Abstract
A series of new 2,4-bis[(substituted-aminomethyl)phenyl]quinoline, 1,3-bis[(substituted-aminomethyl)phenyl]isoquinoline, and 2,4-bis[(substituted-aminomethyl)phenyl]quinazoline derivatives was designed, synthesised, and evaluated in vitro against three protozoan parasites (Plasmodium falciparum, Leishmania donovani, and Trypanosoma brucei brucei). Biological results showed antiprotozoal activity with IC50 values in the µM range. In addition, the in vitro cytotoxicity of these original molecules was assessed with human HepG2 cells. The quinoline 1c was identified as the most potent antimalarial candidate with a ratio of cytotoxic to antiparasitic activities of 97 against the P. falciparum CQ-sensitive strain 3D7. The quinazoline 3h was also identified as the most potent trypanosomal candidate with a selectivity index (SI) of 43 on T. brucei brucei strain. Moreover, as the telomeres of the parasites P. falciparum and Trypanosoma are possible targets of this kind of nitrogen heterocyclic compounds, we have also investigated stabilisation of the Plasmodium and Trypanosoma telomeric G-quadruplexes by our best compounds through FRET melting assays.
Graphical Abstract
Introduction
According to WHO, malaria remains a major public health problem, all the more worrying nowadays as epidemiological data show that no significant progress in reducing malaria cases was registered for the period 2015–2017. Indeed, in 2017, an estimated 219 million cases of malaria occurred worldwide compared with 239 million cases in 2010 and 217 million cases in 2015Citation1. Fortunately, progress in reducing mortality from malaria occurs, since in 2017, indeed 435 000 estimated deaths were globally recorded from malaria, compared with 451,000 estimated deaths in 2016 and 607,000 in 2010Citation1. In this context, even if artemisinin and artemisinin-based combination therapies (ACTs) represent the most effective antimalarial drugs, a resistance of Plasmodium falciparum to artemisinin has been first observed on the Cambodia–Thailand border in 2009Citation2,Citation3, then spread in the Greater Mekong SubregionCitation1. Recent studies have demonstrated that the mechanisms of resistance developed by the parasite against artemisinin affect only one stage of the malaria parasite cycle in humans, the ring stage, resulting in a “partial resistance,” that includes information on the genotype, since in 2013, the identification of the PfKelch13 (K13) mutations has been defined to be associated with reduced susceptibility to artemisininCitation4,Citation5. ACTs composed of artemisinin or its derivatives and various partner drugs (mefloquine (MQ), lumefantrine, amodiaquine, sulfadoxine-pyrimethamine, or piperaquine) are then recommended for 3 dCitation6. These ACTs are also recommended as the first-line treatment for uncomplicated malaria, caused by all Plasmodium species, except for the first trimester of pregnancy.
The increase in global drug resistance in the malaria-endemic areas has significantly reduced the potency of most current used antimalarial compounds. In order to solve this problem, the development of new antimalarial drug candidates with novel potential mechanisms of action is urgently neededCitation7–10. Efforts to discover new 4-aminoquinoline derivatives are ongoing. In fact, it is unlikely that the parasite will be able to evolve resistance to drugs targeting the pathway involved in haemoglobin degradation. Previous studies have shown that modification and modulation of the lateral side chain of chloroquine (CQ) that led to original aminoquinoline compounds avoid the CQ resistance mechanismCitation11–14.
Another strategy is the design and the synthesis of new quinoline-based drugs that could not be recognised by the protein system involved in the drug efflux. By following this strategy, two original series of bisquinoline and bisacridine antiplasmodium drugs were designed and prepared ()Citation15–18. These new derivatives had much lower resistance indices than CQ, indicating that these original heterocyclic pharmacophores are less efficiently excluded by drug-resistant parasites. Recently, high throughput screens (HTS) followed by the design and synthesis of new structures revealed several analogues possessing the 2-anilino quinazoline scaffold, such as the disubstituted quinazolines C-D, BIX-01294, and TM2-115 (). In addition, the quinoline-4-carboxamide series was also identified from a screen against the P. falciparum 3D7 strain leading to the discovery of quinolines E and DDD107498 ()Citation19–23.
Figure 1. The structures of chloroquine (CQ), bisquinoline A, bisacridine B, 2-anilino-4-amino- quinazolines C, D, diamino-quinazolines BIX-01294 and TM2-115, and quinolines E and DDD107498, and newly synthesised 2,4-bis[(substituted-aminomethyl)phenyl]quinoline, 1,3-bis[(substituted-aminomethyl)phenyl]isoquinoline and 2,4-bis[(substituted-aminomethyl)phenyl]quinazoline derivatives 1–3.
![Figure 1. The structures of chloroquine (CQ), bisquinoline A, bisacridine B, 2-anilino-4-amino- quinazolines C, D, diamino-quinazolines BIX-01294 and TM2-115, and quinolines E and DDD107498, and newly synthesised 2,4-bis[(substituted-aminomethyl)phenyl]quinoline, 1,3-bis[(substituted-aminomethyl)phenyl]isoquinoline and 2,4-bis[(substituted-aminomethyl)phenyl]quinazoline derivatives 1–3.](/cms/asset/dc5039f0-495b-48b7-b8e3-f9660129c38c/ienz_a_1706502_f0001_b.jpg)
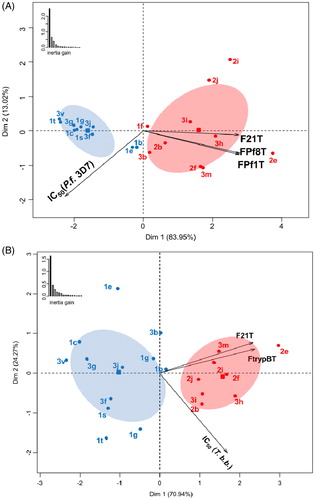
Figure 2. Principal Component Analysis biplots employed in Hierarchical Ascendant Classification (HAC) in relation to P. falciparum (A) and T. brucei brucei variables (B). Colours and confidence ellipses (for α = 0.05) define the attribution of the selected twenty ligands 1–3 based on their IC50 best results to the two groups defined by HAC.
Among other vector-borne parasitic diseases, those caused by parasites of the Trypanosomatidae family are also public health problems. Indeed, leishmaniases, caused by parasites of the Leishmania genus which are transmitted by the bite of infected female phlebotomine sandflies, are among the most neglected parasitic diseases in the world. An estimated 0.7–1 million new cases of leishmaniases per year are reported from nearly 100 endemic countriesCitation24. There are three main clinical forms of leishmaniases: cutaneous (the most common), mucocutaneous and visceral, also known as kala-azar and the most serious form of the disease since this is fatal if left untreated in over 95% of cases. Humans are the main reservoir for visceral leishmaniasis (VL) due to Leishmania donovani. Nevertheless, Leishmania infantum may also cause VL, but the domestic dog is the primary reservoir of this Leishmania species, although other mammalian reservoirs exist and sporadic non-vector transmission routes such as direct transmission between drug users co-infected with HIV through sharing needlesCitation25. VL is mainly characterised by irregular fever, enlargement of the spleen and liver, and anaemia. Most cases occur in Brazil, East Africa, and South-East Asia. An estimated 50,000–90,000 new cases of VL occur worldwide each year, less than half of which are reported to WHOCitation2. In 2017, more than 95% of new cases reported to WHO occurred in only 10 countries: Bangladesh, Brazil, China, Ethiopia, India, Kenya, Nepal, Somalia, South Sudan, and Sudan. A limited number of drugs (meglumine antimoniate, sodium stibogluconate, pentamidine, amphotericin B, and miltefosine), all of which have high toxicities, resistances, and costsCitation24,Citation26,Citation27, can be used to treat leishmaniases, and although efforts have been made by WHO, non-governmental organisations, and manufacturers to improve access to medicines, leishmaniases persist as poverty-related diseases.
Furthermore, another neglected disease caused by Trypanosomatidae parasites of the Trypanosoma genus is the human African trypanosomiasis (HAT), or sleeping sickness, almost invariably fatal unless treated. This infection is transmitted to humans through the bite of an infected tsetse fly. Brain involvement causes various neurological disturbances, including sleep disorders, progression to coma and, ultimately, death. There are two clinical forms: the slowly progressing form (gambiense HAT), caused by infection with Trypanosoma brucei gambiense (currently 98% of cases), and the faster progressing form (rhodesiense HAT), caused by infection with Trypanosoma brucei rhodesiense. As a neglected tropical disease targeted by the WHO for elimination, a historically low number of cases (<1000) was reported in 2018. The recent approval of a new medicine (fexinidazole) for the treatment of gambiense HAT has opened new possibilities for the management of cases and thus led to recent WHO interim guidelines for this treatmentCitation27. A veterinary form of this parasitic disease exists. Named Nagana, it is caused by Trypanosoma brucei brucei which contaminates African livestock, thus having a significant economic impact.
In the course of our work devoted to discovery of new heterocyclic compounds for use in antiprotozoal chemotherapyCitation28–34, we previously prepared a series of substituted 2,9-bis[(substituted-aminomethyl)phenyl]-1,10-phenanthroline derivatives () designed as antimalarial candidates that could bind to P. falciparum DNA G-quadruplexesCitation35. By taking into account our experience in the field of the synthesis of new antiprotozoal heterocyclic compounds, we describe here the design and synthesis of new 2,4-bis[(substituted-aminomethyl)phenyl]quinoline, 1,3-bis[(substituted-aminomethyl)phenyl]isoquinoline and 2,4-bis[(substituted-aminomethyl)phenyl]quinazoline derivatives 1–3 () that could be considered as new bioisoster analogues of our previously described phenanthroline compounds. We report on their in vitro antiplasmodial activity against the CQ -sensitive (3D7) and the CQ -resistant (W2) strains of the malaria parasite P. falciparum. As aza heterocyclic scaffolds are the fundamental units of many antiprotozoal candidates, these quinoline-like derivatives were also tested for in vitro efficacy against medically important protozoans L. donovani and T. brucei brucei.
In addition, the in vitro cytotoxicity of our new bis[(substituted-aminomethyl)phenyl]quinoline-like derivatives was assessed in human HepG2 cells, and an index of selectivity, the ratio of cytotoxic to antiparasitic activity, was determined for each derivative. The telomeres of the different protozoa could constitute attractive drug targetsCitation36–39 and telomerase activity is detected in gametocytes and during the transition to the erythrocytic stage of P. falciparumCitation40. The telomeric 3′G-overhang region of P. falciparum is a repetition of degenerate unit 5′-GGGTTYA-3′ (where Y could be T or C)Citation41 which can fold into intramolecular G-quadruplexCitation42. This difference between parasitic and human (5′-GGGTTA-3′) G-quadruplexes is also observed with L. spp and T. brucei brucei, which augurs the possibility of developing antiparasitic ligands targeting G-quadruplexes found in these protozoal species. Thus, we investigated whether these derivatives could stabilise some parasitic telomeric DNA G-quadruplex structures. Consequently, potential stabilisation of P. falciparum and T. brucei brucei telomeric G-quadruplexes was evaluated using a FRET melting assay.
Experimental
Chemistry
The received commercial reagents were used without additional purification. Melting points were determined with a SM-LUX-POL Leitz hot-stage microscope and are uncorrected. IR spectra were recorded on a NICOLET 380FT-IR spectrophotometer. NMR spectra were recorded with tetramethylsilane as an internal standard using a BRUKER AVANCE 300 spectrometer. Splitting patterns have been designated as follows: s = singlet; bs = broad singlet; d = doublet; t = triplet; q = quartette; qt = quintuplet, dd = double doublet; ddd = double double doublet; and m = multiplet. Analytical TLC was carried out on 0.25 precoated silica gel plates (POLYGRAM SIL G/UV254) and visualisation of compounds after UV light irradiation. Silica gel 60 (70–230 mesh) was used for column chromatography. Microwave experiments were carried out at atmospheric pressure using a focussed microwave reactor (CEM Discover). High-resolution mass spectra (electrospray in positive mode, ESI + or MALDI-TOF MS) were recorded on a Waters Q-TOF Ultima apparatus. Elemental analyses were found within ±0.4% of the theoretical values.
General procedure for 2,4-bis(4-formylphenyl)quinolines (4a–d), 1,3-bis(4-formylphenyl)isoquinolines(5a–c) and 2,4-bis(4-formylphenyl) quinazolines (6a–d)
To a solution of 4.37 mmol of the appropriate 2,4-dichloroquinoline, or 1,3-dichloroisoquinoline or 2,4-dichloroquinazoline, 1.44 g of 3- or 4-formylphenyl boronic acid (9.63 mmol, 2.2 eq.) and 506 mg (0.437 mmol, 0.1 eq.) of tetrakis(triphenylphosphine) palladium in 45 ml of 1,2-dimethoxyethane, 5 ml of 2 M K2CO3 aqueous solution, previously degassed for 10 min with nitrogen, were added at room temperature. Then, the mixture was warmed to reflux and stirred for 24 h under nitrogen positive pressure. The reaction mixture was cooled down to room temperature and the solvent was evaporated under vacuum. The organic layer was extracted with CH2Cl2 and the organic phase was filtered on filter paper, then washed with water (20 ml× 3 times), dried over anhydrous sodium sulphate and activated charcoal, filtered, and evaporated under vacuum. The residue was cooled and triturated with a minimum of EtOH and EtO2 and filtered on sintered glassware to give the crude product. The residue was purified by silica gel column chromatography (CH2Cl2/CH3OH 95:5), then cooled and triturated again in EtOH, filtered on sintered glassware, washed with a minimum of EtOH, EtO2 and petroleum ether and dried under pressure to give the solid product.
2,4-bis(4-Formylphenyl)quinoline (4a)
White crystals (60%); Mp = 186 °C; IR νmax (KBr)/cm−1 1695 (C=O);1H NMR δ (300 MHz, CDCl3) 10.19 (s, 1H, CHO), 10.14 (s, 1H, CHO), 8.41 (d, 2H, J= 9.00 Hz, H-3′′ and H-5′′), 8.33 (dd, 1H, J= 9.00 and 1.60 Hz, H-8), 8.12 (d, 2H, J= 9.00 Hz, H-3′ and H-5′), 8.08 (d, 2H, J= 9.00 Hz, H-2′′ and H-6′′), 7.90 (s, 1H, H-3), 7.87(dd, 1H, J= 9.00 and 1.60 Hz, H-5), 7.83 (ddd, J= 9.00, 7.20 and 1.60 Hz, H-7), 7.77 (d, 2H, J= 9.00 Hz, H-2′ and H-6'), 7.59 (ddd, 1H, J= 9.00, 7.20 and 1.60 Hz, H-6); HRMS-ESI m/z [M + H]+ Calcd for C23H16NO2: 338.1181, Found: 338.1182.
6-Methoxy-2,4-bis(4-formylphenyl)quinoline (4b)
Pale yellow crystals (44.1%); Mp = 212 °C; Rf = 0.84; IR νmax (KBr)/cm−1 1695 (C=O);1H NMR δ (300 MHz, CDCl3) 10.18 (s, 1H, CHO), 10.12 (s, 1H, CHO), 8.38 (d, 2H, J= 8.25 Hz, H-3′′ and H-5′′), 8.20 (d, 1H, J= 9.30 Hz, H-8), 8.12 (d, 2H, J= 8.25 Hz, H-3′ and H-5′), 8.00 (d, 2H, J= 8.25 Hz, H-2′′ and H-6′′), 7.84 (s, 1H, H-3), 7.79 (d, 2H, J= 8.25 Hz, H-2′ and H-6′), 7.47 (dd, 1H, J= 9.30 and 2.70 Hz, H-7), 7.10 (d, 1H, J= 2.70 Hz, H-5), 3.83 (s, 3H, CH3O); 13C NMR δ (75 MHz, CDCl3) 193.4 (CHO), 193.1 (CHO), 160.0 (C-6), 154.2 (C-2), 148.2 (C-4), 148.0 (C-8a), 146.3 (C-4′), 146.0 (C-4′′), 137.8 (C-1′), 137.6 (C-1′′), 133.4 (C-8), 131.5 (C-3′ and C-5′, C-3′′ and C-5′′, C-2′ and C-6′), 129.2 (C-2′′ and C-6′′), 127.9 (C-4a), 124.1 (C-7), 120.8 (C-3), 104.4 (C-5), 56.9 (CH3O); HRMS-ESI m/z [M + H]+ Calcd for C24H18NO3: 368.1287, Found: 368.1289.
7-Methoxy-2,4-bis(4-formylphenyl)quinoline (4c)
Pale yellow crystals (71.4%); Mp = 179 °C; Rf = 0.83; IR νmax (KBr)/cm−1 1696 (C=O); 1H NMR δ (300 MHz, CDCl3) 10.18 (s, 1H, CHO), 10.14 (s, 1H, CHO), 8.39 (d, 2H, J= 8.30 Hz, H-3′′ and H-5′′), 8.11 (d, 2H, J= 8.30 Hz, H-3′ and H-5′), 8.07 (d, 2H, J= 8.30 Hz, H-2′′ and H-6′′), 7.77 (d, 2H, J= 8.30 Hz, H-2′ and H-6′), 7.76 (s, 1H, H-3), 7.75 (d, 1H, J= 9.30 Hz, H-5), 7.63 (d, 1H, J= 2.60 Hz, H-8), 7.23 (dd, 1H, J= 9.30 and 2.60 Hz, H-6), 4.04 (s, 3H, CH3O); HRMS-ESI m/z [M + H]+ Calcd for C24H18NO3: 368.1287, Found: 368.1287.
2,4-bis(3-Formylphenyl)quinoline (4d)
White crystals (66%); Mp = 150 °C; IR νmax (KBr)/cm−11694 (C=O); 1H NMR δ (300 MHz, CDCl3) 10.19 (s, 1H, CHO), 10.18 (s, 1H, CHO), 8.74 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.57 (ddd, 1H, J= 7.80, 1.50 and 1.50 Hz, H-6′), 8.33 (dd, 1H, J= 8.70 and 1.20 Hz, H-8), 8.13 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′′), 8.09 (ddd, 1H, J= 7.80, 1.50 and 1.50 Hz, H-4′), 8.03 (ddd, 1H, J= 7.80, 1.50 and 1.50 Hz, H-4′′), 7.93 (s, 1H, H-3), 7.91–785 (m, 2H, H-5 and H-6′′), 7.83 (ddd, 1H, J= 8.70, 7.20 and 1.20 Hz, H-7), 7.81 (t, 1H, J= 7.80 Hz, H-5′), 7.75 (t, 1H, J= 7.80 Hz, H-5′′), 7.58 (ddd, 1H, J= 8.70, 7.20 and 1.20 Hz, H-6); 13C NMR δ (75 MHz, CDCl3) 193.5 (C=O), 193.1 (C=O), 156.5 (C-2), 150.0 (C-4), 149.5 (C-8a), 141.5 (C-1′′), 140.5 (C-3′), 138.3 (C-3′′), 138.2 (C-1′), 136.7 (C-6′), 134.7 (C-6′′), 131.9 (C-2′ and C-4′′), 131.7 (C-2′′), 131.5 (C-4′), 131.3 (C-7), 131.0 (C-5′′), 130.9 (C-5′), 130.1 (C-8), 128.6 (C-5), 127.0 (C-4a), 126.5 (C-6), 120.3 (C-3); HRMS-ESI m/z [M + H]+ Calcd for C23H16NO2: 338.1181, Found: 338.1182.
1,3-bis(4-Formylphenyl)isoquinoline (5a)
Beige crystals (72%); Mp = 194 °C; IR νmax (KBr)/cm−1 1695 (C=O); 1H NMR δ (300 MHz, CDCl3) 10.20 (s, 1H, CHO), 10.12 (s, 1H, CHO), 8.41 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.26 (s, 1H, H-4), 8.14–8.09 (m, 3H, H-8, H-3′ and H-5′), 8.06–8.00 (m, 5H, H-2′′, H-6′′, H-2′, H-6′ and H-5), 7.79 (ddd, 1H, J= 7.10, 6.90 and 1.50 Hz, H-6), 7.63 (ddd, 1H, J= 7.10, 6.90 and 1.50 Hz, H-7); HRMS-ESI m/z [M + H]+ Calcd for C23H16NO2: 338.1181, Found: 338.1183.
7-Methoxy-1,3-bis(4-formylphenyl)isoquinoline (5b)
Pale-yellow crystals (57%); Mp = 190 °C; IR νmax (KBr)/cm−1 1692 (C=O); 1H NMR δ (300 MHz, CDCl3) 10.19 (s, 1H, CHO), 10.10 (s, 1H, CHO), 8.38 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 8.19 (s, 1H, H-4), 8.13 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 8.04 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.02 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.94 (d, 1H, J= 9.00 Hz, H-5), 7.44 (dd, 1H, J= 9.00 and 2.40 Hz, H-6), 7.36 (d, 1H, J= 2.40 Hz, H-8), 3.87 (s, 3H, CH3O); HRMS-ESI m/z [M + H]+ Calcd for C24H18NO3: 368.1287, Found: 368.1286.
6-Methoxy-1,3-bis(4-formylphenyl)isoquinoline (5c)
White crystals (76%); Mp = 191 °C; IR νmax (KBr)/cm−1 1695 (C=O); 1H NMR δ (300 MHz, CDCl3) 10.20 (s, 1H, CHO), 10.10 (s, 1H, CHO), 8.37 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 8.12 (d, 1H, J= 9.00 Hz, H-8), 8.10 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 8.02 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.00 (s, 1H, H-4), 7.97 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.26 (d, 1H, J= 2.40 Hz, H-5), 7.23 (dd, 1H, J= 9.00 and 2.40 Hz, H-7), 4.02 (s, 3H, CH3O); HRMS-ESI m/z [M + H]+ Calcd for C24H18NO3: 368.1287, Found: 368.1293.
2,4-bis(4-Formylphenyl)quinazoline (6a)
White crystals (53%); Mp = 194 °C; IR νmax (KBr)/cm−1 1698 (C=O); 1H NMR δ (300 MHz, CDCl3) 10.22 (s, 1H, CHO), 10.16 (s, 1H, CHO), 8.89 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.25 (dd, 1H, J= 8.40 and 1.35 Hz, H-8), 8.17 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 8.14 (dd, 1H, J= 8.40 and 1.35 Hz, H-5), 8.10–8.06 (m, 4H, H-2′′, H-6′′, H-2′ and H-6′),8.00 (ddd, 1H, J= 8.40, 7.05 and 1.35 Hz, H-7), 7.67 (ddd, 1H, J= 8.40, 7.05 and 1.35 Hz, H-6); 13C NMR δ (75 MHz, CDCl3) 193.6 (C=O), 193.2 (C=O), 168.6 (C-2), 160.3 (C-4), 153.3 (C-8a), 144.7 (C-1′′), 144.4 (C-1′), 139.0 (C-4′′), 138.5 (C-4′), 135.6 (C-7), 132.2 (C-3′′ and C-5′′), 131.3 (C-2′′, C-6′′, C-3′ and C-5′), 131.0 (C-8), 130.5 (C-2′ and C-6′), 129.6 (C-5), 127.8 (C-6), 123.1 (C-4a); HRMS-ESI m/z [M + H]+ Calcd for C22H15N2O2: 339.1134, Found: 339.1126.
6-Methoxy-2,4-bis(4-formylphenyl)quinazoline (6b)
Pale yellow crystals (32%); Mp = 220 °C; Rf = 0.81; IR νmax (KBr)/cm−1 1697 (C=O); 1H NMR δ (300 MHz, CDCl3) 10.21 (s, 1H, CHO), 10.13 (s, 1H, CHO), 8.83 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.18–8.10 (m, 7H, H-8, H-3′ and H-5′, H-2′′ and H-6′′, H-2′ and H-6′), 7.63 (dd, 1H, J= 9.20 and 2.90 Hz, H-7), 7.31 (d, 1H, J= 2.90 Hz, H-5), 3.89 (s, 3H, CH3O); HRMS-ESI m/z [M + H]+ Calcd for C23H17N2O3: 369.1239, Found: 369.1236.
7-Methoxy-2,4-bis(4-formylphenyl)quinazoline (6c)
Yellow crystals (73%); Mp = 238 °C; Rf = 0.78; IR νmax (KBr)/cm−1 1697 (C=O); 1H NMR δ (300 MHz, CDCl3) 10.20 (s, 1H, CHO), 10.15 (s, 1H, CHO), 8.85 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 8.14 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 8.06 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.04 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.96 (d, 1H, J= 9.30 Hz, H-5), 7.51 (d, 1H, J= 2.55 Hz, H-8), 7.26 (d, 1H, J= 9.30 and 2.55 Hz, H-6), 4.07 (s, 3H, CH3O); HRMS-ESI m/z [M + H]+ Calcd for C23H17N2O3: 369.1239, Found: 369.1231.
