Abstract
Inflammatory bowel disease (IBD) is a chronic immuno-inflammation in gastrointestinal tract. We have evaluated the activity of the compounds to inhibit the adhesion of monocytes to colon epithelial cells is triggered by a pro-inflammatory cytokine, tumour necrosis factor (TNF)-α. The in vitro activity of the compounds, 13b (an ureido-derivative), 14c, 14j, 14k, 14n (thioureido-), 18c and 18d (sulfonamido-), was in correlation with in vivo anti-colitis activity revealed as significant recovery in body- and colon-weights and colon myeloperoxidase level, a biochemical marker of inflammation reflecting neutrophil infiltration. In vivo, TNBS-induced changes in the expression of inflammatory cytokines (TNF-α, IL-6, IL-1β, IL-10, and TGF-β), NLRP3 inflammasome components (NLRP-3, Caspase-1, and IL-18), and epithelial junction molecules (E-cadherin, claudin2/3, and ZO-1) were blocked and recovered by oral administration of the compounds (1 mg/kg). Compound 14n which showed the best efficacy can be a promising lead for orally available therapeutics for pathology of IBD.
1. Introduction
Inflammatory bowel disease (IBD) refers to a chronic inflammation occurring in the gastrointestinal tract with increasing worldwide incidence and prevalence. Two major subtypes of IBD include ulcerative colitis (UC) and Crohn’s disease (CD) with distinct injury site and disease types. The exact cause of IBD is not fully understood, but mounting evidences suggest that its pathogenesis is associated with close interactions between genetic, immune, and environmental factors, resulting in impairment of intestinal epithelial barrier and dysregulation of inflammatory cytokine homeostasis. While a couple of molecular targets have been studied for treatment of IBD, only a few target-based approaches are successful and there are no well-validated molecular targets known so far. The best examples of successful anti-IBD treatment would be anti-tumour necrosis factor-α (anti-TNF-α) antibody therapies such as infliximab and adalimumab used in clinics, showing mucosal healing in CDCitation1. However, some patients do not respond to the drugs, which highlights the necessity of new pharmacological interventions for of IBD. Examples of small molecule inhibitors with good therapeutic outcome are even scarcer. A Janus kinase (JAK) inhibitor, tofacitinib has recently been included as a therapeutic option in the treatment of UC and possibly of CDCitation2. Therefore, medical unmet need for small molecule IBD treatment still remains huge.
Uncontrolled inflammatory response results from an imbalance between the levels of proinflammatory cytokines such as TNF-α, IL-6, IL-1β, and anti-inflammatory cytokines like IL-10 and TGF-βCitation3,Citation4. TNF-α is a master cytokine which induces the production of IL-1β and IL-6 through activation of NF-kB, leading to a vicious cycle of persistent inflammation and deterioration of epithelial functionCitation5,Citation6. IL-10 and TGF-β which downregulate proinflammatory cytokine (TNF-α and IL-6) production and Th1 immune response, also play a crucial role in maintenance of colon mucosa integrity during inflammationCitation7–9. The effects of IL-10 depend on the timing and sources of its secretion. In low to moderate inflammatory condition, IL-10 from dendritic cells or macrophages drives the production of IL-10 by regulatory T cells (Treg), whereas in case of challenging with strong proinflammatory response condition, a large amount of IL-10 is produced from larger populations of cells to minimise pathology during the resolution phase of the challengeCitation10.
During cellular stress or infection, an innate immune response occurs through pattern recognition receptors (PRRs) like toll-like receptors (TLR) and NOD-like receptors. NOD-like receptor protein 3 (NLRP3), one of the most characterised PRRs, forms a multiprotein complex called inflammasome, leading to caspase-1 activation and release of IL-1β and IL-18Citation11. NLRP3 further triggers pyroptosis, an inflammatory programmed cell death, and thus intestinal barrier damageCitation12. Abnormal activation of NLRP3 inflammasome has been linked to IBDCitation13. Based on the findings that inhibition of NLRP3 blocks not only cytokine release but also p’yroptotic cell death, NLRP3 inflammasome is proposed to be a potential target in IBD drug discovery effortsCitation13.
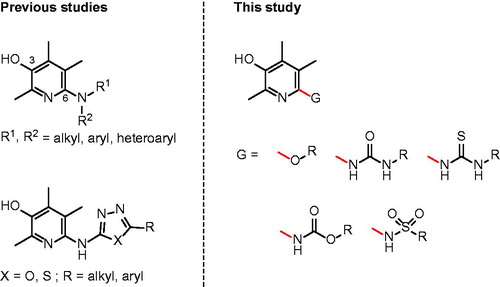
For the last several years, we have conducted a phenotype-based approach to identify small molecule inhibitorsCitation14–19. Based on the notion that immune cells such as monocytes infiltrate and adhere to colon epithelium in the presence of TNF-α, we have developed a cell-based screening assay to monitor the cell adhesion phenotype in the presence of compounds. We recently published a couple of papers on the discovery of 6-aminopyridin-3-ols to inhibit IBD in vitro and in vivo (. Among the many compounds identified as effective in the previous studies, the analogues having (4-propylphenyl)amino, (6-chloropyridin-3-yl)amino, and (5-phenyl-1,3,4-thiadiazol-2-yl)amino substructures at C(6)-position could be of particular excellence. These three compounds showed >75% inhibitory effect against TNF-α-induced cell adhesion between monocyte and colon epithelial cells at 1 μM concentration. Considering that 5-aminosalicylic acid (5-ASA, mesalazine), an active metabolite of sulfasalazine (SSZ) which is widely used to treat IBD in the clinical field, has only 3.5% inhibitory activity at the same drug concentration (1 μM), the in vitro activity of our three compounds can be quite marvellous. Moreover, in vivo efficacy studies using rats with severe colon inflammation induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS) have confirmed that our compounds are certainly effective against IBD. When orally administered at the dose of 1 mg/kg, those compounds showed very good efficacy demonstrated by ameliorating disease parameters such as % of the recovery in colon- and body-weights (up to 79% and 59%, respectively) and myeloperoxidase (MPO) level.
In this study, we made some changes in the functional group at C(6)-position of the pyridin-3-ol ring and examined how these structural changes affect on efficacy against IBD. We considered five types of functional groups (alkoxy-, ureido-, thioureido-, carbamato-, and sulfonamido-group) to replace 6-amino groups, and synthesised several to a dozen new derivatives for each family (.
2. Materials and methods
2.1. Chemistry
Unless noted otherwise, materials were purchased from commercial suppliers and used without further purification. Air- or moisture-sensitive reactions were carried out under an inert gas atmosphere. Reaction progress was monitored by thin-layer-chromatography (TLC) using silica gel F254 plates. The products were purified by flash column chromatography using silica gel 60 (70–230 mesh) or by Biotage ‘Isolera One’ system with indicated solvents. Melting points were determined using a Fischer-Jones melting point apparatus and were not corrected. NMR spectra were obtained using Bruker spectrometers 250 MHz or 400 MHz for 1H-NMR and 62.5 MHz or 100 MHz for 13 C-NMR. Chemical shifts (δ) were expressed in ppm using solvent as an internal standard and coupling constant (J) in hertz. Low-resolution mass spectra (LRMS) were obtained using an Advion Expression CMS, and recorded in a positive ion mode with an electrospray (ESI) source. Described below are detailed methods for synthesising the new compounds introduced in this paper. Other compounds were prepared using the synthetic methods we had previously reportedCitation20.
2.1.1. 5-(Benzyloxy)-3,4,6-trimethylpyridin-2-ol (6)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 2 g, 8.25 mmol) in a mixed solvent of THF (3 ml) and water (100 ml) was added 10% H2SO4 (1.1 ml, 20.63 mmol) followed by addition of NaNO2 (285 mg, 4.13 mmol) at 0 °C. The reaction temperature was gradually increased to room temperature and stirred for 2 h. The reaction mixture was diluted with CH2Cl2; washed with sat. NaHCO3. The aqueous layer separated was extracted with CH2Cl2. The combined CH2Cl2 solution was washed with brine, dried over MgSO4, filtered, and concentrated to give 6 (1.95 g, 98%). Yellow solid; TLC Rf 0.20 (CH2Cl2/MeOH = 20/1); m.p. 198 °C; MS (ESI) m/z 244 [M + H]+; 1H-NMR (CDCl3) δ 13.04 (s, 1H), 7.48–7.32 (m, 5H), 4.70 (s, 2H), 2.28 (s, 3H), 2.17 (s, 3H), 2.10 (s, 3H); 13 C-NMR (CDCl3) δ 163.20, 145.35, 139.06, 136.95, 133.43, 128.75 (2 C), 128.42, 128.20 (2 C), 123.53, 75.83, 13.98, 13.85, 12.49.
2.1.2. 2,5-Bis(benzyloxy)-3,4,6-trimethylpyridine (7a)
To a solution of 5-(benzyloxy)-3,4,6-trimethylpyridin-2-ol (6, 50 mg, 0.21 mmol) in a mixed solvent of DMF (1 ml) and THF (1 ml) was added Ag2CO3 (69 mg, 0.25 mmol) followed by addition of 3-bromo-1-propylbenzeze (38 µL, 0.32 mmol). The mixture was stirred at room temperature for 24 h. After filtration of the reaction mixture through a Celite pad, the filtrate was diluted with CH2Cl2 and water. The aqueous layer separated was extracted with CH2Cl2, and the combined CH2Cl2 solution was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 20/1) to give 7a (60 mg, 88%). White solid; TLC Rf 0.61 (Hexanes/EtOAc = 10/1); m.p. 64 °C; MS (ESI) m/z 334 [M + H]+; 1H-NMR (CDCl3) δ 7.54–7.29 (m, 10H), 5.39 (s, 2H), 4.76 (s, 2H), 2.44 (s, 3H), 2.22 (s, 3H), 2.16 (s, 3H); 13 C-NMR (CDCl3) δ 156.88, 146.70, 144.54, 141.09, 138.43, 137.33, 128.57 (2 C), 128.28 (2 C), 128.10, 127.92 (2 C), 127.68 (2 C), 127.39, 117.07, 74.97, 67.26, 18.97, 12.78, 11.83.
2.1.3. 3,4,6-Trimethylpyridine-2,5-diol (8a)
To a suspension of 10% palladium on activated carbon (5 mg) in MeOH (2 ml) was added 7a (20 mg, 0.06 mmol). The mixture was stirred with hydrogen balloon at room temperature for 1 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 20/1) to give 8a (9 mg, 98%). White solid; TLC Rf 0.27 (CH2Cl2/MeOH = 9/1); m.p. 176 °C; MS (ESI) m/z 154 [M + H]+; 1H-NMR (DMSO-d6) δ 10.96 (s, 1H), 7.51 (s, 1H), 2.07 (s, 3H), 2.03 (s, 3H), 1.90 (s, 3H); 13 C-NMR (DMSO-d6) δ 160.04, 141.97, 134.45, 126.76, 121.30, 13.70, 13.55, 12.27.
2.1.4. 3-(Benzyloxy)-6-butoxy-2,4,5-trimethylpyridine (7 b)
To a solution of 5-(benzyloxy)-3,4,6-trimethylpyridin-2-ol (6, 100 mg, 0.41 mmol) in DMF (4 ml) was added Ag2CO3 (136 mg, 0.49 mmol) followed by addition of 1-iodobutane (70 µL, 0.62 mmol). The mixture was stirred at 40 °C for 2 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated then the residue was diluted with EtOAc and washed with water and brine. The EtOAc solution was dried over MgSO4, filtered and concentrated. The residue was further purified by silica gel column chromatography (Hexanes/EtOAc = 30/1) to give 7 b (87 mg, 71%). Pale yellow solid; TLC Rf 0.29 (Hexanes/EtOAc = 20/1); m.p. 33 °C; MS (ESI) m/z 300 [M + H]+; 1H-NMR (CDCl3) δ 7.55–7.31 (m, 5H), 4.74 (s, 2H), 4.28 (t, J = 6.5 Hz, 2H), 2.41 (s, 3H), 2.20 (s, 3H), 2.11 (s, 3H), 1.76 (dt, J = 14.6, 6.5 Hz, 2H), 1.51 (dq, J = 14.2, 7.3 Hz, 2H), 0.99 (t, J = 7.3 Hz, 3H); 13 C-NMR (CDCl3) δ 157.57, 146.47, 144.59, 140.90, 137.53, 128.69 (2 C), 128.21, 128.06 (2 C), 117.08, 75.09, 65.57, 31.58, 19.62, 19.12, 14.12, 12.87, 11.87.
