Abstract
The synthesis of a novel series of 3-functionalised benzenesulfonamides incorporating phenyl-1,2,3-triazole with an amide linker was achieved by using the “click-tail” approach. The new compounds, including the intermediates, were assayed as inhibitors of human carbonic anhydrase (CA, EC 4.2.1.1) isoforms hCA I and II (cytosolic isoforms) and also for hCA IV and IX (transmembrane isoforms) taking acetazolamide as standard drug. Most of these compounds exhibited excellent activity against all these isoforms. hCA I was inhibited with Kis in the range of 50.8–966.8 nM, while the glaucoma associated hCA II was inhibited with Kis in the range of 6.5–760.0 nM. Isoform hCA IV was inhibited with Kis in the range of 65.3–957.5 nM, whereas the tumor associated hypoxia induced hCA IX was inhibited with Kis in the range of 30.8–815.9 nM. The structure activity relationship study for the 3-functionalised-1-phenyl-1,2,3-triazole sulfamoylbenzamides against these isoforms was also inferred from the results.
1. Introduction
Many approaches for the development of sulfonamide carbonic anhydrase (CA, EC 4.2.1.1) inhibitors have been explored with the aim to achieve better selectivity profiles towards the different human (h) isoforms of the enzyme. CAs are ubiquitous Zn containing metalloenzymes present in all life phyla catalyzing the reversible hydration of carbon dioxide to bicarbonate anion and a proton by using a metal hydroxide nucleophilic mechanism and is being crucial for a variety of physiological and pathological processes such as maintenance pH and CO2 homeostasis, electrolyte secretion, bone resorption/calcification, gluconeogenesis, cell differentiation and proliferation, neurotransmission (in mammals), and virulence and tissue colonization (in pathogens)Citation1. Up until now it has already been reported that sulfonamides, sulfamates, sulfamides, hydroxamates incorporate efficient zinc binding groups (ZBGs) and directly bind to the metal ion within the enzyme cavityCitation2. Thus, from the drug design view point Zn2+ is the interesting target where the classical sulfonamide group (–SO2NH2) is the recognition motif for small molecules. In deprotonated form it binds to the zinc (II) ion in the active site thereby inhibiting the binding of the endogenous substrates (CO2 and H2O) and hence reducing the catalytic ability of the enzyme, similar to the transition state of the endogenous reaction constituting two additional H-bonds with Thr199 residue ()Citation3,Citation4. The binding pattern for the sulfonamide moiety exhibits a common feature among the active site super structure of all the 15 human isoenzymes belonging to α-class. The active site consists of a tetrahedral Zn2+ coordinated to the imidazole side chains of the three histidine residues present at the base of the funnel shaped active site cavity. Till now seven genetically distinct families have been identified the α, β, γ, δ, ζ, η, θ-CAs, which are very much different from the α-CAs mentioned aboveCitation5–7.
Figure 1. Zinc coordination within CA active site showing: (a) hydration of CO2 to HCO3– and (b) a sulfonamide inhibitor bound to the zinc ion and the gate keeping residues Thr199-Glu106, conserved in all α-CAs [4].
![Figure 1. Zinc coordination within CA active site showing: (a) hydration of CO2 to HCO3– and (b) a sulfonamide inhibitor bound to the zinc ion and the gate keeping residues Thr199-Glu106, conserved in all α-CAs [4].](/cms/asset/0ade6722-493d-4763-8b49-0a1e7e0dd8a6/ienz_a_1629432_f0001_c.jpg)
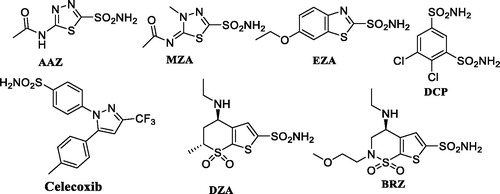
The hCA isoforms differ in their subcellular location, tissue distribution, and molecular and kinetic properties. Basically, four isoforms are cytosolic (I, II, III, and VII), five membrane-bound (IV, IX, XII, XIV, and XV), two mitochondrial (VA and VB), and CAVI is secreted in saliva and milk. Among these CA IV and XV are having GPI (glycosylphosphatidylinositol) tails anchored to the membrane while CAs IX, XII, XIV are transmembrane proteins possessing just one membrane domainCitation8. Despite that, all these five membrane-bound isoforms are commonly termed as extracellular CAs due to having their active sites outside the cell. Many sulfonamide-based drugs () such as acetazolamide (AAZ), methazolamide, ethoxzolamide, dorzolamide, brinzolamide, dichlorophenamide, and celecoxib are used clinically for many years as diuretics, anti-epileptics, anti-glaucoma, or as anti-tumor agentsCitation9,Citation10.
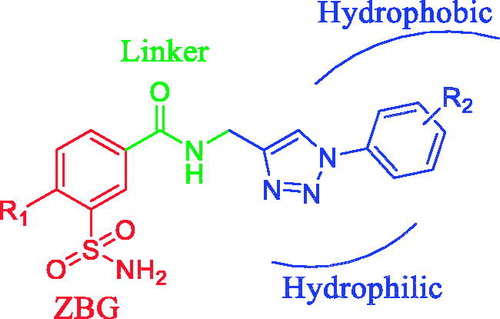
Recently many researchers in this field are focusing on isoenzyme selective sulfonamide inhibitors by using two strategies such as the ring and the tail approach (first described by Supuran’s group)Citation11. The first one consists in modulating the ring directly linked to the sulfonamide moiety and the later one entails attaching different tails to the aromatic/heterocyclic ring bearing the ZBG (. The respective tail moieties of the ligand have the ability to specifically interact with amino acid residues (most variable among various isoenzymes) present at the rim of the active site pocket. Hence, the tail approach is followed mostly. It was also possible to harmonise the physicochemical properties (most crucial for activity) of the CAIs by selecting tails with a diverse chemical natureCitation12. Although diverse types of sulfonamide derivatives have already been reported for CA inhibition, it is necessary to explore this class further for better CA inhibitory profiles.
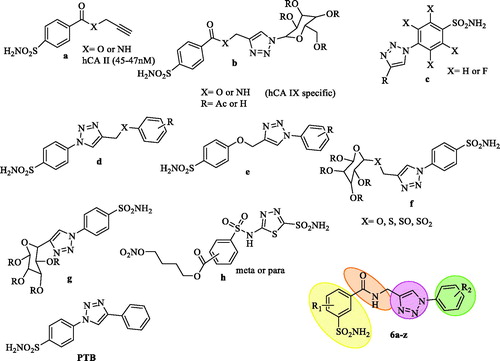
Nowadays, click chemistry is widely used to obtain metallo-enzyme CA inhibitors belonging to sulfonamide and coumarin classes. To synthesise 1,4-disubstituted 1,2,3-triazoles, the copper-catalyzed azide-alkyne cyclooadditions also well known as “click chemistry” has played a pivotal role in medicinal chemistry. The 1,2,3-triazole ring is a bioisostere of the amide bond and maintains high stability under basic as well as acid hydrolysis, reductive and oxidative conditions. It also has high dipole moment and capability of H-bonding in the in-vivo environment. Due to its aromatic character, it may undergo π-stacking interactions with relevant amino acid residues within the enzyme cavity. In recent years, CAIs belonging to sulfonamide and coumarin classes have been obtained by recurrent use of click chemistry. Thus, owing to the versatility of click chemistry in medicinal chemistry and drug discovery, it was combined with the tail approach, together termed as “click tailing” for the first time in 2006, for the development of cell membrane impermeable CA inhibitors. Authors have explored the reversal of CA isoenzyme selectivity from hCA II to hCA IX by tethering a sugar triazole tail on to the CA anchor pharmacophore ()Citation13. Pala et al., recently published two series of benzene and tetrafluorobenzenesulfonamide bearing aliphatic or aromatic moieties eliciting inhibition of the cytosolic hCA I and II in medium potencies and the tumor associated CA IX and XII in nanomolar to subnanomolar potencies ()Citation14. Nocentini et al., have synthesised a library of 1,2,3-triazoles endowed with enhanced flexibility compared to the lead PTB () and the molecules showed potent inhibition against hCA I, II, IX, XII, and some of them were highly effective for anti-glaucoma activityCitation15. Lopez et al., also designed triazoles with and without S-linked glycosyl (), which are again effective against hCA I, II, IX, and XII isoformsCitation16. Recently a novel series of 4-functionalised 1,5-diaryl-1,2,3-triazoles containing benzene sulfonamide moieties described by Pawan et al., showed selectivity towards CA isoforms I, II, IX and XIICitation17. All these triazole derivatives above were designed with a 4-sulfamoyl moiety and were having selectivity for the four CA isoforms. The through literature search shows that there is only one report which has a 3-sulfamoyl moiety coupled with 1,3,4-thiadiazole sulfonamide exhibiting exclusively inhibitory action against hCA II ()Citation18. Apart from this report there are no reports on the 3-sulfamoyl substituted derivatives as CAIs.