2,4-bis(3-Formylphenyl)quinazoline (6d)
White crystals (54%); Mp = 174 °C; IR νmax (KBr)/cm−1 1697 (C=O); 1H NMR δ (300 MHz, CDCl3) 10.21 (s, 1H, CHO), 10.20 (s, 1H, CHO), 9.20 (dd, 1H, J= 1.35 and 1.35 Hz, H-2′), 8.99 (ddd, 1H, J= 7.70, 1.35 and 1.35 Hz, H-6′), 8.42 (dd, 1H, J= 1.35 and 1.35 Hz, H-2′′), 8.24–8.18 (m, 2H, H-8 and H-6′′), 8.15 (ddd, 1H, J= 7.70, 1.35 and 1.35 Hz, H-4′), 8.17–8.07 (m, 1H, H-5), 8.05 (ddd, 1H, J= 7.70, 1.35 and 1.35 Hz, H-4′′), 7.98 (ddd, 1H, J= 8.30, 7.20 and 1.50 Hz, H-7), 7.83 (dd, 1H, J= 7.70 and 7.70 Hz, H-5′′), 7.72 (dd, 1H, J= 7.70 and 7.70 Hz, H-5′), 7.65 (ddd, 1H, J= 8.30, 7.20 and 1.50 Hz, H-6); HRMS-ESI m/z [M + H]+ Calcd for C22H15N2O2: 339.1134, Found: 339.1128.
General procedure for 2,4-bis[(substituted-iminomethyl)phenyl]quinolines (7a-t), 1,3-bis[(substituted-iminomethyl)phenyl]isoquinolines (8a–l), and 2,4-bis[(substituted-iminomethyl)phenyl]quinazolines (9a–v)
To a solution of diamine (0.126 mmol, 2.1 eq.) in ethanol (7 ml) was added 2,4-bis(4-formylphenyl)quinoline 4 or 1,3-bis(4-formylphenyl)isoquinoline 5 or 2,4-bis(4-diformylphenyl)quinazoline 6(0.6 mmol). The reaction mixture was then heated under reflux for 5 h, and then evaporated to dryness under reduced pressure. After cooling, the residue was extracted with dichloromethane (40 ml). The organic layer was dried over sodium sulphate and activated charcoal and evaporated to dryness. Products were then used without further purification.
2,4-Bis{4-[(4-dimethylaminobutyl)iminomethyl]phenyl}quinoline (7a)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.36 (s, 1H, CH=N), 8.32 (s, 1H, CH=N), 8.23 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.21 (dd, 1H, J= 8.50 and 1.35 Hz, H-8), 7.87 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.86 (s, 1H, H-3), 7.82 (dd, 1H, J= 8.50 and 1.35 Hz, H-5), 7.81 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.72 (ddd,1H, J= 8.50, 7.00 and 1.35 Hz, H-7), 7.57 (d, 2H, J= 8.40 Hz, H-2′ and H-6′′), 7.45 (ddd, 1H, J= 8.50, 7.00 and 1.35 Hz, H-6), 3.67 (t, 2H, J= 6.90 Hz, NCH2), 3.64 (t, 2H, J= 6.90 Hz, NCH2), 2.34–2.26 (m. 4H, 2NCH2), 2.21 (s, 6H, N(CH3)2), 2.20 (s, 6H, N(CH3)2), 1.80–1.68 (m, 4H, 2CH2), 1.61–1.52 (m, 4H, 2CH2).
2,4-Bis{4-[(3-dimethylaminopropyl)iminomethyl]phenyl}quinoline (7b)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.42 (s, 1H, CH=N), 8.38 (s, 1H, CH=N), 8.28 (d, 2H, J= 9.00 Hz, H-3′′ and H-5′′), 8.26 (dd, 1H, J= 8.90 and 1.50 Hz, H-8), 7.94 (d, 2H, J= 9.00 Hz, H-3′ and H-5′), 7.89 (dd, 1H, J= 8.90 and 1.50 Hz, H-5), 7.88 (d, 2H, J= 9.00 Hz, H-2′′ and H-6′′), 7.85 (s, 1H, H-3), 7.76 (ddd,1H, J= 8.90, 7.20 and 1.50 Hz, H-7), 7.62 (d, 2H, J= 9.00 Hz, H-2′ and H-6′), 7.50 (ddd, 1H, J= 8.90, 7.20 and 1.50 Hz, H-6), 3.73 (t, 2H, J= 6.90 Hz, NCH2), 3.69 (t, 2H, J= 6.90 Hz, NCH2), 2.46–2.40 (m. 4H, 2NCH2), 2.30 (s, 6H, N(CH3)2), 2.29 (s, 6H, N(CH3)2), 2.00–1.89 (m, 4H, 2CH2).
2,4-Bis{4-[(2-dimethylaminoethyl)iminomethyl]phenyl}quinoline (7c)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.43 (s, 1H, CH=N), 8.40 (s, 1H, CH=N), 8.27 (d, 2H, J= 9.00 Hz, H-3′′ and H-5′′), 8.24 (dd, 1H, J= 8.90 and 1.50 Hz, H-8), 7.92 (d, 2H, J= 9.00 Hz, H-3′ and H-5′), 7.89 (d, 2H, J= 9.00 Hz, H-2′′ and H-6′′),7.86 (dd, 1H, J= 8.90 and 1.50 Hz, H-5), 7.84 (s, 1H, H-3), 7.75 (ddd,1H, J= 8.90, 7.20 and 1.50 Hz, H-7), 7.61 (d, 2H, J= 9.00 Hz, H-2′ and H-6′), 7.49 (ddd, 1H, J= 8.90, 7.20 and 1.50 Hz, H-6), 3.82 (t, 2H, J= 7.00 Hz, NCH2), 3.80 (t, 2H, J= 7.00 Hz, NCH2), 2.71 (t, 2H, J= 7.00 Hz, NCH2), 2.69 (t, 2H, J= 7.00 Hz, NCH2), 2.36 (s, 6H, N(CH3)2), 2.35 (s, 6H, N(CH3)2).
2,4-Bis{4-[(4–(4-methylpiperazin-1-yl)butyl)iminomethyl]phenyl}quinoline (7d)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.39 (s, 1H, CH=N), 8.35 (s, 1H, CH=N), 8.27–8.23 (m, 3H, H-3′′, H-5′′ and H-8), 7.90 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.88 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.87 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.85 (s, 1H, H-3), 7.75 (ddd,1H, J= 8.10, 6.90 and 1.20 Hz, H-7), 7.61 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 7.49 (ddd, 1H, J= 8.10, 6.90 and 1.20 Hz, H-6), 3.69 (t, 2H, J= 6.90 Hz, NCH2), 3.67 (t, 2H, J= 6.90 Hz, NCH2), 2.74–2.35 (m. 20H, 2NCH2 and 8NCH2pip.), 2.29 (s, 3H, NCH3), 2.28 (s, 3H, NCH3), 1.79–1.74 (m, 4H, 2CH2), 1.62–1.58 (m, 4H, 2CH2).
2,4-Bis{4-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}quinoline (7e)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.41 (s, 1H, CH=N), 8.37 (s, 1H, CH=N), 8.27 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.25 (dd, 1H, J= 8.40 and 1.50 Hz, H-8), 7.91 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.88 (dd, 1H, J= 8.40 and 1.50 Hz, H-5), 7.87 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.85 (s, 1H, H-3), 7.76 (ddd,1H, J= 8.40, 6.90 and 1.50 Hz, H-7), 7.63 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.50 (ddd, 1H, J= 8.40, 6.90 and 1.50 Hz, H-6), 3.72 (t, 2H, J= 6.90 Hz, NCH2), 3.70 (t, 2H, J= 6.90 Hz, NCH2), 2.65–2.46 (m. 20H, 2NCH2 and 8NCH2pip.), 2.32 (s, 3H, NCH3), 2.31 (s, 3H, NCH3), 2.00–1.92 (m, 4H, 2CH2).
2,4-Bis{4-[(2–(4-methylpiperazin-1-yl)ethyl)iminomethyl]phenyl}quinoline (7f)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.40 (s, 1H, CH=N), 8.36 (s, 1H, CH=N), 8.25 (d, 2H, J= 9.00 Hz, H-3′′ and H-5′′), 8.23 (dd, 1H, J= 8.50 and 1.40 Hz, H-8), 7.89 (d, 2H, J= 9.00 Hz, H-3′ and H-5′), 7.86 (d, 2H, J= 9.00 Hz, H-2′′ and H-6′′), 7.85 (dd, 1H, J= 8.50 and 1.40 Hz, H-5), 7.83 (s, 1H, H-3), 7.74 (ddd,1H, J= 8.50, 6.90 and 1.20 Hz, H-7), 7.60 (d, 2H, J= 9.00 Hz, H-2′ and H-6′), 7.48 (ddd, 1H, J= 8.50, 6.90 and 1.20 Hz, H-6), 3.85–3.66 (m, 4H, 2NCH2), 2.83–2.72 (m, 4H, 2NCH2), 2.62–2.42 (m. 20H, 2NCH2 and 8NCH2pip.), 2.29 (s, 3H, NCH3), 2.28 (s, 3H, NCH3).
2,4-Bis{4-[(3-morpholinopropyl)iminomethyl]phenyl}quinoline (7g)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.43 (s, 1H, CH=N), 8.39 (s, 1H, CH=N), 8.28 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.26 (dd, 1H, J= 8.00 and 1.50 Hz, H-8), 7.93 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.90 (dd, 1H, J= 8.00 and 1.50 Hz, H-5), 7.88 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.86 (s, 1H, H-3), 7.77 (ddd,1H, J= 8.00, 7.20 and 1.50 Hz, H-7), 7.64 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.51 (ddd, 1H, J= 8.00, 7.20 and 1.50 Hz, H-6), 3.78–3.69 (m, 12H, 2NCH2 and 4 OCH2), 2.52–2.45 (m, 12H, 6NCH2), 2.02–1.90 (m, 4H, 2CH2).
2,4-Bis{4-[(2-morpholinoethyl)iminomethyl]phenyl}quinoline (7h)
Orange oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.43 (s, 1H, CH=N), 8.39 (s, 1H, CH=N), 8.27 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.25 (dd, 1H, J= 8.70 and 1.50 Hz, H-8), 7.93 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.90 (dd, 1H, J= 8.70 and 1.50 Hz, H-5), 7.88 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.86 (s, 1H, H-3), 7.77 (ddd,1H, J= 8.70, 7.10 and 1.50 Hz, H-7), 7.63 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.51 (ddd, 1H, J= 8.70, 7.10 and 1.50 Hz, H-6), 3.86 (t, 2H, J= 6.90 Hz, NCH2), 3.83 (t, 2H, J= 6.90 Hz, NCH2), 3.78 (m, 8H, 4 OCH2), 2.80–2.73 (m, 4H, 2NCH2), 2.62–2.57 (m, 8H, 4NCH2).
6-Methoxy-2,4-bis{4-[(4-dimethylaminobutyl)iminomethyl]phenyl}quinoline (7i)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.35 (s, 1H, CH=N), 8.30 (s, 1H, CH=N), 8.19 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.10 (d, 1H, J= 9.30 Hz, H-8), 7.87 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.82 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.75 (s, 1H, H-3), 7.58 (d, 2H, J = 8.10 Hz, H-2′ and H-6′), 7.35 (dd,1H, J= 9.30 and 2.60 Hz, H-7), 7.10 (m, 1H, H-5), 3.74 (s, 3H, CH3O), 3.67–3.62 (m, 4H, 2NCH2), 2.33–2,25 (m. 4H, 2NCH2), 2.21 (s, 6H, N(CH3)2), 2.19 (s, 6H, N(CH3)2), 1.76–1.70 (m, 4H, 2CH2), 1.56–1.50 (m, 4H, 2CH2).
6-Methoxy-2,4-bis{4-[(3-dimethylaminopropyl)iminomethyl]phenyl}quinoline (7j)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.38 (s, 1H, CH=N), 8.32 (s, 1H, CH=N), 8.20 (d, 2H, J= 8.25 Hz, H-3′′ and H-5′′), 8.11 (d, 1H, J= 9.00 Hz, H-8), 7.89 (d, 2H, J= 8.25 Hz, H-3′ and H-5′), 7.84 (d, 2H, J= 8.25 Hz, H-2′′ and H-6′′), 7.76 (s, 1H, H-3), 7.60 (d, 2H, J = 8.25 Hz, H-2′and H-6′), 7.36 (dd, 1H, J= 9.00 and 2.70 Hz, H-7), 7.12 (d, 1H, J= 2.70 Hz, H-5), 3.74 (s, 3H, CH3O) 3.69 (t, 2H, J= 6.90 Hz, NCH2), 3.65 (t, 2H, J= 6.90 Hz, NCH2), 2.37 (t, 2H, J= 6.90 Hz, NCH2), 2.35 (t, 2H, J= 6.90 Hz, NCH2), 2.24 (s, 6H, N(CH3)2), 2.22 (s, 6H, N(CH3)2), 1.95–1.80 (m, 4H, 2CH2).
6-Methoxy-2,4-bis{4-[(4–(4-methylpiperazin-1-yl)butyl)iminomethyl]phenyl}quinoline (7k)
Pale yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.31 (s, 1H, CH=N), 8.25 (s, 1H, CH=N), 8.16 (d, 2H, J= 8.50 Hz, H-3′′ and H-5′′), 8.07 (d, 1H, J= 9.30 Hz, H-8), 7.84 (d, 2H, J= 8.50 Hz, H-3′ and H-5′), 7.78 (d, 2H, J= 8.50 Hz, H-2′′ and H-6′′), 7.72 (s, 1H, H-3), 7.55 (d, 2H, J= 8.50 Hz, H-2′ and H-6′), 7.32 (dd,1H, J= 9.30 and 2.80 Hz, H-7), 7.07 (d, 1H, J= 2.80 Hz, H-5), 3.70 (s, 3H, CH3O), 3.62 (t, 2H, J= 6.60 Hz, NCH2), 3.58 (t, 2H, J= 6.60 Hz, NCH2), 2.50–2.29 (m, 20H, 2NCH2 and 8NCH2 pip), 2.21 (s, 3H, NCH3), 2.20 (s, 3H, NCH3), 1.75–1.63 (m, 4H, 2CH2), 1.59–1.46 (m, 4H, 2CH2).
6-Methoxy-2,4-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}quinoline (7l)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.35 (s, 1H, CH=N), 8.30 (s, 1H, CH=N), 8.18 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.09 (d, 1H, J= 9.30 Hz, H-8), 7.86 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.81 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.74 (s, 1H, H-3), 7.58 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 7.34 (dd,1H, J= 9.30 and 2.60 Hz, H-7), 7.09 (d, 1H, J= 2.60 Hz, H-5), 3.73 (s, 3H, CH3O), 3.67 (t, 2H, J= 6.60 Hz, NCH2), 3.63 (t, 2H, J= 6.60 Hz, NCH2), 2.47–2.38 (m, 20H, 2NCH2 and 8NCH2 pip), 2.25 (s, 3H, NCH3), 2.24 (s, 3H, NCH3), 1.93–1.85 (m, 4H, 2CH2).
7-Methoxy-2,4-bis{4-[(4-dimethylaminobutyl)iminomethyl]phenyl}quinoline (7m)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.34 (s, 1H, CH=N), 8.31 (s, 1H, CH=N), 8.20 (d, 2H, J= 8.20 Hz, H-3′′ and H-5′′), 7.86 (d, 2H, J= 8.20 Hz, H-3′ and H-5′), 7.83 (d, 2H, J= 8.20 Hz, H-2′′ and H-6′′), 7.72 (d, 1H, J= 9.15 Hz, H-5), 7.66 (s, 1H, H-3), 7.55 (d, 2H, J= 8.20 Hz, H-2′ and H-6′), 7.53 (d, 1H, J= 2,70 Hz, H-8), 7.09 (dd, 1H, J= 9.15 and 2.70 Hz, H-6), 3.94 (s, 3H, CH3O) 3.65 (t, 2H, J= 7.10 Hz, NCH2), 3.63 (t, 2H, J= 7.10 Hz, NCH2), 2.30 (t, 2H, J= 7.10 Hz, NCH2), 2.39 (t, 2H, J= 7.10 Hz, NCH2), 2.20 (s, 6H, N(CH3)2), 2.19 (s, 6H, N(CH3)2), 1.78–1.67 (m, 4H, 2CH2), 1.60–1.48 (m, 4H, 2CH2).
7-Methoxy-2,4-bis{4-[(3-dimethylaminopropyl)iminomethyl]phenyl}quinoline (7n)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.36 (s, 1H, CH=N), 8.33 (s, 1H, CH=N), 8.20 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 7.89–7.83 (m, 4H, H-3′ and H-5′, H-2′′ and H-6′′), 7.73 (d, 1H, J= 9.30 Hz, H-5), 7.67 (d, 1H, J= 2,10 Hz, H-8), 7.57–7.53 (m, 3H, H-2′ and H-6′, H-3), 7.10 (dd, 1H, J= 9.30 and 2.10 Hz, H-6), 3.95 (s, 3H, CH3O) 3.68 (t, 2H, J= 6.90 Hz, NCH2), 3.65 (t, 2H, J= 6.90 Hz, NCH2), 2.36–2,32 (m, 4H, 2NCH2), 2.23 (s, 6H, N(CH3)2), 2.22 (s, 6H, N(CH3)2), 1.95–1.80 (m, 4H, 2CH2).
7-Methoxy-2,4-bis{4-[(4–(4-methylpiperazin-1-yl)butyl)iminomethyl]phenyl}quinoline (7o)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.32 (s, 1H, CH=N), 8.29 (s, 1H, CH=N), 8.19 (d, 2H, J= 8.10 Hz, H-3′′and H-5′′), 7.84 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.81 (d, 2H, J= 8.20 Hz, H-2′′and H-6′′), 7.71 (d, 1H, J= 9.30 Hz, H-5), 7.65 (s, 1H, H-3), 7.54 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 7.52 (d, 1H, J= 2.40 Hz, H-8), 7.09 (dd,1H, J= 9.30 and 2.40 Hz, H-6), 3.94 (s, 3H, CH3O), 3.62 (t, 2H, J= 6.60 Hz, NCH2), 3.61 (t, 2H, J= 6.60 Hz, NCH2), 2.42–2.29 (m, 20H, 2NCH2 and 8NCH2 pip), 2.23 (s, 3H, NCH3), 2.22 (s, 3H, NCH3), 1.71–1.67 (m, 4H, 2CH2), 1.59–1.52 (m, 4H, 2CH2).
7-Methoxy-2,4-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}quinoline (7p)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.34 (s, 1H, CH=N), 8.31 (s, 1H, CH=N), 8.19 (d, 2H, J= 8.20 Hz, H-3′′ and H-5′′), 7.85 (d, 2H, J= 8.20 Hz, H-3′ and H-5′), 7.83 (d, 2H, J= 8.20 Hz, H-2′′and H-6′′), 7.72 (d, 1H, J= 9.25 Hz, H-5), 7.66 (s, 1H, H-3), 7.55 (d, 2H, J= 8.20 Hz, H-2′ and H-6′), 7.53 (d, 1H, J= 2.60 Hz, H-8), 7.10 (dd,1H, J= 9.25 and 2.60 Hz, H-6), 3.95 (s, 3H, CH3O), 3.67 (t, 2H, J= 6.90 Hz, NCH2), 3.64 (t, 2H, J= 6.90 Hz, NCH2), 2.46–2.33 (m, 20H, 2NCH2 and 8NCH2 pip), 2.25 (s, 3H, NCH3), 2.24 (s, 3H, NCH3), 1.93–1.86 (m, 4H, 2CH2).
2,4-Bis{3-[(3-dimethylaminopropyl)iminomethyl]phenyl}quinoline (7q)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.53 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.44 (s, 1H, CH=N), 8.41 (s, 1H, CH=N), 8.31 (ddd, 1H, J= 7.80, 1.50 and 1.50 Hz, H-6′), 8.26 (dd, 1H, J= 8.50 and 1.20 Hz, H-8), 7.94–7.85 (m, 5H, H-2′′, H-4′, H-4′′, H-5 and H-3), 7.76 (ddd,1H, J= 8.50, 7.20 and 1.20 Hz, H-7), 7.65–7.57 (m, 3H, H-5′, H-5′′ and H-6′′), 7.51 (ddd, 1H, J= 8.50, 7.20 and 1.20 Hz, H-6), 3.72 (t, 4H, J= 7.00 Hz, 2NCH2), 2.43 (t, 4H, J= 7.00 Hz, 2NCH2), 2.29 (s, 6H, N(CH3)2), 2.28 (s, 6H, N(CH3)2), 1.97–1.89 (m, 4H, 2CH2).
2,4-Bis{3-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}quinoline (7r)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.48 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.37 (s, 1H, CH=N), 8.34 (s, 1H, CH=N), 8.26 (ddd, 1H, J= 7.80, 1.50 and 1.50 Hz, H-6′), 8.22 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 7.88–7.78 (m, 5H, H-2′′, H-4′, H-4′′, H-5 and H-3), 7.70 (ddd,1H, J= 8.10, 7.20 and 1.50 Hz, H-7), 7.57–7.50 (m, 3H, H-5′, H-5′′ and H-6′′), 7.44 (ddd, 1H, J= 8.10, 7.20 and 1.50 Hz, H-6), 3.67–3.60 (m, 4H, 2NCH2), 2.56–2.33 (m, 20H, 10NCH2), 2.24 (s, 3H, NCH3), 2.23 (s, 3H, NCH3), 1.94–1.82 (m, 4H, 2CH2).
2,4-Bis{3-[(3-morpholinopropyl)iminomethyl]phenyl}quinoline (7s)
Yellow oil (99%); 1H NMR δ (300 MHz, CDCl3) 8.48 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.35 (s, 1H, CH=N), 8.32 (s, 1H, CH=N), 8.25 (ddd, 1H, J= 8.10, 1.50 and 1.50 Hz, H-6′), 8.20 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 7.87–7.65 (m, 5H, H-2′′, H-4′, H-4′′, H-5 and H-3), 7.66 (ddd,1H, J= 8.10, 6.90 and 1.50 Hz, H-7), 7.56–7.48 (m, 3H, H-5′, H-5′′ and H-6′′), 7.44 (ddd, 1H, J= 8.10, 6.90 and 1.50 Hz, H-6), 3.67–3.60 (m, 12H, 2NCH2 and 4OCH2), 2.40–2.35 (m, 12H, 6NCH2), 1.91–1.79 (m, 4H, 2CH2).
2,4-Bis{3-[(2-morpholinoethyl)iminomethyl]phenyl}quinoline (7t)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.48 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.36 (s, 1H, CH=N), 8.33 (s, 1H, CH=N), 8.25 (ddd, 1H, J= 7.80, 1.50 and 1.50 Hz, H-6′), 8.20 (dd, 1H, J= 8.40 and 1.50 Hz, H-8), 7.88–7.76 (m, 5H, H-2′′, H-4′, H-4′′, H-5 and H-3), 7.68 (ddd,1H, J= 8.40, 6.90 and 1.50 Hz, H-7), 7.56–7.49 (m, 3H, H-5′, H-5′′ and H-6′′), 7.43 (ddd, 1H, J= 8.40, 6.90 and 1.50 Hz, H-6), 3.75 (t, 4H, J= 7.20 Hz, 2NCH2), 3.68–3.64 (m, 8H, 4OCH2), 2.70–2.64 (m, 4H, 2NCH2), 2.52–2.47 (m, 8H, 4NCH2).
1,3-Bis{4-[(4-dimethylaminobutyl)iminomethyl]phenyl}isoquinoline (8a)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.37 (s, 1H, CH=N), 8.30 (s, 1H, CH=N), 8.25 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.08 (s, 1H, H-4), 8.06 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 7.91–7.87 (m, 3H, H-5, H-3′ and H-5′), 7.85–7.80 (m, 4H, H-2′′, H-6′′, H-2′, and H-6′), 7.64 (ddd, 1H, J= 8.10, 7.20 and 1.20 Hz, H-6), 7.48 (ddd, 1H, J= 8.10, 7.20 and 1.20 Hz, H-7), 3.70–3.62 (m, 4H, 2NCH2), 2.32 (t, 2H, J= 6.90 Hz, NCH2), 2.29 (t, 2H, J= 6.90 Hz, NCH2), 2.22 (s, 6H, N(CH3)2), 2.21 (s, 6H, N(CH3)2), 1.78–1.68 (m, 4H, 2CH2), 1.62–1.52 (m, 4H, 2CH2).
1,3-Bis{4-[(3-dimethylaminopropyl)iminomethyl]phenyl}isoquinoline (8 b)
Yellow oil (99%); 1H NMR δ (300 MHz, CDCl3) 8.38 (s, 1H, CH=N), 8.31 (s, 1H, CH=N), 8.23 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.10 (s, 1H, H-4), 8.07 (d, 1H, J= 8.00 Hz, H-8), 7.93–7.75 (m, 7H, H-5, H-3′, H-5′, H-2′′, H-6′′, H-2′ and H-6′), 7.66 (t, 1H, J= 8.00 Hz, H-6), 7.49 (t, 1H, J= 8.00 Hz, H-7), 3.68–3.62 (m, 4H, 2NCH2), 2.35–2.24 (m, 4H, 2NCH2), 2.21 (s, 6H, N(CH3)2), 2.18 (s, 6H, N(CH3)2), 1.96–1.78 (m, 4H, 2CH2).