2.1.5. 6-Butoxy-2,4,5-trimethylpyridin-3-ol (8 b)
To a suspension of 10% palladium on activated carbon (11 mg) in MeOH (2 ml) was added 7 b (57 mg, 0.19 mmol). The mixture was stirred with hydrogen balloon at room temperature for 1 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was dissolved in MeOH and filtered through a membrane filter. The filtrate was concentrated to give 8 b (28 mg, 70%). Yellow solid; TLC Rf 0.41 (CH2Cl2/MeOH = 20/1); m.p. 63 °C; MS (ESI) m/z 210 [M + H]+; 1H-NMR (CDCl3) δ 4.23 (t, J = 6.5 Hz, 2H), 4.07 (s, 1H), 2.35 (s, 3H), 2.16 (s, 3H), 2.10 (s, 3H), 1.79–1.65 (m, 2H), 1.47 (dd, J = 15.0, 7.5 Hz, 2H), 0.97 (t, J = 7.3 Hz, 3H); 13 C-NMR (CDCl3) δ 155.76, 143.03, 136.41, 134.63, 117.05, 65.54, 31.62, 19.61, 18.61, 14.12, 12.25, 11.86.
2.1.6. 3-(Benzyloxy)-2,4,5-trimethyl-6-(octyloxy)pyridine (7c)
To a solution of 5-(benzyloxy)-3,4,6-trimethylpyridin-2-ol (6, 100 mg, 0.41 mmol) in DMF (4 ml) was added Ag2CO3 (136 mg, 0.49 mmol) followed by addition of 1-iodooctane (111 µL, 0.62 mmol). The mixture was stirred at 40 °C for 4 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was diluted with EtOAc and washed with water and brine. The EtOAc solution was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 30/1) to give 7c (131 mg, 90%). Pale yellow solid; TLC Rf 0.28 (Hexanes/EtOAc = 20/1); m.p. 31 °C; MS (ESI) m/z 356 [M + H]+; 1H-NMR (CDCl3) δ 7.52–7.32 (m, 5H), 4.73 (s, 2H), 4.27 (t, J = 6.6 Hz, 2H), 2.40 (s, 3H), 2.19 (s, 3H), 2.10 (s, 3H), 1.75 (dd, J = 14.3, 6.6 Hz, 2H), 1.32 (m, 10H), 0.89 (t, J = 6.5 Hz, 3H); 13 C-NMR (CDCl3) δ 157.58, 146.54, 144.60, 140.99, 137.58, 128.71 (2 C), 128.22, 128.08 (2 C), 117.15, 75.14, 65.99, 32.02, 29.58, 29.45 (2 C), 26.42, 22.83, 19.10, 14.24, 12.90, 11.89.
2.1.7. 2,4,5-Trimethyl-6-(octyloxy)pyridin-3-ol (8c)
To a suspension of 10% palladium on activated carbon (20 mg) in MeOH (2 ml) was added 7c (99 mg, 0.28 mmol). The mixture was stirred with hydrogen balloon at room temperature for 1 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was dissolved in MeOH and filtered through a membrane filter. The filtrate was concentrated to give 8c (70 mg, 95%). Pale yellow solid; TLC Rf 0.48 (CH2Cl2/MeOH = 20/1); m.p. 52 °C; MS (ESI) m/z 266 [M + H]+; 1H-NMR (CDCl3) δ 4.22 (t, J = 6.6 Hz, 2H), 4.14 (s, 1H), 2.35 (s, 3H), 2.16 (s, 3H), 2.10 (s, 3H), 1.72 (dd, J = 14.2, 6.7 Hz, 2H), 1.51–1.18 (m, 10H), 0.89 (dd, J = 8.9, 4.4 Hz, 3H); 13 C-NMR (CDCl3) δ 155.75, 143.05, 136.47, 134.77, 117.09, 65.93, 32.00, 29.57, 29.46, 29.44, 26.40, 22.83, 18.58, 14.25, 12.27, 11.87.
2.1.8. 2,4,5-Trimethyl-6-(octyloxy)pyridin-3-ol (7d)
To a solution of 5-(benzyloxy)-3,4,6-trimethylpyridin-2-ol (6, 100 mg, 0.41 mmol) in DMF (4 ml) was added Ag2CO3 (136 mg, 0.49 mmol) followed by addition of 1-iodo-3-methylbutane (71 µL, 0.62 mmol). The mixture was stirred at 50 °C for 20 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was diluted with EtOAc and washed with water and brine. The EtOAc solution was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 30/1) to give 7d (98 mg, 76%). Colourless liquid; TLC Rf 0.30 (Hexanes/EtOAc = 20/1); MS (ESI) m/z 514 [M + H]+; 1H-NMR (CDCl3) δ 7.52–7.33 (m, 5H), 4.72 (s, 2H), 4.29 (t, J = 6.6 Hz, 2H), 2.40 (s, 3H), 2.19 (s, 3H), 2.09 (s, 3H), 1.82 (td, J = 13.3, 6.6 Hz, 1H), 1.65 (q, J = 6.7 Hz, 2H), 0.97 (d, J = 6.6 Hz, 6H); 13 C-NMR (CDCl3) δ 157.52, 146.42, 144.55, 142.42, 137.46, 128.72 (2 C), 128.24, 128.07 (2 C), 117.11, 75.09, 64.33, 38.26, 25.42, 22.88 (2 C), 19.10, 12.91, 11.92.
2.1.9. 6-(Isopentyloxy)-2,4,5-trimethylpyridin-3-ol (8d)
To a suspension of 10% palladium on activated carbon (14 mg) in MeOH (2 ml) was added 7d (72 mg, 0.23 mmol). The mixture was stirred with hydrogen balloon at room temperature for 1 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was dissolved in MeOH and filtered through a membrane filter. The filtrate was concentrated to give 8d (40 mg, 78%). Pale yellow liquid; TLC Rf 0.41 (CH2Cl2/MeOH = 20/1); MS (ESI) m/z 224 [M + H]+; 1H-NMR (CDCl3) δ 4.26 (t, J = 6.6 Hz, 2H), 4.10 (s, 1H), 2.36 (s, 3H), 2.16 (s, 3H), 2.09 (s, 3H), 1.81 (dt, J = 13.3, 6.7 Hz, 1H), 1.63 (q, J = 6.7 Hz, 2H), 0.96 (d, J = 6.6 Hz, 6H); 13 C-NMR (CDCl3) δ 155.72, 143.05, 136.40, 134.75, 117.08, 64.33, 38.34, 25.46, 22.87 (2 C), 18.58, 12.27, 11.88.
2.1.10. 6-(Isopentyloxy)-2,4,5-trimethylpyridin-3-ol (7e)
To a solution of 5-(benzyloxy)-3,4,6-trimethylpyridin-2-ol (6, 100 mg, 0.41 mmol) in DMF (4 ml) was added Ag2CO3 (136 mg, 0.49 mmol) followed by addition of 1-iodocyclopentane (71 µL, 0.62 mmol). The mixture was stirred at 50 °C for 7 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was diluted with EtOAc and washed with water and brine. The EtOAc solution was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 50/1) to give 7e (88 mg, 69%). Colourless liquid; TLC Rf 0.29 (Hexanes/EtOAc = 20/1); MS (ESI) m/z 312 [M + H]+; 1H-NMR (CDCl3) δ 7.52–7.31 (m, 5H), 5.54–5.39 (m, 1H), 4.74 (s, 2H), 2.39 (s, 3H), 2.18 (s, 3H), 2.06 (s, 3H), 1.93 (dd, J = 10.8, 6.3 Hz, 2H), 1.77 (dd, J = 20.6, 5.4 Hz, 4H), 1.63 (dd, J = 10.9, 4.1 Hz, 2H); 13 C-NMR (CDCl3) δ 157.18, 146.30, 144.66, 140.72, 137.60, 128.69 (2 C), 128.19, 128.05 (2 C), 117.49, 75.07, 33.09 (2 C), 29.86, 24.07 (2 C), 19.26, 12.89, 11.9.
2.1.11. 6-(Cyclopentyloxy)-2,4,5-trimethylpyridin-3-ol (8e)
To a suspension of 10% palladium on activated carbon (13 mg) in MeOH (2 ml) was added 7e (63 mg, 0.20 mmol). The mixture was stirred with hydrogen balloon at room temperature for 1 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was dissolved in MeOH and filtered through a membrane filter. The filtrate was concentrated to give 8e (39 mg, 87%). Pale yellow solid; TLC Rf 0.41 (CH2Cl2/MeOH = 20/1); m.p. 79 °C; MS (ESI) m/z 222 [M + H]+; 1H-NMR (CDCl3) δ 5.38 (dt, J = 8.2, 3.0 Hz, 1H), 4.18 (s, 1H), 2.34 (s, 3H), 2.14 (s, 3H), 2.06 (s, 3H), 1.95–1.82 (m, 2H), 1.82–1.67 (m, 4H), 1.66–1.51 (m, 2H); 13 C-NMR (CDCl3) δ 155.33, 142.90, 136.71, 134.56, 117.61, 77.46, 33.03 (2 C), 24.03 (2 C), 18.71, 12.26, 11.92.
2.1.12. 3-(Benzyloxy)-2,4,5-trimethyl-6–(3-phenylpropoxy)pyridine (7f)
To a solution of 5-(benzyloxy)-3,4,6-trimethylpyridin-2-ol (6, 30 mg, 0.12 mmol) in DMF (1 ml) was added Ag2CO3 (41 mg, 0.15 mmol) followed by addition of 3-bromo-1-propylbenzeze (28 µL, 0.19 mmol). The mixture was stirred at 80 °C for 12 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was diluted with EtOAc and washed with water and brine. The EtOAc solution was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 30/1) to give 7f (37 mg, 83%). Colourless liquid; TLC Rf 0.31 (Hexanes/EtOAc = 15/1); MS (ESI) m/z 362 [M + H]+; 1H-NMR (CDCl3) δ 7.54–7.36 (m, 5H), 7.36–7.17 (m, 5H), 4.76 (s, 2H), 4.35 (t, J = 6.3 Hz, 2H), 2.84 (dd, J = 8.6, 6.9 Hz, 2H), 2.42 (s, 3H), 2.23 (s, 3H), 2.15 (s, 3H), 2.14–2.05 (m, 2H); 13 C-NMR (CDCl3) δ 157.35, 146.57, 144.65, 142.21, 140.96, 137.51, 128.69 (2 C), 128.62 (2 C), 128.46 (2 C), 128.21, 128.05 (2 C), 125.88, 117.04, 75.08, 64.95, 32.66, 31.09, 19.12, 12.88, 11.89.
2.1.13. 2,4,5-Trimethyl-6–(3-phenylpropoxy)pyridin-3-ol (8f)
To a suspension of 10% palladium on activated carbon (15 mg) in MeOH (2 ml) was added 7f (77 mg, 0.21 mmol). The mixture was stirred with hydrogen balloon at room temperature for 1 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was dissolved in MeOH and filtered through a membrane filter. The filtrate was concentrated to give 8f (50 mg, 87%). White solid; TLC Rf 0.30 (CH2Cl2/MeOH = 20/1); m.p. 73 °C; MS (ESI) m/z 272 [M + H]+; 1H-NMR (CDCl3) δ 7.35–7.16 (m, 5H), 4.29 (t, J = 6.3 Hz, 3H), 2.89–2.71 (m, 2H), 2.36 (s, 3H), 2.18 (s, 3H), 2.14 (s, 3H), 2.13–2.02 (m, 2H); 13 C-NMR (CDCl3) δ 155.55, 143.15, 142.24, 136.65, 134.92, 128.61 (2 C), 128.44 (2 C), 125.86, 117.04, 65.00, 32.64, 31.14, 18.55, 12.28, 11.87.
2.1.14. 1–(5-(Benzyloxy)-3,4,6-trimethylpyridin-2-yl)-3-propylurea (11a)
To a solution of 5-(benzyloxy)-3,4,6-trimethylpyridin-6-amine (5, 100 mg, 0.41 mmol) in CH2Cl2 (5 ml) was added propyl isocyanate (46 μL, 0.50 mmol). The mixture was stirred at room temperature for 40 h. After concentration of the reaction mixture, the residue was purified by silica gel column chromatography (CHCl3/MeOH = 50/1 to 20/1) to give 13a (125 mg, 92%). White solid; TLC Rf 0.34 (CH2Cl2/MeOH = 30/1); m.p. 175 °C; MS (ESI) m/z 328 [M + H]+; 1H-NMR (CDCl3) δ 9.79 (s, 1H), 7.49–7.33 (m, 5H), 6.69 (s, 1H), 4.74 (s, 2H), 3.35 (td, J = 6.9, 5.6 Hz, 2H), 2.40 (s, 3H), 2.22 (s, 3H), 2.10 (s, 3H), 1.64 (dd, J = 14.3, 7.1 Hz, 2H), 1.02 (t, J = 7.4 Hz, 3H); 13 C-NMR (CDCl3) δ 155.95, 146.87, 146.75, 144.92, 141.54, 137.01, 128.77 (2 C), 128.43, 128.10 (2 C), 115.89, 75.38, 41.73, 23.20, 19.11, 13.31, 12.84, 11.75.
