;25:16–27.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012: globocan 2012. Int J Cancer 2015;136:E359–E86.
- Sledge GW, Mamounas EP, Hortobagyi GN, et al. Past, present, and future challenges in breast cancer treatment. J Clin Oncol 2014;32:1979–86.
- Tate CR, Rhodes LV, Segar HC, et al. Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat. Breast Cancer Res 2012;14:R79.
- Rakha EA, Ellis IO. Triple-negative/basal-like breast cancer: review. Pathology 2009;41:40–7.
- Eliyatkin N, Yalcin E, Zengel B, et al. Molecular classification of breast carcinoma: from traditional, old-fashioned way to a new age, and a new way. J Breast Health 2015;11:59–66.
- Goldhirsch A, Wood WC, Coates AS, Panel members, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol 2011;22:1736–47.
- Damaskos C, Valsami S, Kontos M, et al. Histone deacetylase inhibitors: an attractive therapeutic strategy against breast cancer. Anticancer Res 2017;37:35–46.
- Soheilifar MH, Taheri RA, Zolfaghari Emameh R, et al. Molecular landscape in alveolar soft part sarcoma: implications for molecular targeted therapy. Biomed Pharmacother 2018;103:889–96.
- Sulaiman A, Sulaiman B, Khouri L, et al. Both bulk and cancer stem cell subpopulations in triple-negative breast cancer are susceptible to Wnt, HDAC, and ERα coinhibition. FEBS Lett 2016;590:4606–16.
- Sulaiman A, McGarry S, Lam KM, et al. Co-inhibition of MTORC1, HDAC and ESR1α retards the growth of triple-negative breast cancer and suppresses cancer stem cells. Cell Death Dis 2018;9:815.
- Yao H, He G, Yan S, et al. Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget 2017;8:1913–24.
- Nazir S, Ansari FL, Hussain T, et al. Brine shrimp lethality assay ‘an effective prescreen’: microwave-assisted synthesis, BSL toxicity and 3DQSAR studies-based designing, docking and antitumor evaluation of potent chalcones. Pharm Biol 2013;51:1091–103.
- Reddy Kotha R, G Kulkarni R, Garige AK, et al. Synthesis and cytotoxic activity of new chalcones and their flavonol derivatives. Med Chem 2017;7:353.
- Das M, Manna K. Chalcone scaffold in anticancer armamentarium: a molecular insight. J Toxicol 2016;2016:1–14.
- Mai CW, Yaeghoobi M, Abd-Rahman N, et al. Chalcones with electron-withdrawing and electron-donating substituents: anticancer activity against TRAIL resistant cancer cells, structure–activity relationship analysis and regulation of apoptotic proteins. Eur J Med Chem 2014;77:378–87.
- Iftikhar S, Khan S, Bilal A, et al. Synthesis and evaluation of modified chalcone based P53 stabilizing agents. Bioorg Med Chem Lett 2017;27:4101–6.
- Silva G, Marins M, Chaichanasak N, et al. Trans-chalcone increases P53 activity via DNAJB1/HSP40 induction and CRM1 inhibition. PLoS One 2018;13:e0202263.
- Santos MB, Pinhanelli VC, Garcia MAR, et al. Antiproliferative and pro-apoptotic activities of 2′- and 4′-aminochalcones against tumor canine cells. Eur J Med Chem 2017;138:884–9.
- Konduri SD, Medisetty R, Liu W, et al. Mechanisms of estrogen receptor antagonism toward P53 and its implications in breast cancer therapeutic response and stem cell regulation. Proc Natl Acad Sci USA 2010;107:15081–6.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
- Silva G, Fachin A, Beleboni R, et al. In vitro action of flavonoids in the canine malignant histiocytic cell line DH82. Molecules 2013;18:15448–63.
- Faraji F, Karjoo Z, Moghaddam MV, et al. Challenges related to the immunogenicity of parenteral recombinant proteins: underlying mechanisms and new approaches to overcome it. Int Rev Immunol 2018;37:301–15.
- Gupta SC, Singh R, Pochampally R, et al. Acidosis promotes invasiveness of breast cancer cells through ROS-AKT-NFκβ pathway. Oncotarget 2014;5:12070–82.