1,3-Bis{4-[(4–(4-methylpiperazin-1-yl)butyl)iminomethyl]phenyl}isoquinoline (8c)
Yellow oil (80%); 1H NMR δ (300 MHz, CDCl3) 8.34 (s, 1H, CH=N), 8.27 (s, 1H, CH=N), 8.22 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.06 (s, 1H, H-4), 8.04 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 8.04 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.85 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.80 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.78 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.62 (ddd, 1H, J= 8.10, 7.80 and 1.20 Hz, H-6), 7.46 (ddd, 1H, J= 8.10, 7.80 and 1.20 Hz, H-7), 3.65 (t, 2H, J= 6.90 Hz, NCH2), 3.60 (t, 2H, J= 6.90 Hz, NCH2), 2.45–2.35 (m, 20H, 2NCH2 and 8 NCH2pip.), 2.24 (s, 3H, NCH3), 2.23 (s, 3H, NCH3), 1.75–1.65 (m, 4H, 2CH2), 1.61–1.51 (m, 4H, 2CH2).
1,3-Bis{4-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}isoquinoline (8d)
Yellow oil (99%); 1H NMR δ (300 MHz, CDCl3) 8.36 (s, 1H, CH=N), 8.29 (s, 1H, CH=N), 8.22 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.07 (s, 1H, H-4), 8.05 (d, 1H, J= 8.00 Hz, H-8), 7.88 (d, 1H, J= 8.00 Hz, H-5), 7.87 (d, 2H, J= 8.10 Hz, H-3′, and H-5′), 7.82 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.79 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 7.63 (t, 1H, J= 8.00 Hz, H-6), 7.47 (t, 1H, J= 8.00 Hz, H-7), 3.66 (t, 2H, J= 6.90 Hz, NCH2), 3.61 (t, 2H, J= 6.90 Hz, NCH2), 2.46–2.38 (m, 20H, 2NCH2 and 8 NCH2pip.), 2.25 (s, 3H, NCH3), 2.23 (s, 3H, NCH3), 1.95–1.83 (m, 4H, 2CH2).
7-Methoxy-1,3-bis{4-[(4-dimethylaminobutyl)iminomethyl]phenyl}isoquinoline (8e)
Yellow oil (88%); 1H NMR δ (300 MHz, CDCl3) 8.41 (s, 1H, CH=N), 8.33 (s, 1H, CH=N), 8.23 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.05 (s, 1H, H-4), 7.94–7.81 (m, 7H, H-5, H-3′, H-5′, H-2′′, H-6′′, H-2′ and H-6′), 7.38 (d, 1H, J= 2.40 Hz, H-8), 7.34 (dd, 1H, J= 9.00 and 2.40 Hz, H-6), 3.81 (s, 3H, CH3O), 3.70 (t, 2H, J= 6.90 Hz, NCH2), 3.66 (t, 2H, J= 6.90 Hz, NCH2), 2.35 (t, 2H, J= 6.90 Hz, NCH2), 2.30 (t, 2H, J= 6.90 Hz, NCH2), 2.23 (s, 6H, N(CH3)2), 2.21 (s, 6H, N(CH3)2), 1.81–1.70 (m, 4H, 2CH2), 1.65–1.54 (m, 4H, 2CH2).
7-Methoxy-1,3-bis{4-[(3-dimethylaminopropyl)iminomethyl]phenyl}isoquinoline (8f)
Orange-yellow oil (91%); 1H NMR δ (300 MHz, CDCl3) 8.44 (s, 1H, CH=N), 8.36 (s, 1H, CH=N), 8.25 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.08 (s, 1H, H-4), 7.98–7.88 (m, 6H, H-3′, H-5′, H-2′′, H-6′′, H-2′ and H-6′), 7.84 (d, 1H, J= 8.40 Hz, H-5), 7.41 (d, 1H, J= 2.40 Hz, H-8), 7.37 (dd, 1H, J= 8.40 and 2.40 Hz, H-6), 3.83 (s, 3H, CH3O), 3.73 (t, 2H, J= 7.20 Hz, NCH2), 3.69 (t, 2H, J= 7.20 Hz, NCH2), 2.42 (t, 2H, J= 7.20 Hz, NCH2), 2.39 (t, 2H, J= 7.20 Hz, NCH2), 2.28 (s, 6H, N(CH3)2), 2.26 (s, 6H, N(CH3)2), 2.00–1.87 (m, 4H, 2CH2).
7-Methoxy-1,3-bis{4-[(4–(4-methylpiperazin-1-yl)butyl)iminomethyl]phenyl}isoquinoline (8g)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.30 (s, 1H, CH=N), 8.22 (s, 1H, CH=N), 8.14 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 7.94 (s, 1H, H-4), 7.83 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.78 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.73 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.71 (d, 1H, J= 9.00 Hz, H-5), 7.28 (d, 1H, J= 2.40 Hz, H-8), 7.24 (dd, 1H, J= 9.00 and 2.40 Hz, H-6), 3.71 (s, 3H, CH3O), 3.58 (t, 2H, J= 7.20 Hz, NCH2), 3.54 (t, 2H, J= 7.20 Hz, NCH2), 2.40–2.25 (m, 20H, 2NCH2 and 8NCH2pip.), 2.20 (s, 3H, NCH3), 2.19 (s, 3H, NCH3), 1.72–1.63 (m, 4H, 2CH2), 1.54–1.47 (m, 4H, 2CH2).
7-Methoxy-1,3-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}isoquinoline (8h)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.37 (s, 1H, CH=N), 8.29 (s, 1H, CH=N), 8.20 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.01 (s, 1H, H-4), 7.90–7.78 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-2′, H-6′ and H-5), 7.33 (d, 1H, J= 2.40 Hz, H-8), 7.28 (dd, 1H, J= 8.40 and 2.40 Hz, H-6), 3.76 (s, 3H, CH3O), 3.68–3.61 (m, 4H, 2NCH2), 32.47–2.38 (m, 20H, 2NCH2 and 8NCH2pip.), 2.26 (s, 3H, NCH3), 2.25 (s, 3H, NCH3), 1.96–1.84 (m, 4H, 2CH2).
6-Methoxy-1,3-bis{4-[(4-dimethylaminobutyl)iminomethyl]phenyl}isoquinoline (8i)
Pale-orange oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.35 (s, 1H, CH=N), 8.29 (s, 1H, CH=N), 8.21 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 7.98 (s, 1H, H-4), 7.94 (d, 1H, J= 9.30 Hz, H-8), 7.87 (d, 2H, J= 8.40 Hz,H-3′ and H-5′), 7.79 (d, 4H, J= 8.40 Hz, H-2′′, H-6′′, H-2′ and H-6′), 7.14 (d, 1H, J= 2.50 Hz, H-5), 7.09 (dd, 1H, J= 9.30 and 2.50 Hz, H-7), 3.91 (s, 3H, CH3O),3.65 (t, 2H, J= 6.80 Hz, NCH2), 3.63 (t, 2H, J= 6.80 Hz, NCH2), 2.46–2.38 (m, 4H, 2NCH2), 2.28 (s, 6H, N(CH3)2), 2.26 (s, 6H, N(CH3)2), 1.76–1.67 (m, 4H, 2CH2), 1.64–1.55 (m, 4H, 2CH2).
6-Methoxy-1,3-bis{4-[(3-dimethylaminopropyl)iminomethyl]phenyl}isoquinoline (8j)
Pale-yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.34 (s, 1H, CH=N), 8.28 (s, 1H, CH=N), 8.20 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 7.93 (s, 1H, H-4), 7.91 (d, 1H, J= 9.00 Hz, H-8), 7.86 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.78 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.76 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.09 (d, 1H, J= 2.40 Hz, H-5), 7.06 (dd, 1H, J= 9.00 and 2.40 Hz, H-7), 3.87 (s, 3H, CH3O), 3.66 (t, 2H, J= 7.20 Hz, NCH2), 3.63 (t, 2H, J= 7.20 Hz, NCH2), 2.37–2.30 (m, 4H, 2NCH2), 2.22 (s, 6H, N(CH3)2), 2.21 (s, 6H, N(CH3)2), 1.91–1.85 (m, 4H, 2CH2).
6-Methoxy-1,3-bis{4-[(4–(4-methylpiperazin-1-yl)butyl)iminomethyl]phenyl}isoquinoline (8k)
Orange-yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.29 (s, 1H, CH=N), 8.23 (s, 1H, CH=N), 8.17 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 7.92 (s, 1H, H-4), 7.88 (d, 1H, J= 9.00 Hz, H-8), 7.82 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.74 (d, 4H, J= 8.40 Hz, H-2′′, H-6′′, H-2′ and H-6′), 7.08 (d, 1H, J= 2.40 Hz, H-5), 7.03 (dd, 1H, J= 9.00 and 2.40 Hz, H-7), 3.85 (s, 3H, CH3O), 3.62–3.54 (m, 4H, 2NCH2), 2.48–2.30 (m, 20H, 2NCH2 and 8NCH2pip.), 2.21 (s, 3H, NCH3), 2.19 (s, 3H, NCH3), 1.71–1.64 (m, 4H, 2CH2), 1.56–1.49 (m, 4H, 2CH2).
6-Methoxy-1,3-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}isoquinoline (8l)
Yellow oil (95%); 1H NMR δ (300 MHz, CDCl3) 8.38 (s, 1H, CH=N), 8.33 (s, 1H, CH=N), 8.23 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.01 (s, 1H, H-4), 7.97 (d, 1H, J= 9.20 Hz, H-8), 7.89 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.82 (d, 4H, J= 8.40 Hz, H-2′′, H-6′′, H-2′ and H-6′), 7.18 (d, 1H, J= 2.40 Hz, H-5), 7.11 (dd, 1H, J= 9.20 and 2.40 Hz, H-7), 3.96 (s, 3H, CH3O),3.69 (t, 2H, J= 6.90 Hz, NCH2), 3.66 (t, 2H, J= 6.90 Hz, NCH2), 2.50–2.38 (m, 20H, 2NCH2 and 8NCH2pip.), 2.27 (s, 3H, NCH3), 2.26 (s, 3H, NCH3), 1.99–1.90 (m, 4H, 2CH2).
2,4-Bis{4-[(4-dimethylaminobutyl)iminomethyl]phenyl}quinazoline (9a)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.72 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.39 (s, 1H, CH=N), 8.35 (s, 1H, CH=N), 8.13 (dd, 1H, J= 8.60 and 1.50 Hz, H-8), 8.07 (dd, 1H, J= 8.60 and 1.50 Hz, H-5), 7.96–7.84 (m, 7H, H-3′, H-5′, H-2′′, H-6′′ H-2′, H-6′ and H-7), 7.54 (ddd,1H, J= 8.60, 7.20 and 1.50 Hz, H-6), 3.69 (t, 2H, J= 7.00 Hz, NCH2), 3.67 (t, 2H, J= 7.00 Hz, NCH2), 2.38–2.31 (m. 4H, 2NCH2), 2.26 (s, 6H, N(CH3)2), 2.24 (s, 6H, N(CH3)2), 1.82–1.70 (m, 4H, 2CH2), 1.64–1.52 (m, 4H, 2CH2).
2,4-Bis{4-[(3-dimethylaminopropyl)iminomethyl]phenyl}quinazoline (9b)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.76 (d, 2H, J= 8.80 Hz, H-3′′ and H-5′′), 8.44 (s, 1H, CH=N), 8.39 (s, 1H, CH=N), 8.18 (dd, 1H, J= 8.60 and 1.50 Hz, H-8), 8.12 (dd, 1H, J= 8.60 and 1.50 Hz, H-5), 7.97–7.85 (m, 7H, H-3′, H-5′, H-2′′, H-6′′ H-2′, H-6′ and H-7), 7.58 (ddd,1H, J= 8.60, 7.20 and 1.50 Hz, H-6), 3.74–3.70 (m, 4H, 2NCH2), 2.43 (t, 2H, J= 7.20 Hz, NCH2), 2.41 (t, 2H, J= 7.20 Hz, NCH2), 2.30 (s, 6H, N(CH3)2), 2.29 (s, 6H, N(CH3)2), 1.99–1.92 (m, 4H, 2CH2).
2,4-Bis{4-[(2-dimethylaminoethyl)iminomethyl]phenyl}quinazoline (9c)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.72 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.47 (s, 1H, CH=N), 8.42 (s, 1H, CH=N), 8.18 (dd, 1H, J= 8.10 and 1.50 Hz, H-8), 8.08 (dd, 1H, J= 8.10 and 1.50 Hz, H-5), 7.95–7.88 (m, 7H, H-3′, H-5′, H-2′′, H-6′′ H-2′, H-6′ and H-7), 7.58 (ddd,1H, J= 8.10, 6.90 and 1.50 Hz, H-6), 3.84 (t, 2H, J= 6.90 Hz, NCH2), 3.82 (t, 2H, J= 6.90 Hz, NCH2), 2.73 (t, 2H, J= 6.90 Hz, NCH2), 2.71 (t, 2H, J= 6.90 Hz, NCH2), 2.37 (s, 6H, N(CH3)2), 2.35 (s, 6H, N(CH3)2).
2,4-Bis{4-[(4–(4-methylpiperazin-1-yl)butyl)iminomethyl]phenyl}quinazoline (9d)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.74 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.41 (s, 1H, CH=N), 8.37 (s, 1H, CH=N), 8.18 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 8.18 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.96–7.86 (m, 7H, H-3′, H-5′, H-2′′, H-6′′ H-2′, H-6′ and H-7), 7.60 (ddd,1H, J= 8.10, 6.90 and 1.20 Hz, H-6), 3.72–3.66 (m, 4H, 2NCH2), 2.65–2.38 (m, 20H, 2NCH2 and 8 NCH2pip.), 2.29 (s, 3H, NCH3), 2.28(s, 3H, NCH3), 1.83–1.71 (m, 4H, 2CH2), 1.63–1.58 (m, 4H, 2CH2).
2,4-Bis{4-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}quinazoline (9e)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.75 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.43 (s, 1H, CH=N), 8.38 (s, 1H, CH=N), 8.17 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 8.13 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.96–7.86 (m, 7H, H-3′, H-5′, H-2′′, H-6′′ H-2′, H-6′ and H-7), 7.58 (ddd,1H, J= 8.10, 6.90 and 1.20 Hz, H-6), 3.76–3.67 (m, 4H, 2NCH2), 2.65–2.41 (m, 20H, 2NCH2 and 8 NCH2pip.), 2.31 (s, 3H, NCH3), 2.30 (s, 3H, NCH3), 1.99–1.91 (m, 4H, 2CH2).
2,4-Bis{4-[(3-morpholinopropyl)iminomethyl]phenyl}quinazoline (9f)
Yellow oil (93%); 1H NMR δ (300 MHz, CDCl3) 8.67 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.32 (s, 1H, CH=N), 8.28 (s, 1H, CH=N), 8.06 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 7.99 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.89–7.77 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-2′, H-6′ and H-7), 7.46 (ddd, 1H, J= 8.10, 6.90 and 1.20 Hz, H-6), 3.67–3.57 (m, 12H, 2NCH2 and 4 OCH2), 2.40–2.32 (m, 12H, 6NCH2), 1.92–1.81 (m, 4H, 2CH2).
2,4-Bis{4-[(2-morpholinoethyl)iminomethyl]phenyl}quinazoline (9g)
Yellow oil (88%); 1H NMR δ (300 MHz, CDCl3) 8.76 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.45 (s, 1H, CH=N), 8.41 (s, 1H, CH=N), 8.17 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 8.12 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.97–7.75 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-2′, H-6′ and H-7), 7.60 (ddd, 1H, J= 8.10, 6.90 and 1.20 Hz, H-6), 3.87 (t, 2H, J= 6.90 Hz, NCH2), 3.84 (t, 2H, J= 6.90 Hz, NCH2), 3.78–3.71 (m, 8H, 4OCH2), 2.84–2.74 (m, 4H, 2NCH2), 2.61–2.57 (m, 8H, 4NCH2).
6-Methoxy-2,4-bis{4-[(4-dimethylaminobutyl)iminomethyl]phenyl}quinazoline (9h)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.64 (d, 2H, J= 8.25 Hz, H-3′′ and H-5′′), 8.35 (s, 1H, CH=N), 8.30 (s, 1H, CH=N), 7.99 (s, 1H, J= 9.30 Hz, H-8), 7.90–7.88 (m, 4H, H-3′ and H-5′, H-2′′ and H-6′′), 7.80 (d, 2H, J= 8.25 Hz, H-2′ and H-6′), 7.46 (dd, 1H, J= 9.30 and 2.70 Hz, H-7), 7.25 (d, 1H, J= 2.70 Hz, H-5), 3.76 (s, 3H, CH3O and 2NCH2), 3.65 (t, 2H, J= 7.10 Hz, NCH2), 3.62 (t, 2H, J= 7.10 Hz, NCH2), 2.28 (t, 2H, J= 7.10 Hz, NCH2), 2.27 (t, 2H, J= 7.10 Hz, NCH2), 2.19 (s, 6H, N(CH3)2), 2.18 (s, 6H, N(CH3)2), 1.76–1.68 (m, 4H, 2CH2), 1.57–1.50 (m, 4H, 2CH2).
6-Methoxy-2,4-bis{4-[(3-dimethylaminopropyl)iminomethyl]phenyl}quinazoline (9i)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.66 (d, 2H, J= 8.25 Hz, H-3′′ and H-5′′), 8.39 (s, 1H, CH=N), 8.33 (s, 1H, CH=N), 8.00 (d, 1H, J= 9.15 Hz, H-8), 7.92–7.90 (m, 4H, H-3′ and H-5′, H-2′′ and H-6′′), 7.82 (d, 2H, J= 8.25 Hz, H-2′ and H-6′), 7.48 (dd,1H, J= 9.15 and 2.40 Hz, H-7), 7.28 (d, 1H, J= 2.40 Hz, H-5), 3.77 (s, 3H, CH3O), 3.69–3.62 (m, 4H, 2NCH2), 2.39–2.32 (m, 4H, 2NCH2), 2.23 (s, 6H, N(CH3)2), 2.22 (s, 6H, N(CH3)2), 1.95–1.83 (m, 4H, 2CH2).
6-Methoxy-2,4-bis{4-[(2-dimethylaminoethyl)iminomethyl]phenyl}quinazoline (9j)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.66 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.36 (s, 1H, CH=N), 8.30 (s, 1H, CH=N), 7.95–7.76 (m, 7H, H-8, H-3′ and H-5′, H-2′′ and H-6′′, H-2 and H-6′), 7.41 (dd, 1H, J= 9.10 and 2.80 Hz, H-7), 7.20 (d, 1H, J= 2.80 Hz, H-5), 3.77–3.70 (m, 7H, CH3O and 2NCH2), 2.64 (t, 2H, J= 6.60 Hz, NCH2), 2.62 (t, 2H, J= 6.60 Hz, NCH2), 2.27 (s, 6H, N(CH3)2), 2.26 (s, 6H, N(CH3)2).
6-Methoxy-2,4-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}quinazoline (9k)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.61 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.33 (s, 1H, CH=N), 8.27 (s, 1H, CH=N), 7.96 (d, 1H, J= 9.15 Hz, H-8), 7.89–7.83 (m, 4H, H-3′ and H-5′, H-2′′ and H-6′′), 7.77 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.43 (dd,1H, J= 9.15 and 2.70 Hz, H-7), 7.22 (d, 1H, J= 2.70 Hz, H-5), 3.72 (s, 3H, CH3O), 3.64 (t, 2H, J= 6.60 Hz, NCH2), 3.60 (t, 2H, J= 6.60 Hz, NCH2), 2.50–2.30 (m, 20H, 2NCH2 and 8NCH2 pip.), 2.21 (s, 3H, NCH3), 2.20 (s, 3H, NCH3), 1.90–1.82 (m, 4H, 2CH2).
6-Methoxy-2,4-bis{4-[(2–(4-methylpiperazin-1-yl)ethyl)iminomethyl]phenyl}quinazoline (9l)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.64 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.38 (s, 1H, CH=N), 8.32 (s, 1H, CH=N), 8.00 (d, 1H, J= 9.15 Hz, H-8), 7.90 (S, 4H, H-3′ and H-5′, H-2′′ and H-6′′), 7.80 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.48 (dd,1H, J= 9.15 and 2.70 Hz, H-7), 7.27 (d, 1H, J= 2.70 Hz, H-5), 3.82–3.73 (m, 7H, CH3O and 2NCH2), 2.73 (t, 2H, J= 7.20 Hz, NCH2), 2.71 (t, 2H, J= 7.20 Hz, NCH2), 2.58–2.38 (m, 16H, 8NCH2 pip.), 2.25 (s, 3H, NCH3), 2.24 (s, 3H, NCH3).
7-Methoxy-2,4-bis{4-[(4-dimethylaminobutyl)iminomethyl]phenyl}quinazoline (9m)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.72 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.41 (s, 1H, CH=N), 8.37 (s, 1H, CH=N), 8.00–7.85 (m, 7H, H-5, H3′ and H5′, H-2′′ and H-6′′, H-2′ and H-6′), 7.45 (d, 1H, J= 2.70 Hz, H-8), 7.18 (dd,1H, J= 9.30 and 2.70 Hz, H-6), 4.02 (s, 3H, CH3O), 3.71 (t, 2H, J= 6.90 Hz, NCH2), 3.62 (t, 2H, J= 6.90 Hz, NCH2), 2.35 (t, 2H, J= 6.90 Hz, NCH2), 2.32 (t, 2H, J= 6.90 Hz, NCH2), 2.25 (s, 6H, N(CH3)2), 2.23 (s, 6H, N(CH3)2), 1.81–1.71 (m, 4H, 2CH2), 1.64–1,54 (m, 4H, 2CH2).
7-Methoxy-2,4-bis{4-[(3-dimethylaminopropyl)iminomethyl]phenyl}quinazoline (9n)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.73 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.44 (s, 1H, CH=N), 8.40 (s, 1H, CH=N), 8.00 (d, 1H, J= 9.25 Hz, H-5), 7.97 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.92 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.88 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.46 (d, 1H, J= 2.50 Hz, H-8), 7.19 (dd,1H, J= 9.25 and 2.50 Hz, H-6), 4.04 (s, 3H, CH3O), 3.74 (t, 2H, J= 7.10 Hz, NCH2), 3.71 (t, 2H, J= 7.10 Hz, NCH2), 2.40 (t, 2H, J= 7.10 Hz, NCH2), 2.39 (t, 2H, J= 7.10 Hz, NCH2), 2.28 (s, 6H, N(CH3)2), 2.27 (s, 6H, N(CH3)2),1.96–1.83 (m, 4H, 2CH2).
7-Methoxy-2,4-bis{4-[(2-dimethylaminoethyl)iminomethyl]phenyl}quinazoline (9o)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.69 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.42 (s, 1H, CH=N), 8.38 (s, 1H, CH=N), 7.95–7.84 (m, 7H, H-5, H3′ and H5′, H-2′′ and H-6′′, H-2′ and H-6′), 7.41 (d, 1H, J= 2.40 Hz, H-8), 7.14 (dd,1H, J= 9.30 and 2.40 Hz, H-6), 3.98 (s, 3H, CH3O), 3.80 (t, 2H, J= 7.00 Hz, NCH2), 3.78 (t, 2H, J= 7.00 Hz, NCH2), 2.68 (t, 2H, J= 7.00 Hz, NCH2), 2.67 (t, 2H, J= 7.00 Hz, NCH2), 2.33 (s, 6H, N(CH3)2), 2.32 (s, 6H, N(CH3)2).
7-Methoxy-2,4-bis{4-[(4–(4-methylpiperazin-1-yl)butyl)iminomethyl]phenyl}quinazoline (9p)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.59 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.26 (s, 1H, CH=N), 8.22 (s, 1H, CH=N), 7.84–7.72 (m, 7H, H-5, H3′ and H5′, H-2′′ and H-6′′, H-2′ and H-6′), 7.28 (d, 1H, J= 2.40 Hz, H-8), 7.12 (dd,1H, J= 9.30 and 2.40 Hz, H-6), 3.86 (s, 3H, CH3O), 3.57 (t, 2H, J= 6.60 Hz, NCH2), 3.53 (t, 2H, J= 6.60 Hz, NCH2), 2.50–2.20 (m, 20H, 2NCH2 and 8NCH2 pip.), 2.17 (s, 3H, NCH3), 2.16 (s, 3H, NCH3), 1.66–1.62 (m, 4H, 2CH2), 1.51–1.47 (2CH2).
