Abstract
Testing of an expanded, 800-compound set of analogues of the earlier described Strecker-type α-aminonitriles (selected from publicly available Enamine Ltd. Screening Collection) in thermal shift assay against bovine carbonic anhydrase (bCA) led to further validation of this new class of inhibitors and identification a new, refined chemotype represented by inhibitors with 10-improved potency. 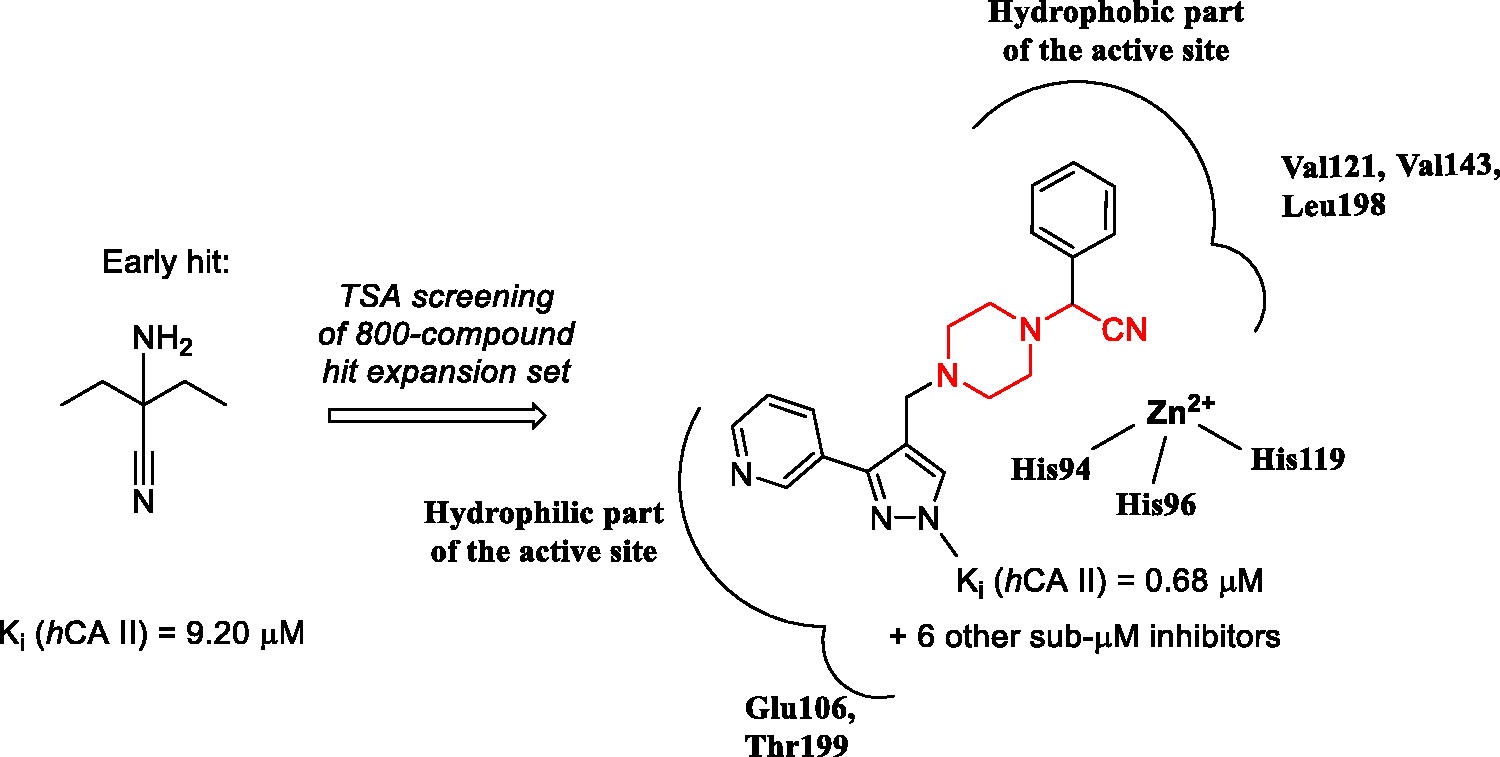
Introduction
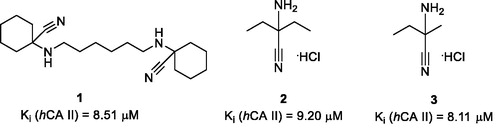
Differential scanning fluorimetry (DSF), also termed thermal shift assay (TSA), is an efficient technique for direct determination of a small molecule’s affinity to a protein targetCitation1. The underlying principle of the method is the ability of a small molecule binding to the protein, in principle, to stabilise or destabilise the tertiary structure of the macromolecule and thus increase or decrease its melting temperature (Tm), respectively. Earlier, we conducted a high-throughput DSF screening of a chemically diverse set of 8000 compounds selected from the Enamine screening collection comprising over 2,000,000 compoundsCitation2 against bovine carbonic anhydrase (bCA), a protein most closely resembling isoform II of the human carbonic anhydrase (hCA II). This led to the discovery of three compounds (1–3) that produced noticeable thermal shift of bCA Tm (ΔTm) which did not belong to any of then-known classes of carbonic anhydrase inhibitors and which we dubbed as Strecker α-aminonitriles considering they could be obtained from various ketones via the Strecker reaction. Besides the TSA biophysical readout, these compounds were found, by testing in biochemical stopped-flow kinetics CO2 hydration assay, to inhibit hCA II (for which bCA was initially selected as a surrogate protein) in the single-digit micromolar range ()Citation3.