Figure 3. 1,2,3-Triazole containing sulfonamide molecules as CAIs based on “Click Tailing” approach.
Thus in the present study we decided to synthesise a novel series of 1,2,3-triazole derivatives linked to a 3-sulfamoyl moiety with an amide linker via the “click tailing” approach and to test them as CA inhibitors.
2. Materials and methods
2.1. General
All the commercially available reagents were used without further purification. Solvents were dried and distilled wherever necessary prior to use using standard methods. All the air and moisture sensitive reactions were performed under inert conditions using clean and dried glassware and syringe technique to transfer solutions. Reactions were monitored by TLC using Merck silica gel 60 F-254 plates. Purification was performed by column chromatography on silica gel (60–120 mesh) using a mixture of DCM and methanol as eluent. Melting points were acquired on Stuart digital melting point apparatus/SMP 30 in open capillary tubes and uncorrected. Nuclear Magnetic Resonance (1H NMR and 13C NMR) spectra were recorded by using an Avance bruker 500 and 125 MHz spectrometer in DMSO-d6 as solvent and tetramethylsilane as internal standard. Chemical shifts are reported as δ values in parts per million (ppm) and coupling constants (J) are expressed in Hz. Multiplicities are described as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), dd (doublet of doublets). HRMS were determined with Agilent QTOF mass spectrometer 6540 series instrument and were performed in the ESI techniques at 70 eV.
2.2. Chemistry
2.2.1. Synthesis of 3-(chlorosulfonyl)benzoic acid derivatives (2a–d)
To the stirred chlorosulfonic acid (13.6 ml, 204.5 mmol) at 0 °C, 4-Substituted benzoic acid derivatives (1a–d) (5 g, 40.9 mmol) were added portion wise and then stirred at 110 °C for 5 to 8 h. After completion of the reaction (monitored by TLC) it was cooled to RT and then the reaction mixture is poured into crushed ice (200 g) with vigorous stirring. The solid obtained was filtered off and the residue collected and washed with 50 ml water and dried in vacuo to obtain desired intermediate (2a–d) as white solid with 70–85% yield.
2.2.2. Synthesis of 3-(sulfamoyl)benzoic acids (3a–d)
To the ice-cold solution of ammonium hydroxide (25% in water) (5 ml, 122.7 mmol, 30.0 equiv) 3-(chlorosulfonyl)benzoic acid derivatives (2a–d) (0.5 g, 4.09 mmol, 1.0 equiv) were added portion wise and stirred for 2–3 h at room temperature. After the reaction was completed (monitored by TLC) the solvent was removed under reduced pressure. The residue was suspended in 5 ml of water and quenched with 2–5 ml of Conc. HCl. The precipitate obtained was collected by vacuum filtration and was washed with 10 ml of water and dried to obtain 3a–d as white solid with 85–95% yield.
2.2.3. Synthesis of N-(prop-2-yn-1-yl)-3-sulfamoylbenzamide (4a–d)
To the stirred solution of 3-(sulfamoyl)benzoic acid derivatives 3a–d (0.5 g, 2.5 mmol) in dry DMF (5 ml), EDCI (2.75 mmol), and HOBt (2.75 mmol) were added under inert conditions and the resultant solution stirred for 30 min at room temperature. This was followed by addition of propagyl amine (2.75 mmol) and the resultant solution was stirred at room temperature until the reaction was completed (monitored by TLC). After completion of the reaction as indicated by TLC, the reaction mixture was quenched with ice and the precipitate obtained is filtered and washed with ice cold water. The crude product was purified by column chromatography using alumina as the stationary phase and DCM: Methanol (97:3) as eluent to afford the products as white solid in 70–80% yield.
2.2.4. Synthesis of N-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamides (6a-z) via click chemistry
N-(prop-2-yn-1-yl)-3-sulfamoylbenzamides 4a–d (0.08 g, 0.34 mmol) and phenyl azides (5a–m) (0.37 mmol) were dissolved in tBuOH/H2O (1:1, 5 ml) followed by the addition of CuSO4.5H2O (0.07 mmol) and sodium ascorbate (0.14 mmol). The resultant solution was kept for stirring till completion of the reaction (TLC monitoring). Solvents were removed under vacuum and the residue was purified by column chromatography using silica gel (60–120 mesh) as the stationary phase and methanol in DCM (0–5%) as the mobile phase. The pure products (6a–z) were collected in 52–98% yield.
2.2.4.1. 3-Sulfamoylbenzoic acid (3a):
White solid, Yield 95%; 1H NMR (500 MHz, DMSO) δ 13.42 (s, 1H), 8.40 (t, J = 1.7 Hz, 1H), 8.15 (dd, J = 7.7, 1.1 Hz, 1H), 8.06 (dd, J = 7.9, 1.3 Hz, 1H), 7.72 (dd, J = 9.7, 5.8 Hz, 1H), 7.51 (s, 2H). 13C NMR (125 MHz, DMSO) δ 166.67, 145.09, 132.83, 132.00, 130.17, 130.07, 126.91.
2.2.4.2. 4-Chloro-3-sulfamoylbenzoic acid (3b)
White solid, Yield 85%; 1H NMR (500 MHz, DMSO) δ 13.44 (s, 1H), 8.36 (dt, J = 10.0, 5.0 Hz, 1H), 8.23–8.17 (m, 1H), 7.86 (s, 2H), 7.56 (dt, J = 14.7, 7.4 Hz, 1H). 13C NMR (125 MHz, DMSO) δ 165.91, 136.02 (d, J = 9.9 Hz), 132.34 (d, J = 15.4 Hz), 130.21, 127.78 (d, J = 3.4 Hz), 118.32, 118.22 (d, J = 22.1 Hz).
2.2.4.3. 4-Fluoro-3-sulfamoylbenzoic acid (3c)
White solid, Yield 87%; 1H NMR (500 MHz, DMSO) δ 13.46 (s, 1H), 8.39–8.32 (m, 1H), 8.23–8.15 (m, 1H), 7.88 (s, 2H), 7.56 (dt, J = 15.4, 7.7 Hz, 1H). 13C NMR (125 MHz, DMSO) δ 165.90, 160.10, 136.04, 135.97, 132.40, 132.28, 130.21, 127.79, 118.30, 118.13.
2.2.4.4. 4-Methoxy-3-sulfamoylbenzoic acid (3d)
White solid, Yield 92%; 1H NMR (500 MHz, DMSO) δ 12.94 (s, 1H), 8.32 (t, J = 3.1 Hz, 1H), 8.17–8.08 (m, 1H), 7.32 (d, J = 8.7 Hz, 1H), 7.23 (s, 2H), 3.99 (s, 3H). 13C NMR (125 MHz, DMSO) δ 166.62, 159.85, 135.49, 131.74, 129.54, 122.79, 113.20, 57.07. HRMS (ESI) m/z: [M + Na]+ calculated for C8H9NNaO5S 254.0099, found 254.0098.
2.2.4.5. N-(prop-2-yn-1-yl)-3-sulfamoylbenzamide (4a)
White solid, Yield 80%; 1H NMR (500 MHz, DMSO) δ 9.19 (t, J = 5.4 Hz, 1H), 8.33 (t, J = 1.7 Hz, 1H), 8.10–8.03 (m, 1H), 8.01–7.96 (m, 1H), 7.69 (dd, J = 14.2, 6.4 Hz, 1H), 7.45 (s, 2H), 4.09 (dd, J = 5.5, 2.5 Hz, 2H), 3.15 (t, J = 2.5 Hz, 1H). 13C NMR (125 MHz, DMSO) δ 165.31, 144.96, 135.00, 130.68, 129.71, 128.85, 125.32, 81.50, 73.49, 29.14. HRMS (ESI) m/z: [M + Na]+ calculated for C10H10N2NaO3S 261.0310, found 261.0310.
2.2.4.6. 4-Chloro-N-(prop-2-yn-1-yl)-3-sulfamoylbenzamide (4b)
White solid, Yield 76%; 1H NMR (500 MHz, DMSO) δ 9.26 (t, J = 5.4 Hz, 1H), 8.48 (dd, J = 5.4, 2.1 Hz, 1H), 8.05 (dd, J = 8.2, 2.1 Hz, 1H), 7.78 (t, J = 6.1 Hz, 1H), 7.72 (s, 2H), 4.07 (ddd, J = 12.3, 5.5, 2.4 Hz, 2H), 3.16 (t, J = 2.4 Hz, 1H). 13C NMR (125 MHz, DMSO) δ 164.51, 141.67, 133.92, 133.24, 132.21, 132.00, 128.68, 81.37, 73.62, 29.19. HRMS (ESI) m/z: [M + H]+ calculated for C10H10ClN2O3S+ 273.0095, found 273.0010.