7-Methoxy-2,4-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}quinazoline(9q)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.70 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.40 (s, 1H, CH=N), 8.36 (s, 1H, CH=N), 7.96 (d, 1H, J= 9.30 Hz, H-5), 7.93 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.88 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.85 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.43 (d, 1H, J= 2.70 Hz, H-8), 7.16 (dd,1H, J= 9.30 and 2.70 Hz, H-6), 4.00 (s, 3H, CH3O), 3.73–3.65 (m, 4H, 2NCH2), 2.49–2,42 (m, 20H, 2NCH2 and 8NCH2 pip.), 2.28 (s, 3H, NCH3), 2.27 (s, 3H, NCH3), 1.99–1.87 (m, 4H, 2CH2).
7-Methoxy-2,4-bis{4-[(2–(4-methylpiperazin-1-yl)ethyl)iminomethyl]phenyl}quinazoline (9r)
Orange oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.68 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.39 (s, 1H, CH=N), 8.35 (s, 1H, CH=N), 7.94–7.82 (m, 7H, H-5, H3′ and H5′, H-2′′ and H-6′′, H-2′ and H-6′), 7.41 (d, 1H, J= 2.60 Hz, H-8), 7.14 (dd,1H, J= 9.25 and 2.60 Hz, H-6), 3.98 (s, 3H, CH3O), 3.82 (t, 2H, J= 7.20 Hz, NCH2), 3.79 (t, 2H, J= 7.20 Hz, NCH2), 2.76 (t, 2H, J= 7.20 Hz, NCH2), 2.73 (t, 2H, J= 7.20 Hz, NCH2), 2.65–2,30 (m, 16H, 8NCH2 pip), 2.28 (s, 3H, NCH3), 2.27 (s, 3H, NCH3).
2,4-Bis{3-[(3-dimethylaminopropyl)iminomethyl]phenyl}quinazoline (9s)
Pale-yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.94 (dd, 1H, J= 1.40 and 1.40 Hz, H-2′), 8.77 (ddd, 1H, J= 7.80, 1.40 and 1.40 Hz, H-6′), 8.48 (s, 1H, CH=N), 8.45 (s, 1H, CH=N), 8.21–8.17 (m, 2H, H-2′′ and H-8), 8.11 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 8.03–7.90 (m, 4H, H-6′′, H-4′, H-4′′ and H-7), 7.67 (dd,1H, J= 7.80 and 7.80 Hz, H-5′′), 7.62–7.56 (m, 2H, H-5′ and H-6), 3.74–3.69 (m, 4H, 2NCH2), 2.43–2.33 (m, 4H, 2NCH2), 2.27 (s, 6H, N(CH3)2), 2.26 (s, 6H, N(CH3)2), 1.98–1.87 (m, 4H, 2CH2).
2,4-Bis{3-[(3–(4-methylpiperazin-1-yl)propyl)iminomethyl]phenyl}quinazoline(9t)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.93 (dd, 1H, J= 1.35 and 1.35 Hz, H-2′), 8.76 (ddd, 1H, J= 7.80, 1.35 and 1.35 Hz, H-6′), 8.47 (s, 1H, CH=N), 8.44 (s, 1H, CH=N), 8.21–8.18 (m, 2H, H-2′′ and H-8), 8.11 (dd, 1H, J= 8.40 and 1.50 Hz, H-5), 8.02–7.90 (m, 4H, H-6′′, H-4′, H-4′′ and H-7), 7.67 (dd,1H, J= 7.80 and 7.80 Hz, H-5′′), 7.62–7.56 (m, 2H, H-5′ and H-6), 3.76–3.68 (m, 4H, 2NCH2), 2.50–2.38 (m, 20H, 2NCH2 and 8NCH2pip.), 2.30 (s, 3H, NCH3), 2.29 (s, 3H, NCH3), 1.98–1.90 (m, 4H, 2CH2).
2,4-Bis{3-[(3-morpholinopropyl)iminomethyl]phenyl}quinazoline (9u)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.93 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.76 (ddd, 1H, J= 7.80, 1.50 and 1.50 Hz, H-6′), 8.46 (s, 1H, CH=N), 8.43 (s, 1H, CH=N), 8.20–8.16 (m, 2H, H-2′′ and H-8), 8.09 (dd, 1H, J= 8.60 and 1.20 Hz, H-5), 8.00–7.91 (m, 4H, H-6′′, H-4′, H-4′′ and H-7), 7.66 (dd,1H, J= 7.80 and 7.80 Hz, H-5′′), 7.61–7.55 (m, 2H, H-5′ and H-6), 3.75–3.68 (m, 12H, 2NCH2 and 4OCH2), 2.48–2.37 (m, 12H, 2NCH2 and 4NCH2morph.), 1.96–1.87 (m, 4H, 2CH2).
2,4-Bis{3-[(2-morpholinoethyl)iminomethyl]phenyl}quinazoline (9v)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.95 (dd, 1H, J= 1.35 and 1.35 Hz, H-2′), 8.76 (ddd, 1H, J= 7.80, 1.35 and 1.35 Hz, H-6′), 8.49 (s, 1H, CH=N), 8.46 (s, 1H, CH=N), 8.21–8.18 (m, 2H, H-2′′ and H-8), 8.10 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 8.02–7.91 (m, 4H, H-6′′, H-4′, H-4′′ and H-7), 7.68 (dd,1H, J= 7.80 and 7.80 Hz, H-5′′), 7.63–7.57 (m, 2H, H-5′ and H-6), 3.85 (t, 4H, J= 6.90 Hz, NCH2), 3.77–3.72 (m, 8H, 4OCH2), 2.79–2.73 (m, 4H, 2NCH2), 2.61–2.57–1.87 (m, 8H, 4NCH2).
General procedure for 2,4-bis[(substituted-aminomethyl)phenyl]quinolines (1a-t), 1,3-bis[(substituted-aminomethyl)phenyl]isoquinolines (2a–l), and 2,4-bis[(substituted-aminomethyl)phenyl]quinazolines (3a–v)
To a solution of compound 7–9 (0.4 mmol) in methanol (10 ml) was added portion-wise at 0 °C sodium borohydride (3.2 mmol, 8 eq.). The reaction mixture was then stirred at room temperature for 1 h and subsequently heated under reflux for 1 h. Then it was evaporated to dryness under reduced pressure. After cooling, the residue was triturated in water and extracted with dichloromethane (40 ml). The organic layer was separated, dried over sodium sulphate and activated charcoal and evaporated to dryness. Oils were used without further purification to give compounds 1–3.
2,4-Bis{4-[(4-dimethylaminobutyl)aminomethyl]phenyl}quinoline (1a)
Yellow oil (89%); 1H NMR δ (300 MHz, CDCl3) 8.23 (dd, 1H, J= 8.10 and 1.50 Hz, H-8), 8.15 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 7.91 (dd, 1H, J= 8.10 and 1.50 Hz, H-5), 7.80 (s, 1H, H-3), 7.72 (ddd,1H, J= 8.10, 6.70 and 1.50 Hz, H-7), 7.52–7.43 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-3′′, H-5′′ and H-6), 3.91 (s, 2H, NCH2), 3.87 (s, 2H, NCH2),2.73 (t, 2H, J= 6.70 Hz, NCH2), 2.67 (t, 2H, J= 6.70 Hz, NCH2), 2.29 (t, 2H, J= 6.70 Hz, NCH2), 2.26 (t, 2H, J = 6.70 Hz, NCH2), 2.22 (s, 6H, N(CH3)2), 2.20 (s, 6H, N(CH3)2), 1.58–1.52 (m, 8H, 4CH2).13C NMR δ (75 MHz, CDCl3) 158.0 (C-2), 150.3 (C-4), 150.2 (C-8a), 143.1 (C-1′′), 142.2 (C-1′), 139.7 (C-4′′), 138.4 (C-4′), 131.4 (C-7), 131.0(C-3′′ and C-5′′), 130.9 (C-8), 130.0 (C-3′ and C-5′), 129.7(C-2′′ and C-6′′), 129.0 (C-2′ and C-6′), 127.6 (C-5),127.2 (C-4a), 127.0 (C-6), 120.6 (C-3), 61.0 (NCH2), 55.0 (NCH2), 54.8 (NCH2), 50.8 (NCH2), 50.6 (NCH2), 46.8 (2N(CH3)2), 29.4 (2CH2), 26.9 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C35H48N5: 538.391, Found: 538.250.
2,4-Bis{4-[(3-dimethylaminopropyl)aminomethyl]phenyl}quinoline (1b)
Pale-yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.22 (dd, 1H, J= 8.40 and 1.50 Hz, H-8), 8.15 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.91 (dd, 1H, J= 8.40 and 1.50 Hz, H-5), 7.80 (s, 1H, H-3), 7.71 (ddd,1H, J= 8.40, 6.90 and 1.50 Hz, H-7), 7.55–7.42 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-3′′, H-5′′ and H-6), 3.91 (s, 2H, NCH2), 3.88 (s, 2H, NCH2), 2.76 (t, 2H, J= 6.90 Hz, NCH2), 2.71 (t, 2H, J= 6.90 Hz, NCH2), 2.37 (t, 2H, J= 6.90 Hz, NCH2), 2.33 (t, 2H, J = 6.90 Hz, NCH2), 2.24 (s, 6H, N(CH3)2), 2.22 (s, 6H, N(CH3)2), 1.80–1.68 (m, 4H, 2CH2). 13C NMR δ (75 MHz, CDCl3) 158.0 (C-2), 150.3 (C-4), 150.2 (C-8a), 142.6 (C-1′′), 141.9 (C-1′), 139.9 (C-4′′), 138.4 (C-4′), 131.5 (C-7), 131.0 (C-3′′ and C-5′′), 130.9 (C-8), 130.0 (C-3′ and C-5′), 129.7 (C-2′′ and C-6′′), 129.0 (C-2′ and C-6′), 127.6 (C-5), 127.1(C-4a), 127.0 (C-6), 120.6 (C-3), 59.6 (NCH2), 55.0 (NCH2), 54.9 (NCH2), 49.5 (NCH2), 49.2 (NCH2), 46.9 (2N(CH3)2), 29.3 (CH2), 29.0 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C33H44N5: 510.360, Found: 510.384.
2,4-Bis{4-[(2-dimethylaminoethyl)aminomethyl]phenyl}quinoline (1c)
Yellow oil (76%); 1H NMR δ (300 MHz, CDCl3) 8.23 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 8.16 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.91 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.80 (s, 1H, H-3), 7.72 (ddd,1H, J= 8.10, 6.90 and 1.20 Hz, H-7), 7.52–7.42 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-3′′, H-5′′ and H-6), 3.94 (s, 2H, NCH2), 3.90 (s, 2H, NCH2), 2.79 (t, 2H, J= 6.30 Hz, NCH2), 2.72 (t, 2H, J= 6.30 Hz, NCH2), 2.50 (t, 2H, J= 6.30 Hz, NCH2), 2.46 (t, 2H, J= 6.30 Hz, NCH2), 2.25 (s, 6H, N(CH3)2), 2.22 (s, 6H, N(CH3)2). 13C NMR δ (75 MHz, CDCl3) 156.7 (C-2), 149.0 (C-4), 148.8 (C-8a), 141.6 (C-1′′), 140.7 (C-1′), 138.3 (C-4′′), 137.0 (C-4′), 130.0 (C-7), 129.6 (C-3′′ and C-5′′), 129.7 (C-8), 128.6 (C-3′ and C-5′), 128.4 (C-2′′ and C-6′′), 127.6 (C-2′ and C-6′), 126.2 (C-5), 125.8 (C-4a), 125.6 (C-6), 119.2 (C-3), 59.0 (NCH2), 53.7 (NCH2), 53.6 (NCH2), 46.7 (NCH2), 46.4 (NCH2), 45.5 (2 N(CH3)2); MALDI-TOF MS m/z [M + H]+ Calc for C31H40N5: 482.328, Found: 482.584.
2,4-Bis{4-[(4–(4-methylpiperazin-1-yl)butyl)aminomethyl]phenyl}quinoline (1d)
Yellow oil (59%); 1H NMR δ (300 MHz, CDCl3) 8.21 (dd, 1H, J= 8.40 and 1.20 Hz, H-8), 8.15 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 7.91 (dd, 1H, J= 8.40 and 1.20 Hz, H-5), 7.80 (s, 1H, H-3), 7.72 (ddd,1H, J= 8.40, 6.60 and 1.20 Hz, H-7), 7.55–7.43 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-2′, H-6′ and H-6), 3.90 (s, 2H, NCH2), 3.87 (s, 2H, NCH2), 2.73 (t, 2H, J= 6.90 Hz, NCH2), 2.66 (t, 2H, J= 6.90 Hz, NCH2), 2.55–2.32 (m. 20H, 2NCH2 and 8 NCH2pip.), 2.28 (s, 3H, NCH3), 2.27 (s, 3H, NCH3), 1.61–1.51 (m, 8H, 4CH2). 13C NMR δ (75 MHz, CDCl3) 158.1 (C-2), 150.3 (C-4), 150.2 (C-8a), 143.1 (C-1′′), 142.2 (C-1′), 139.7 (C-4′′), 138.4 (C-4′), 131.4 (C-7), 131.0 (C-3′′ and C-5′′), 130.9 (C-8), 129.9 (C-3′ and C-5′), 129.7 (C-2′′ and C-6′′), 129.0 (C-2′ and C-6′), 127.6 (C-5), 127.2 (C-4a), 127.0 (C-6), 120.6 (C-3), 59.9 (NCH2), 56.5 (NCH2), 55.1 (NCH2), 54.6 (NCH2), 50.8 (NCH2), 47.4 (2NCH3), 29.5 (2CH2), 26.1 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C41H58N7: 648.475, Found: 648.451.
2,4-Bis{4-[(3–(4-methylpiperazin-1-yl)propyl)aminomethyl]phenyl}quinoline (1e)
Yellow oil (78%); 1H NMR δ (300 MHz, CDCl3) 8.21(dd, 1H, J= 8.40 and 1.50 Hz, H-8), 8.15 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 7.88(dd, 1H, J= 8.40 and 1.50 Hz, H-5), 7.79 (s, 1H, H-3), 7.71 (ddd,1H, J= 8.40, 6.90 and 1.50 Hz, H-7), 7.54–7.41 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-2′, H-6′ and H-6), 3.90 (s, 2H, NCH2), 3.87 (s, 2H, NCH2),) , 2.76 (t, 2H, J= 6.90 Hz, NCH2), 2.70 (t, 2H, J= 6.90 Hz, NCH2), 2.48–2.37 (m. 20H, 2NCH2 and 8NCH2pip.), 2.26 (s, 3H, NCH3), 2.25 (s, 3H, NCH3), 1.81–1.68 (m, 4H, 2CH2). 13C NMR δ (75 MHz, CDCl3) 158.0 (C-2), 150.3 (C-4), 150.2 (C-8a), 142.7 (C-1′′), 142.0 (C-1′), 139.8 (C-4′′), 138.4 (C-4′), 131.4 (C-7), 131.0 (C-3′′ and C-5′′), 130.9 (C-8), 130.0 (C-3′ and C-5′), 129.7 (C-2′′ and C-6′′), 129.0 (C-2′ and C-6′), 127.6 (C-5), 127.1 (C-4a), 127.0 (C-6), 120.6 (C-3), 58.4 (NCH2), 56.5 (NCH2), 55.0 (NCH2), 54.9 (NCH2), 54.6 (NCH2), 49.7 (NCH2), 49.5 (NCH2), 47.4 (2NCH3), 28.2 (CH2), 28.0 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C39H54N7: 620.444, Found: 620.560.
2,4-Bis{4-[(2–(4-methylpiperazin-1-yl)ethyl)aminomethyl]phenyl}quinoline (1f)
Orange oil (65%); 1H NMR δ (300 MHz, CDCl3) 8.23 (dd, 1H, J= 8.50 and 1.20 Hz, H-8), 8.16 (d, 2H, J= 8.10 Hz, H-3′′′′ and H-5′′), 7.92 (dd, 1H, J= 8.50 and 1.20 Hz, H-5), 7.81 (s, 1H, H-3), 7.81 (ddd,1H, J= 8.50, 6.70 and 1.20 Hz, H-7), 7.54–7.44 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-2′, H-6′ and H-6),) , 3.94 (s, 2H, NCH2), 3.91 (s, 2H, NCH2), 2.81 (t, 2H, J= 6.90 Hz, NCH2), 2.74 (t, 2H, J= 6.90 Hz, NCH2), 2.61–2.40 (m. 20H, 2NCH2 and 8NCH2pip.), 2.30 (s, 3H, NCH3), 2.29 (s, 3H, NCH3). 13C NMR δ (75 MHz, CDCl3) 158.0 (C-2), 150.3 (C-4), 150.2 (C-8a), 142.7 (C-1′′), 142.0 (C-1′), 139.9 (C-4′′), 138.5 (C-4′), 131.5 (C-7), 131.0 (C-3′′ and C-5′′), 130.9 (C-8), 130.1 (C-3′ and C-5′), 129.8 (C-2′′ and C-6′′), 129.0 (C-2′ and C-6′), 127.6 (C-5), 127.2 (C-4a), 127.0 (C-6), 120.6 (C-3), 59.0 (NCH2), 58.9(NCH2), 56.5 (NCH2), 55.0 (NCH2), 54.9 (NCH2), 54.5 (NCH2), 47.4 (NCH3), 47.1 (NCH2), 46.7 (NCH2); MALDI-TOF MS m/z [M + H]+ Calc for C37H50N7: 592.413, Found: 592.525.
2,4-Bis{4-[(3-morpholinopropyl)aminomethyl]phenyl}quinoline (1g)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.21 (dd, 1H, J= 8.40 and 1.20 Hz, H-8), 8.14 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 7.89 (dd, 1H, J= 8.40 and 1.20 Hz, H-5), 7.79 (s, 1H, H-3), 7.70(ddd,1H, J= 8.40, 6.90 and 1.20 Hz, H-7), 7.53–7.41 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-2′, H-6′ and H-6), 3.88 (s, 2H, NCH2), 3.84 (s, 2H, NCH2), 3.69 (t, 2H, J= 4.50 Hz, OCH2), 3.67 (t, 2H, J= 4.50 Hz, OCH2), 2.74 (t, 2H, J= 6.90 Hz, NCH2), 2.68 (t, 2H, J= 6.90 Hz, NCH2), 2.45–2.35 (m, 12H, 6NCH2), 1.85 (bs, 2H, 2NH), 1.78–1.64 (m, 4H, 2CH2). 13C NMR δ (75 MHz, CDCl3) 158.0 (C-2), 150.3 (C-4), 150.2 (C-8a), 143.2 (C-1′′), 142.3 (C-1′), 139.6 (C-4′′), 138.3 (C-4′), 131.4 (C-7), 131.0 (C-3′′ and C-5′′), 130.9 (C-8), 129.8 (C-3′ and C-5′), 129.6 (C-2′′ and C-6′′), 128.9 (C-2′ and C-6′), 127.6 (C-5), 127.1 (C-4a), 127.0 (C-6), 120.5 (C-3), 68.4 (OCH2), 58.8 (NCH2), 55.2 (NCH2), 49.5 (NCH2), 49.3 (NCH2), 28.1 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C37H48N5O2: 594.381, Found: 594.465.
2,4-Bis{4-[(2-morpholinoethyl)aminomethyl]phenyl}quinoline (1h)
Pale-yellow oil (76%); 1H NMR δ (300 MHz, CDCl3) 8.22 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 8.15 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 7.89 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.79 (s, 1H, H-3), 7.71 (ddd,1H, J= 8.10, 6.90 and 1.20 Hz, H-7), 7.54–7.41 (m, 7H, H-3′, H-5′, H-2′′, H-6′′, H-2′, H-6′ and H-6), 3.90 (s, 2H, NCH2), 3.87 (s, 2H, NCH2), 3.69 (t, 2H, J= 4.70 Hz, OCH2), 3.68 (t, 2H, J= 4.70 Hz, OCH2), 2.77 (t, 2H, J= 6.20 Hz, NCH2), 2.70 (t, 2H, J= 6.20 Hz, NCH2), 2.53 (t, 2H, J= 6.20 Hz, NCH2), 2.47 (t, 2H, J= 6.20 Hz, NCH2), 2.45–2.39 (m, 8H, 4NCH2), 2.20 (bs, 2H, 2NH). 13C NMR δ (75 MHz, CDCl3) 158.0 (C-2), 150.3 (C-4), 150.2 (C-8a), 143.1 (C-1′′), 142.2 (C-1′), 139.7 (C-4′′), 138.4 (C-4'), 131.4 (C-7), 131.0 (C-3′′ and C-5′′), 130.9 (C-8), 129.9 (C-3′ and C-5′), 129.7 (C-2′′ and C-6′′), 129.0 (C-2′ and C-6′), 127.6 (C-5), 127.1 (C-4a), 127.0 (C-6), 120.6 (C-3), 68.4 (OCH2), 63.6 (NCH2), 59.7 (NCH2), 59.6 (NCH2), 55.1 (NCH2), 55.0 (NCH2), 54.9 (NCH2), 46.8 (NCH2), 46.5 (NCH2); MALDI-TOF MS m/z [M + H]+ Calc for C35H44N5O2: 566.350, Found: 566.488.
6-Methoxy-2,4-bis{4-[(4-dimethylaminobutyl)aminomethyl]phenyl}quinoline (1i)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.06 (m, 3H, H-2′′ and H-6′′, H-8), 7.69 (s, 1H, H-3), 7.46–7.37 (m, 6H, H-2′ and H-6′, H-3′′ and H-5′′, H-3′ and H-5′), 7.32–7.28 (m, 1H, H-7), 7.14–7.13 (m, 1H, H-5), 3.83 (s, 2H, NCH2), 3.78 (s, 2H, NCH2), 3.70 (s, 3H, CH3O), 2.70–2.56 (m, 4H, 2NCH2), 2.24–2.19 (m, 4H, 2NCH2), 2.15 (s, 6H, N(CH3)2), 2.13 (s, 6H, N(CH3)2), 1.52–1.46 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 159.0 (C-6), 155.7 (C-2), 148.9 (C-4), 146.2 (C-8a), 142.7 (C-4′), 142.1 (C-4′′), 139.7 (C-1′), 138.6 (C-1′′), 132.9 (C-8), 130.7 (C-3′ and C-5′), 129.8 (C-3′′and C-5′′), 129.7 (C-2′ and C-6′), 128.6 (C-2′′ and C-6′′), 127.9 (C-4a), 123.1 (C-7), 120.8 (C-3), 105.0 (C-5), 61.0 (2NCH2), 56.8 (CH3O), 55.0 (2NCH2), 50.9 (NCH2), 50.6 (NCH2), 46.8 (2N(CH3)2), 29.3 (2CH2), 26.9 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C36H48N5O: 566.386, Found: 566.285.
6-Methoxy-2,4-bis{4-[(3-dimethylaminopropyl)aminomethyl]phenyl}quinoline (1j)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.07 (m, 3H, H-2′′and H-6′′, H-8), 7.71 (s, 1H, H-3), 7.49 (d, 2H, J= 8.20 Hz, H-2′ and H-6′), 7.45 (d, 2H, J= 8.20 Hz, H-3′′and H-5′′), 7.41 (d, 2H, J= 8.20 Hz, H-3′ and H-5′), 7.32 (dd, 1H, J= 9.30 and 2.70 Hz, H-7), 7.16 (d, 1H, J= 2.70 Hz, H-5), 3.85 (s, 2H, NCH2), 3.81 (s, 2H, NCH2), 3.73 (s, 3H, CH3O), 2.71 (t, 2H, J= 7.10 Hz, NCH2), 2.64 (t, 2H, J= 7.10 Hz, NCH2), 2.32 (t, 2H, J= 7.10 Hz, NCH2), 2.27 (t, 2H, J = 7.10 Hz, NCH2), 2.19 (s, 6H, N(CH3)2), 2.17 (s, 6H, N(CH3)2), 1.74–1.60 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 159.0 (C-6), 155.7 (C-2), 148.9 (C-4), 146.2 (C-8a), 142.8 (C-4′), 142.2 (C-4′′), 139.7 (C-1′), 138.6 (C-1′′), 133.9 (C-8), 130.7 (C-3′ and C-5′), 129.8 (C-3′′and C-5′′), 129.7 (C-2′ and C-6′), 128.6 (C-2′′and C-6′′), 127.9 (C-4a), 123.1 (C-7), 120.9 (C-3), 105.0 (C-5), 59.5 (NCH2), 59.4 (NCH2), 56.8 (CH3O), 55.2 (NCH2), 55.1 (NCH2), 49.5 (NCH2), 49.2 (NCH2), 47.0 (N(CH3)2), 46.9 (N(CH3)2), 29.5 (CH2), 29.4 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C34H44N5O: 538.354, Found: 538.372.