Figure 1. Strecker α-aminonitrile hCA II inhibitory hits 1–3 discovered earlier via the DSF screening.
As it was confirmed that compounds 1–3 acted as true inhibitors (and sources of adventitious cyanide anion which is known to inhibit hCA), we postulated that α-aminonitriles in general may act as suicide in situ donors of CN- anion (similarly to N-cyanosulfonamides reported by Supuran et al.Citation4) and thus hCA inhibition in general (and hCA II in particular) can be expected from any representatives of this chemotype with sufficient complementarity (affinity) to the enzyme’s active site. In order to verify this hypothesis and also to discover more potent inhibitors belonging to this class of compounds as well as to establish structure-activity relationships (SAR), we undertook more focussed screening of 800 publicly available α-aminonitriles selected from the Enamine Ltd. screening collectionCitation2. This led, after subsequent biochemical testing of the strongest ‘thermal shifters’, to the discovery of a series of submicromolar inhibitors of hCA II, an established target for glaucoma and diuretic drugsCitation5. Herein, we present the results obtained in the course of these efforts.
Materials and methods
Chemical compounds
The 800 α-aminonitriles (for the full list, see Supplemental Material) for the follow-up DSF screening were selected by substructure search and obtained directly from the Enamine screening collectionCitation2. Their identity and purity was confirmed by 1H NMR spectroscopy prior to biochemical testing in CA inhibition assay.
Differential scanning fluorimetry (thermal shift) assay
Thermal shift assay was carried out using ViiA™ 7 Real-Time PCR System equipped with 384-well block (Applied Biosystems, USA). The TSA procedure was adopted from the literatureCitation6–8 and was modified in order to allow measuring the bCA melting temperature on interaction with various compounds, including the known CA inhibitor azetazolamide (AZ)Citation9, which was used in this study as a reference bCA binder at 10 and 20 µM concentration.
For 800-compound screening, the test reactions were set up in the following buffer: 10 mM NaH2PO4/Na2HPO4, pH 7.0, 10 mM NaCl. The total volume of the reaction mixture per well was 10 µL. Carbonic anhydrase (Sigma Aldrich Cat# C3934) in 300 µg/mL concentration was pre-mixed with environment-sensitive SYPRO orange dye (Invitrogen, Cat# S6650) at final concentration in the reaction of 10 ×, with regard to the stock concentration stated by the vendor. The mixtures were pre-incubated for 1 h at 4 °C with 20 µM concentrations of the compounds (and 1% final concentration of DMSO), placed into MicroAmp® Optical 384-Well Reaction Plate (ThermoFisher, Cat# 4309849). The reaction mixture was kept at room temperature for 15 min to ensure full protein-compound interactions. The temperature was raised at 1.6 °C/s rate to 40 °C without signal reading. Starting from 40 °C up to 85 °C the heating rate was set to 0.05 °C/s with constant fluorescence reading, using 470/623 nm filter set. The raw data of dye fluorescence intensity change upon melting of the protein were obtained from the instrument ViiA 7 RUO software. Further data processing and visualisation was performed by custom-made Microsoft Excel scripts. The peak of the first derivative for the fluorescence curve was used to define melting temperature Tm. Tm for DMSO control wells, having only protein, dye and 1% DMSO was used as a To to determine melting temperature shifts (ΔTm). All measurements were made in quadruplicates.