2.1.15. 1–(5-Hydroxy-3,4,6-trimethylpyridin-2-yl)-3-propylurea (13a)
To a suspension of 10% palladium on activated carbon (21 mg) in CH2Cl2 (4 ml) was added 11a (105 mg, 0.32 mmol). The mixture was stirred with hydrogen balloon at room temperature for 2 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 20/1) to give 13a (58 mg, 75%). White solid; TLC Rf 0.26 (CH2Cl2/MeOH = 20/1); m.p. 176 °C; MS (ESI) m/z 239 [M + H]+; 1H-NMR (DMSO-d6) δ 8.88 (s, 1H), 8.20 (s, 1H), 7.71 (s, 1H), 3.14 (dd, J = 12.4, 6.7 Hz, 2H), 2.29 (s, 3H), 2.12 (s, 3H), 2.05 (s, 3H), 1.56–1.41 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H); 13 C-NMR (DMSO-d6) δ 155.50, 144.40, 143.36, 138.73, 135.53, 118.36, 40.75, 22.78, 19.13, 13.08, 12.66, 11.39.
2.1.16. 1–(5-(Benzyloxy)-3,4,6-trimethylpyridin-2-yl)-3–(4-nitrophenyl)urea (11k)
To a solution of 5-(benzyloxy)-3,4,6-trimethylpyridin-6-amine (5, 50 mg, 0.21 mmol) in CH2Cl2 (2 ml) was added 4-nitrophenyl isocyanate (41 mg, 0.25 mmol). The mixture was stirred at room temperature for 4 h. The reaction mixture was diluted Et2O and the precipitate was filtered. The filter cake was washed with Et2O and acetone to give 11k (73 mg, 87%). White solid; TLC Rf 0.33 (CH2Cl2/MeOH = 40/1); m.p. 227 °C; MS (ESI) m/z 429 [M + Na]+; 1H-NMR (DMSO-d6) δ 11.77 (s, 1H), 8.72 (s, 1H), 8.21 (d, J = 8.4 Hz, 2H), 7.77 (d, J = 8.5 Hz, 2H), 7.60–7.32 (m, 5H), 4.81 (s, 2H), 2.44 (s, 3H), 2.22 (s, 3H), 2.16 (s, 3H); 13 C-NMR (DMSO-d6) δ 152.21, 147.72, 145.66, 145.12, 144.96, 141.49, 141.38, 136.80, 128.22 (2 C), 127.98 (2 C), 127.90, 124.87 (2 C), 120.88, 118.06 (2 C), 74.34, 18.59, 13.18, 12.75.
2.1.17. 1–(4-Aminophenyl)-3–(5-hydroxy-3,4,6-trimethylpyridin-2-yl)urea (13k)
To a suspension of 10% palladium on activated carbon (15 mg) in MeOH (3 ml) was added 11k (75 mg, 0.18 mmol). The mixture was stirred with hydrogen balloon at room temperature for 12 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 20/1) to give 13k (14 mg, 26%). White solid; TLC Rf 0.35 (CH2Cl2/MeOH = 20/1); m.p. 200 °C (decomp.); MS (ESI) m/z 309 [M + Na]+; 1H-NMR (DMSO-d6) δ 11.03 (s, 1H), 8.34 (s, 1H), 7.94 (s, 1H), 7.15 (d, J = 8.7 Hz, 2H), 6.52 (d, J = 8.7 Hz, 2H), 4.77 (s, 2H), 2.36 (s, 3H), 2.14 (s, 3H), 2.10 (s, 3H); 13 C-NMR (DMSO-d6) δ 153.01, 144.74, 144.09, 142.99, 138.81, 135.83, 128.50, 120.61 (2 C), 118.97, 114.17 (2 C), 19.20, 13.19, 12.76.
2.1.18. 1–(5-(Benzyloxy)-3,4,6-trimethylpyridin-2-yl)thiourea (12a)
To a solution of N-((5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)carbamothioyl)benzamide (12n, 100 mg, 0.25 mmol) in a mixed solvent of THF (1 ml) and water (1 ml) was added NaOH (20 mg, 0.49 mmol). The mixture was refluxed for 17 h. The reaction mixture was cooled to room temperature and diluted with water. The pH of the reaction mixture was adjusted to around 7 using 2 M HCl. The precipitate formed was collected by filtration and the filter cake was washed with water to give 12a (43 mg, 58%). Ivory solid; TLC Rf 0.22 (Hex/EtOAc = 2/1); m.p. 165 °C; MS (ESI) m/z 302 [M + H]+; 1H-NMR (CDCl3) δ 11.26 (s, 1H), 7.96 (s, 1H), 7.46 – 7.35 (m, 5H), 6.84 (s, 1H), 4.76 (s, 2H), 2.40 (s, 3H), 2.24 (s, 3H), 2.18 (s, 3H); 13 C-NMR (CDCl3) δ 148.10, 148.00, 146.49, 145.99, 142.17, 136.69, 128.83 (2 C), 128.58, 128.14 (2 C), 116.92, 75.41, 19.25, 13.43, 12.99.
2.1.19. 1–(5-Hydroxy-3,4,6-trimethylpyridin-2-yl)thiourea (14a)
To a solution of 12a (34 mg, 0.11 mmol) in CH2Cl2 (1 ml), was added pentamethylbenzene (50 mg, 0.34 mmol) followed by dropwise addition of boron trichloride (1 M in CH2Cl2, 0.2 ml) at 0 °C. The reaction mixture was stirred at 0 °C for 15 min then quenched with CHCl3/MeOH solution (9/1, 1.5 ml) and stirred at room temperature for 30 min. After concentration of the reaction mixture, 2 ml of a mixed solvent (CH2Cl2/MeOH = 40/1) was added to the residue and precipitate formed was filtered, washed with CH2Cl2 and collected to give 14a (23.6 mg, 99%). White solid; TLC Rf 0.35 (CH2Cl2/MeOH = 9/1); m.p. 189 °C; MS (ESI) m/z 212 [M + H]+; 1H-NMR (CD3OD) δ 2.35 (s, 3H), 2.22 (s, 3H), 2.18 (s, 3H); 13 C-NMR (CD3OD) δ 147.61, 144.33, 141.39, 137.55, 129.24, 128.91, 19.08, 12.98, 12.77.
2.1.20. Methyl (5-benzyloxy-3,4,6-trimethylpyridin-2-yl)carbamate (15a)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 70 mg, 0.29 mmol) in acetone (1 ml) was added K2CO3 (120 mg, 0.87 mmol) followed by dropwise addition of methyl chloroformate (112 µL, 1.45 mmol) at 0 °C. The mixture was stirred at room temperature for 24 h. The reaction mixture was concentrated and then diluted with CH2Cl2 and water. The aqueous layer separated was extracted with CH2Cl2. The combined CH2Cl2 solution was washed with brine and dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 30/1 to 20/1) to give 15a (63 mg, 73%). Pale yellow solid; TLC Rf 0.29 (CH2Cl2/MeOH = 15/1); m.p. 117 °C; MS (ESI) m/z 301 [M + H]+; 1H-NMR (CDCl3) δ 7.50–7.32 (m, 5H), 6.96 (s, 1H), 4.77 (s, 2H), 3.76 (s, 3H), 2.43 (s, 3H), 2.25 (s, 3H), 2.16 (s, 3H); 13 C-NMR (CDCl3) δ 155.16, 150.60, 148.10, 143.55, 141.69, 136.96, 128.78 (2 C), 128.43, 128.05 (2 C), 126.58, 75.01, 52.71, 19.19, 14.98, 13.32.
2.1.21. Methyl (5-hydroxy-3,4,6-trimethylpyridin-2-yl)carbamate (16a)
To a suspension of 10% palladium on activated carbon (12 mg) in a mixed solvent of CHCl3 (1.5 ml) and MeOH (1.5 ml) was added 15a (55 mg, 0.18 mmol). The mixture was stirred with hydrogen balloon at room temperature for 1 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 20/1 to 5/1) to give 16a (37 mg, 96%). White solid; TLC Rf 0.19 (CH2Cl2/MeOH = 15/1); m.p. 210 °C; MS (ESI) m/z 233 [M + Na]+; 1H-NMR (DMSO-d6) δ 9.02 (s, 1H), 8.53 (s, 1H), 3.57 (s, 3H), 2.27 (s, 3H), 2.11 (s, 3H), 2.00 (s, 3H); 13 C-NMR (DMSO-d6) δ 155.20, 147.63, 141.39, 140.32, 133.75, 126.18, 51.47, 19.15, 14.14, 12.51.
2.1.22. Methyl (5-hydroxy-3,4,6-trimethylpyridin-2-yl)carbamate (15 b)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 70 mg, 0.29 mmol) in acetone (1 ml) was added K2CO3 (200 mg, 1.45 mmol) followed by dropwise addition of butyl chloroformate (184 µL, 1.45 mmol) at 0 °C. The mixture was stirred at room temperature for 24 h. The reaction mixture was concentrated then diluted with CH2Cl2 and washed with sat. NaHCO3 and brine. The CH2Cl2 layer was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 30/1 to 20/1) to give 15 b (90 mg, 91%). White solid; TLC Rf 0.33 (CH2Cl2/MeOH = 15/1); m.p. 81 °C; MS (ESI) m/z 399 [M + H]+; 1H-NMR (CDCl3) δ 7.51–7.33 (m, 5H), 7.08 (s, 1H), 4.77 (s, 2H), 4.14 (t, J = 6.6 Hz, 2H), 2.43 (s, 3H), 2.24 (s, 3H), 2.16 (s, 3H), 1.65 (dt, J = 14.8, 6.9 Hz, 2H), 1.40 (dq, J = 14.2, 7.2 Hz, 2H), 0.94 (t, J = 7.3 Hz, 3H); 13 C-NMR (CDCl3) δ 154.83, 150.52, 147.90, 143.80, 141.66, 137.01, 128.75 (2 C), 128.38, 128.03 (2 C), 126.53, 75.02, 65.43, 31.11, 19.18, 19.08, 15.01, 13.85, 13.28.
2.1.23. Butyl (5-hydroxy-3,4,6-trimethylpyridin-2-yl)carbamate (16 b)
To a suspension of 10% palladium on activated carbon (16 mg) in MeOH (5 ml) was added 15 b (79 mg, 0.23 mmol). The mixture was stirred with hydrogen balloon at room temperature for 1 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was dissolved in MeOH and filtered through a membrane filter and concentrated to give 16 b (54 mg, 93%). White solid; TLC Rf 0.24 (CH2Cl2/MeOH = 15/1); m.p. 166 °C; MS (ESI) m/z 253 [M + H]+; 1H-NMR (DMSO-d6) δ 8.95 (s, 1H), 3.98 (t, J = 6.5 Hz, 2H), 2.27 (s, 3H), 2.11 (s, 3H), 1.99 (s, 3H), 1.55 (dt, J = 14.5, 6.6 Hz, 2H), 1.34 (dq, J = 14.1, 7.1 Hz, 2H), 0.89 (t, J = 7.3 Hz, 3H); 13 C-NMR (DMSO-d6) δ 154.94, 147.82, 141.42, 140.33, 133.76, 126.26, 63.68, 30.80, 19.32, 18.65, 14.30, 13.70, 12.65.
2.1.24. Isobutyl (5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)carbamate (15c)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 30 mg, 0.12 mmol) in acetone (1 ml) was added K2CO3 (86 mg, 0.62 mmol) followed by dropwise addition of isobutyl chloroformate (24 µL, 0.19 mmol) at 0 °C. The mixture was stirred at room temperature for 24 h. The reaction mixture was concentrated and then diluted with CH2Cl2. It was washed with sat. NaHCO3 and brine. The CH2Cl2 solution was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 40/1) to give 15c (40 mg, 87%). White solid; TLC Rf 0.29 (CH2Cl2/MeOH = 15/1); m.p. 70 °C; MS (ESI) m/z 343 [M + H]+; 1H-NMR (CDCl3) δ 7.52–7.34 (m, 5H), 7.28 (s, 1H), 4.77 (s, 2H), 3.93 (d, J = 6.6 Hz, 2H), 2.44 (s, 3H), 2.24 (s, 3H), 2.17 (s, 3H), 2.04–1.87 (m, 1H), 0.94 (d, J = 6.7 Hz, 6H); 13 C-NMR (CDCl3) δ 154.89, 150.50, 147.89, 143.87, 141.61, 137.01, 128.73 (2 C), 128.37, 128.02 (2 C), 126.53, 75.00, 71.63, 29.80, 28.09, 19.11 (d, 2 C), 15.00, 13.27.
2.1.25. Isobutyl (5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)carbamate (16c)
To a suspension of 10% palladium on activated carbon (18 mg) in MeOH (5 ml) was added 15c (91 mg, 0.27 mmol). The mixture was stirred with hydrogen balloon at room temperature for 6 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 30/1 to 20/1) to give 16c (34 mg, 51%). White solid; TLC Rf 0.35 (CH2Cl2/MeOH = 15/1); m.p. 166 °C; MS (ESI) m/z 253 [M + H]+; 1H-NMR (DMSO-d6) δ 8.89 (s, 1H), 8.44 (s, 1H), 3.78 (d, J = 6.6 Hz, 2H), 2.28 (s, 3H), 2.12 (s, 3H), 2.01 (s, 3H), 1.86 (dt, J = 13.3, 6.6 Hz, 1H), 0.89 (d, J = 6.7 Hz, 6H); 13 C-NMR (DMSO-d6) δ 154.85, 147.54, 141.30, 140.38, 133.66, 126.12, 69.84, 27.59, 19.10, 18.80 (2 C), 14.11, 12.47.