2.2.4.7. 4-Fluoro-N-(prop-2-yn-1-yl)-3-sulfamoylbenzamide (4c)
White solid, Yield 70%; 1H NMR (500 MHz, DMSO) δ 9.21 (t, J = 5.4 Hz, 1H), 8.33 (dd, J = 7.0, 2.2 Hz, 1H), 8.14 (ddd, J = 8.5, 4.5, 2.3 Hz, 1H), 7.77 (s, 2H), 7.56 (t, J = 9.2 Hz, 1H), 4.08 (dd, J = 5.4, 2.5 Hz, 2H), 3.21–3.09 (m, 1H). 13C NMR (125 MHz, DMSO) δ 164.39, 159.20, 133.79, 133.72, 132.21, 132.09, 130.65, 128.58, 117.85, 117.67, 81.44, 73.54, 73.50, 29.18. HRMS (ESI) m/z: [M + H]+ calculated for C10H10FN2O3S+ 257.0391, found 257.0397.
2.2.4.8. 4-Methoxy-N-(prop-2-yn-1-yl)-3-sulfamoylbenzamide (4d)
White solid, Yield 79%; 1H NMR (500 MHz, DMSO) δ 9.03 (t, J = 5.4 Hz, 1H), 8.31 (dd, J = 12.1, 2.2 Hz, 1H), 8.19–7.98 (m, 1H), 7.37–7.27 (m, 1H), 7.17 (s, 2H), 4.09–4.02 (m, 2H), 3.97 (d, J = 3.7 Hz, 3H), 3.20–3.07 (m, 1H). 13C NMR (125 MHz, DMSO) δ 164.96, 158.75, 133.17, 131.60, 127.80, 125.72, 112.87, 81.77, 73.33, 56.97, 29.02. HRMS (ESI) m/z: [M + H]+ calculated for C11H13N2O4S+ 269.0591, found 269.0591.
2.2.4.9. N-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6a)
White solid; yield: 98%, m.p: 214–216 °C; 1H NMR (500 MHz, DMSO) δ 9.32 (t, J = 5.4 Hz, 1H), 8.70 (s, 1H), 8.37 (s, 1H), 8.12 (d, J = 7.8 Hz, 1H), 7.98 (d, J = 7.8 Hz, 1H), 7.91 (d, J = 7.7 Hz, 2H), 7.68 (dd, J = 17.5, 9.8 Hz, 1H), 7.59 (t, J = 7.9 Hz, 2H), 7.47 (dd, J = 14.1, 6.7 Hz, 1H), 7.44 (s, 2H), 4.64 (d, J = 5.5 Hz, 2H).13C NMR (125 MHz, DMSO) δ 165.56, 146.40, 144.92, 137.17, 135.32, 130.78, 130.34, 129.58, 129.03, 128.71, 125.38, 121.71, 120.46, 35.45. HRMS (ESI) m/z: [M + H]+ calculated for C16H16N5O3S 358.0968, found 358.0976.
2.2.5.0. 3-Sulfamoyl-N-((1–(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (6b)
White solid; yield: 98%, m.p: 230–231 °C; 1H NMR (500 MHz, DMSO) δ 9.34 (t, J = 5.6 Hz, 1H), 8.73 (s, 1H), 8.37 (s, 1H), 8.11 (d, J = 7.8 Hz, 1H), 7.98 (d, J = 7.8 Hz, 1H), 7.69 (t, J = 7.8 Hz, 1H), 7.44 (s, 2H), 7.20 (s, 2H), 4.64 (d, J = 5.6 Hz, 2H), 3.87 (s, 6H), 3.71 (s, 3H).13C NMR(125 MHz, DMSO) δ 165.49, 154.00, 146.19, 144.94, 137.85, 135.28, 133.06, 130.76, 129.61, 128.74, 125.39, 98.56, 60.67, 56.81, 35.41. HRMS (ESI) m/z: [M + H]+ calculated for C19H22N5O6S 448.1285, found 448.1293.
2.2.5.1. N-((1–(3-nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6c)
White solid; yield: 92%, m.p: 212–213 °C; 1H NMR (500 MHz, DMSO) δ 9.36 (t, J = 5.6 Hz, 1H), 8.97 (s, 1H), 8.74 (t, J = 2.1 Hz, 1H), 8.44–8.40 (m, 1H), 8.37 (t, J = 1.6 Hz, 1H), 8.34–8.29 (m, 1H), 8.14–8.10 (m, 1H), 8.00–7.96 (m, 1H), 7.89 (t, J = 8.2 Hz, 1H), 7.70 (t, J = 7.8 Hz, 1H), 7.44 (s, 2H), 4.66 (d, J = 5.6 Hz, 2H).13C NMR (125 MHz, DMSO) δ 165.57, 149.07, 147.01, 144.95, 137.73, 135.26, 132.00, 130.77, 129.60, 128.76, 126.46, 125.39, 123.47, 122.20, 115.13, 35.42. HRMS (ESI) m/z: [M + H]+ calculated for C16H15N6O5S 403.0819, found 403.0826.
2.2.5.2. N-((1–(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6d)
White solid, yield: 96%, m.p: 209–210 °C; 1H NMR (500 MHz, DMSO) δ 9.34 (t, J = 5.5 Hz, 1H), 8.77 (s, 1H), 8.37 (t, J = 1.5 Hz, 1H), 8.11 (d, J = 7.9 Hz, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.88–7.78 (m, 2H), 7.72–7.59 (m, 2H), 7.44 (s, 2H), 7.33 (tt, J = 10.9, 5.5 Hz, 1H), 4.64 (d, J = 5.6 Hz, 2H).13C NMR (125 MHz, DMSO) δ 165.56, 146.66, 144.93, 135.28, 132.29 (d, J = 9.1 Hz), 130.77, 129.59, 128.74, 125.38, 121.88, 116.32 (d, J = 2.9 Hz), 115.81, 115.64, 107.99, 107.78, 35.41. HRMS (ESI) m/z: [M + H]+ calculated for C16H15N5O3S 376.0874, found 376.0883.
2.2.5.3. N-((1–(3-cyanophenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6e)
White solid, yield: 87%, m.p: 238–240 °C; 1H NMR (500 MHz, DMSO) δ 9.37 (t, J = 5.6 Hz, 1H), 8.83 (s, 1H), 8.49–8.43 (m, 1H), 8.37 (t, J = 1.6 Hz, 1H), 8.34–8.28 (m, 1H), 8.12 (d, J = 7.9 Hz, 1H), 7.97 (dd, J = 17.3, 7.8 Hz, 2H), 7.80 (t, J = 8.0 Hz, 1H), 7.69 (dd, J = 17.6, 9.8 Hz, 1H), 7.50–7.40 (m, 2H), 4.65 (d, J = 5.6 Hz, 2H).13C NMR (125 MHz, DMSO) δ 165.57, 146.94, 144.95, 137.56, 135.25, 132.61, 131.75, 130.76, 129.60, 125.38, 125.02, 123.75, 121.92, 118.27 113.29, 35.42. HRMS (ESI) m/z: [M + H]+ calculated for C17H15N6O3S 383.0921, found 383.0928.
2.2.5.4. N-((1–(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6f)
White solid, yield: 91%, m.p: 219–220 °C; 1H NMR (500 MHz, DMSO) δ 9.31 (t, J = 5.6 Hz, 1H), 8.35 (t, J = 1.6 Hz, 1H), 8.32 (s, 1H), 8.11–8.08 (m, 1H), 7.98–7.95 (m, 1H), 7.68 (t, J = 7.8 Hz, 1H), 7.60 (dd, J = 7.8, 1.6 Hz, 1H), 7.54–7.50 (m, 1H), 7.43 (s, 2H), 7.31 (dd, J = 8.4, 0.8 Hz, 1H), 7.13 (td, J = 7.7, 1.1 Hz, 1H), 4.64 (d, J = 5.6 Hz, 2H), 3.85 (s, 3H).13C NMR (125 MHz, DMSO) δ 165.59, 152.05, 144.96 (d, J = 12.8 Hz), 135.39, 131.08, 130.76, 129.59, 128.69, 126.13, 125.35, 121.36, 113.55, 56.61, 35.36. HRMS (ESI) m/z: [M + H]+ calculated for C17H18N5O4S 388.1074, found 388.1082.
2.2.5.5. N-((1–(2,3-dimethylphenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6g)
White solid; yield: 86%, m.p: 205–206 °C; 1H NMR (500 MHz, DMSO) δ 9.30 (t, J = 5.5 Hz, 1H), 8.36 (s, 1H), 8.27 (s, 1H), 8.09 (t, J = 9.4 Hz, 1H), 7.97 (d, J = 7.8 Hz, 1H), 7.68 (t, J = 7.8 Hz, 1H), 7.44 (s, 2H), 7.39 (d, J = 7.6 Hz, 1H), 7.28 (t, J = 7.7 Hz, 1H), 7.20 (d, J = 7.8 Hz, 1H), 4.65 (d, J = 5.5 Hz, 2H), 2.34 (s, 3H), 1.96 (s, 3H).13C NMR (125 MHz, DMSO) δ 165.59, 145.21, 144.91, 138.91, 136.95, 135.40, 132.64, 131.48, 130.78, 129.57, 128.69, 126.62, 125.37, 124.36, 35.42, 20.34, 14.40. HRMS (ESI) m/z: [M + H]+ calculated for C18H20N5O3S 386.1281, found 386.1287.