- Sonehara N, Lacerda J, Jardim-Perassi B, et al. Melatonin regulates tumor aggressiveness under acidosis condition in breast cancer cell lines. Oncol Lett 2018;17:1635–45.
- Seba V, Silva G, Santos M, et al. Chalcone derivatives 4′-amino-1-naphthyl-chalcone (D14) and 4′-amino-4-methyl-1-naphthyl-chalcone (D15) suppress migration and invasion of osteosarcoma cells mediated by P53 regulating EMT-related genes. Int J Mol Sci 2018;19:2838.
- Thompson HW, Mera R, Prasad C. The analysis of variance (ANOVA). Nutr Neurosci 1999;2:43–55.
- Ullah A, Ansari FL, Ihsan-ul-Haq, et al. Combinatorial synthesis, lead identification, and antitumor study of a chalcone-based positional-scanning library. Chem Biodivers 2007;4:203–14.
- Jain U, Bhatia R, Rao A, et al. Design and development of halogenated chalcone derivatives as potential anticancer agents. Trop J Pharm Res 2014;13:73.
- Singh RK, Lange TS, Kim KK, et al. Isothiocyanate NB7M causes selective cytotoxicity, pro-apoptotic signalling and cell-cycle regression in ovarian cancer cells. Brit J Cancer 2008;99:1823–31.
- Brown N. Bioisosteres and scaffold hopping in medicinal chemistry. Mol Inform 2014;33:458–62.
- Hashim AI, Zhang X, Wojtkowiak JW, et al. Imaging PH and metastasis. NMR Biomed 2011;24:582–91.
- Gatenby RA, Smallbone K, Maini PK, et al. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer 2007;97:646–53.
- Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic PH microenvironment. Mol Pharm 2011;8:2032–8.
- Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 2018;25:486–541.
- Ramirez-Tagle R, Escobar C, Romero V, et al. Chalcone-induced apoptosis through caspase-dependent intrinsic pathways in human hepatocellular carcinoma cells. Int J Mol Sci 2016;17:260.
- Zhang S, Li T, Zhang L, et al. A novel chalcone derivative S17 induces apoptosis through ROS dependent DR5 up-regulation in gastric cancer cells. Sci Rep 2017;7(1):9873.
- Hsu YL, Kuo PL, Tzeng WS, Lin CC. Chalcone inhibits the proliferation of human breast cancer cell by blocking cell cycle progression and inducing apoptosis. Food Chem. Toxicol 2006;44:704–13.
- Bortolotto LFB, Barbosa FR, Silva G, et al. Cytotoxicity of trans-chalcone and licochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomed Pharmacother 2017;85:425–33.
- Muller PAJ, Vousden KH. Mutant P53 in cancer: new functions and therapeutic opportunities. Cancer Cell 2014;25:304–17.
- Wang XB, Peng W, Yi Z, et al. [Expression and prognostic value of transcriptional factor sp1 in breast cancer]. Ai Zheng 2007;26:996–1000.
- Vizcaíno C, Mansilla S, Portugal J. Sp1 transcription factor: a long-standing target in cancer chemotherapy. Pharmacol Therapeut 2015;152:111–24.
- Beishline K, Azizkhan-Clifford J. Sp1 and the ‘hallmarks of cancer’. FEBS J 2015;282:224–58.
- Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent pro-apoptotic functions of P53. Curr Opin Cell Biol 2005;17:631–6.
- Bailey ST, Shin H, Westerling T, et al. Estrogen receptor prevents P53-dependent apoptosis in breast cancer. P Natl Acad Sci 2012;109:18060–5.
- Sayeed A, Konduri SD, Liu W, et al. Estrogen receptor alpha inhibits p53-mediated transcriptional repression: implications for the regulation of apoptosis. Cancer Res 2007;67:7746–55.
- Zhang Y, Srinivasan B, Xing C, Lu J. A new chalcone derivative (E)-3-(4-methoxyphenyl)-2-methyl- 1-(3,4,5-trimethoxyphenyl)prop-2-En-1-one suppresses prostate cancer involving P53-mediated cell cycle arrests and apoptosis. Anticancer Res 2012;32:3689–98.