Carbonic anhydrase inhibition assay
An Applied Photophysics stopped-flow instrument was used for assaying the CA catalysed CO2 hydration activityCitation10. Phenol red (at a concentration of 0.2 mM) was used as an indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as a buffer, and 20 mM Na2SO4 (for maintaining constant ionic strength), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The non-catalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionized water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were combined and pre-incubated for 15 min at room temperature prior to running the assay, in order to allow for the formation of the ezyme–inhibitor complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3 and the Cheng-Prusoff equation, as reported earlierCitation11, and represent the mean from at least three different determinations. Recombinant hCA II was obtained in-house as reported earlierCitation12–16.
Results and discussion
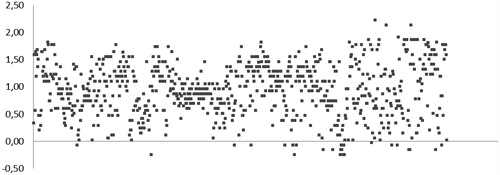
Considering that amino acetonitrile moiety (characteristic of the Strecker-type α-aminonitrile chemotype) was postulated as the pharmacophore for the recently discoveredCitation3 class of carbonic anhydrase inhibitors, we selected 800 compounds featuring this motif (see Supplemental Data) from the Enamine, Ltd. Screening CollectionCitation2 and screened this set at 20 µM concentration against bCA for the ability to shift the enzyme’s melting temperature (Tm) using acetazolamide (AZ)Citation9 as the positive comparator (producing a > 5 °C shift in bCA TmCitation3).
As it follows from the graphical representation of the TSA screening data obtained for the 800 compounds (), none of the compounds produced an equally strong thermal shift compared to AZ. However, quite a few compounds (precisely, 375) populated the >1.0 °C area. In order to select compounds from this subset of ‘stronger shifters’ for subsequent biochemical testing of carbonic anhydrase inhibition, the value of the thermal shift (ΔTm,°C) as well as the quality of the melting curve obtained (evaluated by visual inspection) were taken into account. With these two criteria, a total of 47 compounds were selected and tested in stopped-flow CO2 hydration assay for their ability to inhibit hCA II. The Ki values presented in clearly demonstrate that for a substantial number of compounds, the effect on the enzyme’s melting point determined by TSA, did not translate into potent (or any) inhibition of hCA II.
Table 1. hCA II inhibition (Ki) data of 48 compounds selected from the initial 800-compound screening set based on their bCA thermal shift (ΔTm, °C) values.
A substantial number of TSA hits displayed biochemical hCA II inhibition in the same single-digit micromolar Ki range as the initial hits (1–3). Reassuringly, however, seven compounds displayed Ki values in the submicromolar range, which represented a 10-fold improvement compared to inhibitory potency of 1–3 towards hCA II. Moreover, these compounds, while belonging to the general Strecker α-aminonitrile class, turned out to be structurally distinct from the initial hits 1–3 as they all are based on a new N-(cyanomethyl)piperazine scaffold ().
Figure 3. Most potent, N-(cyanomethyl)piperazine hCA II inhibitors discovered in the course of this study.
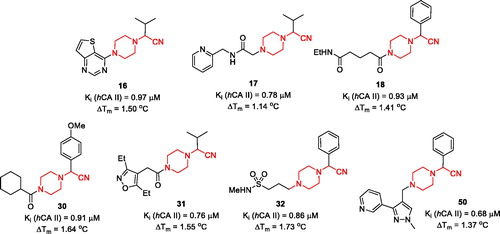
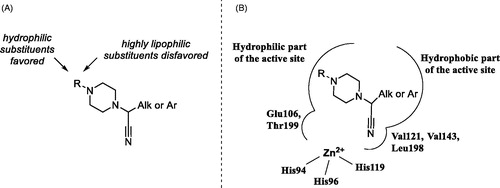
It is also evident that while all frontrunner compounds (16–18, 30–32 and 50) bear a hydrophobic aliphatic or aromatic substituent on the same carbon atom as the cyano group, the N4 position of the piperazine ring is substituted with a relatively polar group. Moreover, examination of the SAR information contained in the total TSA screening data (Supplemental Information), reveals that hydrophobic groups at N4 reduce the compound’s affinity to the target (as indirectly measured by ΔTm). Such an active chemotype topology appear to be in line with the known distinct architecture of the carbonic anhydrase active site where two halves – hydrophobic and hydrophilic are clearly delineatedCitation5. Considering the fact that in order to exert its inhibitory potency (hypothesized to include suicide donation of the cyanide anion in situCitation3), the inhibitor’s molecule must have complementarity to its active site, such hydrophilic/hydrophobic dichotomy of the most active inhibitors matches that of the hCA II active site ().