6-Methoxy-2,4-bis{4-[(4–(4-methylpiperazin-1-yl)butyl)aminomethyl]phenyl}quinoline (1k)
Pale yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.03 (d, 2H, J= 8.20 Hz, H-2′′and H-6′′), 8.02 (d, 1H, J= 9.15 Hz, H-8), 7.66 (s, 1H, H-3), 7.45 (d, 2H, J= 8.20 Hz, H-2′ and H-6′), 7.41 (d, 2H, J= 8.20 Hz, H-3′′and H-5′′), 7.36 (d, 2H, J= 8.20 Hz, H-3′ and H-5′), 7.28 (dd, 1H, J= 9.15 and 2.70 Hz, H-7), 7.11 (d, 1H, J= 2.70 Hz, H-5), 3.81 (s, 2H, NCH2), 3.76 (s, 2H, NCH2), 3.69 (s, 3H, CH3O), 2.65–2.22 (m, 24H, 4NCH2 and 8NCH2 pip.), 2.18 (s, 3H, NCH3), 2.17 (s, 3H, NCH3), 1.49–1.42 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 159.0 (C-6), 155.6 (C-2), 148.9 (C-4), 146.2 (C-8a), 142.7 (C-4′), 142.2 (C-4′′), 139.6 (C-1′), 138.5 (C-1′′), 132.8 (C-8), 130.7 (C-3′ and C-5′), 129.8 (C-3′′and C-5′′), 129.7 (C-2′ and C-6′), 128.5 (C-2′′and C-6′′), 127.9 (C-4a), 123.0 (C-7), 120.8 (C-3), 105.0 (C-5), 59.8 (2NCH2), 56.7 (CH3O), 56.5 (2NCH2 pip.), 55.0 (2NCH2), 54.5 (2NCH2 pip.), 50.9 (NCH2), 50.5 (NCH2), 47.4 (2NCH3), 29.4 (2CH2), 26.1 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C42H60N7O: 678.486, Found: 678.487.
6-Methoxy-2,4-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)aminomethyl]phenyl}quinoline (1l)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.10 (d, 2H, J= 8.25 Hz, H-2′′ and H-6′′), 8.07 (d, 1H, J= 9.10 Hz, H-8), 7.72 (s, 1H, H-3), 7.51 (d, 2H, J= 8.25 Hz, H-2′ and H-6′), 7.47 (d, 2H, J= 8.25 Hz, H-3′′ and H-5′′), 7.41 (d, 2H, J= 8.25 Hz, H-3′ and H-5′), 7.34 (dd, 1H, J= 9.10 and 2.80 Hz, H-7), 7.17 (d, 1H, J= 2.80 Hz, H-5), 3.86 (s, 2H, NCH2), 3.82 (s, 2H, NCH2), 3.75 (s, 3H, CH3O), 2.73 (t, 2H, J= 6.70 Hz, NCH2), 2.65 (t, 2H, J= 6.70 Hz, NCH2), 2.45–2.34 (m, 16H, 8NCH2 pip), 2.24 (s, 3H, NCH3), 2.23 (s, 3H, NCH3), 1.78–1.63 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 159.1 (C-6), 155.8 (C-2), 148.9 (C-4), 146.2 (C-8a), 142.7 (C-4′), 142.1 (C-4′′), 139.7 (C-1′), 138.6 (C-1′′), 132.8 (C-8), 130.7 (C-3′ and C-5′), 129.8 (C-3′′and C-5′′), 129.7 (C-2′ and C-6′), 128.6 (C-2′′and C-6′′), 128.0 (C-4a), 123.1 (C-7), 120.9 (C-3), 105.0 (C-5), 58.4 (2NCH2), 56.8 (CH3O), 56.5 (2NCH2 pip.), 55.1 (2NCH2), 54.6 (2NCH2 pip.), 49.8 (NCH2), 49.4 (NCH2), 47.4 (2NCH3), 28.3 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C40H56N7O: 650.454, Found: 650.442.
7-Methoxy-2,4-bis{4-[(4-dimethylaminobutyl)aminomethyl]phenyl}quinoline (1m)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.07 (d, 2H, J= 8.40 Hz, H-2′′and H-6′′), 7.73 (d, 1H, J= 9.25 Hz, H-5), 7.60 (s, 1H, H-3), 7.49 (d, 1H, J= 2.60 Hz, H-8), 7.42–7.38 (m, 6H, H-2′ and H-6′, H-3′′ and H-5′′, H-3′ and H-5′), 7.03 (dd, 1H, J= 9.25 and 2.60 Hz, H-6), 3.90 (s, 3H, CH3O), 3.81 (s, 2H, NCH2), 3.79 (s, 2H, NCH2), 2.64 (t, 2H, J= 6.60 Hz, NCH2), 2.59 (t, 2H, J= 6.60 Hz, NCH2), 2.21 (t, 2H, J= 6.60 Hz, NCH2), 2.19 (t, 2H, J= 6.60 Hz, NCH2), 2.15 (s, 6H, N(CH3)2), 2.13 (s, 6H, N(CH3)2), 1.50–1.44 (m, 8H, 4CH2); MALDI-TOF MS m/z [M + H]+ Calc for C36H50N5O: 568.402, Found: 568.860.
7-Methoxy-2,4-bis{4-[(3-dimethylaminopropyl)aminomethyl]phenyl}quinoline (1n)
Pale yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.09 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.75 (d, 1H, J= 9.15 Hz, H-5), 7.62 (s, 1H, H-3), 7.51 (d, 1H, J= 2.40 Hz, H-8), 7.46–7.40 (m, 6H, H-2′ and H-6′, H-3′′ and H-5′′, H-3′ and H-5′), 7.32 (dd, 1H, J= 9.15 and 2.40 Hz, H-6), 3.92 (s, 3H, CH3O), 3.84 (s, 2H, NCH2), 3.82 (s, 2H, NCH2), 2.70 (t, 2H, J= 7.20 Hz, NCH2), 2.64 (t, 2H, J= 7.20 Hz, NCH2), 2.31 (t, 2H, J= 7.20 Hz, NCH2), 2.28 (t, 2H, J= 7.20 Hz, NCH2), 2.19 (s, 6H, N(CH3)2), 2.17 (s, 6H, N(CH3)2), 1.73–1.60 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 162.0 (C-7), 158.4 (C-2), 152.0 (C-4), 150.2 (C-8a), 142.9 (C-4′), 142.2 (C-4′′), 139.8 (C-1′), 138.4 (C-1′′), 130.9 (C-3′ and C-5′), 129.9 (C-3′′and C-5′′), 129.6 (C-2′ and C-6′), 128.9 (C-2′′and C-6′′), 128.1 (C-8), 122.2 (C-4a), 120.6 (C-6), 118.6 (C-3), 109.3 (C-5), 59.4 (2NCH2), 56.9 (CH3O), 55.0 (2NCH2), 49.4 (NCH2), 49.2 (NCH2), 46.9 (2N(CH3)2), 29.3 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C34H46N5O: 540.370, Found: 540.292.
7-Methoxy-2,4-bis{4-[(4–(4-methylpiperazin-1-yl)butyl)aminomethyl]phenyl}quinoline (1o)
Yellow oil (73%); 1H NMR δ (300 MHz, CDCl3) 8.13 (d, 2H, J= 8.10 Hz, H-2′′and H-6′′), 7.81 (d, 1H, J= 9.25 Hz, H-5), 7.67 (s, 1H, H-3), 7.57 (d, 1H, J= 2.60 Hz, H-8), 7.51 (s, 4H, H-2′ and H-6′, H-3′′ and H-5′′), 7.47 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.13 (dd, 1H, J= 9.25 and 2.60 Hz, H-6), 4.00 (s, 3H, CH3O), 3.91 (s, 2H, NCH2), 3.88 (s, 2H, NCH2), 2.74 (t, 2H, J= 6.60 Hz, NCH2), 2.68 (t, 2H, J= 6.60 Hz, NCH2), 2.47–2.36 (m, 20H, 2NCH2 and 8NCH2 pip), 2.29 (s, 3H, NCH3), 2.28 (s, 3H, NCH3), 1.62–1.53 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 162.7 (C-7), 157.1 (C-2), 150.7 (C-4), 148.3 (C-8a), 141.6 (C-4′), 140.7 (C-4′′), 138.6 (C-1′), 137.2 (C-1′′), 129.6 (C-3′ and C-5′), 128.6 (C-3′′ and C-5′′), 128.3 (C-2′ and C-6′), 127.6 (C-2′′ and C-6′′), 126.8 (C-8), 120.9 (C-4a), 119.2 (C-6), 117.3 (C-3), 107.9 (C-5), 58.5 (2NCH2), 55.6 (CH3O), 55.1 (2NCH2 pip.), 53.7 (NCH2), 53.6 (NCH2), 53.2 (2NCH2 pip.), 49.4 (NCH2), 49.2 (NCH2), 46.0 (2NCH3), 28.1 (2CH2), 24.8 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C42H60N7O: 678.486, Found: 678.697.
7-Methoxy-2,4-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)aminomethyl]phenyl}quinoline (1p)
Yellow oil (92%); 1H NMR δ (300 MHz, CDCl3) 8.03 (d, 2H, J= 8.25 Hz, H-2′′ and H-6′′), 7.69 (d, 1H, J= 9.20 Hz, H-5), 7.56 (s, 1H, H-3), 7.45 (d, 1H, J= 2.50 Hz, H-8), 7.39 (s, 4H, H-2′ and H-6′, H-3′′ and H-5′′), 7.36 (d, 2H, J= 8.25 Hz, H-3′ and H-5′), 7.00 (dd, 1H, J= 9.20 and 2.50 Hz, H-6), 3.86 (s, 3H, CH3O), 3.78 (s, 2H, NCH2), 3.75 (s, 2H, NCH2), 2.64 (t, 2H, J= 6.75 Hz, NCH2), 2.58 (t, 2H, J= 6.75 Hz, NCH2), 2.37–2.28 (m, 16H, 8NCH2 pip), 2.17 (s, 3H, NCH3), 2.16 (s, 3H, NCH3), 1.69–1.57 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 162.0 (C-7), 158.3 (C-2), 152.0 (C-4), 150.1 (C-8a), 143.0 (C-4′), 142.2 (C-4′′), 139.7 (C-1′), 138.4 (C-1′′), 130.8 (C-3′ and C-5′), 129.8 (C-3′′ and C-5′′), 129.5 (C-2′ and C-6′), 128.8 (C-2′′ and C-6′′), 128.0 (C-8), 122.1 (C-4a), 120.5 (C-6), 118.5 (C-3), 109.2 (C-5), 58.3 (2NCH2), 56.8 (CH3O), 56.5 (2NCH2 pip.), 55.0 (2NCH2), 54.6 (2NCH2 pip.), 49.6 (NCH2), 49.4 (NCH2), 47.4 (2NCH3), 28.3 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C40H56N7O: 650.455, Found: 650.475.
2,4-Bis{3-[(3-dimethylaminopropyl)aminomethyl]phenyl}quinoline (1q)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.22 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 8.14 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.06 (ddd, 1H, J= 7.20, 1.50 and 1.50 Hz, H-6′), 7.87 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.83 (s, 1H, H-3), 7.70 (ddd,1H, J= 8.10, 6.90 and 1.20 Hz, H-7), 7.51–7.28 (m, 7H, H-6, H-2′′, H-6′′, H-4′, H-4′′, H-5′ and H-5′′), 3.90 (s, 2H, NCH2), 3.89 (s, 2H, NCH2), 2.72 (t, 2H, J= 7.20 Hz, NCH2), 2.70 (t, 2H, J= 7.20 Hz, NCH2), 2.31 (t, 4H, J= 7.20 Hz, 2NCH2), 2.19 (s, 6H, N(CH3)2), 2.18 (s, 6H, N(CH3)2), 1.69 (qt, 4H, J= 7.20 Hz, 2CH2); 13C NMR δ (75 MHz, CDCl3) 158.2 (C-2), 150.6 (C-4), 150.1 (C-8a), 142.4 (C-1′′ and C-3′), 141.1 (C-3′′), 139.8 (C-1′), 131.4 (C-6′), 130.8 (C-6′′), 130.5 (C-2′ and C-4′′), 130.3 (C-2′′), 130.0 (C-4′), 129.5 (C-7 and C-5′′), 128.7 (C-5’), 127.6 (C-8 and C-5), 127.2 (C-4a), 127.0 (C-6), 120.8 (C-3), 59.4 (NCH2), 55.5 (NCH2), 55.3 (NCH2), 49.4 (NCH2), 46.9 (NCH3), 29.4 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C33H44N5: 510.360, Found: 510.391.
2,4-Bis{3-[(3–(4-methylpiperazin-1-yl)propyl)aminomethyl]phenyl}quinoline (1r)
Pale-yellow oil (88%); 1H NMR δ (300 MHz, CDCl3) 8.22 (dd, 1H, J= 8.40 and 1.20 Hz, H-8), 8.14 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.06 (ddd, 1H, J= 7.20, 1.50 and 1.50 Hz, H-6′), 7.88 (dd, 1H, J= 8.40 and 1.20 Hz, H-5), 7.83 (s, 1H, H-3), 7.72 (ddd,1H, J= 8.40, 7.20 and 1.20 Hz, H-7), 7.51–7.36 (m, 7H, H-6, H-2′′, H-6′′, H-4′, H-4′′, H-5′ and H-5′′), 3.91 (s, 2H, NCH2), 3.90 (s, 2H, NCH2), 2.76–2.69 (m, 4H, 2NCH2), 2.70 (t, 2H, J= 7.20 Hz, NCH2), 2.31 (t, 4H, J= 7.20 Hz, 2NCH2), 2.19 (s, 6H, N(CH3)2), 2.18 (s, 6H, N(CH3)2), 2.57–2.28 (m, 20H, 10NCH2), 2.22 (s, 3H, NCH3), 2.19 (s, 3H, NCH3), 1.72 (qt, 4H, J= 6.90 Hz, 2CH2); 13C NMR δ (75 MHz, CDCl3) 158.2 (C-2), 150.6 (C-4), 150.1 (C-8a), 142.4 (C-1′′ and C-3′), 141.1 (C-3′′), 139.8 (C-1′), 131.4 (C-6′), 130.9 (C-6′′), 130.5 (C-2′) 130.4 (C-4′′), 130.3 (C-2′′), 130.0 (C-4′ and C-7), 129.5 (C-5′′), 128.6 (C-5′ and C-8), 127.6 (C-5), 127.3 (C-4a), 127.0 (C-6), 120.8 (C-3), 58.4 (NCH2), 56.5 (NCH2), 55.4 (NCH2), 55.3 (NCH2), 54.6 (NCH2), 49.8 (NCH2), 49.6 (NCH2), 47.4 (NCH3), 28.3 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C39H54N7: 620.444, Found: 620.924.
2,4-Bis{3-[(3-morpholinopropyl)aminomethyl]phenyl}quinoline (1s)
Yellow oil (90%); 1H NMR δ (300 MHz, CDCl3) 8.22 (dd, 1H, J= 8.10 and 1.00 Hz, H-8), 8.15 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.05 (ddd, 1H, J= 7.50, 1.50 and 1.50 Hz, H-6′), 7.87 (dd, 1H, J= 8.10 and 1.00 Hz, H-5), 7.82 (s, 1H, H-3), 7.71 (ddd,1H, J= 8.10, 6.90 and 1.00 Hz, H-7), 7.52–7.39 (m, 7H, H-6, H-2′′, H-6′′, H-4′, H-4′′, H-5′ and H-5′′), 3.89 (s, 2H, NCH2), 3.88 (s, 2H, NCH2), 3.65 (t, 4H, J= 4.80 Hz, 2OCH2), 3.63 (t, 4H, J= 4.80 Hz, 2OCH2), 2.75–2.68 (m, 4H, 2NCH2), 2.41–2.36 (m, 12H, 6NCH2), 1.71 (qt, 4H, J= 7.20 Hz, 2CH2); 13C NMR δ (75 MHz, CDCl3) 158.1 (C-2), 150.6 (C-4), 150.1 (C-8a), 142.3 (C-1′′ and C-3′), 141.1 (C-3′′), 139.8 (C-1′), 131.4 (C-6′), 130.9 (C-6′′), 130.5 (C-2′ and C-4′′), 130.3 (C-2′′), 130.0 (C-4′), 129.5 (C-5′′ and C-7), 128.7 (C-5′), 127.7 (C-5 and C-8), 127.2 (C-4a), 127.0 (C-6), 120.7 (C-3), 68.3 (OCH2), 58.8 (NCH2), 55.4 (NCH2), 55.1 (NCH2), 49.5 (NCH2), 49.4 (NCH2), 27.9 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C37H48N5O2: 594.381, Found: 594.324.
2,4-Bis{3-[(2-morpholinoethyl)aminomethyl]phenyl}quinoline (1t)
Orange oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.26 (dd, 1H, J= 8.40 and 1.20 Hz, H-8), 8.18 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.09 (ddd, 1H, J= 7.40, 1.50 and 1.50 Hz, H-6′), 7.91 (dd, 1H, J= 8.40 and 1.20 Hz, H-5), 7.85 (s, 1H, H-3), 7.75 (ddd,1H, J= 8.40, 6.90 and 1.20 Hz, H-7), 7.54–7.44 (m, 7H, H-6, H-2′′, H-6′′, H-4′, H-4′′, H-5′ and H-5′′), 3.96 (s, 2H, NCH2), 3.95 (s, 2H, NCH2), 3.68 (t, 4H, J= 4.80 Hz, 2OCH2), 3.67 (t, 4H, J= 4.80 Hz, 2OCH2), 2.79 (t, 2H, J= 6.60 Hz, NCH2), 2.77 (t, 2H, J= 6.60 Hz, NCH2), 2.54 (t, 2H, J= 6.60 Hz, NCH2), 2.53 (t, 2H, J= 6.60 Hz, NCH2), 2.44–2.40 (m, 8H, 6NCH2), 2.22 (bs, 2H, 2NH); 13C NMR δ (75 MHz, CDCl3) 156.8 (C-2), 149.2 (C-4), 148.8 (C-8a), 140.9 (C-1′′ and C-3′), 139.8 (C-3′′), 138.5 (C-1′), 130.1 (C-6′), 129.6 (C-6′′), 129.2 (C-2′ and C-4′′), 128.9 (C-2′′), 128.6 (C-4′), 128.2 (C-5′′ and C-7), 127.4 (C-5′), 126.3 (C-5 and C-8), 125.9 (C-4a), 125.6 (C-6), 119.4 (C-3), 67.0 (OCH2), 58.2 (NCH2), 53.7 (NCH2), 45.4 (NCH2), 45.2 (NCH2); MALDI-TOF MS m/z [M + H]+ Calc for C35H44N5O2: 566.349, Found: 566.339.
1,3-Bis{4-[(4-dimethylaminobutyl)aminomethyl]phenyl}isoquinoline (2a)
Yellow oil (88%); 1H NMR δ (300 MHz, CDCl3) 8.15 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.10 (d, 1H, J= 8.40 Hz, H-8), 8.01 (s, 1H, H-4), 7.86 (d, 1H, J= 8.40 Hz, H-5), 7.76 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.62 (t, 1H, J= 8.40 Hz, H-6), 7.48 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.45 (t, 1H, J= 8.40 Hz, H-7), 7.41 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 3.88 (s, 2H, NCH2), 3.83 (s, 2H, NCH2), 2.69 (t, 2H, J= 6.90 Hz, NCH2), 2.64 (t, 2H, J= 6.90 Hz, NCH2), 2.29–2.22 (m, 4H, 2NCH2), 2.20 (s, 6H, N(CH3)2), 2.18 (s, 6H, N(CH3)2), 1.55–1.48 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 161.5 (C-3), 151.3 (C-1), 142.3 (C-4’), 142.0 (C-4′′), 139.9 (C-1′), 139.7 (C-1′′), 139.2 (C-4a), 131.7 (C-2′ and C-6′), 131.3 (C-6), 129.8 (C-2′′ and C-6′′), 129.4 (C-3′ and C-5′), 128.9 (C-7), 128.8 (C5), 128.4 (C-3′′ and C-5′′), 128.1 (C-8), 127.1 (C-8a), 116.7 (C-4), 61.1 (2NCH2), 55.1 (NCH2), 50.7 (NCH2), 50.6 (NCH2), 46.8 (2 N(CH3)2), 29.3 (2CH2), 26.9 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C35H48N5: 538.391, Found: 538.389.
1,3-Bis{4-[(3-dimethylaminopropyl)aminomethyl]phenyl}isoquinoline (2b)
Yellow oil (83%); 1H NMR δ (300 MHz, CDCl3) 8.17 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.13 (d, 1H, J= 8.10 Hz, H-8), 8.05 (s, 1H, H-4), 7.91 (d, 1H, J= 8.10 Hz, H-5), 7.78 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.67 (t, 1H, J= 8.10 Hz, H-6), 7.51 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.47 (t, 1H, J= 8.10 Hz, H-7), 7.44 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 3.92 (s, 2H, NCH2), 3.86 (s, 2H, NCH2), 2.75 (t, 2H, J= 6.90 Hz, NCH2), 2.70 (t, 2H, J= 6.90 Hz, NCH2), 2.36 (t, 2H, J= 6.90 Hz, NCH2), 2.33 (t, 2H, J= 6.90 Hz, NCH2), 2.24 (s, 6H, N(CH3)2), 2.22 (s, 6H, N(CH3)2), 1.79–1.65 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 161.5 (C-3), 151.4 (C-1), 142.3 (C-4′), 142.1 (C-4′′), 139.9 (C-1′), 139.7 (C-1′′), 139.2 (C-4a), 131.7 (C-2′ and C-6′), 131.4 (C-6), 129.8 (C-2′′ and C-6′′), 129.4 (C-3′ and C-5′), 128.9 (C-7), 128.8 (C5), 128.5 (C-3′′ and C-5′′), 128.2 (C-8), 127.1 (C-8a), 116.8 (C-4), 59.5 (2NCH2), 55.2 (2NCH2), 49.3 (2NCH2), 46.9 (2NCH2), 29.4 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C33H44N5: 510.359, Found: 510.360.
1,3-Bis{4-[(4–(4-methylpiperazin-1-yl)butyl)aminomethyl]phenyl}isoquinoline (2c)
Yellow oil (65%); 1H NMR δ (300 MHz, CDCl3) 8.14 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.09 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 8.00 (s, 1H, H-4), 7.86 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.74 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.61 (ddd, 1H, J= 8.10, 7.90 and 1.20 Hz, H-6), 7.46 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.45 (ddd, 1H, J= 8.10, 7.90 and 1.20 Hz, H-7), 7.39 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 3.87 (s, 2H, NCH2), 3.81 (s, 2H, NCH2), 2.66 (t, 2H, J= 6.80 Hz, NCH2), 2.62 (t, 2H, J= 6.80 Hz, NCH2), 2.45–2.28 (m, 20H, 2NCH2 and 8 NCH2pip.), 2.24 (s, 3H, NCH3), 2.23 (s, 3H, NCH3), 1.57–1.48 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 161.5 (C-3), 151.3 (C-1), 142.3 (C-4′), 142.1 (C-4′′), 139.9 (C-1′), 139.6 (C-1′′), 139.2 (C-4a), 131.6 (C-2′ and C-6′), 131.3 (C-6), 129.8 (C-2′′ and C-6′′), 129.3 (C-3′ and C-5′), 128.8 (C-7), 128.7 (C5), 128.4 (C-3′′ and C-5′′), 128.1 (C-8), 127.1 (C-8a), 116.7 (C-4), 59.9 (NCH2), 56.5 (NCH2pip.), 55.1 (NCH2), 54.6 (NCH2pip.), 50.7 (NCH2), 50.6 (NCH2), 47.4 (NCH3), 29.5 (CH2), 26.1 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C41H58N7: 648.475, Found: 648.473.
1,3-Bis{4-[(3–(4-methylpiperazin-1-yl)propyl)aminomethyl]phenyl}isoquinoline (2d)
Yellow oil (87%); 1H NMR δ (300 MHz, CDCl3) 8.17 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.12 (d, 1H, J= 8.10 Hz, H-8), 8.04 (s, 1H, H-4), 7.91 (d, 1H, J= 8.10 Hz, H-5), 7.77 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.66 (t, 1H, J= 8.10 Hz, H-6), 7.50 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.48 (t, 1H, J= 8.10 Hz, H-7), 7.42 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 3.90 (s, 2H, NCH2), 3.85 (s, 2H, NCH2), 2.75 (t, 2H, J= 6.90 Hz, NCH2), 2.69 (t, 2H, J= 6.90 Hz, NCH2), 2.50–2.37 (m, 20H, 2NCH2 and 8 NCH2pip.), 2.27 (s, 3H, NCH3), 2.26 (s, 3H, NCH3), 1.80–1.67 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 161.6 (C-3), 151.3 (C-1), 142.3 (C-4′), 142.1 (C-4′′), 139.9 (C-1′), 139.7 (C-1′′), 139.2 (C-4a), 131.7 (C-2′ and C-6′), 131.4 (C-6), 129.8 (C-2′′ and C-6′′), 129.3 (C-3′ and C-5′), 128.9 (C-7), 128.8 (C5), 128.4 (C-3′′ and C-5′′), 128.2 (C-8), 127.1 (C-8a), 116.7 (C-4), 58.4 (NCH2), 56.5 (NCH2pip.), 55.1 (NCH2), 54.7 (NCH2pip.), 49.6 (NCH2), 49.4 (NCH2), 47.4 (NCH3), 28.4 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C39H54N7: 620.444, Found: 620.441.