2.2.5.6. N-((1–(2,4-dimethylphenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6h)
White solid, yield: 89%, m.p: 189–191 °C; 1H NMR (500 MHz, DMSO) δ 9.29 (t, J = 5.4 Hz, 1H), 8.35 (d, J = 1.5 Hz, 1H), 8.29 (s, 1H), 8.10 (d, J = 7.8 Hz, 1H), 7.97 (d, J = 8.3 Hz, 1H), 7.68 (t, J = 7.8 Hz, 1H), 7.43 (s, 2H), 7.27 (d, J = 7.7 Hz, 2H), 7.19 (d, J = 8.0 Hz, 1H), 4.64 (d, J = 5.5 Hz, 2H), 2.36 (s, 3H), 2.11 (s, 3H). 13C NMR (125 MHz, DMSO) δ 165.56, 145.17, 144.91, 139.78, 135.39, 134.46, 133.12, 132.23, 130.78, 129.57, 128.69, 127.83, 126.18, 125.37, 125.06, 35.41, 21.07, 17.82. HRMS (ESI) m/z: [M + H]+ calculated for C18H20N5O3S 386.1281, found 386.1288.
2.2.5.7. 4-Chloro-N-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6i)
White solid, yield: 83%, m.p: 199–201 °C; 1H NMR (500 MHz, DMSO) δ 9.38 (t, J = 5.5 Hz, 1H), 8.70 (s, 1H), 8.51 (d, J = 2.1 Hz, 1H), 8.10 (dd, J = 8.3, 2.2 Hz, 1H), 7.90 (dd, J = 8.5, 0.9 Hz, 2H), 7.76 (t, J = 10.0 Hz, 1H), 7.69 (s, 2H), 7.59 (dd, J = 10.8, 5.0 Hz, 2H), 7.47 (dd, J = 17.5, 10.1 Hz, 1H), 4.63 (d, J = 5.5 Hz, 2H).13C NMR (125 MHz, DMSO) δ 164.77, 146.28, 141.63, 137.16, 133.68 (d, J = 19.8 Hz), 132.08, 130.34, 129.04, 128.79, 121.73, 120.45, 35.49. HRMS (ESI) m/z: [M + H]+ calculated for C16H15ClN5O3S 392.0579, found 392.0586.
2.2.5.8. 4-Chloro-3-sulfamoyl-N-((1–(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)-benzamide (6j)
White solid, yield: 95%, m.p: 249–251 °C; 1H NMR (500 MHz, DMSO) δ 9.41 (t, J = 5.5 Hz, 1H), 8.73 (s, 1H), 8.51 (d, J = 2.0 Hz, 1H), 8.10 (dd, J = 8.3, 2.1 Hz, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.69 (s, 2H), 7.20 (s, 2H), 4.63 (d, J = 5.5 Hz, 2H), 3.87(s, 6H), 3.71 (s, 3H).13C NMR (125 MHz, DMSO) δ 164.72, 154.01, 146.06, 141.66, 137.89, 133.80, 133.57, 133.06, 132.09 (d, J = 4.4 Hz), 128.79, 122.05, 98.59, 60.68, 56.82, 35.45. HRMS (ESI) m/z: [M + H]+ calculated for C19H21ClN5O6S 482.0896, found 482.0899.
2.2.5.9. 4-Chloro-N-((1–(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide(6k)
White solid, yield: 90%, m.p: 172–174 °C; 1H NMR (500 MHz, DMSO) δ 9.37 (t, J = 5.5 Hz, 1H), 8.59 (s, 1H), 8.51 (d, J = 2.1 Hz, 1H), 8.09 (dd, J = 8.3, 2.2 Hz, 1H), 7.82–7.75 (m, 3H), 7.69 (s, 2H), 7.15–7.08 (m, 2H), 4.61 (d, J = 5.5 Hz, 2H), 3.82 (s, 3H).13C NMR (125 MHz, DMSO) δ 164.76, 159.69, 146.01, 141.64, 133.69 (d, J = 14.6 Hz), 132.07, 130.62, 128.79, 122.11, 121.71, 115.35, 56.04, 35.50. HRMS (ESI) m/z: [M + H]+ calculated for C17H17ClN5O4S 422.0684, found 422.0690.
2.2.6. 4-Chloro-N-((1–(4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide(6l)
White solid, yield: 83%, m.p: 189–190 °C; 1H NMR (500 MHz, DMSO) δ 9.39 (t, J = 5.6 Hz, 1H), 8.69 (s, 1H), 8.51 (d, J = 2.2 Hz, 1H), 8.10 (dd, J = 8.3, 2.2 Hz, 1H), 7.98–7.91 (m, 2H), 7.78 (d, J = 8.3 Hz, 1H), 7.70 (s, 2H), 7.47–7.39 (m, 2H), 4.62 (d, J = 5.6 Hz, 2H).13C NMR (125 MHz, DMSO) δ 164.76, 163.03, 146.35, 141.64, 133.76, 133.58, 132.07, 128.78, 122.79 (d, J = 8.8 Hz), 121.98, 117.25, 117.07, 35.46. HRMS (ESI) m/z: [M + H]+ calculated for C16H14 ClFN5O3S 410.0484, found 410.0492.
2.2.6.1. 4-Chloro-3-sulfamoyl-N-((1–(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide(6m)
White solid, yield: 92%, m.p: 178–180 °C; 1H NMR (500 MHz, DMSO) δ 9.42 (t, J = 5.6 Hz, 1H), 8.87 (s, 1H), 8.52 (d, J = 2.1 Hz, 1H), 8.18 (d, J = 8.5 Hz, 2H), 8.10 (dd, J = 8.3, 2.2 Hz, 1H), 8.03–7.92 (m, 2H), 7.82–7.74 (m, 1H), 7.70 (s, 2H), 4.64 (d, J = 5.5 Hz, 2H).13C NMR (125 MHz, DMSO) δ 164.79, 146.78, 141.65, 139.91, 133.80, 133.54, 132.08, 128.78, 127.64, 122.00, 120.89, 35.44. HRMS (ESI) m/z: [M + H]+ calculated for C17H14ClF3N5O3S 460.0452, found 460.0343.
2.2.6.2. 4-Fluoro-N-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6n)
White solid, yield: 87%, m.p: 192–194 °C; 1H NMR (500 MHz, DMSO) δ 9.35 (t, J = 5.3 Hz, 1H), 8.70 (s, 1H), 8.37 (dd, J = 6.9, 2.0 Hz, 1H), 8.24–8.14 (m, 1H), 7.90 (d, J = 7.8 Hz, 2H), 7.76 (s, 2H), 7.64–7.52 (m, 3H), 7.48 (t, J = 7.4 Hz, 1H), 4.63 (d, J = 5.5 Hz, 2H).13C NMR (125 MHz, DMSO) δ 164.65, 146.36, 137.16, 133.81 (d, J = 9.6 Hz), 132.11 (d, J = 15.0 Hz), 131.00, 130.34, 129.03, 128.65, 121.71, 120.45, 117.97–117.71 (m), 117.60 (d, J = 22.3 Hz), 35.48. HRMS (ESI) m/z: [M + H]+ calculated for C16H15FN5O3S 376.0874, found 376.0881.
2.2.6.3. 4-Fluoro-N-((1–(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6o)
White solid, yield: 85%,m.p: 209–211 °C; 1H NMR (500 MHz, DMSO) δ 9.33 (t, J = 5.4 Hz, 1H), 8.59 (s, 1H), 8.37 (dd, J = 7.0, 2.2 Hz, 1H), 8.18 (ddd, J = 8.4, 4.5, 2.3 Hz, 1H), 7.83–7.78 (m, 2H), 7.76 (s, 2H), 7.59–7.51 (m, 1H), 7.16–7.08 (m, 2H), 4.61 (d, J = 5.5 Hz, 2H), 3.82 (s, 3H).13C NMR (125 MHz, DMSO) δ 164.63, 159.67, 146.08, 133.81 (d, J = 9.3 Hz), 132.10 (d, J = 15.0 Hz), 130.99 (d, J = 3.5 Hz), 130.61, 128.64, 122.10, 121.68, 117.69, 117.52, 115.34, 56.03, 35.48. HRMS (ESI) m/z: [M + H]+ calculated for C17H17FN5O4S 406.0980, found 406.0988.