2.1.26. 2-Chloroethyl (5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)carbamate (15d)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 70 mg, 0.29 mmol) in acetone (1 ml) was added K2CO3 (200 mg, 1.45 mmol) followed by dropwise addition of 2-chloroethyl chloroformate (149 µL, 1.45 mmol) at 0 °C. The mixture was stirred at room temperature for 9 h. The reaction mixture was concentrated. The residue was diluted with CH2Cl2 and washed with sat. NaHCO3 and brine. The CH2Cl2 layer was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 50/1 to 30/1) to give 15d (84 mg, 83%). White solid; TLC Rf 0.21 (CH2Cl2/MeOH = 30/1); m.p. 128 °C; MS (ESI) m/z 349 [M + H]+; 1H-NMR (CDCl3) δ 7.51–7.34 (m, 5H), 4.78 (s, 2H), 4.44–4.34 (m, 2H), 3.78–3.66 (m, 2H), 2.44 (s, 3H), 2.25 (s, 3H), 2.17 (s, 3H); 13 C-NMR (CDCl3) δ 154.05, 150.70, 148.22, 143.22, 141.80, 136.90, 128.79 (2 C), 128.45, 128.05 (2 C), 126.62, 75.02, 65.05, 42.06, 19.14, 14.96, 13.33.
2.1.27. 2-Chloroethyl (5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)carbamate (16d)
To a suspension of 15d (75 mg, 0.22 mmol) in CH2Cl2 (2 ml), was added pentamethylbenzene (96 mg, 0.65 mmol) followed by dropwise addition of boron trichloride (1 M in CH2Cl2, 0.32 ml) at 0 °C. The reaction mixture was stirred at 0 °C for 30 min, The mixture was then quenched with a mixed solvent of CHCl3/MeOH (9/1, 1.5 ml) and stirred at room temperature for 30 min. After concentration of the reaction mixture, the residue was purified by silica gel column chromatography (CHCl3/MeOH = 30/1 to 10/1) to give 16d (51 mg, 92%). White solid; TLC Rf 0.18 (CH2Cl2/MeOH = 30/1); m.p. 163 °C; MS (ESI) m/z 259 [M + H]+; 1H-NMR (DMSO-d6) δ 9.11 (s, 1H), 8.48 (s, 1H), 4.30–4.22 (m, 2H), 3.85–3.78 (m, 2H), 2.28 (s, 3H), 2.12 (s, 3H), 2.02 (s, 3H); 13 C-NMR (DMSO-d6) δ 154.28, 147.70, 141.39, 140.01, 133.74, 126.29, 64.06, 42.99, 19.11, 14.08, 12.48.
2.1.28. 2-Methoxyethyl (5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)carbamate (15e)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 70 mg, 0.29 mmol) in acetone (2 ml) was added K2CO3 (200 mg, 1.45 mmol) followed by dropwise addition of 2-methoxyethyl chloroformate (168 µL, 1.45 mmol) at 0 °C. The mixture was stirred at room temperature for 17 h and then concentrated. The residue was diluted with CH2Cl2 and washed with sat. NaHCO3 and brine. The CH2Cl2 layer was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 40/1) to give 15e (72 mg, 72%). White solid; TLC Rf 0.22 (CH2Cl2/MeOH = 30/1); m.p. 98 °C; MS (ESI) m/z 345 [M + H]+; 1H-NMR (CDCl3) δ 7.50–7.35 (m, 5H), 6.94 (s, 1H), 4.77 (s, 2H), 4.35–4.27 (m, 2H), 3.67–3.58 (m, 2H), 3.41 (s, 3H), 2.44 (s, 3H), 2.24 (s, 3H), 2.16 (s, 3H); 13 C-NMR (CDCl3) δ 154.41, 150.65, 148.01, 143.46, 141.81, 136.98, 128.78 (2 C), 128.43, 128.06 (2 C), 126.51, 75.06, 70.90, 64.57, 59.11, 19.15, 14.91, 13.32.
2.1.29. 2-Methoxyethyl (5-hydroxy-3,4,6-trimethylpyridin-2-yl)carbamate (16e)
To a suspension of 10% palladium on activated carbon (19 mg) in MeOH (5 ml) was added 15e (95 mg, 0.28 mmol). The mixture was stirred with hydrogen balloon at room temperature for 1 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was dissolved in MeOH and filtered through a membrane filter. The filtrate was concentrated to give 16e (70 mg, 99%). White solid; TLC Rf 0.16 (CH2Cl2/MeOH = 30/1); m.p. 147 °C; MS (ESI) m/z 255 [M + H]+; 1H-NMR (DMSO-d6) δ 9.01 (s, 1H), 8.52 (br s, 1H), 4.11 (dd, J = 5.4, 3.9 Hz, 2H), 3.52 (dd, J = 5.4, 4.0 Hz, 2H), 3.27 (s, 3H), 2.28 (s, 3H), 2.12 (s, 3H), 2.00 (s, 3H); 13 C-NMR (DMSO-d6) δ 154.74, 147.70, 141.40, 140.31, 133.77, 126.35, 70.29, 63.20, 58.05, 19.31, 14.25, 12.63.
2.1.30. N-(5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)methanesulfonamide (17a)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 50 mg, 0.21 mmol) in pyridine (2 ml) was added methanesulfonyl chloride (48 µL, 0.62 mmol). The mixture was stirred at room temperature for 24 h and then concentrated. The residue was diluted with CH2Cl2 and washed with brine. The CH2Cl2 layer was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 40/1 to 20/1) to give 17a (34 mg, 52%). White solid; TLC Rf 0.35 (Hexanes/EtOAc = 1/1); m.p. 122 °C; MS (ESI) m/z 321 [M + H]+; 1H-NMR (CDCl3) δ 9.23 (br, s, 1H), 7.52–7.29 (m, 5H), 4.75 (s, 2H), 3.23 (s, 3H), 2.33 (s, 3H), 2.23 (s, 3H), 2.15 (s, 3H); 13 C-NMR (CDCl3) δ 147.81, 145.88, 144.54, 140.41, 136.48, 128.86 (2 C), 128.66, 128.23 (2 C), 123.99, 75.68, 42.92, 17.18, 13.77, 13.74.
2.1.31. N-(5-Hydroxy-3,4,6-trimethylpyridin-2-yl)methanesulfonamide (18a)
To a suspension of 10% palladium on activated carbon (5 mg) in a mixed solvent of MeOH (2.5 ml) and CH2Cl2 (2.5 ml) was added 17a (26 mg, 0.08 mmol). The mixture was stirred with hydrogen balloon at room temperature for 1 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was dissolved in MeOH and filtered through a membrane filter and concentrated to give 18a (19 mg, 100%). Pale yellow solid; TLC Rf 0.2 (CH2Cl2/MeOH = 20/1); m.p. 142 °C; MS (ESI) m/z 231 [M + H]+; 1H-NMR (DMSO-d6) δ 9.00 (s, 2H), 3.20 (s, 3H), 2.30 (s, 3H), 2.11 (s, 3H), 2.10 (s, 3H); 13 C-NMR (DMSO-d6) δ 147.22, 141.11, 140.84, 134.54, 124.95, 42.20, 19.48, 14.07, 12.69.
2.1.32. N-(5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)-4-methylbenzenesulfonamide (17 b)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 100 mg, 0.41 mmol) in pyridine (2 ml) was added p-toluenesulfonyl chloride (87 mg, 0.45 mmol). The mixture was stirred at room temperature for 2 h and then concentrated. The residue was diluted with CH2Cl2 and washed with brine. The CH2Cl2 layer was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 3/1) to give 17 b (82 mg, 50%). White solid; TLC Rf 0.34 (Hexanes/EtOAc = 2/1); m.p. 198 °C; MS (ESI) m/z 397 [M + H]+; 1H-NMR (CDCl3) δ 7.83 (d, J = 8.3 Hz, 2H), 7.43–7.33 (m, 5H), 7.24 (d, J = 8.0 Hz, 2H), 4.72 (s, 2H), 2.39 (s, 3H), 2.25 (s, 3H), 2.21 (s, 3H), 2.16 (s, 3H); 13 C-NMR (CDCl3) δ 149.33, 146.34, 144.21, 142.36, 140.62, 136.70, 136.15, 129.26 (2 C), 128.88 (2 C), 128.76, 128.29 (2 C), 126.54 (2 C), 125.87, 75.93, 21.62, 15.88, 14.06, 13.85.
2.1.33. N-(5-Hydroxy-3,4,6-trimethylpyridin-2-yl)-4-methylbenzenesulfonamide (18 b)
To a suspension of 10% palladium on activated carbon (26 mg) in MeOH (10 ml) was added 17 b (130 mg, 0.33 mmol). The mixture was stirred with hydrogen balloon at room temperature for 2 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was dissolved in MeOH and filtered through a membrane filter and concentrated to give 18 b (86 mg, 86%). White solid; TLC Rf 0.21 (Hexanes/EtOAc = 1/1); m.p. 135 °C; MS (ESI) m/z 307 [M + H]+; 1H-NMR (DMSO-d6) δ 9.13 (br, s, 2H), 7.72 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 8.2 Hz, 2H), 2.36 (s, 3H), 2.12 (s, 3H), 2.10 (s, 3H), 2.09 (s, 3H); 13 C-NMR (DMSO-d6) δ 146.78, 141.98, 140.59, 140.41, 139.53, 134.66, 128.71 (2 C), 127.32 (2 C), 124.58, 20.95, 18.72, 14.05, 12.61.
2.1.34. N-(5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)naphthalene-1-sulphonamide (17c)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 50 mg, 0.21 mmol) in CH2Cl2 (1 ml) was added 1-napthalenesulfonyl chloride (70 mg, 0.31 mmol) followed by addition of triethylamine (57 µL, 0.41 mmol). The mixture was stirred at room temperature for 70 h. The reaction mixture was diluted with CH2Cl2 and washed with sat. NaHCO3 and brine. The CH2Cl2 layer was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 15/1 to 2/1) to give 17c (54 mg, 61%). Pale yellow solid; TLC Rf 0.31 (Hexanes/EtOAc = 2/1);) ; m.p. 153 °C; MS (ESI) m/z 433 [M + H]+; 1H-NMR (CDCl3) δ 11.85 (br s, 1H), 9.03 (d, J = 8.5 Hz, 1H), 8.26 (dd, J = 7.3, 1.0 Hz, 1H), 7.96 (d, J = 8.2 Hz, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.65 (ddd, J = 8.5, 6.9, 1.4 Hz, 1H), 7.56 (dd, J = 11.0, 4.0 Hz, 1H), 7.50–7.43 (m, 1H), 7.43–7.29 (m, 5H), 4.69 (s, 2H), 2.23 (s, 3H), 2.18 (s, 3H), 2.12 (s, 3H); 13 C-NMR (CDCl3) δ 160.04, 150.54, 147.41, 143.15, 139.48, 136.05, 134.45, 132.93, 128.90 (2 C), 128.82, 128.75, 128.47, 128.31 (2 C), 127.54, 126.78, 126.70, 126.63, 126.43, 124.06, 76.11, 15.30, 14.19, 13.68.
2.1.35. N-(5-Hydroxy-3,4,6-trimethylpyridin-2-yl)naphthalene-1-sulphonamide (18c)
To a suspension of 10% palladium on activated carbon (22 mg) in MeOH (5 ml) was added 17c (109 mg, 0.25 mmol). The mixture was stirred with hydrogen balloon at room temperature for 8 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 2/1 to 1/1) to give 18c (77 mg, 89%). Pale yellow solid; TLC Rf 0.32 (CH2Cl2/MeOH = 15/1); m.p. 95 °C; MS (ESI) m/z 343 [M + H]+; 1H-NMR (CDCl3) δ 8.92 (d, J = 8.5 Hz, 1H), 8.17 (dd, J = 7.3, 0.9 Hz, 1H), 7.90 (d, J = 8.2 Hz, 1H), 7.83 (d, J = 7.9 Hz, 1H), 7.68–7.56 (m, 1H), 7.52 (dd, J = 11.0, 4.0 Hz, 1H), 7.47–7.35 (m, 1H), 2.27 (s, 3H), 2.14 (s, 3H), 2.04 (s, 3H); 13 C-NMR (CDCl3) δ 148.37, 144.31, 141.04, 139.26, 134.40, 132.99, 129.12, 128.60, 128.56, 127.52, 126.63, 126.46, 126.42, 125.67, 124.04, 15.50, 13.93, 13.59.