7-Methoxy-1,3-bis{4-[(4-dimethylaminobutyl)aminomethyl]phenyl}isoquinoline (2e)
Orange oil (85%); 1H NMR δ (300 MHz, CDCl3) 8.13 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 7.97 (s, 1H, H-4), 7.81 (d, 1H,J= 9.00 Hz, H-5), 7.79 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.51 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.43 (d, 1H, J= 2.40 Hz, H-8), 7.41 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 7.32 (dd, 1H, J= 9.00 and 2.40 Hz, H-6), 3.91 (s, 2H, NCH2), 3.85 (s, 2H, NCH2), 3.81 (s, 3H, CH3O), 2.73 (t, 2H, J= 6.90 Hz, NCH2), 2.67 (t, 2H, J= 6.90 Hz, NCH2), 2.31–2.24 (m, 4H, 2NCH2), 2.22 (s, 6H, N(CH3)2), 2.20 (s, 6H, N(CH3)2), 1.59–1.48 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 159.9 (C-1), 159.6 (C-7), 149.7 (C-3), 142.1 (C-4′), 141.6 (C-4′′), 140.2 (C-1′), 139.8 (C-1′′), 134.8 (C-4a and C-8a), 131.4 (C-2′ and C-6′), 130.4 (C-5), 129.8 (C-2′′ and C-6′′), 129.5 (C-3′ and C-5′), 128.1 (C-3′′ and C-5′′), 124.2 (C-6), 116.7 (C-4), 106.6 (C-8), 61.1 (NCH2), 56.8 (OCH3), 55.2 (NCH2), 55.1 (NCH2), 50.8 (NCH2), 50.6 (NCH2), 46.8 (2 N(CH3)2), 29.4 (2CH2), 27.0 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C36H50N5O: 568.401, Found: 568.435.
7-Methoxy-1,3-bis{4-[(3-dimethylaminopropyl)aminomethyl]phenyl}isoquinoline (2f)
Yellow oil (70%); 1H NMR δ (300 MHz, CDCl3) 8.14 (d, 2H, J= 8.30 Hz, H-3′′ and H-5′′), 8.00 (s, 1H, H-4), 7.84 (d, 1H, J= 8.90 Hz, H-5), 7.81 (d, 2H, J= 8.30 Hz, H-3′ and H-5′), 7.51 (d, 2H, J= 8.30 Hz, H-2′′ and H-6′′), 7.44 (d, 1H, J= 2.40 Hz, H-8), 7.42 (d, 2H, J= 8.30 Hz, H-2′ and H-6′), 7.35(dd, 1H, J= 8.90 and 2.40 Hz, H-6), 3.93 (s, 2H, NCH2), 3.86 (s, 2H, NCH2), 3.84 (s, 3H, CH3O), 2.77 (t, 2H, J= 6.90 Hz, NCH2), 2.70 (t, 2H, J= 6.90 Hz, NCH2), 2.37 (t, 2H, J= 6.90 Hz, NCH2), 2.31 (t, 2H, J= 6.90 Hz, NCH2), 2.25 (s, 6H, N(CH3)2), 2.23 (s, 6H, N(CH3)2), 1.80–1.66 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 159.9 (C-1), 159.6 (C-7), 149.8 (C-3), 142.2 (C-4′), 141.8 (C-4′′), 140.2 (C-1′), 139.8 (C-1′′), 134.8 (C-4a and C-8a), 131.4 (C-2′ and C-6′), 130.3 (C-5), 129.8 (C-2′′ and C-6′′), 129.4 (C-3′ and C-5′), 128.2 (C-3′′ and C-5′′), 124.3 (C-6), 116.7 (C-4), 106.7 (C-8), 59.4 (NCH2), 56.8 (OCH3), 55.3 (NCH2), 49.4 (NCH2), 49.2 (NCH2), 46.9 (2 N(CH3)2), 29.4 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C34H46N5O: 540.370, Found: 540.367.
7-Methoxy-1,3-bis{4-[(4–(4-methylpiperazin-1-yl)butyl)aminomethyl]phenyl}isoquinoline (2g)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.10 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 7.94 (s, 1H, H-4), 7.78 (d, 1H, J= 9.00 Hz, H-5), 7.76 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.47 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.40 (d, 1H, J= 2.40 Hz, H-8),7.38 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 7.29 (dd, 1H, J= 9.00 and 2.40 Hz, H-6), 3.87 (s, 2H, NCH2), 3.81 (s, 2H, NCH2), 3.78 (s, 3H, CH3O), 2.70 (t, 2H, J= 6.60 Hz, NCH2), 2.63 (t, 2H, J= 6.60 Hz, NCH2), 2.45–2.28 (m, 20H, 2NCH2 and 8NCH2pip.), 2.24 (s, 3H, NCH3), 2.23 (s, 3H, NCH3), 1.55–1.45 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 159.9 (C-1), 159.5 (C-7), 149.7 (C-3), 142.0 (C-4′), 141.5 (C-4′′), 140.2 (C-1′), 139.8 (C-1′′), 134.8 (C-4a and C-8a), 131.4 (C-2′ and C-6′), 130.2 (C-5), 129.8 (C-2′′ and C-6′′), 129.4 (C-3′ and C-5′), 128.2 (C-3′′ and C-5′′), 124.2 (C-6), 116.6 (C-4), 106.6 (C-8), 59.8 (NCH2), 56.8 (OCH3), 56.4 (NCH2), 55.1 (NCH2), 55.0 (NCH2), 54.5 (NCH2pip.), 50.7 (NCH2), 50.5 (NCH2), 47.4 (NCH3), 29.4 (2CH2), 26.1 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C42H60N7O: 678.486, Found: 678.492.
7-Methoxy-1,3-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)aminomethyl]phenyl}isoquinoline (2h)
Yellow oil (98%); 1H NMR δ (300 MHz, CDCl3) 8.13 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 7.98 (s, 1H, H-4), 7.82 (d, 1H, J= 9.00 Hz, H-5), 7.79 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.50 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.42 (d, 1H, J= 2.40 Hz, H-8), 7.41 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 7.33 (dd, 1H, J= 9.00 and 2.40 Hz, H-6), 3.91 (s, 2H, NCH2), 3.83 (s, 2H, NCH2), 3.82 (s, 3H, CH3O), 2.76 (t, 2H, J= 6.60 Hz, NCH2), 2.72 (t, 2H, J= 6.60 Hz, NCH2), 2.48–2.30 (m, 20H, 2NCH2 and 8NCH2pip.), 2.28 (s, 3H, NCH3), 2.27 (s, 3H, NCH3), 1.81–1.67 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 159.9 (C-1), 159.6 (C-7), 149.8 (C-3), 142.1 (C-4′), 141.6 (C-4′′), 140.2 (C-1′), 139.8 (C-1′′), 134.8 (C-4a and C-8a), 131.4 (C-2′ and C-6′), 130.3 (C-5), 129.8 (C-2′′ and C-6′′), 129.4 (C-3′ and C-5′), 128.2 (C-3′′ and C-5′′), 124.2 (C-6), 116.7 (C-4), 106.7 (C-8), 58.4 (NCH2), 56.8 (OCH3), 56.5 (NCH2pip.), 55.2 (NCH2), 55.1 (NCH2), 54.6 (NCH2pip.), 49.7 (NCH2), 49.4 (NCH2), 47.4 (NCH3), 28.2 (2CH2), 28.1 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C40H56N7O: 650.454, Found: 650.451.
6-Methoxy-1,3-bis{4-[(4-dimethylaminobutyl)aminomethyl]phenyl}isoquinoline (2i)
Yellow oil (95%); 1H NMR δ (300 MHz, CDCl3) 8.14 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 8.00 (d, 1H, J= 9.30 Hz, H-8), 7.94 (s, 1H, H-4), 7.74 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.48 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.42 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.15 (d, 1H, J= 2.40 Hz, H-5), 7.09 (dd, 1H, J= 9.30 and 2.40 Hz, H-7), 3.94 (s, 3H, CH3O),) , 3.89 (s, 2H, NCH2), 3.84 (s, 2H, NCH2),2.70 (t, 2H, J= 6.90 Hz, NCH2), 2.66 (t, 2H, J= 6.90 Hz, NCH2), 2.28–2.19 (m, 4H, 2NCH2), 2.21 (s, 6H, N(CH3)2), 2.20 (s, 6H, N(CH3)2), 1.57–1.51 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 161.9 (C-3), 161.0 (C-6), 151.9 (C-1), 142.2 (C-4′), 142.0 (C-4′′), 141.2 (C-4a), 140.0 (C-1′), 139.9 (C-1′′), 131.6 (C-2′ and C-6′), 130.7 (C-7), 129.8(C-2′′ and C-6′′), 129.4 (C-3′ and C-5′), 128.5 (C-3′′ and C-5′′), 122.8 (C-8a), 120.9 (C-5), 116.2 (C-4), 106.3 (C-8), 61.1 (2NCH2), 56.8 (OCH3), 55.1 (2NCH2), 50.6 (2NCH2), 46.8 (2 N(CH3)2), 29.3 (2CH2), 26.9 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C36H50N5O: 568.401, Found: 568.407.
6-Methoxy-1,3-bis{4-[(3-dimethylaminopropyl)aminomethyl]phenyl}isoquinoline (2j)
Pale-yellow oil (96%); 1H NMR δ (300 MHz, CDCl3) 8.11 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 7.96 (d, 1H, J= 9.00 Hz, H-8), 7.89 (s, 1H, H-4), 7.71 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.45 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.38 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.10 (d, 1H, J= 2.40 Hz, H-5), 7.04 (dd, 1H, J= 9.00 and 2.40 Hz, H-7), 3.88 (s, 3H, CH3O),) , 3.86 (s, 2H, NCH2), 3.81 (s, 2H, NCH2), 2.70 (t, 2H, J= 6.90 Hz, NCH2), 2.65 (t, 2H, J= 6.90 Hz, NCH2), 2.32 (t, 2H, J= 6.90 Hz, NCH2), 2.29 (t, 2H, J= 6.90 Hz, NCH2), 2.20 (s, 6H, N(CH3)2), 2.18 (s, 6H, N(CH3)2), 1.71–1.63 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 161.9 (C-3), 160.9 (C-6), 151.9 (C-1), 142.1 (C-4′), 141.9 (C-4′′), 141.2 (C-4a), 140.0 (C-1′), 139.8 (C-1′′), 131.5 (C-2′ and C-6′), 130.6 (C-7), 129.7 (C-2′′ and C-6′′), 129.3 (C-3′ and C-5′), 128.4 (C-3′′ and C-5′′), 122.8 (C-8a), 119.9 (C-5), 116.2 (C-4), 106.2 (C-8), 59.4 (2NCH2), 56.7 (OCH3), 55.1 (2NCH2), 49.1 (2NCH2), 46.9 (2 N(CH3)2), 29.4 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C34H46N5O: 540.370, Found: 540.368.
6-Methoxy-1,3-bis{4-[(4–(4-methylpiperazin-1-yl)butyl)aminomethyl]phenyl}isoquinoline (2k)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.11 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 7.97 (d, 1H, J= 9.30 Hz, H-8), 7.91 (s, 1H, H-4), 7.71 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.44 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.38 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 7.12 (d, 1H, J= 2.40 Hz, H-5), 7.06 (dd, 1H, J= 9.30 and 2.40 Hz, H-7), 3.91 (s, 3H, CH3O),) , 3.86 (s, 2H, NCH2), 3.81 (s, 2H, NCH2), 2.67–2.62 (m, 4H, 2NCH2), 2.41–2.30 (m, 20H, 2NCH2 and 8NCH2pip.), 2.24 (s, 3H, NCH3), 2.23 (s, 3H, NCH3), 1.53–1.48 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 161.9 (C-3), 161.0 (C-6), 151.9 (C-1), 142.3 (C-4′), 142.1 (C-4′′), 141.2 (C-4a), 140.0 (C-1′), 139.8 (C-1′′), 131.6 (C-2′ and C-6′), 130.7 (C-7), 129.7 (C-2′′ and C-6′′), 129.3 (C-3′ and C-5′), 128.4 (C-3′′ and C-5′′), 122.8 (C-8a), 120.9 (C-5), 116.2 (C-4), 106.2 (C-8), 59.9 (NCH2), 56.8 (OCH3), 55.2 (NCH2), 54.6 (NCH2pip.), 50.7 (NCH2), 50.6 (NCH2), 47.4 (NCH3), 29.5 (2CH2), 26.1 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C42H60N7O: 678.486, Found: 678.485.
6-Methoxy-1,3-bis{4-[(3–(4-methylpiperazin-1-ylpropyl)aminomethyl]phenyl}isoquinoline (2l)
Yellow oil (59%); 1H NMR δ (300 MHz, CDCl3) 8.14 (d, 2H, J= 8.20 Hz, H-3′′ and H-5′′), 8.00 (d, 1H, J= 9.20 Hz, H-8), 7.95 (s, 1H, H-4), 7.74 (d, 2H, J= 8.20 Hz, H-3′ and H-5′), 7.48 (d, 2H, J= 8.20 Hz, H-2′′ and H-6′′), 7.42 (d, 2H, J= 8.20 Hz, H-2′ and H-6′), 7.16 (d, 1H, J= 2.50 Hz, H-5), 7.10 (dd, 1H, J= 9.20 and 2.50 Hz, H-7), 3.96 (s, 3H, CH3O),) , 3.89 (s, 2H, NCH2), 3.84 (s, 2H, NCH2), 2.73 (t, 2H, J= 6.90 Hz, NCH2), 2.69 (t, 2H, J= 6.90 Hz, NCH2), 2.47 (bs, 2H, 2NH), 2.46–2.38 (m, 16H, 8NCH2pip.), 2.44 (t, 2H, J= 6.90 Hz, NCH2), 2.41 (t, 2H, J= 6.90 Hz, NCH2), 2.27 (s, 3H, NCH3), 2.26 (s, 3H, NCH3), 1.79–1.67 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 162.0 (C-3), 161.0 (C-6), 152.0 (C-1), 142.2 (C-4′), 142.1 (C-4′′), 141.2 (C-4a), 140.0 (C-1′), 139.8 (C-1′′), 131.6 (C-2′ and C-6′), 130.7 (C-7), 129.7 (C-2′′ and C-6′′), 129.3 (C-3′ and C-5′), 128.4 (C-3′′ and C-5′′), 122.8 (C-8a), 120.9 (C-5), 116.2 (C-4), 106.2 (C-8), 58.4 (NCH2), 56.8 (OCH3), 56.5 (NCH2pip.), 55.1 (NCH2), 54.6 (NCH2), 49.5 (NCH2), 47.4 (NCH3), 28.3 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C40H56N7O: 650.454, Found: 650.451.
2,4-Bis{4-[(4-dimethylaminobutyl)aminomethyl]phenyl}quinazoline (3a)
Yellow oil (83%); 1H NMR δ (300 MHz, CDCl3) 8.69 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 8.17 (dd, 1H, J= 8.40 and 1.20 Hz, H-8), 8.14 (dd, 1H, J= 8.40 and 1.20 Hz, H-5), 7.91 (ddd, 1H, J= 8.40, 7.00 and 1.20 Hz, H-7), 7.89 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.63 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.57 (ddd, 1H, J= 8.40, 7.00 and 1.20 Hz, H-6), 7.55 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 3.99 (s, 2H, NCH2), 3.96 (s, 2H, NCH2), 2.79 (t, 2H, J= 6.90 Hz, NCH2), 2.77 (t, 2H, J= 6.90 Hz, NCH2), 2.37–2.32 (m, 4H, 2NCH2), 2.26 (s, 6H, N(CH3)2), 2.19 (s, 6H, N(CH3)2), 1.76–1.52 (m, 8H, 4CH2). 13C NMR δ (75 MHz, CDCl3) 169.5 (C-2), 161.5 (C-4), 153.3 (C-8a), 144.2 (C-4′′), 143.9 (C-4′), 138.4 (C-1′′), 137.7 (C-1′), 134.8 (C-7), 131.7 (C-3′′and C-5′′), 130.4 (C-8), 130.1 (C-3', C-5',C-2'', C-6'', C-2' and C-6'), 128.4 (C-5), 128.2 (C-6), 123.0 (C-4a), 61.0 (NCH2), 55.1 (NCH2), 50.7 (NCH2), 50.6(NCH2), 46.8 (2 N(CH3)2), 29.3 (2CH2), 26.8 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C34H47N6: 539.386, Found: 539.245.
2,4-Bis{4-[(3-dimethylaminopropyl)aminomethyl]phenyl}quinazoline (3b)
Yellow oil (97%); IR νmax (KBr)/cm−1 3290 (NH), 1610 (C = N); 1H NMR δ (300 MHz, CDCl3) 8.64 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 8.13 (d, 2H, J= 9.00 Hz, H-8 and H-5), 7.90–7.84 (m, 3H, H-7, H-2′′ and H-6′′), 7.57–7.46 (m, 5H, H-3′, H-5′, H-6, H-3′′ and H-5′′), 3.93 (s, 2H, NCH2), 3.87 (s, 2H, NCH2), 2.75 (t, 2H, J= 6.90 Hz, NCH2), 2.70 (t, 2H, J= 6.90 Hz, NCH2), 2.36 (t, 2H, J= 6.90 Hz, NCH2), 2.33 (t, 2H, J= 6.90 Hz, NCH2), 2.24 (s, 6H, N(CH3)2), 2.22 (s, 6H, N(CH3)2), 1.79–1.70 (m, 4H, 2CH2). 13C NMR δ (75 MHz, CDCl3) 169.5 (C-2), 161.5 (C-4), 153.4 (C-8a), 144.3 (C-4′′), 144.0 (C-4′), 138.4 (C-1′′), 137.7 (C-1′), 134.9 (C-7), 131.7 (C-3′′ and C-5′′), 130.5 (C-8), 130.1 (C-3′, C-5′), 129.6 (C-2′′, C-6′′, C-2′ and C-6′), 128.4 (C-5), 128.2 (C-6), 123.0 (C-4a), 59.5 (NCH2), 55.2 (NCH2), 49.4 (NCH2), 49.2 (NCH2), 47.00 (2N(CH3)2), 29.4 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C32H43N6: 511.355, Found: 511.400.
2,4-Bis{4-[(2-dimethylaminoethyl)aminomethyl]phenyl}quinazoline (3c)
Yellow oil (85%); 1H NMR δ (300 MHz, CDCl3) 8.64 (d, 2H, J= 8.20 Hz, H-2′ and H-6′), 8.14–8.10 (m, 2H, H-8 and H-5), 7.88–7.83 (m, 3H, H-7, H-2′′ and H-6′′), 7.56 (d, 2H, J= 8.20 Hz, H-3′ and H-5′), 7.49–7.45 (m, 3H, H-6, H-3′′ and H-5′′), 3.95 (s, 2H, NCH2), 3.90 (s, 2H, NCH2), 2.77 (t, 2H, J= 6.40 Hz, NCH2), 2.71 (t, 2H, J= 6.40 Hz, NCH2), 2.47 (t, 2H, J= 6.40 Hz, NCH2), 2.44 (t, 2H, J= 6.40 Hz, NCH2), 2.24 (s, 6H, N(CH3)2), 2.19 (s, 6H, N(CH3)2).13C NMR δ (75 MHz, CDCl3) 169.5 (C-2), 161.5 (C-4), 153.4 (C-8a), 144.4 (C-4′′), 144.0 (C-4′), 138.3 (C-1′′), 137.7 (C-1'), 134.8 (C-7), 131.7 (C-3′′ and C-5′′), 130.4(C-8), 130.1 (C-3′, C-5′), 129.7 (C-2′′ and C-6′′), 129.6 (C-2′ and C-6′), 128.4 (C-5), 128.2 (C-6), 123.0 (C-4a), 60.5 (NCH2), 55.2 (NCH2), 48.2 (NCH2), 49.2 (NCH2), 47.8 (NCH2), 47.0 (N(CH3)2),46.9 (N(CH3)2); MALDI-TOF MS m/z [M + H]+ Calc for C30H39N6: 483.324, Found: 483.397.
2,4-Bis{4-[(4–(4-methylpiperazin-1-yl)butyl)aminomethyl]phenyl}quinazoline (3d)
Yellow oil (73%); 1H NMR δ (300 MHz, CDCl3) 8.63 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 8.15–8.10 (m, 2H, H-8 and H-5), 7.87 (ddd, 1H, J= 8.10, 7.20 and 1.50 Hz, H-7), 7.84 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.54 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.52 (ddd, 1H, J= 8.10, 7.20 and 1.50 Hz, H-6), 7.45 (d, 2H, J= 8.40 Hz, H-3′′ and H-5′′), 3.91 (s, 2H, NCH2), 3.87 (s, 2H, NCH2), 2.71–2.55 (m, 4H, 2NCH2), 2.46–2.34 (m, 20H, 2NCH2 and 8NCH2pip.), 2.27 (s, 3H, NCH3), 2.26 (s, 3H, NCH3), 1.57–1.50 (m, 8H, 4CH2). 13C NMR δ (75 MHz, CDCl3) 169.5 (C-2), 161.5 (C-4), 153.3 (C-8a), 144.3 (C-4′′), 143.9 (C-4'), 138.4 (C-1′′), 137.7 (C-1′), 134.9 (C-7), 131.7 (C-3′′ and C-5′′), 130.5 (C-8), 130.1 (C-3' and C-5'), 129.6 (C-2′′, C-6′′, C-2′ and C-6′), 128.7 (C-5), 128.3 (C-6), 123.0 (C-4a), 59.8 (NCH2), 56.5 (NCH2), 55.1 (NCH2), 54.6 (NCH2), 50.7 (NCH2), 50.6 (NCH2), 47.4 (2NCH3), 29.4 (2CH2), 26.1 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C40H57N8: 649.471, Found: 649.291.
2,4-Bis{4-[(3–(4-methylpiperazin-1-yl)propyl)aminomethyl]phenyl}quinazoline (3e)
Yellow oil (70%); 1H NMR δ (300 MHz, CDCl3) 8.62 (d, 2H, J= 8.25 Hz, H-2′ and H-6′), 8.13–8.09 (m, 2H, H-8 and H-5), 7.87 (ddd, 1H, J= 8.10, 6.90 and 1.20 Hz, H-7), 7.83 (d, 2H, J= 8.25 Hz, H-2′′ and H-6′′), 7.53 (d, 2H, J= 8.25 Hz, H-3' and H-5′), 7.51 (ddd, 1H, J= 8.10, 6.90 and 1.20 Hz, H-6), 7.45 (d, 2H, J= 8.25 Hz, H-3′′ and H-5′′), 3.90 (s, 2H, NCH2), 3.86 (s, 2H, NCH2), 2.73 (t, 2H, J= 6.90 Hz, NCH2), 2.68 (t, 2H, J= 6.90 Hz, NCH2), 2.46–2.35 (m, 20H, 2NCH2 and 8NCH2pip.), 2.26 (s, 3H, NCH3), 2.25 (s, 3H, NCH3), 1.77–1.68 (m, 4H, 2CH2). 13C NMR δ (75 MHz, CDCl3) 169.5 (C-2), 161.5 (C-4), 153.3 (C-8a), 144.2 (C-4′′), 143.9 (C-4′), 138.4 (C-1′′), 137.7 (C-1′), 134.9 (C-7), 131.7 (C-3′′ and C-5′′), 130.4 (C-8), 130.1 (C-3′ and C-5′), 129.6 (C-2′′, C-6′′, C-2′ and C-6′), 128.6 (C-5), 128.2 (C-6), 123.0 (C-4a), 58.4 (NCH2), 56.5 (NCH2), 55.1 (NCH2), 54.6 (NCH2), 49.6 (NCH2), 49.5(NCH2), 47.4 (2NCH3), 28.2 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C38H53N8: 621.439, Found: 621.626.
2,4-Bis{4-[(3-morpholinopropyl)aminomethyl]phenyl}quinazoline (3f)
Yellow oil (95%); 1H NMR δ (300 MHz, CDCl3) 8.66 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.14 (dd, 1H, J= 8.10 and 1.20 Hz, H-8), 8.11 (dd, 1H, J= 8.10 and 1.20 Hz, H-5), 7.91 (ddd,1H, J= 8.10, 7.20 and 1.20 Hz, H-7), 7.86 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.59 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 7.54 (ddd,1H, J= 8.10, 7.20 and 1.20 Hz, H-6), 7.51 (d, 2H, J= 8.10 Hz, H-2' and H-6'), 3.97 (s, 2H, NCH2), 3.94 (s, 2H, NCH2), 3.68 (t, 4H, J= 4.50 Hz, 2OCH2), 3.68 (t, 4H, J= 4.50 Hz, 2OCH2), 2.81 (t, 2H, J= 6.60 Hz, NCH2), 2.80 (t, 2H, J= 6.60 Hz, NCH2), 2.49–2.40 (m, 12H, 6NCH2), 1.85–1.75 (m, 4H, 2CH2). 13C NMR δ (75 MHz, CDCl3) 169.4 (C-2), 161.2 (C-4), 153.3 (C-8a), 142.6 (C-4′′), 142.0 (C-4′), 138.9 (C-1′′), 138.0 (C-1'), 135.0 (C-7), 131.8 (C-3′′ and C-5′′), 130.5 (C-8), 130.3 (C-3′ and C-5′), 130.0 (C-2′′ and C-6′′), 129.8 (C-2'′ and C-6′), 128.4 (C-5), 128.3 (C-6), 123.0 (C-4a), 68.3 (OCH2), 58.8 (NCH2), 58.7 (NCH2), 55.1 (NCH2), 54.7 (NCH2), 54.5 (NCH2), 49.2 (NCH2), 27.4 (CH2), 26.8 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C36H47N6O2: 595.376, Found: 595.326.