2.2.6.4. 4-Fluoro-3-sulfamoyl-N-((1–(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (6p)
White solid, yield: 79%, m.p: 157–158 °C; 1H NMR (500 MHz, DMSO) δ 9.38 (t, J = 5.6 Hz, 1H), 8.87 (s, 1H), 8.37 (dd, J = 7.0, 2.3 Hz, 1H), 8.23–8.14 (m, 3H), 7.96 (t, J = 9.9 Hz, 2H), 7.76 (s, 2H), 7.55 (dt, J = 18.9, 9.5 Hz, 1H), 4.64 (d, J = 5.6 Hz, 2H). 13C NMR (125 MHz, DMSO) δ 164.67, 146.85, 139.91, 133.81 (d, J = 9.3 Hz), 132.13 (d, J = 15.0 Hz), 130.92 (d, J = 3.4 Hz), 129.20, 128.94, 128.64, 127.65 (d, J = 3.9 Hz), 121.98, 120.89, 117.94–117.77 (m), 117.63 (d, J = 22.1 Hz), 35.43. HRMS (ESI) m/z: [M + H]+ calculated for C17H14F4N5O3S 444.0748, found 444.0757.
2.2.6.5. 4-Fluoro-N-((1–(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6q)
White solid, yield: 76%, m.p: 230–232 °C; 1H NMR (500 MHz, DMSO) δ 9.36 (t, J = 5.5 Hz, 1H), 8.77 (s, 1H), 8.37 (dd, J = 7.0, 2.2 Hz, 1H), 8.19 (ddd, J = 8.5, 4.5, 2.3 Hz, 1H), 7.88–7.78 (m, 2H), 7.76 (s, 2H), 7.68–7.60 (m, 1H), 7.58–7.52 (m, 1H), 7.33 (td, J = 8.4, 2.2 Hz, 1H), 4.62 (d, J = 5.6 Hz, 2H).13C NMR (125 MHz, DMSO) δ 164.65, 146.62, 138.38 (d, J = 10.7 Hz), 133.81 (d, J = 9.4 Hz), 132.29 (d, J = 9.3 Hz), 130.94 (d, J = 3.4 Hz), 128.64, 121.88, 118.07–117.72 (m), 117.62 (d, J = 22.1 Hz), 116.32 (d, J = 3.0 Hz), 115.81, 115.64, 107.99, 107.78, 35.44. HRMS (ESI) m/z: [M + H]+ calculated for C16H14F2N5O3S 394.0780, found 394.0786.
2.2.6.6. 4-Fluoro-3-sulfamoyl-N-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (6r)
White solid, yield: 71%, m.p: 175–177 °C; 1H NMR (500 MHz, DMSO) δ 9.34 (t, J = 5.5 Hz, 1H), 8.65 (s, 1H), 8.37 (dd, J = 7.0, 2.2 Hz, 1H), 8.19 (ddd, J = 8.5, 4.5, 2.3 Hz, 1H), 7.82–7.69 (m, 4H), 7.57 (dd, J = 22.9, 13.2 Hz, 1H), 7.38 (d, J = 8.2 Hz, 2H), 4.61 (d, J = 5.5 Hz, 2H), 2.37 (s, 3H).13C NMR (125 MHz, DMSO) δ 164.63, 146.22, 138.63, 134.93, 133.81 (d, J = 9.3 Hz), 132.11 (d, J = 15.2 Hz), 130.98 (d, J = 3.4 Hz), 130.68, 128.65, 121.58, 120.32, 117.69, 117.51, 35.48, 21.01. HRMS (ESI) m/z: [M + H]+ calculated for C17H17FN5O3S 390.1031, found 390.1037.
2.2.6.7. N-((1–(3-cyanophenyl)-1H-1,2,3-triazol-4-yl)methyl)-4-fluoro-3-sulfamoylbenzamide (6s)
White solid, yield: 69%, m.p: 249–251 °C; 1H NMR (500 MHz, DMSO) δ 9.40 (t, J = 5.6 Hz, 1H), 8.82 (s, 1H), 8.48–8.43 (m, 1H), 8.38 (dd, J = 7.0, 2.2 Hz, 1H), 8.30 (ddd, J = 8.3, 2.2, 0.9 Hz, 1H), 8.19 (ddd, J = 8.5, 4.5, 2.3 Hz, 1H), 7.97–7.92 (m, 1H), 7.79 (dd, J = 13.4, 5.3 Hz, 1H), 7.77 (s, 2H), 7.57 (dd, J = 18.6, 8.9 Hz, 1H), 4.63 (d, J = 5.6 Hz, 2H).13C NMR (125 MHz, DMSO) δ 164.67, 146.91, 137.55, 133.79 (d, J = 9.4 Hz), 132.61, 131.75, 128.65, 125.02, 123.75, 121.92, 118.27, 117.64 (d, J = 21.9 Hz), 117.46–117.19 (m), 116.12–115.96 (m), 113.29, 35.45. HRMS (ESI) m/z: [M + H]+ calculated for C17H14FN6O3S 401.0827, found 401.0833.
2.2.6.8. N-((1–(4-bromophenyl)-1H-1,2,3-triazol-4-yl)methyl)-4-fluoro-3-sulfamoylbenzamide (6t)
White solid, yield: 62%, m.p: 153–155 °C; 1H NMR (500 MHz, DMSO) δ 9.36 (t, J = 5.4 Hz, 1H), 8.74 (s, 1H), 8.37 (dd, J = 6.9, 1.9 Hz, 1H), 8.22 – 8.15 (m, 1H), 7.89 (d, J = 8.8 Hz, 2H), 7.79 (d, J = 8.8 Hz, 2H), 7.76 (s, 2H), 7.56 (t, J = 9.2 Hz, 1H), 4.59 (t, J = 19.7 Hz, 2H).13C NMR (125 MHz, DMSO) δ 164.65, 146.61, 136.35, 133.80 (d, J = 9.3 Hz), 133.23, 132.12 (d, J = 15.0 Hz), 130.94 (d, J = 3.4 Hz), 128.64, 122.36, 121.70 (d, J = 13.7 Hz), 117.70, 117.53, 35.45. HRMS (ESI) m/z: [M + 2]+ calculated for C17H13BrFN5O3S 453.9979, found 455.9962.
2.2.6.9. 4-Methoxy-N-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6u)
White solid, yield: 58%, m.p: 210–212 °C; 1H NMR (500 MHz, DMSO) δ 9.18 (t, J = 5.6 Hz, 1H), 8.68 (s, 1H), 8.34 (d, J = 2.3 Hz, 1H), 8.14 (dd, J = 8.7, 2.3 Hz, 1H), 7.90 (dd, J = 8.5, 1.0 Hz, 2H), 7.58 (dd, J = 7.9 Hz, 2H), 7.48 (t, J = 7.4 Hz, 1H), 7.29 (d, J = 8.8 Hz, 1H), 7.16 (s, 2H), 4.61 (d, J = 5.5 Hz, 2H), 3.98 (s, 3H).13C NMR (125 MHz, DMSO) δ 165.24, 158.65, 146.63, 137.18, 133.22, 131.61, 130.33, 129.00, 127.88, 126.09, 121.66, 120.44, 112.76, 56.97, 35.37. HRMS (ESI) m/z: [M + H]+ calculated for C17H18N5O4S 388.1074, found 388.1079.
2.2.7. N-((1–(4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-4-methoxy-3-sulfamoylbenzamide (6v)
White solid, yield: 52%, m.p: 222–224 °C; 1H NMR (500 MHz, DMSO) δ 9.18 (t, J = 5.5 Hz, 1H), 8.66 (s, 1H), 8.33 (d, J = 2.1 Hz, 1H), 8.13 (dd, J = 8.7, 2.1 Hz, 1H), 8.01–7.85 (m, 2H), 7.48–7.37 (m, 2H), 7.29 (d, J = 8.7 Hz, 1H), 7.16 (s, 2H), 4.60 (d, J = 5.5 Hz, 2H), 3.96 (s, 3H).13C NMR (125 MHz, DMSO) δ 165.24, 158.68, 146.70, 133.73 (d, J = 2.8 Hz), 133.22, 131.61, 127.87, 126.07, 122.79 (d, J = 8.7 Hz), 121.92, 117.23, 117.05, 112.77, 56.97, 35.34. HRMS (ESI) m/z: [M + H]+ calculated for C17H17FN5O4S 406.0980, found 406.0987.
2.2.7.1. 4-Methoxy-3-sulfamoyl-N-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide (6w)
White solid, yield: 53%, m.p: 211–213 °C; 1H NMR (500 MHz, DMSO) δ 9.16 (t, J = 5.5 Hz, 1H), 8.62 (s, 1H), 8.33 (d, J = 2.0 Hz, 1H), 8.13 (dd, J = 8.6, 2.0 Hz, 1H), 7.78 (d, J = 8.3 Hz, 2H), 7.38 (d, J = 8.4 Hz, 2H), 7.29 (d, J = 8.7 Hz, 1H), 7.16 (s, 2H), 4.59 (d, J = 5.5 Hz, 2H), 3.96 (s, 3H), 2.37 (s, 3H).13C NMR (125 MHz, DMSO) δ 165.23, 158.67, 146.49, 138.60, 134.94, 133.22, 131.61, 130.67, 127.88, 126.10, 121.53, 120.32, 112.76, 56.97, 35.37, 21.01. HRMS (ESI) m/z: [M + H]+ calculated for C18H20N5O4S 402.1231, found 402.1238.