2.1.36. N-(5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)-4-(trifluoromethyl)benzenesulfonamide (17d)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 150 mg, 0.62 mmol) in pyridine (3 ml) was added 4-(trifluoromethyl)benzenesulfonyl chloride (227 mg, 0.93 mmol). The mixture was stirred at room temperature for 24 h and then concentrated. The residue was diluted with CH2Cl2 and washed with sat. NaHCO3 and brine. The CH2Cl2 layer was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 3/1) to give 17d (231 mg, 83%). White solid; TLC Rf 0.33 (Hexanes/EtOAc = 3/1); m.p. 131 °C; MS (ESI) m/z 451 [M + H]+; 1H-NMR (CDCl3) δ 11.72 (s, 1H), 8.07 (d, J = 8.2 Hz, 2H), 7.69 (d, J = 8.3 Hz, 2H), 7.45–7.32 (m, 5H), 4.74 (s, 2H), 2.27 (s, 3H), 2.24 (s, 3H), 2.16 (s, 3H); 13 C-NMR (CDCl3) δ 150.14, 147.69, 143.80, 135.98, 135.23, 133.21 (q, J = 32.9 Hz), 128.90 (2 C), 128.84, 128.31 (2 C), 126.70 (4 C), 126.27, 125.81 (q, J = 3.7 Hz), 121.47, 76.10, 15.52, 14.23, 13.69.
2.1.37. N-(5-Hydroxy-3,4,6-trimethylpyridin-2-yl)-4-(trifluoromethyl)benzenesulfonamide (18d)
To a suspension of 10% palladium on activated carbon (27 mg) in MeOH (5 ml) was added 17d (146 mg, 0.32 mmol). The mixture was stirred with hydrogen balloon at room temperature for 4 h. After filtration of the reaction mixture through a Celite pad, the filtrate was concentrated. The residue was dissolved in MeOH and filtered through a membrane filter and concentrated to give 18d (82 mg, 70%). White solid; TLC Rf 0.32 (CH2Cl2/MeOH = 15/1); m.p. 165 °C; MS (ESI) m/z 361 [M + H]+; 1H-NMR (DMSO-d6) δ 10.17 (s, 1H), 8.55 (s, 1H), 8.03 (d, J = 8.3 Hz, 2H), 7.93 (d, J = 8.4 Hz, 2H), 2.12 (s, 3H), 2.10 (s, 3H), 2.07 (s, 3H); 13 C-NMR (DMSO-d6) δ 146.89, 146.42, 140.31, 131.62 (dd, J = 64.2, 32.1 Hz), 128.17 (2 C), 125.83, 125.58 (q, J = 3.8 Hz), 124.84 (2 C), 121.50, 18.40, 13.93, 12.66.
2.1.38. N-(5-(benzyloxy)-3,4,6-trimethylpyridin-2-yl)-4-nitrobenzenesulfonamide (17e)
To a solution of 5-benzyloxy-3,4,6-trimethylpyridin-6-amine (5, 100 mg, 0.41 mmol) in pyridine (2 ml) was added 4-nitrobenzenesulfonyl chloride (110 mg, 0.50 mmol). The mixture was stirred at room temperature for 4 h and then concentrated. The residue was diluted with CH2Cl2 and washed with sat. NaHCO3 and brine. The CH2Cl2 layer was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 4/1) to give 17e (126 mg, 71%). Yellow solid; TLC Rf 0.25 (Hexanes/EtOAc = 2/1); m.p. 62 °C; MS (ESI) m/z 428 [M + H]+; 1H-NMR (CDCl3) δ 11.86 (br s, 1H), 8.25 (d, J = 8.7 Hz, 2H), 8.10 (d, J = 8.8 Hz, 2H), 7.49–7.31 (m, 5H), 4.74 (s, 2H), 2.28 (s, 3H), 2.24 (s, 3H), 2.14 (s, 3H); 13 C-NMR (CDCl3) δ 150.09, 149.30, 148.24, 143.85, 135.85, 135.09, 135.07, 128.86 (2 C), 128.83, 128.27 (2 C), 127.28 (2 C), 126.36, 123.97 (2 C), 76.10, 15.44, 14.27, 13.63.
2.1.39. N-(5-Hydroxy-3,4,6-trimethylpyridin-2-yl)-4-nitrobenzenesulfonamide (18e)
To a suspension of 17e (74 mg, 0.17 mmol) in CH2Cl2 (2 ml), was added pentamethylbenzene (77 mg, 0.52 mmol) followed by dropwise addition of boron trichloride (1 M in CH2Cl2, 0.35 ml) at 0 °C. The reaction mixture was stirred at 0 °C for 15 min then quenched with a mixed solvent of CHCl3/MeOH (9/1, 1.5 ml) and stirred at room temperature for 30 min. After concentration of the reaction mixture, the residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 20/1) to give 18e (47 mg, 81%). Yellow solid; TLC Rf 0.29 (Hexanes/EtOAc = 2/1); m.p. 199 °C; MS (ESI) m/z 388 [M + H]+; 1H-NMR (CD3OD) δ 8.36 − 8.27 (m, 2H), 8.09 − 8.00 (m, 2H), 2.28 (s, 3H), 2.19 (s, 3H), 2.10 (s, 3H); 13 C-NMR (CD3OD) δ 150.91, 150.53, 147.15, 144.33, 141.49, 137.52, 129.19 (2 C), 127.54, 124.85 (2 C), 16.80, 14.01, 13.35.
2.1.40. 2-Bromo-5-methoxy-3,4,6-trimethylpyridine (19)
To a solution of 6-bromo-2,4,5-trimethylpyridin-3-ol (3, 216 mg, 1.0 mmol) of CH3CN (10 ml) were added K2CO3 (207 mg, 1.5 mmol) and iodomethane (0.12 ml, 2.0 mmol). The mixture was stirred at 50 °C for 16 h and then cooled to room temperature. It was diluted with EtOAc and washed with 1 M HCl, water and brine successively. The EtOAc solution was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 9/1) to give 19 (154 mg, 67%). Yellow oil; TLC Rf 0.37 (Hexanes/EtOAc = 9/1); MS (ESI) m/z 230 [M + H]+; 1H-NMR (CDCl3) δ 3.67 (s, 3H), 2.44 (s, 3H), 2.30 (s, 3H), 2.24 (s, 3H); 13 C-NMR (CDCl3) δ 152.76, 150.07, 141.29, 137.88, 131.95, 60.33, 18.88, 18.77, 13.50.
2.1.41. N-(diphenylmethylene)-5-methoxy-3,4,6-trimethylpyridin-2-amine (20)
To a suspension of 19 (155 mg, 0.67 mmol) in toluene (5 ml) were added BINAP (42 mg, 0.07 mmol), Pd2(dba)3 (31 mg, 0.03 mmol), NaOBut (71 mg, 0.74 mmol), and benzophenone imine (0.11 ml, 0.67 mmol). The mixture was stirred at 115 °C for 4 h and then cooled to room temperature. It was diluted with EtOAc and washed with water and brine. The EtOAc layer was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (Hexanes/EtOAc = 4/1) to give 20 (170 mg, 77%). Yellow solid; TLC Rf 0.15 (Hexanes/EtOAc = 4/1); m.p. 95 °C; MS (ESI) m/z 331 [M + H]+; 1H-NMR (CDCl3) δ 7.80 − 7.36 (m, 10H), 3.66 (s, 3H), 2.52 (s, 3H), 2.29 (s, 3H), 2.10 (s, 3H); 13 C-NMR (CDCl3) δ 169.36, 156.96, 149.30, 146.80, 139.56, 139.23, 136.89, 130.80, 129.60, 128.89, 128.56, 127.98, 127.53, 119.56, 60.36, 18.74, 13.79, 12.37.
2.1.42. 5-Methoxy-3,4,6-trimethylpyridin-2-amine (21)
A solution of 20 (168 mg, 0.51 mmol) in a mixed solvent of THF/MeOH (1/10, 5.5 ml) was cooled to 0 °C. A mixed solution of CH3COCl/MeOH (1/10, 1 ml) was slowly added to the mixture. The reaction mixture was stirred at room temperature for 18 h and then concentrated. The residue was diluted with EtOAc and water. The EtOAc layer separated was extracted with water. To the combined aqueous solution was added 1 M NaOH until the pH of the solution reached 10. After extraction of the mixture with EtOAc, the EtOAc solution was dried over MgSO4, filtered and concentrated. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 30/1) to give 21 (54 mg, 64%). Grey solid; TLC Rf 0.15 (CH2Cl2/MeOH = 20/1); m.p. 84 °C; MS (ESI) m/z 167 [M + H]+; 1H-NMR (DMSO-d6) δ 5.22 (s, 2H), 3.52 (s, 3H), 2.17 (s, 3H), 2.06 (s, 3H), 1.91 (s, 3H); 13 C-NMR (CDCl3) δ 152.25, 146.62, 145.79, 139.72, 113.51, 60.53, 18.45, 13.05, 12.42.
2.1.43. 1–(3,4-Dichlorophenyl)-3–(5-methoxy-3,4,6-trimethylpyridin-2-yl)thiourea (22a)
To a solution of 21 (25 mg, 0.15 mmol) in EtOH (2 ml) was added 3,4-dichlorophenyl isothiocyanate (24 μL, 0.16 mmol). The reaction mixture was stirred at room temperature for 48 h and then concentrated. The residue was purified by recrystallization over EtOH to give 22a (39 mg, 70%). White solid; TLC Rf 0.63 (Hexanes/EtOAc = 4/1); m.p. 176 °C; MS (ESI) m/z 370 [M + H]+; 1H-NMR (DMSO-d6) δ 13.05 (s, 1H), 9.54 (s, 1H), 8.22 (s, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.59 (t, J = 7.6 Hz, 1H), 7.55 − 7.47 (m, 1H), 3.66 (s, 3H), 2.41 (s, 3H), 2.21 (s, 3H), 2.19 (s, 3H); 13 C-NMR (DMSO-d6) δ 178.41, 149.52, 145.53, 145.11, 141.92, 139.20, 130.49, 130.22, 126.54, 124.63, 123.60, 121.50, 60.21, 18.43, 13.23, 12.67.
2.1.44. 1–(5-Methoxy-3,4,6-trimethylpyridin-2-yl)-3–(3-(trifluoromethyl)phenyl)thiourea (22 b)
To a solution of 21 (25 mg, 0.15 mmol) in EtOH (2 ml) was added 3-(trifluoromethyl)phenyl isothiocyanate (24 μL, 0.16 mmol). Reaction mixture was stirred at room temperature for 48 h and then concentrated. The residue was purified with silica flash column chromatography (Hexanes/EtOAc = 4/1) to give 22 b (30 mg, 54%). White solid; TLC Rf 0.46 (Hexanes/EtOAc = 4/1); m.p. 140 °C; MS (ESI) m/z 370 [M + H]+; 1H-NMR (DMSO-d6) δ 13.05 (s, 1H), 9.54 (s, 1H), 8.22 (s, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.59 (t, J = 7.6 Hz, 1H), 7.55 − 7.47 (m, 1H), 3.66 (s, 3H), 2.41 (s, 3H), 2.21 (s, 3H), 2.19 (s, 3H); 13 C-NMR (DMSO-d6) δ 178.52, 149.47, 145.59, 145.06, 141.90, 139.91, 129.67, 128.98 (q, J = 31.6 Hz), 127.27, 125.37, 122.67, 121.27, 119.61, 60.20, 18.42, 13.20, 12.66.
2.2. Materials for biology
RPMI-1640, foetal bovine serum (FBS), penicillin/streptomycin and trizol reagent were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). Trypsin/EDTA was purchased from Clonetics, Inc. (Walkersville, MD, USA). Recombinant human TNF-α was procured from R & D system Inc, (Minneapolis, MN, USA). BCECF/AM (acetoxymethyl ester) was received from Molecular probes (Eugene, Oregon, USA). Antibodies directed against TNF-α, IL-1β, Claudin-2, NLRP3, Claudin-3 and Caspase-1 were purchased from Abcam (Cambridge, MA, USA). Antibodies against IL-6 and IL-10 were obtained from Abbiotec (San Diego, CA, USA). E-cadherin, ZO-1, IL-18 and I-κB antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) while NF-κB, TGFβ and phospho-I-κB antibodies were purchased from Cell Signalling Technology Inc. (Beverly, MA, USA).
2.3. Cell culture
HT-29 human colonic epithelial cell line and U937 human pre-monocytic cell line were obtained from the American Type Culture Collection (Manassas, VA, USA). HT-29 cells and U937 cells were cultured in RPMI-1640 media containing 10% FBS, 100 IU/ml of penicillin and 100 μg/mL of streptomycin. Cells were maintained at 37 °C in 5% CO2 in humidifier incubator.