2,4-Bis{4-[(2-morpholinoethyl)aminomethyl]phenyl}quinazoline (3g)
Yellow oil (78%); 1H NMR δ (300 MHz, CDCl3) 8.65 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 8.14 (dd, 1H, J= 8.40 and 1.50 Hz, H-8), 8.12 (dd, 1H, J= 8.40 and 1.50 Hz, H-5), 7.87 (ddd,1H, J= 8.40, 7.20 and 1.50 Hz, H-7), 7.86 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.58–7.46 (m, 5H, H-2′′, H-6′′,H-6, H-2′ and H-6′), 3.95 (s, 2H, NCH2), 3.91 (s, 2H, NCH2), 3.74–3.68 (m, 8H, 4OCH2), 2.79 (t, 2H, J= 6.30 Hz, NCH2), 2.73 (t, 2H, J= 6.30 Hz, NCH2), 2.57 (t, 2H, J= 6.30 Hz, NCH2), 2.51 (t, 2H, J= 6.30 Hz, NCH2), 2.47–2.38 (m, 8H, 4NCH2), 2.20 (bs, 2H, 2NH). 13C NMR δ (75 MHz, CDCl3) 169.5 (C-2), 161.5 (C-4), 153.4 (C-8a), 144.3 (C-4′′), 143.9 (C-4′), 138.4 (C-1′′), 137.7 (C-1′), 134.9 (C-7), 131.7 (C-3′′ and C-5′′), 130.5 (C-8), 130.1 (C-3′ and C-5′), 129.6 (C-2′′ and C-6′′), 129.5 (C-2′ and C-6′), 128.4 (C-5), 128.3 (C-6), 123.0 (C-4a), 68.4 (OCH2), 59.6 (NCH2), 55.1 (NCH2), 55.0 (NCH2), 46.8 (NCH2), 46.5 (NCH2); MALDI-TOF MS m/z [M + H]+ Calc for C34H43N6O2: 567.345, Found: 567.326.
6-Methoxy-2,4-bis{4-[(4-dimethylaminobutyl)aminomethyl]phenyl}quinazoline (3h)
Yellow oil (96%); 1H NMR δ (300 MHz, CDCl3) 8.58 (d, 2H, J= 8.10 Hz, H-2′′ and H-6′′), 8.00 (d, 1H, J= 9.20 Hz, H-8), 7.84 (d, 2H, J= 8.10 Hz, H-2′ and H-6′), 7.53 (d, 2H, J= 8.10 Hz, H-3′′ and H-5′′), 7.49 (dd, 1H, J= 9.20 and 2.80 Hz, H-7), 7.44 (d, 2H, J= 8.10 Hz, H-3′ and H-5′), 7.36 (d, 1H, J= 2.80 Hz, H-5), 3.90 (s, 2H, NCH2), 3.86 (s, 2H, NCH2), 3.81 (s, 3H, CH3O), 2.71 (t, 2H, J= 7.00 Hz, NCH2), 2.65 (t, 2H, J= 7.00 Hz, NCH2), 2.26 (t, 2H, J= 7.00 Hz, NCH2), 2.24 (t, 2H, J= 7.00 Hz, NCH2), 2.20 (s, 6H, N(CH3)2), 2.17 (s, 6H, N(CH3)2), 1.56–1.48 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 167.6 (C-6), 159.7 (C-2), 159.4 (C-4), 149.5 (C-8a), 143.3 (C-4′), 143.1 (C-4′′), 138.7 (C-1′), 138.1 (C-1′′), 131.9 (C-8), 131.3 (C-3′ and C-5′), 129.8 (C-3′′ and C-5′′, C-2′ and C-6′, C-2′′ and C-6′′), 127.5 (C-7), 123.7 (C-4a), 105.7 (C-5), 60.9 (NCH2), 60.8 (NCH2), 57.0 (CH3O), 54.9 (NCH2), 54.8 (NCH2), 50.7 (NCH2), 50.4 (NCH2), 46.7 (N(CH3)2), 46.5 (N(CH3)2), 29.1 (2CH2), 26.9 (2CH2); MALDI-TOF MS m/z [M + H+] Calc for C35H49N6O: 569.397, Found: 569.332.
6-Methoxy-2,4-bis{4-[(3-dimethylaminopropyl)aminomethyl]phenyl}quinazoline (3i)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.55 (d, 2H, J= 8.25 Hz, H-2′′ and H-6′′), 7.96 (d, 1H, J= 9.30 Hz, H-8), 7.80 (d, 2H, J= 8.25 Hz, H-2′ and H-6′), 7.48 (d, 2H, J= 8.25 Hz, H-3′′ and H-5′′), 7.43 (dd, 1H, J= 9.30 and 2.80 Hz, H-7), 7.40 (d, 2H, J= 8.25 Hz, H-3′ and H-5′), 7.31 (d, 1H, J= 2.80 Hz, H-5), 3.85 (s, 2H, NCH2), 3.81 (s, 2H, NCH2), 3.75 (s, 3H, CH3O), 2.69 (t, 2H, J= 7.10 Hz, NCH2), 2.64 (t, 2H, J= 7.10 Hz, NCH2), 2.30 (t, 2H, J= 7.10 Hz, NCH2), 2.26 (t, 2H, J= 7.10 Hz, NCH2), 2.18 (s, 6H, N(CH3)2), 2.16 (s, 6H, N(CH3)2), 1.72–1.59 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 167.6 (C-6), 159.8 (C-2), 159.2 (C-4), 149.4 (C-8a), 143.9 (C-4′), 143.8 (C-4′′), 138.4 (C-1′), 137.9 (C-1′′), 131.9 (C-8), 131.3 (C-3′ and C-5′), 129.7 (C-3′′ and C-5′′), 129.5 (C-2′ and C-6′, C-2′′ and C-6′′), 127.4 (C-7), 123.6 (C-4a), 105.7 (C-5), 59.4 (2NCH2), 56.9 (CH3O), 55.1 (2NCH2), 49.4 (NCH2), 49.2 (NCH2), 46.9 (2N(CH3)2), 29.4 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C33H45N6O: 541.365, Found: 541.376.
6-Methoxy-2,4-bis{4-[(2-dimethylaminoethyl)aminomethyl]phenyl}quinazoline (3j)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.56 (d, 2H, J= 8.15 Hz, H-2′′ and H-6′′), 7.97 (d, 1H, J= 9.00 Hz, H-8), 7.82 (d, 2H, J= 8.15 Hz, H-2′ and H-6'), 7.51 (d, 2H, J= 8.15 Hz, H-3′′ and H-5′′), 7.44 (dd, 1H, J= 9.00 and 2.60 Hz, H-7), 7.42 (d, 2H, J= 8.15 Hz, H-3′ and H-5′), 7.32 (d, 1H, J= 2.60 Hz, H-5), 3.89 (s, 2H, NCH2), 3.84 (s, 2H, NCH2), 3.76 (s, 3H, CH3O), 2.73 (t, 2H, J= 7.10 Hz, NCH2), 2.66 (t, 2H, J= 6.00 Hz, NCH2), 2.43 (t, 2H, J= 6.00 Hz, NCH2), 2.39 (t, 2H, J= 6.00 Hz, NCH2), 2.19 (s, 6H, N(CH3)2), 2.15 (s, 6H, N(CH3)2); 13C NMR δ (75 MHz, CDCl3) 167.7 (C-6), 159.8 (C-2), 159.3 (C-4), 149.4 (C-8a), 143.9 (C-4′), 143.7 (C-4′′), 138.4 (C-1′), 137.9 (C-1′′), 131.9 (C-8), 131.3 (C-3′ and C-5′), 129.7 (C-3′′ and C-5′′, C-2′ and C-6′), 129.6 (C-2′′ and C-6′′), 127.5 (C-7), 123.7 (C-4a), 105.6 (C-5), 60.4 (2NCH2), 56.9 (CH3O), 55.2 (2NCH2), 48.2 (NCH2), 47.8 (NCH2), 46.9 (2 N(CH3)2); MALDI-TOF MS m/z [M + H]+ Calc for C31H41N6O: 513.334, Found: 513.316.
6-Methoxy-2,4-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)aminomethyl]phenyl}quinazoline (3k)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.54 (d, 2H, J= 8.30 Hz, H-2′′ and H-6′′), 7.97 (d, 1H, J= 9.20 Hz, H-8), 7.81 (d, 2H, J= 8.30 Hz, H-2′ and H-6′), 7.49 (d, 2H, J= 8.30 Hz, H-3′′ and H-5′′), 7.47 (dd, 1H, J= 9.20 and 2.80 Hz, H-7), 7.39 (d, 2H, J= 8.30 Hz, H-3′ and H-5′), 7.33 (d, 1H, J= 2.80 Hz, H-5), 3.86 (s, 2H, NCH2), 3.80 (s, 2H, NCH2), 3.77 (s, 3H, CH3O), 2.70 (t, 2H, J= 6.75 Hz, NCH2), 2.63 (t, 2H, J= 6.75 Hz, NCH2), 2.50–2.30 (m, 20H, 2NCH2 and 8NCH2 pip.), 2.22 (s, 3H, NCH3), 2.21 (s, 3H, NCH3), 1.75–1.61 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 167.6 (C-6), 159.8 (C-2), 159.3 (C-4), 149.5 (C-8a), 143.9 (C-4′), 143.8 (C-4′′), 138.4 (C-1'), 137.9 (C-1′′), 131.9 (C-8), 131.3 (C-3′ and C-5′), 129.7 (C-3′′ and C-5′′, C-2′ and C-6′), 129.5 (C-2′′ and C-6′′), 127.4 (C-7), 123.7 (C-4a), 105.7 (C-5), 58.4 (2NCH2), 56.9 (CH3O), 56.5 (2NCH2 pip.), 55.1 (2NCH2), 54.6 (2NCH2 pip.), 49.7 (NCH2), 49.4 (NCH2), 47.4 (2NCH3), 28.4 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C39H55N8O: 651.450, Found: 651.394.
6-Methoxy-2,4-bis{4-[(2–(4-methylpiperazin-1-yl)ethyl)aminomethyl]phenyl}quinazoline (3l)
Yellow oil (96%); 1H NMR δ (300 MHz, CDCl3) 8.59 (d, 2H, J= 8.20 Hz, H-2′′ and H-6′′), 8.04 (d, 1H, J= 9.20 Hz, H-8), 7.87 (d, 2H, J= 8.20 Hz, H-2′ and H-6′), 7.55 (d, 2H, J= 8.30 Hz, H-3′′ and H-5′′), 7.52 (dd, 1H, J= 9.20 and 2.80 Hz, H-7), 7.44 (d, 2H, J= 8.20 Hz, H-3' and H-5'), 7.39 (d, 1H, J= 2.80 Hz, H-5), 3.94 (s, 2H, NCH2), 3.88 (s, 2H, NCH2), 3.84 (s, 3H, CH3O), 2.79 (t, 2H, J= 6.00 Hz, NCH2), 2.71 (t, 2H, J= 6.00 Hz, NCH2), 2.58–2.44 (m, 20H, 2NCH2 and 8NCH2 pip.), 2.28 (s, 3H, NCH3), 2.27 (s, 3H, NCH3); 13C NMR δ (75 MHz, CDCl3) 167.7 (C-6), 159.8 (C-2), 159.3 (C-4), 149.5 (C-8a), 143.7 (C-4′, C-4′′), 138.5 (C-1′), 138.0 (C-1′′), 132.0 (C-8), 131.3 (C-3′ and C-5′), 129.7 (C-3′′ and C-5′′, C-2′ and C-6′, C-2′′ and C-6′′), 127.5 (C-7), 123.7 (C-4a), 105.7 (C-5), 59.1 (2NCH2), 57.0 (CH3O), 56.5 (2NCH2 pip.), 55.1 (NCH2), 54.8 (NCH2), 54.5 (2NCH2 pip.), 47.4 (2NCH2), 47.2 (NCH2), 46.8 (NCH3); MALDI-TOF MS m/z [M + H]+ Calc for C37H53N8O: 625.434, Found: 625.593.
7-Methoxy-2,4-bis{4-[(4-dimethylaminobutyl)aminomethyl]phenyl}quinazoline (3 m)
Pale yellow oil (89%); 1H NMR δ (300 MHz, CDCl3) 8.61 (d, 2H, J= 7.95 Hz, H-2′′ and H-6′′), 7.99 (d, 1H, J= 9.15 Hz, H-5), 7.82 (d, 2H, J= 7.95 Hz, H-2′ and H-6′), 7.53 (d, 2H, J= 7.95 Hz, H-3′′ and H-5′′), 7.46 (d, 2H, J= 7.95 Hz, H-3′ and H-5′), 7.41 (d, 1H, J= 2.10 Hz, H-8), 7.13 (dd, 1H, J= 9.15 and 2.10 Hz, H-6), 3.99 (s, 3H, CH3O), 3.91 (s, 2H, NCH2), 3.87 (s, 2H, NCH2), 2.70 (t, 2H, J= 6.60 Hz, NCH2), 2.66 (t, 2H, J= 6.60 Hz, NCH2), 2.30–2.25 (m, 4H, 2NCH2), 2.21 (s, 6H, N(CH3)2), 2.19 (s, 6H, N(CH3)2), 1.57–1.52 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 168.3 (C-7), 165.1 (C-2), 162.1 (C-4), 155.8 (C-8a), 143.7 (C-4′, C-4′′), 138.7 (C-1′), 137.9 (C-1′′), 131.6 (C-3′ and C-5′), 130.1 (C-3′′ and C-5′′), 129.7 (C-5, C-2' and C-6', C-2′′ and C-6′′), 121.4 (C-6), 118.4 (C-4a), 108.0 (C-8), 60.9 (2NCH2), 57.1 (CH3O), 55.0 (2NCH2), 50.6 (NCH2), 50.1 (NCH2), 46.7 (2 N(CH3)2), 29.7 (2CH2), 26.9 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C35H49N6O: 569.397, Found: 569.455.
7-Methoxy-2,4-bis{4-[(3-dimethylaminopropyl)aminomethyl]phenyl}quinazoline (3n)
Yellow oil (96%); 1H NMR δ (300 MHz, CDCl3) 8.59 (d, 2H, J= 8.20 Hz, H-2'' and H-6''), 7.98 (d, 1H, J= 9.20 Hz, H-5), 7.80 (d, 2H, J= 8.20 Hz, H-2′ and H-6'), 7.51 (d, 2H, J= 8.20 Hz, H-3′′ and H-5′′), 7.44 (d, 2H, J= 8.20 Hz, H-3′ and H-5′), 7.40 (d, 1H, J= 2.50 Hz, H-8), 7.11 (dd, 1H, J= 9.20 and 2.50 Hz, H-6), 3.97 (s, 3H, CH3O), 3.89 (s, 2H, NCH2), 3.85 (s, 2H, NCH2), 2.71 (t, 2H, J= 7.05 Hz, NCH2), 2.67 (t, 2H, J= 7.05 Hz, NCH2), 2.33 (t, 2H, J= 7.05 Hz, NCH2), 2.30 (t, 2H, J= 7.05 Hz, NCH2), 2.22 (s, 6H, N(CH3)2), 2.20 (s, 6H, N(CH3)2), 1.76–1.63 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 168.3 (C-7), 165.0 (C-2), 162.1 (C-4), 155.8 (C-8a), 144.2 (C-4′), 143.8 (C-4′′), 138.5 (C-1′), 137.8 (C-1′′), 131.6 (C-3′ and C-5′), 130.0 (C-3′′ and C-5′′), 129.7 (C-5), 129.5 (C-2′ and C-6′, C-2′′ and C-6′′), 121.4 (C-6), 118.4 (C-4a), 107.8 (C-8), 59.4 (NCH2), 59.3 (NCH2), 57.1 (CH3O), 55.2 (NCH2), 55.1 (NCH2), 49.3 (NCH2), 49.2 (NCH2), 46.9 (N(CH3)2), 46.8 (N(CH3)2), 29.4 (CH2), 29.3 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C33H45N6O: 541.36, Found: 541.476.
7-Methoxy-2,4-bis{4-[(2-dimethylaminoethyl)aminomethyl]phenyl}quinazoline (3o)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.60 (d, 2H, J= 8.25 Hz, H-2'' and H-6''), 7.98 (d, 1H, J= 9.20 Hz, H-5), 7.79 (d, 2H, J= 8.25 Hz, H-2' and H-6'), 7.51 (d, 2H, J= 8.25 Hz, H-3'' and H-5''), 7.45 (d, 2H, J= 8.25 Hz, H-3' and H-5'), 7.38 (d, 1H, J= 2.50 Hz, H-8), 7.10 (dd, 1H, J= 9.20 and 2.50 Hz, H-6), 3.96 (s, 3H, CH3O), 3.90 (s, 2H, NCH2), 3.87 (s, 2H, NCH2), 2.72 (t, 2H, J= 6.10 Hz, NCH2), 2.68 (t, 2H, J= 6.10 Hz, NCH2), 2.44 (t, 2H, J= 6.10 Hz, NCH2), 2.41 (t, 2H, J= 6.10 Hz, NCH2), 2.20 (s, 6H, N(CH3)2), 2.17 (s, 6H, N(CH3)2); 13C NMR δ (75 MHz, CDCl3) 168.3 (C-7), 165.0 (C-2), 162.1 (C-4), 155.8 (C-8a), 144.3 (C-4′), 143.8 (C-4′′), 138.4 (C-1′), 137.8 (C-1′′), 131.5 (C-3′ and C-5′), 130.0 (C-3′′ and C-5′′), 129.6 (C-5, C-2′ and C-6′, C-2′′ and C-6′′), 121.3 (C-6), 118.4 (C-4a), 108.0 (C-8), 60.5 (2NCH2), 57.1 (CH3O), 55.2 (2NCH2), 48.1 (NCH2), 47.7 (NCH2), 46.9 (2 N(CH3)2); MALDI-TOF MS m/z [M + H]+ Calc for C31H41N6O: 513.334, Found: 513.456.
7-Methoxy-2,4-bis{4-[(4–(4-methylpiperazin-1-yl)butyl)aminomethyl]phenyl}quinazoline (3p)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.52 (d, 2H, J= 8.30 Hz, H-2′′ and H-6′′), 7.89 (d, 1H, J= 9.30 Hz, H-5), 7.72 (d, 2H, J= 8.30 Hz, H-2′ and H-6′), 7.44 (d, 2H, J= 8.30 Hz, H-3′′ and H-5′′), 7.38 (d, 2H, J= 8.30 Hz, H-3′ and H-5′), 7.30 (d, 1H, J= 2.30 Hz, H-8), 7.02 (dd, 1H, J= 9.30 and 2.30 Hz, H-6), 3.88 (s, 3H, CH3O), 3.81 (s, 2H, NCH2), 3.78 (s, 2H, NCH2), 2.60 (t, 2H, J= 6.60 Hz, NCH2), 2.57 (t, 2H, J= 6.60 Hz, NCH2), 2.40–2,21 (m, 20H, 2NCH2 and 8NCH2 pip.), 2.17 (s, 3H, NCH3), 2.16 (s, 3H, NCH3), 1.48–1.44 (m, 8H, 4CH2); 13C NMR δ (75 MHz, CDCl3) 168.1 (C-7), 164.9 (C-2), 161.8 (C-4), 155.7 (C-8a), 144.5 (C-4′), 143.2 (C-4′′), 138.5 (C-1′), 137.8 (C-1′′), 131.5 (C-3′ and C-5′), 130.0 (C-3′′ and C-5′′), 129.6 (C-5, C-2′ and C-6′, C-2′′ and C-6′′), 121.3 (C-6), 118.2 (C-4a), 107.9 (C-8), 59.7 (2NCH2), 57.0 (CH3O), 56.4 (2NCH2 pip.), 54.8 (2NCH2), 54.5 (2NCH2 pip.), 50.5 (NCH2), 50.4 (NCH2), 47.3 (2NCH3), 29.2 (2CH2), 26.0 (2CH2); MALDI-TOF MS m/z [M + H]+ Calc for C41H59N8O: 679.481, Found: 679.506.
7-Methoxy-2,4-bis{4-[(3–(4-methylpiperazin-1-yl)propyl)aminomethyl]phenyl}quinazoline (3q)
Yellow oil (97%); 1H NMR δ (300 MHz, CDCl3) 8.55 (d, 2H, J= 8.20 Hz, H-2′′ and H-6′′), 7.92 (d, 1H, J= 9.10 Hz, H-5), 7.75 (d, 2H, J= 8.20 Hz, H-2′ and H-6′), 7.46 (d, 2H, J= 8.20 Hz, H-3′′ and H-5′′), 7.39 (d, 2H, J= 8.20 Hz, H-3′ and H-5′), 7.34 (d, 1H, J= 2.50 Hz, H-8), 7.05 (dd, 1H, J= 9.10 and 2.50 Hz, H-6), 3.92 (s, 3H, CH3O), 3.84 (s, 2H, NCH2), 3.80 (s, 2H, NCH2), 2.67 (t, 2H, J= 7.05 Hz, NCH2), 2.63 (t, 2H, J= 7.05 Hz, NCH2), 2.38–2.34 (m, 20H, 2NCH2 and 8NCH2 pip.), 2.21 (s, 3H, NCH3), 2.20 (s, 3H, NCH3), 1.71–1.63 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 168.3 (C-7), 165.0 (C-2), 162.2 (C-4), 155.8 (C-8a), 144.3 (C-4′), 143.9 (C-4′′), 138.4 (C-1′), 137.8 (C-1′′), 131.5 (C-3′ and C-5′), 130.0 (C-3′′ and C-5′′), 129.7 (C-5), 129.4 (C-2′ and C-6′, C-2′′ and C-6′′), 121.3 (C-6), 118.3 (C-4a), 107.9 (C-8), 58.4 (NCH2), 58.3 (NCH2), 57.1 (CH3O), 56.5 (2NCH2 pip.), 55.1 (NCH2), 55.0 (NCH2), 54.6 (2NCH2 pip.), 49.5 (NCH2), 49.4 (NCH2), 47.4 (2NCH3), 28.4 (CH2), 28.3 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C39H55N8O: 651.450, Found: 651.654.
7-Methoxy-2,4-bis{4-[(2–(4-methylpiperazin-1-yl)ethyl)aminomethyl]phenyl}quinazoline (3r)
Yellow oil (89%); 1H NMR δ (300 MHz, CDCl3) 8.62 (d, 2H, J= 8.40 Hz, H-2′′ and H-6′′), 7.99 (d, 1H, J= 9.30 Hz, H-5), 7.83 (d, 2H, J= 8.40 Hz, H-2′ and H-6′), 7.53 (d, 2H, J= 8.40 Hz, H-3′′and H-5′′), 7.46 (d, 2H, J= 8.40 Hz, H-3′ and H-5′), 7.43 (d, 1H, J= 2.70 Hz, H-8), 7.14 (dd, 1H, J= 9.30 and 2.70 Hz, H-6), 4.01 (s, 3H, CH3O), 3.93 (s, 2H, NCH2), 3.90 (s, 2H, NCH2), 2.77 (t, 2H, J= 6.90 Hz, NCH2), 2.72 (t, 2H, J= 6.90 Hz, NCH2), 2.56–2,41 (m, 20H, 2NCH2 and 8NCH2 pip.), 2.29 (s, 3H, NCH3), 2.27 (s, 3H, NCH3); 13C NMR δ (75 MHz, CDCl3) 168.4 (C-7), 165.1 (C-2), 162.1 (C-4), 155.8 (C-8a), 144.3 (C-4′), 143.7 (C-4′′), 138.5 (C-1′), 137.9 (C-1′′), 131.6 (C-3′ and C-5′), 130.1 (C-3′′ and C-5′′), 129.7 (C-5), 129.6 (C-2′ and C-6′, C-2′′and C-6′′), 121.4 (C-6), 118.4 (C-4a), 108.0 (C-8), 59.1 (2NCH2), 57.1 (CH3O), 56.6 (2NCH2 pip.), 55.1 (2NCH2), 54.5 (2NCH2 pip.), 47.4 (2NCH3), 47.2 (NCH2), 46.9 (NCH2); MALDI-TOF MS m/z [M + H]+ Calc for C37H51N8O: 623.418, Found: 623.325.