2.2.7.2. N-((1–(2,4-dimethylphenyl)-1H-1,2,3-triazol-4-yl)methyl)-4-methoxy-3-sulfamoylbenzamide (6x)
White solid, yield: 59%, m.p: 244–246 °C; 1H NMR (500 MHz, DMSO) δ 9.14 (t, J = 5.6 Hz, 1H), 8.31 (t, J = 6.8 Hz, 1H), 8.26 (s, 1H), 8.13 (dd, J = 8.7, 2.3 Hz, 1H), 7.33–7.22 (m, 3H), 7.18 (dd, J = 7.6, 6.6 Hz, 1H), 7.15 (s, 2H), 4.61 (d, J = 5.5 Hz, 2H), 3.96 (s, 3H), 2.36 (s, 3H), 2.11 (s, 3H).13C NMR (125 MHz, DMSO) δ 165.23, 158.66, 145.41, 139.75, 134.46, 133.16 (d, J = 12.6 Hz), 132.22, 131.60, 127.84 (d, J = 5.5 Hz), 126.17, 125.01, 112.76, 56.97, 35.32, 21.07, 17.82. HRMS (ESI) m/z: [M + H]+ calculated for C19H22N5O4S 416.1387, found 416.1395.
2.2.7.3. N-((1–(4-bromophenyl)-1H-1,2,3-triazol-4-yl)methyl)-4-methoxy-3-sulfamoylbenzamide (6y)
White solid, yield: 54%, m.p: 228–230 °C; 1H NMR (500 MHz, DMSO) δ 9.18 (t, J = 5.5 Hz, 1H), 8.71 (s, 1H), 8.32 (t, J = 9.8 Hz, 1H), 8.13 (dd, J = 8.7, 2.2 Hz, 1H), 7.89 (t, J = 5.8 Hz, 2H), 7.82–7.72 (m, 2H), 7.28 (t, J = 11.6 Hz, 1H), 7.16 (s, 2H), 4.60 (d, J = 5.5 Hz, 2H), 3.97 (s, 3H). 13C NMR (125 MHz, DMSO) δ 165.24, 158.69, 136.37, 133.22, 131.61, 127.87, 126.06, 122.37, 121.67 (d, J = 13.6 Hz), 112.77, 56.98, 35.34. HRMS (ESI) m/z: [M + 2]+ calculated for C17H17BrN5O4S 466.0179, found 468.0162.
2.2.7.4. 4-Methoxy-N-((1–(2-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)-3-sulfamoylbenzamide (6z)
White solid, yield: 59%, m.p: 268–270 °C; 1H NMR (500 MHz, DMSO) δ 9.16 (t, J = 5.6 Hz, 1H), 8.32 (d, J = 2.3 Hz, 1H), 8.29 (s, 1H), 8.12 (dt, J = 14.1, 7.1 Hz, 1H), 7.60 (dd, J = 7.9, 1.6 Hz, 1H), 7.52 (ddd, J = 8.5, 7.6, 1.7 Hz, 1H), 7.29 (ddd, J = 31.9, 15.8, 5.9 Hz, 2H), 7.15 (s, 2H), 7.12 (dd, J = 7.7, 1.1 Hz, 1H), 4.60 (d, J = 5.6 Hz, 2H), 3.96 (s, 3H), 3.85 (s, 3H).13C NMR (125 MHz, DMSO) δ 165.25, 158.66, 152.03, 145.25, 133.20, 131.60, 131.05, 130.79, 127.84, 126.28, 126.12, 125.29, 121.36, 113.54, 112.79, 56.96, 56.60, 35.27. HRMS (ESI) m/z: [M + H]+ calculated for C18H20N5O5S 418.1180, found 418.1186.
2.3. CA inhibition assay
An SX.18 MV-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activityCitation19. Phenol Red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 10 mM Hepes (pH 7.4) as buffer, 0.1 M Na2SO4 or NaClO4 (for maintaining constant the ionic strength; these anions are not inhibitory in the used concentration), following the CA-catalyzed CO2 hydration reaction for a period of 5–10 s. Saturated CO2 solutions in water at 25 °C were used as substrate. Stock solutions of inhibitors were prepared at a concentration of 10 μM (in DMSO-water 1:1, v/v) and dilutions up to 0.01 nM done with the assay buffer mentioned above. At least seven different inhibitor concentrations have been used for measuring the inhibition constant. Inhibitor and enzyme solutions were preincubated together for 10 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. Triplicate experiments were done for each inhibitor concentration, and the values reported throughout the paper are the mean of such results. The inhibition constants were obtained by non-linear least-squares methods using the Cheng-Prusoff equation, as reported earlierCitation20–24, and represent the mean from at least three different determinations. All CA isozymes used here were recombinant proteins obtained as reported earlier by our groupCitation10c,Citation25,Citation26.
3. Result and discussion
3.1. Chemistry
The present work was aimed at designing molecules, which target specifically CA I, II, IX, XII based on previously reported data. The synthesis of the designed 3-functionalised benzenesulfonamide linked triazoles (6a–z) was performed according to the general synthetic route as illustrated in Scheme 1. Commercially available 4-substituted benzoic acid (1a–d) were treated with chlorosulfonic acid at 110 °C to afford the 3-(chlorosulfonyl)benzoic acids (2a–d), which were treated with ammonium hydroxide solution at 0 °C to get the corresponding 3-sulfamoylbenzoic acids (3a–d)Citation23.
Scheme 1. Synthesis of 1,2,3-triazole 3-sulfamoylbenzamide hybrids (6a–z). Reagent and reaction conditions: (i) HSO3Cl, 110 °C, 6–8 h, 70–80%; (ii) NH4OH sol., 0 °C, 2 h; (iii) Propargyl amine, EDCI, HOBt, anhydrous DMF, rt, 16–24 h, 74–85%; (iv) substituted phenyl azides, CuSO4, Sodium ascorbate, tBuOH: H2O (1:1), 40 °C, 4–6 h, 52–98%Citation30.
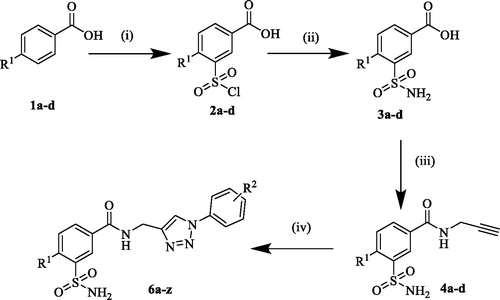
In the next step, the 3-sulfamoyl benzoic acids (3a–d) were coupled with propargyl amine in presence of EDCI and HOBt in the presence of dry DMF to afford the corresponding sulfamoylbenzamide alkyne intermediates (4a–d)Citation24. In the final step the sulfamoylbenzamide alkyne intermediates (4a–d) were subjected to click chemistry reaction with substituted azide intermediates in the presence of CuSO4, sodium ascorbate in t-BuOH and H2O (1:1) solvent system to afford the desired target derivatives (6a–z)Citation13 in good to excellent yields. The phenyl azide intermediates were previously prepared from the corresponding anilines through diazotization, using concentrated HCl, NaNO2, and NaN3Citation27,Citation28.
3.2. CA inhibition
The newly synthesised compounds 3-sulfamoylbenzamide linked 1,2,3-triazoles () (6a–z) as well as the intermediates (3a–d) and (4a–d) (listed in ) were screened for their CA inhibitory activities against four physiologically significant isoforms, the cytosolic hCA I (associated with edema) and II (associated with glaucoma) as well as the membrane bound hCA IV (associated with glaucoma and retinitis pigmentosa) and transmembrane hCA IX (associated with tumors) by means of the stopped flow carbon dioxide assaysCitation29 in comparison to AAZ as standard CAI ().
Table 1. List of synthesised compounds 3a-d, 4a-d and 6a-z.
Table 2. Inhibition of hCA isoforms I, II, IV and IX with compounds 6a–z, 3a–d, 4a–d, and AAZ as standard inhibitor
The results of the CA inhibitory assay are discussed below
The cytosolic isoform hCA I was strongly inhibited by all the synthesised compounds with KI ranging between 50.8 nM and 9.755 μM range. Among all the synthesised compounds only three compounds 6k, 6l, and 6o were found to be more potent hCA I inhibitors with KI 50.8–86.7 nM compared to the standard AAZ (KI = 250 nM), in-fact the compound 6k is almost four fold more active than that of AAZ. The remaining compounds including the intermediates 3a–d and 4a–d were showing the weakest inhibition (KI ranging between 312.1 and 9755 nM). It was also observed that the 4-chloro and 4-fluoro substituted 3-benzenesulfonamide derivatives were exhibiting the better inhibition as compared to the methoxy derivatives ().
The cytosolic isoform hCA II was strongly inhibited by some of the synthesised compounds with KI ranging between 6.5 nM and 0.760 μM. Compounds 6l and 6m showed excellent inhibition with KI 6.5 and 7.8 nM, respectively, compared to the standard AAZ (KI = 12.1 nM). The compounds 6a–6f, 6u-6z, 3a, 3b, 4d were found to be the weakest inhibitors with KI 1.382–9.647 μM and the remaining were found weak inhibitors with KI < 800 nM. The results show that the 4-chloro substituted 3-benzenesulfonamide derivatives were more potent inhibitors of hCA II ().