2.4. Measurements of monocyte adhesion to epithelial cell layer
Adhesion of monocytes to colon epithelial cell layer was measured by using U937 pre-monocytic cells prelabeled with 2′,7′-bis(2-carboxyethyl)-5(6)-carboxylfluorescein acetoxymethyl ester (BCECF/AM, 10 µg/ml) as previously reportedCitation21,Citation22 with slight modification. HT-29 cells (2 × 105 cells/well) cultured in 48-well plates were pre-treated with compounds for 1 h. The prelabeled U937 cells were added on the monolayer of HT-29 cells, and they were treated with TNF-α (10 ng/mL) for 3 h at 37 °C. Non adhering U937 cells were removed by washing thrice with PBS. Cells were lysed with 0.1% Triton X-100 in Tris (0.1 M), and fluorescence intensity was measured using Fluostar Optima microplate reader (BMG LABTECH GmbH, Germany) at an excitation and emission wavelengths of 485 and 520 nm, respectively.
2.5. TNBS-induced colitis in rats
Sprague-Dawley female rats (seven weeks old) were purchased from Orient Bio Inc (Gyeonggi, South Korea) and were acclimatised to the lab condition for 1 week by giving food and water provided ad libitum. Rats were fasted for 24 h before TNBS injection (0.8 ml of 5% TNBS in 50% ethanol) into the lumen of the colon (8 cm proximal to the anus through the rectum) through an polyethylene catheter fitted onto a 1 ml syringe. Right after TNBS injection, rats were kept in vertical position for 1 min before being returned to the cage. Next day, sulfasalazine (300 mg/kg) or compounds (1 mg/kg) in corn oil was administered for up to 5 days. The doses of the drug were selected based on the previous studiesCitation18,Citation19. On the sixth day, all the rats were sacrificed and macroscopic observations were evaluated for ulceration and severity of colitis. The colon tissues from 5 to 7 cm proximal to rectum were excised. The colon tissues were stored at −80 °C to examine the protein expression of inflammatory mediators, junctional molecules, and transcription factors.
The study protocol of the animal experiment was reviewed and approved beforehand by the Institutional Animal Care and Use Committee of Yeungnam University and were performed following the institutional guidelines of the Institute of Laboratory Animal Resources (1996), and of Yeungnam University for the care and use of animals (2009).
2.6. Measurement of myeloperoxidase level
Colon myeloperoxidase (MPO) level was measured by using MPO Rat ELISA kit (HK105-01, Hycult biotechnology, Netherland). Colon tissues were weighed, washed in cold 1× PBS (pH: 7.4), and homogenised in 500 μL of ice-cold lysis buffer (pH 7.4, 200 mM NaCl, 5 mM EDTA, 10 mM Tris, 10% glycerol, 1 mM PMSF, 1 µg/mL leupeptide, 28 µg/mL aprotinin) by using a Bead Blaster (Benchmark scientific, Edison, NJ, USA). MPO amount in the supernatant was measured by detecting the absorbance at 450 nm in microplate reader (Spectrostar Nano, BMG LABTECH, Ortenberg, Germany).
2.7. Protein extraction and Western blotting
Total protein from the colon was extracted by using radioimmunoprecipitation assay (RIPA) buffer (150 mM Sodium chloride, 1% Triton X-100, 0.5% Sodium deoxycholate, 0.1% SDS and 50 mM Tris adjusted to pH 8.0) containing 1X proteases and phosphatase inhibitor (Thermo Scientific, Rockford, USA). Cytosolic and nuclear proteins were extracted by using manufacturer’s instruction given by NE-PER nuclear and cytoplasmic extraction reagent kit (#78833, Thermo Scientific, Rockford, USA). Protein concentrations were measured using a BCA protein assay kit (Pierce, Rockford, IL, USA). Equal amounts of total proteins were then separated by SDS-PAGE and transferred onto Hybond ECL nitrocellulose membranes (Amersham Life Science, Buckinghamshire, UK) at 200 mA for 1 h. Blocking was done using 5% BSA in Tris-buffered saline (TBS)-Tween 20 (TBS-T) at 20 °C for 1 h. The membranes were incubated for 16 h at 4 °C with primary antibody diluted in TBS containing 3% BSA. After incubation, the membranes were washed three times with TBS-T. Next, the membrane was incubated for 1 h at 20 °C with horseradish peroxidase-conjugated secondary antibody in TBS. Immunoreactive proteins were visualised using an enhanced chemiluminescence (ECL) kit (Pierce, Rockford, IL, USA) and digitally processed using an LAS-4000 mini (Fuji, Japan). The membranes were stripped and re-probed with an actin antibody as a loading control.
2.8. Statistical analysis
Data are presented as the mean ± SEM, and were analysed by one way ANOVA followed by the Newman–Keuls comparison method using GraphPad Prism software (version 5.0, San Diego, CA, USA). P values less than 0.05 were considered statistically significant.
3. Results
3.1. Design and synthesis
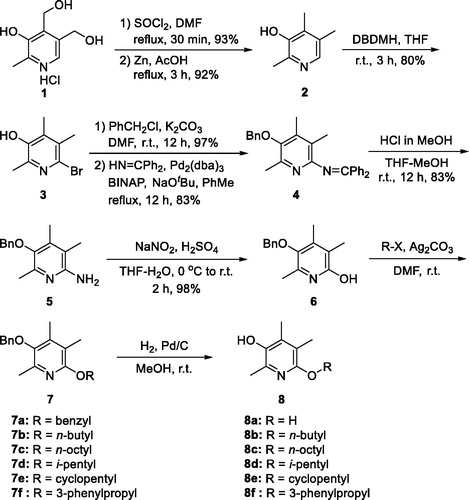
At first, we were curious about the effect of heteroatom at C(6)-position on activity and replaced nitrogen with oxygen to give 6-alkoxy analogues 8. Synthesis of 8 was conducted via intermediate 5 which has been reported by us (Scheme 1)Citation15. Briefly, two primary hydroxy groups of pyridoxine·HCl (1) was converted to chlorides using SOCl2 and catalytic amount of DMF. Then, two benzyl chloride groups were reductively removed with Zn and acetic acid under refluxing conditions to give 2,4,5-trimethylpyridin-3-ol (2). The C(6)-position was brominated using 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) and the phenolic OH group of 3 was protected as benzyloxy group. Bromide on C(6)-position was replaced with benzophenone imine under Buchwald–Hartwig amination conditions to afford 4. The imine group was then methanolyzed to give the free amino compound (5). The amino group of 5 was converted to hydroxy group via diazonium ion to give 6, which was then treated with various alkyl halides to give alkoxy compounds 7. Lastly, the benzyl protective group was removed by catalytic hydrogenolysis to give 6-alkoxy-2,4,5-trimethylpyridin-3-ol analogues 8. The dihydroxy compound 8a was obtained from the dibenzyloxy compound 7a. A direct approach to obtain 8a from 6 under the standard catalytic hydrogenolysis reaction conditions gave poor yield.
Unfortunately, all alkoxy analogues (8a–8f) showed modest activity (29–67%) in our cell adhesion assay (. Compound 8a (67% inhibition) and 8f (56% inhibition) showed 2.9- and 2.1-fold higher activity than the corresponding nitrogen counterparts whose inhibition level was 23% and 27%, respectively. Contrarily, compound 8e (29% inhibition) showed 2.5-fold less activity than the corresponding nitrogen counterpart (73% inhibition)Citation18. The rest of alkoxy analogues with alkyl groups (8 b, 8c, 8d) did not show distinct activity from 6-alkylamino analogues. It seems that nitrogen atom does not necessarily be replaced and might be better to remain on C(6)-position for the better topological polar surface area (tPSA) and calculated partition coefficient (cLogP). By locating nitrogen back to C(6)-position, about 10% of both tPSA and cLogP values can be restored compared to the alkoxy analogues. Therefore, we decided to explore structural diversity with nitrogen atom on the position. 
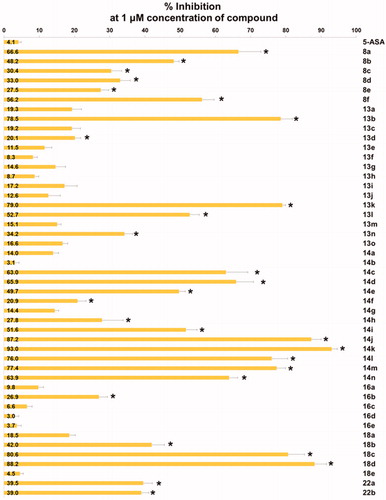
Figure 2. Inhibitory activity of the compounds against TNF-α-induced monocyte adhesion to colon epithelial cells. Data are shown as mean ± SEM of at least three independent experiments. *p < 0.05 compared to TNF-α-induced HT-29 cells.
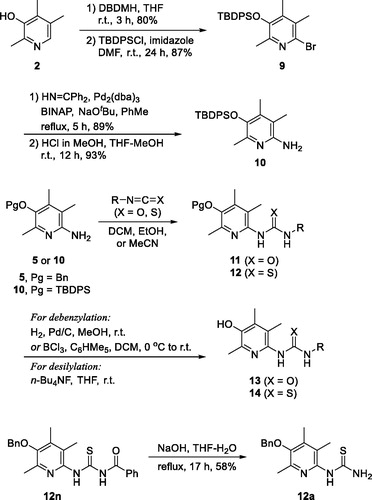
Now that we have reported that 6-(substituted)amino-2,4,5-trimethylpyridin-3-ols have high activity against colitis in vitro and in vivo, structural exploration with nitrogen on C(6)-position was designed to install ureido-, thioureido-, carbamato- and sulfonamido-linkage between pyridine ring and R group.
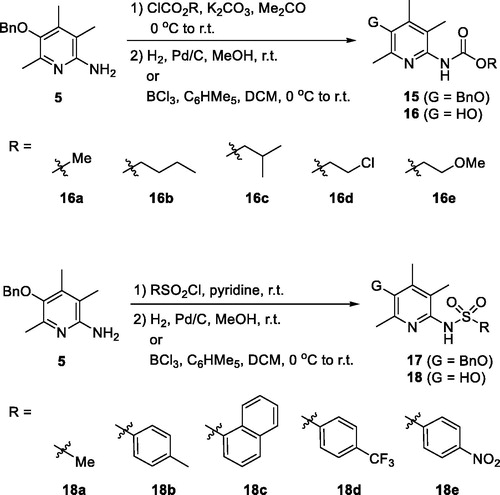
Described in Scheme 2 is the general scheme for the preparation of urea and thiourea analogues, 13 and 14. Isocyanates (R − N = C = O)/isothiocyanates (R − N = C = S) were employed for the construction of urea/thiourea moieties. Two key intermediates, 5 and 10, were used for the coupling reactions and the TBDPS-protected intermediate (10) was prepared by the synthetic procedure which we reported beforeCitation20. In short, compound 10 was prepared from 2,4,5-trimethylpyridin-3-ol (2) by the following sequence; bromination of C(6)-position, protection of − OH with TBDPS group, palladium-catalyzed amination with benzophenone imine, and acidic methanolysis of the imine group. The coupled products with isocyanates and isothiocyanates, 11 and 12, were then transformed the final compounds, 13 and 14, by deprotection of O-benzyl or O-silyl group. For 14a compound with − NH2 group, the precursor, O-benzyl protected compound 12a, was obtained from N-benzoyl thiourea compound 12n by a debenzoylation under basic hydrolysis conditions.
Scheme 2. Synthesis of ureido- and thioureido-analogues, 13 and 14.
For the synthesis of sulfonamido- (16) and carbamato-analogues (18), the key intermediate 5 was treated with either chloroformates or sulphonyl chlorides to give 15 and 17. They were then debenzylated to afford 16 and 18, respectively (Scheme 3).
3.2. Inhibitory effects of 2,4,5-trimethylpyridin-3-ol derivatives on TNF-α-induced adhesion of monocytes to colonic epithelial cells
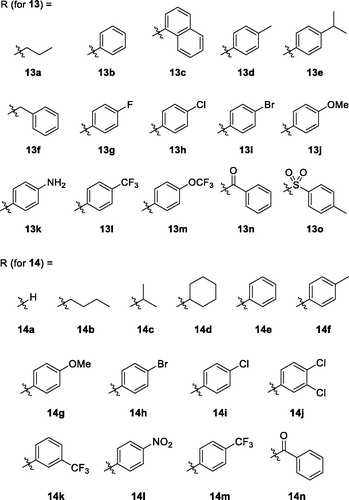
In vitro activity data of the alkoxy- (8), ureido- (13), thioureido- (14), carbamato- (16) and sulfonamido-analogues (18) are listed in . As stated above, alkoxy analogues (8) showed activity in a somewhat similar range to the nitrogen counterparts. The best activity (67%) was seen in compound 8a which has no substituent on C(6)-oxygen. Activity of urea (13) and thiourea (14) analogues are also in a similar range. Among the compounds with the same R group, the phenyl group is more active in urea analogue (13 b: 76.3%) than in thiourea analogue (14e: 49.7%). Both p-tolyl and p-methoxyphenyl groups showed similar and low activity both in urea analogues (13d: 20.1%, 13j: 12.6%, respectively) and thiourea analogues (14f: 19.2%, 14 g: 13.3%, respectively). IC50 values of some compounds of high % inhibition were measured to be 0.75 μM (8f), 0.41 μM (13 b), 0.40 μM (14n), 0.32 μM (18d), and 0.23 μM (18c). The order of these values is quite lined with the order of % inhibition measured at the fixed single concentration (1 μM).