Conclusion
Testing of an expanded, 800-compound set of analogues of the earlier described Strecker-type α-aminonitriles in thermal shift assay against bovine carbonic anhydrase (bCA) led not only to further validation of this new class of inhibitors but also to refinement of the active chemotype, which became possible after biochemical testing of 47 selected TSA hits for hCA II inhibition in stopped-flow CO2 hydration assay. The active chemotype can be defined as N1-(cyanomethyl)piperazine bearing two other substituents (hydrophilic and hydrophobic) on the opposite sides of the piperazine core. Such a dichotomy of the newly identified pharmacophore appears to be in line with the known bipolar character of the enzyme’s active site. Further studies are underway to investigate the validity of this hypothesis.
Supplemental Material
Download PDF (4.6 MB)Acknowledgements
The authors are grateful to Dr. Vladimir Sharoyko for helpful discussions regarding the TSA data interpretation. We thank the Research Centre for Magnetic Resonance of Saint Petersburg State University Research Park for screening compound quality control using 1H NMR spectroscopy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cummings MD, Farnum MA, Nelen MI. Universal screening methods and applications of ThermoFluor®. J Biomol Screen 2006;11:854–63.
- The screening and the building block collection of Enamine, Ltd. can be obtained at https://www.enaminestore.com/catalog
- Krasavin M, Stavniichuk R, Zozulya S, et al. Discovery of strecker-type α-aminonitriles as a new class of human carbonic anhydrase inhibitors using differential scanning fluorimetry. J Enzyme Inhib Med Chem 2016;31:1707–11.
- Supuran CT, Scozzafava A, Briganti F. Carbonic anhydrase inhibitors: N-cyanosulfonamides, a new class of high affinity isozyme II and IV inhibitors. J Enzyme Inh 1999;14:289–306.
- Krasavin M, Korsakov M, Dorogov M, et al. Probing the ‘bipolar nature of the carbonic anhydrase active site: aromatic sulfonamides containing 1,3-oxazol-5-yl moiety as picomolar inhibitors of cytosolic CA I and CA II isoforms. Eur J Med Chem 2015;101:334–47.
- Lo MC, Aulabaugh A, Jin G, et al. Evaluation of fluorescence-based thermal shift assays for hit identification in drug discovery. Anal Biochem 2004;332:153–9.
- Matulis D, Kranz JK, Salemme FR, Todd MJ. Thermodynamic stability of carbonic anhydrase: measurements of binding affinity and stoichiometry using ThermoFluor. Biochemistry 2005;44:5258–66.
- Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2007;2:2212–21.
- Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68.
- Khalifah RG, Edsall JT. Carbon dioxide hydration activity of carbonic anhydrase: kinetics of alkylated anhydrases B and C from humans. Proc Natl Acad Sci USA 1972;69:172–6.
- Maresca A, Temperini C, Vu H, et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009;131:3057–62.
- Temperini C, Innocenti A, Scozzafava A, et al. The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J Med Chem 2010;53:850–4.
- Alterio V, Hilvo M, Di Fiore A, et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc Natl Acad Sci USA 2009;106:16233–8.
- Casey JR, Morgan PE, Vullo D, et al. Carbonic anhydrase inhibitors. Design of selective, membrane-impermeant inhibitors targeting the human tumor-associated isozyme IX. J Med Chem 2004; 47:2337–47.
- Casini A, Scozzafava A, Mincione F, et al. Carbonic anhydrase inhibitors: water-soluble 4-sulfamoylphenylthioureas as topical intraocular pressure-lowering agents with long-lasting effects. J Med Chem 2000;43:4884–92.
- Winum JY, Scozzafava A, Montero JL, Supuran CT. Design of zinc binding functions for carbonic anhydrase inhibitors. Curr Pharm Des 2008;14:615–21.