The membrane bound isoform hCA IV was weakly inhibited by all of the synthesised molecules with KI ranging between 78.8 nM and 2.470 μM except one compound 6l, which was found to be most potent with KI 65.3 nM compared to the reference drug AAZ (KI = 74 nM). Twelve compounds 6i, 6k-6p, 6r, 6t, 3c, 4b, and 4c were showing inhibition with KI < 1000 nM. Whereas, the other compounds were showing very weak inhibition with KI > 2000 nM ().
The tumor associated isoform hCA IX was weakly inhibited by all the synthesised compounds with KI ranging between 30.8 nM and 9.674 μM as compared to the standard AAZ (KI = 25.8 nM). From the results it is found the the 4-chloro and 4-fluoro substituted 3-benzenesulfonamide derivatives 6i, 6k-6o, and 6q-6t were showing moderate inhibition ranging between 30.8 and 84.5 nM ().
Surprisingly, the structure activity relationship (SAR) studies revealed that the derivatives containing –Cl, –F substitution on the C-4 position of 3-sulfamoylbenzamide moiety (6i–6t) were exhibiting strong inhibition against all four isoforms hCA I, II, IV, and IX, whereas all the intermediates i.e. the benzoic acid derivatives 3a–3d and N-propargyl benzamide 4a–4d derivatives showed very poor inhibition, except 4b, which showed moderate inhibition against hCA II and IV with KI 0.073 and 0.078 μM, respectively. The compound 6 l showed excellent inhibition against hCA I, II, IV as compared to AAZ the standard drug used in these assays.
It was also observed from SAR studies that the substitution on the phenyl ring of 1,2,3-triazole moiety led to a dramatic change of inhibition potency. The 4-substituted –F, –CF3, –OMe (6k, 6l, 6m, and 6o) showed strong inhibition against hCA I, II, IV. Hence, in a broader sense the compounds were excellent inhibitors if the 3-sulfamoyl phenyl ring was substituted with electron withdrawing groups. Furthermore, while comparing with the 4-functionalised benzenesulfonamides, the 3-functionalised benzenesulfonamides were more selective towards hCA I, II, IV than towards hCA IX.
4. Conclusions
In conclusion, a series of new 1,2,3-triazole derivatives containing primary 3-benzenesulfonamide moiety (6a–6z) were synthesised using click tailing approach to develop CA inhibitors via covalent linking to the selected fragment onto the CA pharmacophore. All the synthesised compounds were assayed as CA inhibitors against pharmacologically relevant isoforms i.e. cytosolic isoforms hCA I, II; membrane bound isoform hCA IV and transmembrane isoform hCA IX. The synthesised compounds showed inhibition in low to medium nanomolar range. The newly synthesised compounds showed weak inhibitory potency against hCA IX whereas compound 6l showed excellent inhibition against hCA I, II, and IV with KI values of 50.8, 6.5, and 65.3 nM with compared to AAZ as standard drug with KI = 250, 12.1, 74 nM, respectively. Furthermore, the results of hCA inhibition clearly indicate that few of the compounds containing electron withdrawing substitution on both the phenyl rings (6k, 6l, 6m, and 6o) showed strong inhibitory activity against three isoforms hCA I, II, IV. Hence, it may be concluded that the newly synthesised 1,2,3-triazole 3-sulfamoylbenzamide derivatives exhibit potent hCA inhibitory properties.
Acknowledgements
Authors are thankful to DoP, Ministry of Chemicals and Fertilizers, Goverment of India, New Delhi, for the award of a Research Fellowship.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- (a) Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. (b) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60. (c) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88. (d) Supuran CT, Alterio V, Di Fiore A, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev 2018;38:1799–836.
- (a) Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Invest Drugs 2018;27:963–70. (b) Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709–12. (c) Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin Ther Pat 2018;28:713–21.
- (a) Tuccinardi T, Bertini S, Granchi C, et al. Salicylaldoxime derivatives as new leads for the development of carbonic anhydrase inhibitors. Bioorg Med Chem 2013;21:1511–5. (b) Mishra CB, Kumari S, Angeli A, et al. Design, synthesis and biological evaluation of N-(5-methyl-isoxazol-3-yl/1,3,4-thiadiazol-2-yl)-4-(3-substitutedphenylureido) benzenesulfonamides as human carbonic anhydrase isoenzymes I, II, VII and XII inhibitors. J Enzyme Inhib Med Chem 2016;31:174–9.
- (a) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. (b) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- (a) Angapelly S, Ramya PVS, Angeli A, et al. Discovery of 4-sulfamoyl-phenyl-beta-lactams as a new class of potent carbonic anhydrase isoforms I, II, IV and VII inhibitors: the first example of subnanomolar CA IV inhibitors. Bioorg Med Chem 2017;25:539–44. (b) Nocentini A, Supuran CT. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: a patent review (2008-2018). Expert Opin Ther Pat 2018;28:729–40.
- (a) Angeli A, Alaa AM, Aziz A, et al. Synthesis and carbonic anhydrase inhibition of polycyclic imides incorporating N-benzenesulfonamide moieties. Bioorg Med Chem 2017;25:5373–9. (b) Köhler K, Hillebrecht A, Schulze Wischeler J, et al. Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem Int Ed Engl 2007;46:7697–9.
- (a) Del Prete S, Vullo D, Zoccola D, et al. Kinetic properties and affinities for sulfonamide inhibitors of an α-carbonic anhydrase (CruCA4) involved in coral biomineralization in the Mediterranean red coral Corallium rubrum. Bioorg Med Chem 2017;25:3525–350. (b) Supuran CT. Carbonic anhydrase activators. Future Med Chem 2018;10:561–73.
- (a) Supuran CT. Carbonic anhydrases as drug targets - an overview. Curr Top Med Chem 2007;7:825–33. (b) Clare BW, Supuran CT. Carbonic anhydrase activators. 3: structure‐activity correlations for a series of isozyme II activators. J Pharm Sci 1994;83:768–73. (c) De Simone G, Supuran CT. (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29.
- (a) Vullo D, Franchi M, Gallori E, et al. Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozyme IX with aromatic and heterocyclic sulfonamides. Bioorg Med Chem Lett 2003;13:1005–9. (b) Nocentini A, Supuran CT, Winum JY. Benzoxaborole compounds for therapeutic uses: a patent review (2010-2018). Expert Opin Ther Pat 2018;28:493–504.
- (a) Abdoli M, Angeli A, Bozdag M, et al. Synthesis and carbonic anhydrase I, II, VII, and IX inhibition studies with a series of benzo[d]thiazole-5- and 6- sulfonamides. J Enzyme Inhib Med Chem 2017;32:1071–8. (b) Alterio V, Esposito D, Monti SM, et al. Crystal structure of the human carbonic anhydrase II adduct with 1-(4-sulfamoylphenyl-ethyl)-2,4,6-triphenylpyridinium perchlorate, a membrane-impermeant, isoform selective inhibitor. J Enzyme Inhib Med Chem 2018;33:151–7. (c) Krasavin M, Korsakov M, Ronzhina O, et al. Primary mono- and bis-sulfonamides obtained via regiospecific sulfochlorination of N-arylpyrazoles: inhibition profile against a panel of human carbonic anhydrases. J Enzyme Inhib Med Chem 2017;32:920–34.
- (a) Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors. Synthesis of water-soluble, topically effective, intraocular pressure-lowering aromatic/heterocyclic sulfonamides containing cationic or anionic moieties: is the tail more important than the ring? J Med Chem 1999;42:2641–50. (b) Menabuoni L, Scozzafava A, Mincione F, et al. Carbonic anhydrase inhibitors. Water-soluble, topically effective intraocular pressure lowering agents derived from isonicotinic acid and aromatic/heterocyclic sulfonamides: is the tail more important than the ring? J Enzyme Inhib 1999;14:457–74.
- (a) Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72. (b) Winum JY, Temperini C, El Cheikh K, et al. Carbonic anhydrase inhibitors: clash with Ala65 as a means for designing inhibitors with low affinity for the ubiquitous isozyme II, exemplified by the crystal structure of the topiramate sulfamide analogue. J Med Chem 2006;49:7024–31. (c) Supuran CT. Carbonic anhydrase inhibitors in the treatment and prophylaxis of obesity. Expert Opin Ther Pat 2003;13:1545–50. (d) Borras J, Scozzafava A, Menabuoni L, et al. Synthesis of water-soluble, topically effective intraocular pressure lowering aromatic/heterocyclic sulfonamide containing 8-quinoline-sulfonyl moieties: is the tail more important than the ring? Bioorg Med Chem 1999;7:2397–406.
- Wilkinson BL, Bornaghi LF, Houston TA, et al. A novel class of carbonic anhydrase inhibitors: glycoconjugate benzene sulfonamides prepared by “clicktailing”. J Med Chem 2006;49:6539–48.