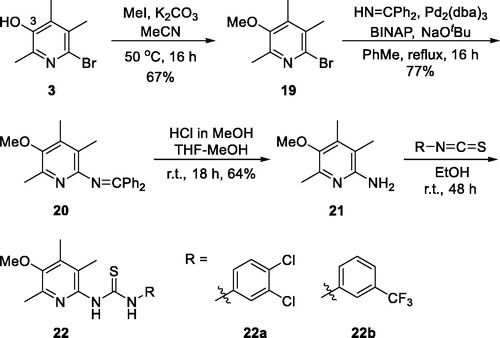
Next, we tried to investigate the role –OH group on C(3)-position on the activity. The hydroxy group is protected as methoxy group as shown in structure 22 to study if the OH group is essential (Scheme 4). Compounds 22a and 22 b are 3-methoxy analogues of 14j and 14k, respectively, which showed excellent activity in our in vitro assay as shown in . The intermediate 3 is methylated with iodomethane and K2CO3 to give 3-methoxy compound 19. Then, benzophenone imine was coupled under Buchwald-Hartwig amination conditions to afford imino compound 20 which in turn was hydrolysed to free amino compound 21. The amino group of 21 was coupled with isothiocyanates to give 22a and 22 b. As shown in , unfortunately, 3-methoxy analogues 22a and 22 b showed more than 2-fold less activity than corresponding 3-hydroxy analogues, 14j and 14k, respectively. It might imply that free hydroxyl group at 3-position is essential to the activity by contributing some sort of hydrogen bonding to a binding partner which is responsible for the activity. Further detail of the role of hydroxyl group will be investigated in subsequent studies.
3.3. Anti-inflammatory effects of seven 2,4,5-trimethylpyridin-3-ol derivatives on TNBS-induced rat colitis
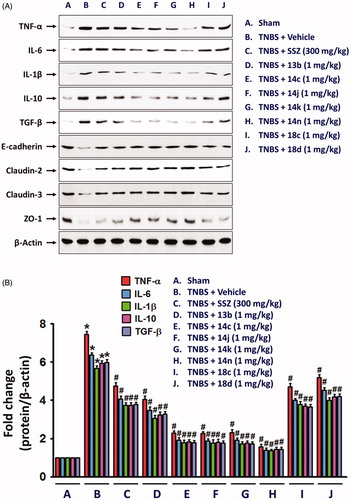
Next, we evaluated in vivo anti-inflammatory activity using TNBS-induced rat colitis model. Seven pyridin-3-ol compounds, 13 b, 14c, 14j, 14k, 14n, 18c, and 18d, were selected for the in vivo efficacy evaluation along with sulfasalazine (SSZ) as the positive control drug. Colitis was induced by rectal administration of TNBS. Our seven pyridin-3-ol compounds along with SSZ were orally administered once a day for five days starting one-day after administration of TNBS at a dose of 1 mg/kg (for seven pyridin-3-ols) and 300 mg/kg (for SSZ), respectively. After treatment with TNBS, the rats showed signs of colitis, such as body weight decrease, bloody diarrhoea, and sluggish and weak movement. Compared to the sham-operated control group, the body weight of the TNBS-treated rats was significantly reduced and then increased gradually. Colon tissues in the TNBS-treated lesion site showed significant inflammation, as revealed by oedema and adhesion in gross morphology examination and increased wet weight per colon length. As a macroscopic marker to represent a disease phenotype, we measured the recovery level in body- and colon-weights upon drug treatment (. Oral administration of rats with compounds, 13 b, 14c, 14j, 14k, 14n, 18c, or 18d (1 mg/kg) significantly restored TNBS-induced body weight loss (. The recovery rate by the compounds (1 mg/kg) ranged from 25% to 98.9%, among which compound 14n was the most excellent. The recovery rate of SSZ (300 mg/kg), a positive control, was 48.8%. Colon weight recovery was between 50.3% and 94.9%, which was better than the body weight recovery in most cases of oral administration of the compounds including sulfasalazine (. Compound 14n (1 mg/kg) was the most effective in colon weight recovery as in the case of body weight recovery. In addition, the myeloperoxidase (MPO) level in colon tissues, which is directly related to neutrophil infiltration into tissues and serves as a biochemical marker of inflammationCitation23, was examined. TNBS treatment induced a tremendous increase in MPO activity (), and the increase was significantly suppressed by oral administration with compounds (1 mg/kg). The order of the compounds having excellent effects was 14n, 14k, 14c, 14j, 13 b, 18c, and 18d, demonstrating the significant efficacy of the new scaffold.
Figure 3. Recovery effects of compounds on TNBS-induced rat colitis, body and colon weights, and MPO activity. Colitis was induced by rectal administration of TNBS, and sham group received vehicle (50% ethanol) in the same route. Compounds (1 mg/kg) and SSZ (300 mg/kg) were given orally 1 day after TNBS treatment. Data represent the mean ± SEM for five rats per group. (A) Body weight was recorded daily from day 0 to day 5, and body weight recovery was calculated based on the last day measurement. Colon wet weight (distal 5–6 cm segment) was measured right after dissection of the colon. #p < 0.05 compared to TNBS-treated group. $p < 0.05 compared to SSZ-treated group. (B) MPO level of colon tissues *p < 0.05 compared to sham-operated control group. #p < 0.05 compared to TNBS-treated group.
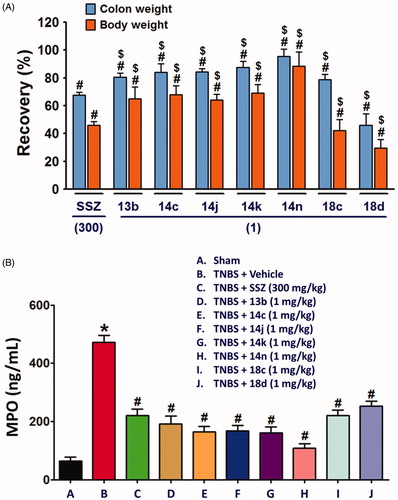
Figure 4. Inhibitory effects of compounds on expressions of inflammatory cytokines and epithelial junction molecules. Total protein extracts from colon tissues homogenates were used for detecting the protein expression of inflammatory mediators, TNFα, IL-6, and IL-1β, and junction molecules, E-cadherin, claudin-2, claudin-3, and ZO-1. The bar graphs represent the mean ± SEM of protein expression quantified in three independent experiments. *p < 0.05, compared with vehicle-treated control group. #p < 0.05, compared with the TNBS-treated group.
3.4. Suppressive effects of seven 2,4,5-trimethylpyridin-3-ol derivatives on TNBS-induced expression changes of inflammatory cytokines, junction molecules, inflammasome, and nuclear transcription factor-κB
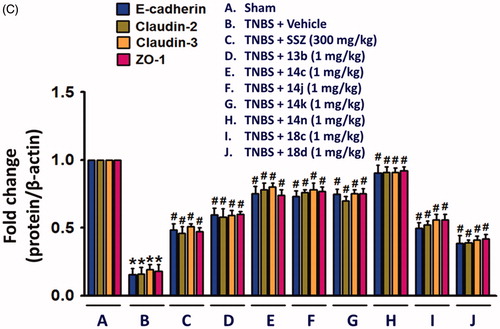
Although the fundamental molecular targets of the compounds responsible for the phenotype are yet to be investigated, we further examined in this study the inhibitory effects of the compounds on the expression levels of cytokines responsible for the inflammatory response. It will provide more consistency to our mechanism based on the assay against TNF-α-induced cell adhesion. In rat colon tissues, TNBS treatment significantly increased the expression of TNF-α, IL-6 and IL-1β. The expression level of IL-10 and TGF-β which are known to be secreted by M2 macrophages for termination of inflammation and tissue repairCitation24,Citation25, were also significantly increased in the inflamed colon tissues (. In contrast, level of colon epithelial junction molecules, E-cadherin, Claudin-2, Claudin-3 and ZO-1 was significantly reduced by TNBS (. However, oral administration of compounds, 13b, 14c, 14j, 14k, 14n, 18c, or 18d (1 mg/kg) significantly restored the TNBS-altered protein expressions of junctional molecules. All these changes consistently and strongly support the anti-IBD activity of our compounds. Indeed, the inhibitory effects of compounds, 14c, 14j, 14k, and 14n (1 mg/kg) was much stronger than that of 300 mg/kg SSZ.
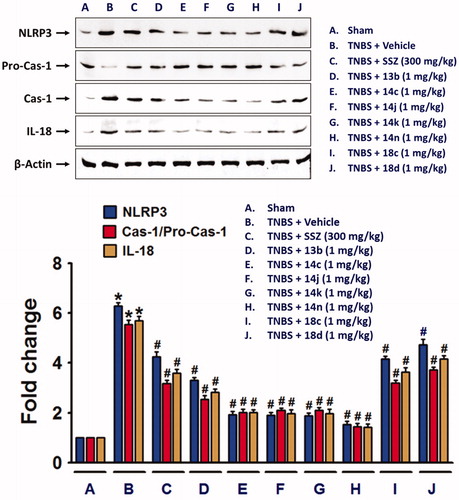
Next, we also examined whether NLRP3 inflammasome, which plays an important role in IL-1β productionCitation26, was suppressed by the compounds. TNBS-induced alteration of NLRP3 inflammasome components, NLRP3, pro-caspase-1Citation27, and cleaved and active caspase-1, were blocked by compounds, 13b, 14c, 14j, 14k, 14n, 18c, or 18d (. Similar to the effects of the compounds on cytokine expressions, 14n exerted the strongest effect, which was followed by 14c, 14j, 14k, 13b, 18c, and 18d. Consistent to the result of NLRP3 inflammasome activation, IL-18 level was significantly increased in the colon of TNBS-treated rats, and compounds, 13b, 14c, 14j, 14k, 14n, 18c, and 18d significantly inhibited the TNBS-induced IL-18 expression.
Figure 5. NLRP3 inflammasome formation in TNBS-treated rat colon was suppressed by the compounds. Total protein extracts from colon tissue homogenates were used for detection of NLRP3 inflammasome components. The bar graphs represent the mean ± SEM of protein expression quantified in three independent experiments. *p < 0.05, compared with vehicle-treated control group. #p < 0.05, compared with the TNBS-treated group.
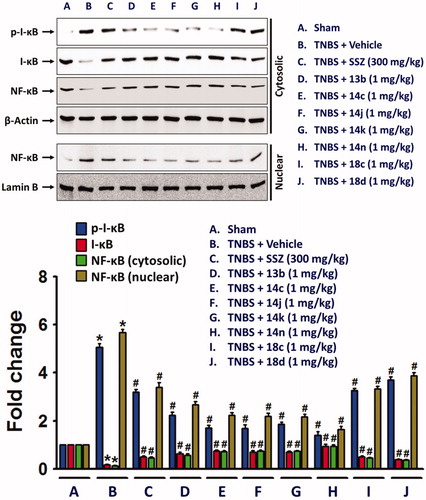
In the colon of TNBS-treated rats, nuclear factor-kappa B (NF-κB), a transcription factor associated with expression of inflammatory cytokines and NLRP3 inflammasome was increased, while cytosolic NF-κB level was decreased in along with inhibitory-κB (I-κB) phosphorylation. However, the compounds significantly suppressed the nuclear translocation of NF-κB (.
Figure 6. Inhibitory effects of the compounds on the nuclear translocation of NF-κB in along with increase in cytosolic phospho-I-κB. Cytosolic and nuclear proteins extracted from colon tissue homogenates were used for detection of phospho-I-κB, I-κB, and NF-κB. The bar graphs represent the mean ± SEM of protein expression quantified in three independent experiments. *p < 0.05, compared with vehicle-treated control group. #p < 0.05, compared with the TNBS-treated group.
4. Discussion
TNF-α is a critical player in the pathogenesis of IBD, which is supported by the most efficacious effects of anti-TNF-α biologics in the clinicCitation28. Based on the notion, we examined anti-IBD activity of various compounds against TNF-α-induced monocyte adhesion to colonic epithelial cells, an in vitro model of initial phase of colitis. In the previous reports, we found that 6-amino-2,4,5-trimethylpyridin-3-ol scaffold was effective against TNF-α-induced cell adhesion between monocyte and colon epithelial cells and against severe inflammation of colon induced by TNBS. Among the 6-aminopyridin-3-ols prepared, compounds A − C showed excellent efficacy in in vivo experimentsCitation18,Citation19. 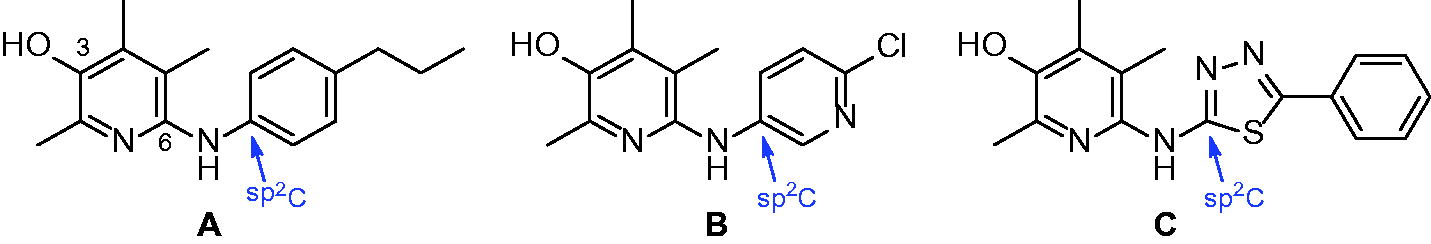
Structurally speaking, these strong analogues (A − C) contain some sort of sp2-carbon (indicated with arrows) attached to C(6)-nitrogen. In this study, we decided to further explore the chemical space of the substituents on C(6)-position. First, we wondered if nitrogen is essential on C(6)-position for activity and decided to replace it with another heteroatom, oxygen. The resulting alkoxy analogues (8a–8f) showed low to mediocre range activity in our in vitro assay and it seems that nitrogen is quite necessary for the position. Therefore, the next approach we decided to conduct based on the activity of the alkoxy-analogues is to keep nitrogen on C(6)-position and instead introduce carbonyl-like functional groups attached to C(6)-nitrogen as an alteration of sp2-carbon shown in previous papers. This type of analogues include ureido (11), thioureido (12), carbamato (16) and sulfonamido (18) analogues. As a result, many compounds shown in this paper were found to have very strong activity. In vitro, some compounds including thiourea and sulphonamide analogues showed up to 93% inhibition against TNF-α-induced cell adhesion at 1 μM concentration. It is the highest in vitro inhibitory activity measured with pyridin-3-ol analogues we synthesised so far. The order of the compounds having excellent in vitro effects was 14n, 14k, 14c, 14j, 13b, 18c, and 18d. Such in vitro activity of the compounds was consistent with in vivo anti-colitis efficacy (1 mg/kg) on colitis signs and biochemical inflammatory markers, which was much better than SSZ (300 mg/kg). Among high activity compounds, 14n, a thiourea analogue, showed the best in vivo activity represented by macroscopic parameters such as almost complete recovery of colon and body weights (95% and 99%, respectively) in TNBS-induced colitis rat model.
Although the exact aetiology of IBD has not yet been clarified, a defective innate immune response seems to play a critical role in the chronic gut inflammationCitation29. Our current study showing an increase in the expressions of TNF-α, IL-6, and IL-1β, referred to as troika of pro-inflammatory cytokines in colon tissues of TNBS-treated rats, was consistent with many previous studies in IBD patients as well as animal models of IBDCitation30,Citation31. TNBS treatment also significantly increased colonic levels of IL-10 and TGF-β, known as anti-inflammatory cytokines. Although the expression level of IL-10 in experimental colitis is contradictoryCitation32, IL-10 level may be related to the inflammatory phase, increased at the initial phase and returned to baseline level in the resolution phase of inflammationCitation33,Citation34. Oral treatments with the compounds significantly suppressed TNBS-induced expressions of IL-10, in addition to TNF-α, IL-6, and IL-1β in colon tissues, indicating that an anti-colitis activity of the compounds led to the resolution phase of TNBS-induced inflammation. In the case of TGF-β, its production is upregulated by bacteria, viruses, cytokines, and dying cells, in an autocrine or paracrine mannerCitation35,Citation36. TGF-β suppresses inflammatory responses similar to IL-10, although previous reports on TGF-β expression in IBD have shown contradictory results depending on active or inactive IBDCitation37,Citation38, TGF-β levels are elevated in inflamed area of gut tissues in IBDCitation39. Consistently, our current results also showed that TNBS induced TGF-β increase in the colon, and oral administration of the compounds significantly reduced the level of TGF-β with the best effects of 14n, 14k, 14c, and 14j.
The innate immune system is activated by the pattern recognition receptors (PRRs) which recognise pathogen-associated molecular patterns (PAMPs) or endogenous danger-associated molecular patterns (DAMPs) generated during cellular injury or tissue damageCitation40–42. PRRs comprise the membrane-bound toll-like receptors (TLRs) and the cytosolic sensory protein complexes such as NOD-like receptors (NLR)Citation43. NLRP3, an NLR family member, has been highlighted as a contributing factor in the pathogenesis of IBDCitation44. NLRP3 mediates the assembly and activation of the inflammasome, a multi-protein complex, which recognises cellular stresses and responds to them by activating caspase-1. Activated caspase-1 converts pro-IL-1β and pro-IL-18 into their active forms, IL-1β and IL-18, and induces pyroptosis, an inflammatory form of cell deathCitation45. In the present study, a significant increase in NLRP3 and caspase-1 in colon tissues by TNBS treatment are correlated to a high-level increase in IL-1β and IL-18. In addition, the correlation between casapase-1 increase and the significant reduction in junction molecules, E-cadherin, claudins, and ZO-1 in TNBS-treated colon implicates TNBS-induced mucosal damage through pyroptosis. Inhibitory effects of compounds, 14n, 14k, 14c, 14j, 13 b, 18c, and 18d on TNBS-induced changes in inflammatory cytokine expression, casapse-1 and junction molecules suggested that anti-colitis effect of the compounds was associated with both anti-inflammatory and mucosal healing activities.
5. Conclusion
In summary, synthetic pyridinols 14n, 14k, 14c, 14j, 13b, 18c, and 18d compounds showed strong inhibitory effects against TNF-α action in vitro and TNBS-induced colitis in vivo. The compound 14n, which showed the highest inhibitory activity against colitis, may be a promising lead compound to develop anti-IBD therapeutics.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Funding
References
- Dave M, Loftus EV. Jr. Mucosal healing in inflammatory bowel disease-a true paradigm of success? Gastroenterol Hepato 2012;8:29–38.
- Theodorou E, Cao P, Karagiannis F, et al. P135 Biological evidence of tofacitinib efficacy in crohn's disease. Gastroenterology 2018;154:S70–S71.
- Xu XR, Liu CQ, Feng BS, Liu ZJ. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroentero 2014;20:3255–64.
- Ahluwalia B, Moraes L, Magnusson MK, Öhman L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroentero 2018;53:379–89.
- Sanchez-Muñoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroentero 2008;14:4280–8.
- Taylor RA, Leonard MC. Curcumin for inflammatory bowel disease: a review of human studies. Altern Med Rev 2011;16:152–6.
- Liu SP, Dong WG, Wu DF, et al. Protective effect of angelica sinensis polysaccharide on experimental immunological colon injury in rats. World J Gastroentero 2003;9:2786–90.
- Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-β and IL-10 in the regulation of experimental colitis. J Immunol 2002;168:900–8.
- Jarry A, Bossard C, Bou-Hanna C, et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J Clin Invest 2008;118:1132–42.
- Couper KN, Blount DG, Riley EM. IL-10: The master regulator of immunity to infection. J Immunol 2008;180:5771–7.
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol 2013;13:397–411.
- Zhen Y, Zhang H. NLRP3 inflammasome and inflammatory bowel disease. Front Immunol 2019;10:276.
- Perera AP, Fernando R, Shinde T, et al. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep 2018;8:8618.
- Kim DG, Kang Y, Lee H, et al. 6-Amino-2,4,5-trimethylpyridin-3-ols: a new general synthetic route and antiangiogenic activity. Eur J Med Chem 2014;78:126–39.
- Lee H, Banskota S, Kim DG, et al. Synthesis and antiangiogenic activity of 6-amido-2,4,5-trimethylpyridin-3-ols. Bioorg Med Chem Lett 2014;24:3131–6.
- Lee H, Kim DG, Banskota S, et al. Pyridoxine-derived bicyclic amido-, ureido-, and carbamato-pyridinols: synthesis and antiangiogenic activities. Org Biomol Chem 2014;12:8702–10.
- Shah S, Lee C, Choi H, et al. 5-Hydroxy-7-azaindolin-2-one, a novel hybrid of pyridinol and sunitinib: design, synthesis and cytotoxicity against cancer cells. Org Biomol Chem 2016;14:4829–41.
- Banskota S, Kang HE, Kim DG, et al. In vitro and in vivo inhibitory activity of 6-amino-2,4,5-trimethylpyridin-3-ols against inflammatory bowel disease. Bioorg Med Chem Lett 2016;26:4587–91.
- Park SW, Banskota S, Gurung P, et al. Synthesis and evaluation of 6-heteroarylamino-2,4,5-trimethylpyridin-3-ols as inhibitors of TNF-α-induced cell adhesion and inflammatory bowel disease. MedChemComm 2018;9:1305–10.
- Bae D, Gautam J, Jang H, et al. Protective effects of 6-ureido/thioureido-2,4,5-trimethylpyridin-3-ols against 4-hydroxynonenal-induced cell death in adult retinal pigment epithelial-19 cells. Bioorg Med Chem Lett 2018;28:107–12.
- Thapa D, Lee JS, Park SY, et al. Clotrimazole ameliorates intestinal inflammation and abnormal angiogenesis by inhibiting interleukin-8 expression through a nuclear factor-κB-dependent manner. J Pharmacol Exp Ther 2008;327:353–64.
- Thapa D, Lee JS, Park MA, et al. Inhibitory effects of clotrimazole on TNF-α-induced adhesion molecule expression and angiogenesis. Arch Pharm Res 2009;32:593–603.
- Seibel J, Molzberger AF, Hertrampf T, et al. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr 2009;48:213–20.
- Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016;44:450–62. 25.
- Zhang C, Shu W, Zhou G, et al. Anti-TNF-α therapy suppresses proinflammatory activities of mucosal neutrophils in inflammatory bowel disease. Mediat Inflamm 2018;2018:1.
- Weinheimer-Haus EM, Mirza RE, Koh TJ. Nod-like receptor protein-3 inflammasome plays an important role during early stages of wound healing. PloS One 2015;10:e0119106.
- Luo X, Yu Z, Deng C, et al. Baicalein ameliorates tnbs-induced colitis by suppressing tlr4/myd88 signaling cascade and nlrp3 inflammasome activation in mice. Sci Rep 2017;7:16374.
- Adegbola SO, Sahnan K, Warusavitarne J, et al. Anti-tnf therapy in crohn's disease. Int J Mol Sci 2018;19:2244.
- Asquith M, Powrie F. An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer. J Exp Med 2010;207:1573–7.
- Tateishi H, Mitsuyama K, Toyonaga A, et al. Role of cytokines in experimental colitis: relation to intestinal permeability. Digestion 1997;58:271–81.
- Stevens C, Walz G, Singaram C, et al. Tumor necrosis factor-α, interleukin-1β, and interleukin-6 expression in inflammatory bowel disease. Digest Dis Sci 1992;37:818–26.
- Kucharzik T, Stoll R, Lügering N, Domschke W. Circulating antiinflammatory cytokine IL‐10 in patients with inflammatory bowel disease (IBD). Clin Exp Immunol 2008;100:452–6.
- Fiorucci S, Antonelli E, Distrutti E, et al. NCX-1015, a nitric-oxide derivative of prednisolone, enhances regulatory T cells in the lamina propria and protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. Proc Natl Acad Sci USA 2002;99:15770–5.
- de Oliveira GAL, de la Lastra CA, Rosillo MÁ, et al. Preventive effect of bergenin against the development of TNBS-induced acute colitis in rats is associated with inflammatory mediators inhibition and NLRP3/ASC inflammasome signaling pathways. Chem Biol Interact 2019;297:25–33.
- Kashiwagi I, Morita R, Schichita T, et al. Smad2 and Smad3 inversely regulate TGF-β autoinduction in Clostridium butyricum-activated dendritic cells. Immunity 2015;43:65–79.
- Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature 2009;458:78–82.
- Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology 1996;110:975–84.
- McCabe RP, Secrist H, Botney M, et al. Cytokine mRNA expression in intestine from normal and inflammatory bowel disease patients. Clin Immunol Immunop 1993;66:52–8.
- Ihara S, Hirata Y, Koike K. TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota. J Gastroenterol 2017;52:777–87.
- Strober W, Ludviksson BR, Fuss IJ. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn disease. Ann Intern Med 1998;128:848–56.
- Amarante-Mendes GP, Adjemian S, Branco LM, et al. Pattern recognition receptors and the host cell death molecular machinery. Front Immunol 2018;9:2379.
- Suresh R, Mosser DM. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv Physiol Educ 2013;37:284–91.
- Ranson N, Kunde D, Eri R. Regulation and sensing of inflammasomes and their impact on intestinal health. Int J Mol Sci 2017;18:2379.
- Sutterwala FS, Ogura Y, Szczepanik M, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 2006;24:317–27.
- Lamkanfi M, Dixit VM. The inflammasomes. PLoS Pathogens 2009;5:e1000510.