- Pala N, Micheletto L, Sechi M, et al. Carbonic anhydrase inhibition with benzenesulfonamides and tetrafluoro-benzenesulfonamides obtained via Click Chemistry. ACS Med Chem Lett 2014;5:927–30.
- Nocentini A, Ferraroni M, Carta F, et al. Benzenesulfonamides incorporating flexible triazole moieties are highly effective carbonic anhydrase inhibitors: synthesis and kinetic, crystallographic, computational, and intraocular pressure lowering investigations. J Med Chem 2016;59:10692–704.
- Lopez M, Salmon AJ, Supuran CT, Poulsen SA. Carbonic anhydrase inhibitors developed through “click tailing”. Curr Pharm Des 2010;16:3277–87.
- (a) Vats L, Sharma V, Angeli A, et al. Synthesis of novel 4-functionalized 1,5-diaryl-1,2,3-triazoles containing benzene sulfonamide moiety as carbonic anhydrase I, II, IV and IX inhibitors. Eur J Med Chem 2018;150:678–86. (b) Supuran CT. Carbon-versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors? J Enzyme Inhib Med Chem 2018;33:485–95. (c) Pastorekova S, Casini A, Scozzafava A, et al. Carbonic anhydrase inhibitors: the first selective, membrane-impermeant inhibitors targeting the tumor-associated isozyme IX. Bioorg Med Chem Lett 2004;14:869–73.
- (a) Mincione F, Benedini F, Biondi S, et al. Synthesis and crystallographic analysis of new sulfonamides incorporating NO-donating moieties with potent antiglaucoma action. Bioorg Med Chem Lett 2011;21:3216–21. (b) Melis C, Meleddu R, Angeli A, et al. Isatin: a privileged scaffold for the design of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2017;32:68–73. (c) Gul HI, Mete E, Eren SE, et al. Designing, synthesis and bioactivities of 4-[3-(4-hydroxyphenyl)-5-aryl-4,5-dihydro-pyrazol-1-yl]benzenesulfonamides. J Enzyme Inhib Med Chem 2017;32:169–75. (d) Gul HI, Mete E, Taslimi P, et al. Synthesis, carbonic anhydrase I and II inhibition studies of the 1,3,5-trisubstituted-pyrazolines. J Enzyme Inhib Med Chem 2017;32:189–92.
- (a) Bozdag M, Ferraroni M, Carta F, et al. Structural insights on carbonic anhydrase inhibitory action, isoform selectivity, and potency of sulfonamides and coumarins incorporating arylsulfonylureido groups. J Med Chem 2014;57:9152–67. (b) Grandane A, Tanc M, Mannelli LDC, et al. Substituted sulfocoumarins are selective carbonic anhdydrase IX and XII inhibitors with significant cytotoxicity against colorectal cancer cells. J Med Chem 2015;58:3975–83. (c) Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors: perfluoroalkyl/aryl-substituted derivatives of aromatic/heterocyclic sulfonamides as topical intraocular pressure-lowering agents with prolonged duration of action. J Med Chem 2000;43:4542–51. (d) Supuran CT, Clare BW. Carbonic anhydrase inhibitors. Part 57. Quantum chemical QSAR of a group of 1,3,4-thiadiazole and 1,3,4-thiadiazoline disulfonamides with carbonic anhydrase inhibitory properties. Eur J Med Chem 1999;34:41–50.
- (a) Akdemir A, Monte CD, Carradori S, Supuran CT. Computational investigation of the selectivity of salen and tetrahydrosalen compounds towards the tumor-associated hCA XII isozyme. J Enzyme Inhib Med Chem 2015;30:114–8. (b) Korkmaz N, Obaidi OA, Senturk M, et al. Synthesis and biological activity of novel thiourea derivatives as carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2015;30:75–80. (c) Rehman SU, Chohan ZH, Gulnaz F, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. J Enzyme Inhib Med Chem 2005;20:333–40.
- (a) Gocer H, Akincioglu AS, Gulcin GI, Supuran CT. Carbonic anhydrase and acetylcholinesterase inhibitory effects of carbamates and sulfamoylcarbamates. J Enzyme Inhib Med Chem 2015;30:316–20. (b) Alafeefy AM, Ceruso M, Al-Tamimi AMS, et al. Inhibition studies of quinazoline-sulfonamide derivatives against the γ-CA (PgiCA) from the pathogenic bacterium, Porphyromonas gingivalis. J Enzyme Inhib Med Chem 2015;30:592–6.
- (a) Ceruso M, Bragagni M, AlOthman Z, et al. New series of sulfonamides containing amino acid moiety act as effective and selective inhibitors of tumor-associated carbonic anhydrase XII. J Enzyme Inhib Med Chem 2015;30:430–4. (b) Emameh RZ, Syrjanen L, Barker H, et al. Drosophila melanogaster: a model organism for controlling Dipteran vectors and pests. J Enzyme Inhib Med Chem 2015;30:505–13. (c) Briganti F, Pierattelli R, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Part 37. Novel classes of carbonic anhydrase inhibitors and their interaction with the native and cobalt-substituted enzyme: kinetic and spectroscopic investigations. Eur J Med Chem 1996;31:1001–10. (d) Carta F, Scozzafava A, Supuran CT. Sulfonamides (RSO2NH2): a patent review 2008-2012. Expert Opin Ther Pat 2012;22:747–58.
- (a) Supuran CT, Barboiu M, Luca C, et al. Carbonic anhydrase activators. Part 14. Syntheses of mono and bis pyridinium salt derivatives of 2-amino-5 (2-aminoethyl)- and 2-amino-5(3-aminopropyl)-1,3,4-thiadiazole and their interaction with isozyme II. Eur J Med Chem 1996;31:597–606. (b) Carta F, Aggarwal M, Maresca A, et al. Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. J Med Chem 2012;55:1721–30. (c) Nishimori I, Vullo D, Innocenti A, et al. Carbonic anhydrase inhibitors. The mitochondrial isozyme VB as a new target for sulfonamide and sulfamate inhibitors. J Med Chem 2005;48:7860–6.
- Lill AP, Rodl CB, Steinhilber D, et al. Development and evaluation of ST-1829 based on 5-benzylidene-2-phenylthiazolones as promising agent for anti-leukotriene therapy. Eur J Med Chem 2015;89:503–23.
- (a) Güzel-Akdemir Ö, Angeli A, Demir K, et al. Novel thiazolidinone-containing compounds, without the well-known sulphonamide zinc-binding group acting as human carbonic anhydrase IX inhibitors. J Enzyme Inhib Med Chem 2018;33:1299–308. (b) Ramya PVS, Angapelly S, Angeli A, et al. Discovery of curcumin inspired sulfonamide derivatives as a new class of carbonic anhydrase isoforms I, II, IX, and XII inhibitors. J Enzyme Inhib Med Chem 2017;32:1274–81. (c) Akocak S, Lolak N, Bua S, Supuran CT. Discovery of novel 1,3-diaryltriazene sulfonamides as carbonic anhydrase I, II, VII, and IX inhibitors. J Enzyme Inhib Med Chem 2018;33:1575–80. (d) El-Gazzar MG, Nafie NH, Nocentini A, et al. Carbonic anhydrase inhibition with a series of novel benzenesulfonamide-triazole conjugates. J Enzyme Inhib Med Chem 2018;33:1565–74.
- (a) Entezari Heravi Y, Sereshti H, Saboury AA, et al. 3D QSAR studies, pharmacophore modeling, and virtual screening of diarylpyrazole-benzenesulfonamide derivatives as a template to obtain new inhibitors, using human carbonic anhydrase II as a model protein. J Enzyme Inhib Med Chem 2017;32:688–700. (b) D'Ascenzio M, Guglielmi P, Carradori S, et al. Open saccharin-based secondary sulfonamides as potent and selective inhibitors of cancer-related carbonic anhydrase IX and XII isoforms. J Enzyme Inhib Med Chem 2017;3:51–59.
- Tars K, Vullo D, Kazaks A, et al. Sulfocoumarins (1,2-benzoxathiine 2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J Med Chem 2013;56:293–300.
- (a) Tanc M, Carta F, Bozdag M, et al. 7-Substituted-sulfocoumarins are isoform-selective, potent carbonic anhydrase II inhibitors. Bioorg Med Chem 2013;21:4502–10. (b) Nocentini A, Ceruso M, Carta F, Supuran CT, 7-Aryl-triazolyl-substituted sulfocoumarins are potent, selective inhibitors of the tumor-associated carbonic anhydrase IX and XII. J Enzyme Inhib Med Chem 2016;31:1226–33. (c) Pustenko A, Stepanovs D, Žalubovskis R, et al. 3H-1,2-benzoxathiepine 2,2-dioxides: a new class of isoform-selective carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2017;32:767–75.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- Derosa J, Cantu AL, Boulous MN, et al. Palladium(II)-catalyzed directed anti-hydrochlorination of unactivated alkynes with HCl. J Am Chem Soc 2017;139:5183–93.