2,4-Bis{3-[(3-dimethylaminopropyl)aminomethyl]phenyl}quinazoline (3 s)
Yellow oil (60%); 1H NMR δ (300 MHz, CDCl3) 8.21 (dd, 1H, J= 8.20 and 1.20 Hz, H-8), 8.17–8.13 (m, 2H, H-6′ and H-2′), 7.91 (dd, 1H, J= 8.20 and 1.20 Hz, H-5), 7.80 (dd, 1H, J= 1.40 and 1.40 Hz, H-2′′), 7.72 (ddd, 1H, J= 8.20, 7.00 and 1.20 Hz, H-7), 7.55–7.43 (m, 6H, H-6, H-6′′, H-4′, H-4′′, H-5′ and H-5′′), 3.90 (s, 2H, NCH2), 3.87 (s, 2H, NCH2), 2.73–2.64 (m, 4H, 2NCH2), 2.45–2.32 (m, 4H, 2NCH2), 2.28 (s, 6H, N(CH3)2), 2.27 (s, 6H, N(CH3)2), 1.61–1.52 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 158.1 (C-2), 150.3 (C-4), 150.2 (C-8a), 143.1 (C-3′′), 142.2 (C-3′), 139.7 (C-1′′), 138.4 (C-1′), 131.4 (C-6′), 131.0 (C-6′′), 130.9 (C-2′ and C-2′′), 129.9 (C-4′ and C-4′′), 129.7 (C-5′ and C-5′′), 129.0 (C-8), 127.6 (C-7), 127.1 (C-4a), 127.0 (C-5), 120.6 (C-6), 59.9 (NCH2), 56.5 (NCH2), 55.1 (NCH2), 54.6 (NCH2), 50.8 (NCH2), 50.6 (NCH2), 47.4 (NCH3), 29.5 (CH2), 26.1 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C32H43N6: 511.355, Found: 511.755.
2,4-Bis{3-[(3–(4-methylpiperazin-1-yl)propyl)aminomethyl]phenyl}quinazoline(3t)
Pale-yellow oil (88%); 1H NMR δ (300 MHz, CDCl3) 8.56–8.52 (m, 2H, H-6′ and H-2′), 8.10 (dd, 1H, J= 8.40 and 1.20 Hz, H-8), 8.06 (dd, 1H, J= 8.40 and 1.20 Hz, H-5), 7.84 (ddd, 1H, J= 8.40, 7.20 and 1.20 Hz, H-7), 7.81 (dd, 1H, J= 1.40 and 1.40 Hz, H-2′′), 7.73–7.69 (m, 1H, H-6′′), 7.52–7.43 (m, 5H, H-6, H-4′, H-4′′, H-5′ and H-5′′), 3.90 (s, 2H, NCH2), 3.88 (s, 2H, NCH2), 2.70 (t, 2H, J= 6.60 Hz, NCH2), 2.68 (t, 2H, J= 6.60 Hz, NCH2), 2.19 (s, 3H, NCH3), 2.17 (s, 3H, NCH3), 1.72–1.66 (m, 4H, 2CH2); 13C NMR δ (75 MHz, CDCl3) 169.8 (C-2), 161.5 (C-4), 153.2 (C-8a), 142.4 (C-3′′), 142.0 (C-3′), 139.7 (C-1′′), 139.0 (C-1′), 134.9 (C-8), 131.7 (C-6′), 131.0 (C-6′′ and C-7), 130.4(C-2′ and C-2′′), 130.0 (C-4′ and C-4′′), 129.6 (C-5′′), 128.8 (C-5′), 128.4 (C-5 and C-6), 123.1 (C-4a), 58.4 (NCH2), 56.5 (NCH2), 55.4 (NCH2), 55.2 (NCH2), 54.6 (NCH2), 49.6 (NCH2), 47.3 (NCH3), 28.3 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C38H53N8: 621.439, Found: 621.478.
2,4-Bis{3-[(3-morpholinopropyl)aminomethyl]phenyl}quinazoline (3 u)
Yellow oil (82%); 1H NMR δ (300 MHz, CDCl3) 8.58 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′), 8.56 (ddd, 1H, J= 7.80, 1.50 and 1.50 Hz, H-6′), 8.13 (dd, 1H, J= 8.70 and 1.10 Hz, H-8), 8.08 (dd, 1H, J= 8.70 and 1.10 Hz, H-5), 7.87 (ddd, 1H, J= 8.70, 7.20 and 1.10 Hz, H-7), 7.81 (dd, 1H, J= 1.50 and 1.50 Hz, H-2′′), 7.76–7.71 (m, 1H, H-6′′), 7.55–7.45 (m, 5H, H-6, H-4′, H-4′′, H-5′ and H-5′′), 3.93 (s, 2H, NCH2), 3.91 (s, 2H, NCH2), 3.69 (t, 4H, J= 4.80 Hz, 2OCH2), 3.64 (t, 4H, J= 4.80 Hz, 2OCH2), 2.75–2.66 (m, 4H, 2NCH2), 2.42–2.31 (m, 12H, 2NCH2 and 4NCH2morph.), 1.71 (qt, 4H, J= 6.90 Hz, 2CH2); 13C NMR δ (75 MHz, CDCl3) 169.8 (C-2), 161.6 (C-4), 153.3 (C-8a), 142.4 (C-3′′), 142.1 (C-3′), 139.7 (C-1′′), 139.1 (C-1′), 135.0 (C-8), 131.7 (C-6′), 131.1 (C-6′′), 131.0(C-7), 130.5 (C-2′′), 130.2 (C-2′), 130.0 (C-4′′), 129.9 (C-4′), 129.6 (C-5′′), 128.8 (C-5′), 128.4 (C-5 and C-6), 123.1 (C-4a), 63.3 (OCH2), 58.8 (NCH2), 58.7 (NCH2), 55.5 (NCH2), 55.3 (NCH2), 55.1 (NCH2), 49.4 (NCH2), 28.1 (CH2), 28.0 (CH2); MALDI-TOF MS m/z [M + H]+ Calc for C36H47N6O2: 595.376, Found: 595.398.
2,4-Bis{3-[(2-morpholinoethyl)aminomethyl]phenyl}quinazoline (3v)
Yellow oil (66%); 1H NMR δ (300 MHz, CDCl3) 8.60–8.56 (m, 2H, H-2′ and H-6′), 8.14 (dd, 1H, J= 8.40 and 1.20 Hz, H-8), 8.08 (dd, 1H, J= 8.40 and 1.20 Hz, H-5), 7.88 (ddd, 1H, J= 8.40, 7.20 and 1.20 Hz, H-7), 7.82 (dd, 1H, J= 1.40 and 1.40 Hz, H-2′′), 7.76–7.72 (m, 1H, H-6′′), 7.56–7.46 (m, 5H, H-6, H-4′, H-4′′, H-5′ and H-5′′), 3.96 (s, 2H, NCH2), 3.94 (s, 2H, NCH2), 3.67–3.62 (m, 8H, 4OCH2), 2.79–2.71 (m, 4H, 2NCH2), 2.53–2.36 (m, 12H, 2NCH2 and 4NCH2morph.); 13C NMR δ (75 MHz, CDCl3) 169.8 (C-2), 161.5 (C-4), 153.3 (C-8a), 142.4 (C-3′′), 142.1 (C-3′), 139.7 (C-1′′), 139.1 (C-1′), 135.0 (C-8), 131.8 (C-6′), 131.0 (C-6′′), 130.5 (C-7), 130.1 (C-2′ and C-2′′), 130.0 (C-4′ and C-4′′), 129.6 (C-5′′), 128.9 (C-5′), 128.4 (C-5 and C-6), 123.1 (C-4a), 68.3 (OCH2), 59.6 (NCH2), 55.1 (NCH2), 46.8 (NCH2), 46.5 (NCH2); MALDI-TOF MS m/z [M + H]+ Calc for C34H43N6O2: 567.345, Found: 567.377.
General procedure for 2,4-bis[(substituted-aminomethyl)phenyl]quinolines (1a–t ⋅ m(COOH)2), 1,3-bis[(substituted-aminomethyl)phenyl]isoquinolines (2a–l ⋅ m(COOH)2), and 2,4-bis[(substituted-aminomethyl)phenyl]quinazolines oxalate salts (3a–v ⋅ m(COOH)2)
To a solution of compounds 1–3 (0.3 mmol) in isopropanol (11 ml) was added oxalic acid (2.4 mmol, 8 eq.). The reaction mixture was heated under reflux for 30 min. The precipitate was filtered, washed with isopropanol then with diethyl ether, and dried under reduced pressure to give the oxalate salts of 1–3.
In vitro antiplasmodial activity
The in vitro antiplasmodial activities were tested over concentrations ranging from 39 to 40 µM against culture-adapted P. falciparum reference strains 3D7 and W2. The former strain is susceptible to CQ but displays a decreased susceptibility to MQ; the latter is considered resistant to CQ. The parasites were cultivated in RPMI medium (Sigma-Aldrich, Lyon, France) supplemented with 0.5% Albumax I (Life Technologies Corporation, Paisley, UK), hypoxanthine (Sigma-Aldrich), and gentamicin (Sigma-Aldrich) with human erythrocytes and were incubated at 37 °C in a candle jar, as described previouslyCitation43 .The P. falciparum drug susceptibility test was carried out in 96-well flat-bottom sterile plates in a final volume of 250 µl. After 48 h incubation period with the drugs, quantities of DNA in treated and control cultures of parasites in human erythrocytes were quantified using the SYBR Green I (Sigma-Aldrich) fluorescence-based methodCitation44,Citation45. Briefly, after incubation, plates were frozen at −20 °C until use. Plates were then thawed for 2 h at room temperature, and 100 µl of each homogenised culture was transferred to a well of a 96-well flat-bottom sterile black plate (Nunc, Inc., Rochester, NY) that contained 100 µl of the SYBR Green I lysis buffer (2xSYBR Green, 20 mM Tris base pH 7.5, 5 mM EDTA, 0.008% w/v saponin, 0.08% w/v Triton X-100). Negative controls treated with solvent (typically DMSO or H2O), and positive controls (CQ and MQ) were added to each set of experiments. Plates were incubated for 1 h at room temperature and then read on a fluorescence plate reader (Tecan, Grödig, Austria) using excitation and emission wavelengths of 485 and 535 nm, respectively. IC50 values were calculated by non-linear regression analysis of data from dose-response curves, using TableCurve 2D version 5.0 software (Systat Software, San Jose, CA). IC50 values are reported as means calculated from three independent experimentsCitation46.
In vitro antileishmanial activity
L. donovani (MHOM/IN/00/DEVI) used in this study was provided by the CNR Leishmania (Montpellier, France). The effects of the tested compounds on the growth of L. donovani (MHOM/IN/00/DEVI) promastigotes were assessed by MTT assayCitation47. Briefly, promastigotes in log-phase in Schneider’s medium supplemented with 20% foetal calf serum (FCS), 2 mM L-glutamine and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin), were incubated at an average density of 106 parasites/ml in sterile 96-well plates with various concentrations of compounds dissolved in DMSO (final concentration less than 0.5% v/v), in duplicate. Appropriate controls treated with DMSO and amphotericin B (reference drug purchased from Sigma-Aldrich) were added to each set of experiments. After 72 h incubation period at 27 °C, parasite metabolic activity was determined. Each well was microscopically examined for precipitate formation. To each well was added 20 µl of 5 mg/ml MTT [3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)] solution followed by 4 h incubation time. The enzyme reaction was stopped by addition of 100 µl of 50% isopropanol/10% sodium dodecyl sulfateCitation48. Plates were vigorously shaken (300 rpm) for 10 min, and the absorbance was measured at 570 nm in a BIO-TEK ELx808 Absorbance Microplate Reader. The IC50 was defined as the concentration of drug required to inhibit by 50% of the metabolic activity of L. donovani promastigotes compared to the control. IC50 of the parasite’s growth (half maximal inhibitory concentration or IC50 values) were then calculated from the obtained experimental results using a previously described regression programmeCitation46.
In vitro antitrypanosomal activity
The effects of the tested compounds on the growth of T. brucei brucei were assessed using an Alamar Blue® assay described by Räz et al.Citation49 T. brucei brucei AnTat 1.9 (IMTA, Antwerpen, Belgium) was cultured in MEM with Earle’s salts, supplemented according to the protocol of Baltz et al.Citation50 with the following modifications: 0.5 mM mercaptoethanol (Sigma Aldrich), 1.5 mM L-cysteine (Sigma Aldrich), 0.05 mM bathocuproine sulphate (Sigma Aldrich), and 20% heat-inactivated horse serum (Gibco, France) at 37 °C and 5% CO2. Samples were incubated at an average density of 2000 parasites/well in sterile 96-wells plates (Fisher, France) with various concentrations of compounds dissolved in 0.9% NaCl. All doses were tested in duplicate. Appropriate controls treated with solvents 0.9% NaCl or DMSO or with suramin, pentamidine, eflornithine, and fexinidazole (reference drugs purchased from Sigma Aldrich and Fluorochem, UK) were added to each set of experiments. After 69 h incubation period at 37 °C, 10 µl of the viability marker Alamar Blue (Fisher) was added to each well, and the plates were incubated for 5 h. The plates were read in a PerkinElmer ENSPIRE (Germany) microplate reader using an excitation wavelength of 530 nm and an emission wavelength of 590 nm. The IC50 was defined as the concentration of drug necessary to inhibit by 50% the activity of T. brucei brucei compared to the control. IC50 values were calculated using a nonlinear regression analysis of dose-response curves performed using GraphPad Prism software (La Jolla, CA). IC50 values were calculated from three independent experiments.
Cytotoxicity evaluation
A cytotoxicity evaluation was performed using the method reported by MosmannCitation48 with slight modifications to determine the cytotoxic concentrations 50% (CC50) and using doxorubicin as a cytotoxic reference compound. These assays were performed in human HepG2 cells purchased from ATCC (ref HB-8065). These cells are a commonly used human hepatocarcinoma-derived cell line that has characteristics similar to those of primary hepatocytes. These cells express many hepatocyte-specific metabolic enzymes, thus enabling the cytotoxicity of tested product metabolites to be evaluated. Briefly, cells in 100 µL of complete RPMI medium, [RPMI supplemented with 10% FCS, 1% L-glutamine (200 mM), penicillin (100 U/ml), and streptomycin (100 µg/ml)] were inoculated at 37 °C into each well of 96-well plates in a humidified chamber in 6% CO2. After 24 h, 100 µl of medium with test compound at various concentrations dissolved in DMSO (final concentration less than 0.5% v/v) were added, and the plates were incubated for 72 h at 37 °C. Duplicate assays were performed for each sample. Each well was microscopically examined for precipitate formation before the medium was aspirated from the wells. After aspiration, 100 µl of MTT solution (0.5 mg/ml in medium without FCS) were then added to each well. Cells were incubated for 2 h at 37 °C. The MTT solution was removed, and DMSO (100 µl) was added to dissolve the resulting blue formazan crystals. Plates were shaken vigorously (300 rpm) for 5 min. The absorbance was measured at 570 nm with 630 nm as reference wavelength in a BIO-TEK ELx808 Absorbance Microplate Reader. DMSO was used as blank and doxorubicin (Sigma Aldrich) as positive control. Cell viability was calculated as percentage of control (cells incubated without compound). The CC50 was determined from the dose-response curve using the TableCurve 2D version 5.0 software (Systat Software, San Jose, CA)
FRET melting experiments
The best bioactive compounds (1b–c, 1e–g, 1s–t, 2b, 2e–f, 2i–j, 3b, 3f–j, 3m, and 3v) have been selected for the subsequent FRET melting experiments. These were performed with dual-labelled oligonucleotides mimicking the Plasmodium telomeric sequences FPf1T [FAM-5′(GGGTTTA)3-GGG3′-TAMRA] and FPf8T [FAM-5′ (GGGTTCA)3GGG3′-TAMRA], the Trypanosoma 9 and 11 chromosomic sequence FTrypBT (also named FEBR1T) [FAM-5′GGGCAGGGGGTGATGGGGAGGAGCCAGGG3′-TAMRA], the human telomeric sequence F21T [FAM-(GGGTTA)3-GGG3′-TAMRA], and the human duplex sequence FdxT [FAM5′-TATAGCTATA-hexaethyleneglycol-TATAGCTATA3′-TAMRA]Citation51,Citation52. The oligonucleotides were pre-folded in 10 mM lithium cacodylate buffer (pH 7.2), with 10 mM KCl and 90 mM LiCl (K+condition). The FAM emissions were recorded at 516 nm using a 492-nm excitation wavelength in the absence and presence of a single compound as a function of temperature (25–95 °C) in 96-well microplates by using a Stratagene MX3000P real-time PCR device at a rate of 1 °C·min−1. Data were normalised between 0 and 1, and the required temperature for half-denaturation of oligonucleotides corresponding to the emission value of 0.5 was taken as the Tm. Each experiment was performed in duplicate with 0.2 µM of labelled oligonucleotide and 2 µM of compound under K+ condition. For each compound, three independent experiments were carried out.
Data analysis
The 20 compounds (1b–c, 1e–g, 1s–t, 2b, 2e–f, 2i–j, 3b, 3f–j, 3m, and 3v), previously selected for FRET assays due to their IC50 best results, have been then submitted to a statistical multivariate analysis in order to check consistent compound types. In particular, a Hierarchical Ascendant Classification (HAC) was performed on the first three principal components (accounting for the 97% of the total variance) of a Principal Component Analysis based on the FPf1T, FPf8T, F21T, and IC50 (against the P. falciparum 3D7 strain) variables. As well, the same analysis was carried out on FtryBT, F21T,l and IC50 (against T. brucei brucei strain) variables. To this end, a Ward’s minimum variance clustering based on Euclidean distances was performed, and the number of retained clusters (Q) was chosen according to the growth of inertia, selecting Q in order to maximise the difference in inertia between (Q − 1, Q) and (Q, Q + 1). This analysis was chosen according to the normal data distribution evaluated with the Shapiro-Wilk normality test. All the analyses were performed within the R 3.0.1 programming environment using functions from the “FactoMineR,” and “lmtest” packagesCitation53–55.
Results and discussion
Chemistry
Novel 2,4-bis[(substituted-aminomethyl)phenyl]quinoline, 1,3-bis[(substituted-aminomethyl)phenyl]isoquinoline and 2,4-bis[(substituted-aminomethyl)phenyl]quinazoline derivatives 1–3 were prepared starting from the commercially substituted 2,4-dichloroquinolines, 1,3-dichloroisoquinolines, or 1,4-dichloroquinazolines (Scheme 1). The intermediate bis-(formylphenyl)-quinolines, -isoquinolines, or -quinazolines 4–6 were synthesised by a double-Suzuki-Miyaura cross-coupling reaction of dichloro-derivatives with 3-, or 4-formylphenylboronic acids in the presence of Pd(PPh3)4 as a catalyst and in the presence of sodium carbonateCitation35,Citation56–58. Condensation of primary amines with these latter dialdehydes 4–6 afforded the di-imines 7–9, which were immediately reduced into the 2,4-bis[(substituted-aminomethyl)phenyl]quinolines 1a–t, 1,3-bis[(substituted-aminomethyl)phenyl]isoquinolines 2a–l and 2,4-bis[(substituted-aminomethyl)phenyl]quinazolines 3a–v using sodium borohydride as reductive agent in refluxing methanol as previously described by our teamCitation35. All compounds were extensively characterised (Supplementary Material).
Scheme 1. Synthesis of 2,4-bis[(substituted-aminomethyl)phenyl]quinoline, 1,3-bis[(substituted-aminomethyl)phenyl]isoquinoline and 2,4-bis[(substituted-aminomethyl)phenyl]quinazoline derivatives 1–3.
![Scheme 1. Synthesis of 2,4-bis[(substituted-aminomethyl)phenyl]quinoline, 1,3-bis[(substituted-aminomethyl)phenyl]isoquinoline and 2,4-bis[(substituted-aminomethyl)phenyl]quinazoline derivatives 1–3.](/cms/asset/27012f7c-de7d-4da7-9abe-48428372e04a/ienz_a_1706502_sch0001_b.jpg)
All quinolines, isoquinolines and quinazolines 1–3 were then converted into ammonium oxalate salts by treatment with oxalic acid in refluxing isopropanol. These oxalate salts were found less hygroscopic than the hydrochloride ones and also soluble in water. summarises the physical properties of the new synthesised 1–3 oxalates.
Table 1. Physical properties of amines 1a–t, 2a–l, and 3a–v.
In vitro antimalarial activity
All the new quinoline, isoquinoline, and quinazoline derivatives 1–3 were evaluated for their antimalarial activity in vitro by incubation with P. falciparum CQ-resistant strain W2 (IC50 CQ = 0.40 µM, IC50 MQ = 0.016 µM) and the strain 3D7, which is CQ sensitive and which has decreased sensitivity to MQ (IC50 CQ = 0.11 µM, MQ = 0.06 µM). As shown in , these new derivatives 1–3 showed IC50 values between 0.13 and 11.50 µM against W2 and between 0.032 and 10.43 µM against 3D7 P. falciparum strains. These biological results could be analysed concerning the position on the phenyl ring of these polyamino side chains, the nature of the terminal amine, and also the influence of the length of the carbon chain between the two amino functions of the side-chain.
Table 2. In vitro sensitivity of P. falciparum, L. donovani and T. brucei brucei strains to compounds 1a–t, 2a–l and 3a–v and cytotoxicity of these compounds in HepG2 cells.
Against the P. falciparum CQ-sensitive strain 3D7, compound 1c bearing dimethylaminoethylamino side chains at position 4 of each of the benzyl moieties was found to be the most active derivative with an IC50 of 0.032 µM. In the non-substituted quinoline series, derivative 1c bearing dimethylaminoethylamino side chains at position 4 of the benzyl rings displayed better activity than the analogues substituted with dimethylaminobutylamino or dimethylaminopropylamino side chains (IC50=0.0.32 µM for 1c versus IC50=0.47 and 4.08 µM for 1b and 1a, respectively). In comparison, when we replaced the dimethylamino function by a methylpiperazine moiety (compounds 1d–f), the quinoline 1f bearing a C2 side chains showed a decrease in antimalarial activity; i.e. IC50=0.69 µM for 1d and 0.47 µM for 1e versus 2.42 µM for 1f). In addition, the quinolines 1g–h which are disubstituted with C3 or C2 morpholino chains on position 4 of its benzyl moieties, exhibited similar behaviour in the biological activity; i.e. morpholinopropylamino compound 1g was found more active (up to 13.5 times) than its morpholinoethylamino analogue 1h with IC50 of 0.099 versus 1.34 µM. The same observations could be done against the P. falciparum CQ-resistant strain W2 with the biological results obtained for these quinolines 1a–h.
The introduction of a methoxy group substituted in position 6 or 7 of the quinoline ring (compounds 1i–p) did not increase the antimalarial activity but seemed to decrease it when compared to the non-substituted quinoline (1a–h and 1q–t). In these subseries in which the quinoline moiety was substituted by a methoxy, the biological activity was found more interesting with a substituted-aminopropylamino side chain (derivatives 1j, 1l, and 1n) compared to a substituted-aminobutylamino side chain (derivatives 1i, 1n, and 1 m), excepted for compounds 1o versus 1p (IC50=2.41 µM for 1o versus IC50=4.34 µM for 1p). Quinoline derivatives with polyamino side chains at position 3 of the benzyl moieties (compounds 1q–t) were significantly less active than quinolines 1a–h substituted in the position 4 of the benzyl rings, with the exception of compound 1t which showed a better activity (IC50=0.23 µM) than its counterpart bearing the morpholinoethylamino side chains at position 4 (compound 1 h) for which the IC50 was found to be 1.34 µM.
Against the 3D7 strain, the non-substituted isoquinolines 2a–d were generally the most active compounds in these series, except for 2d (IC50=5.77 µM) when compared to 2h (IC50=1.81 µM), and also for 2b (IC50=1.82 µM) versus 2f (IC50=1.47 µM). Thus, introduction of a methoxy substituent on the isoquinoline heterocyclic skeleton was not beneficial, meanly in position 6 (compounds 2i–l) for which the IC50 were found between 7.21 and 9.66 µM. Moreover, quinolines 1 generally exhibited better antimalarial activities against the P. falciparum 3D7 strain than their isoquinolines analogues 2, with the exception of compound 2a which showed better antimalarial activity than 1a (IC50=4.08 µM for 1a versus IC50=1.74 µM for 2a). Against the P. falciparum CQ-resistant strain W2, compound 2h bearing methylpiperazinylpropylamino side chains at position 4 of each of the benzyl moieties was found to be the most active with an IC50 of 1.28 µM.
Among the newly substituted quinazolines 3a–r, all derivatives bearing aminoethylamino side chains at position 4 of the benzyl rings generally displayed better activities against 3D7 and W2 strains than their analogues substituted with aminopropylamino or aminobutylamino side chains (for example: IC50=0.56 µM for 3j versus IC50=3.84 and 4.05 µM against 3D7 strain for 3h and 3i, respectively; IC50=1.23 µM for 3j versus IC50=3.68 and 4.34 µM against W2 strain for 3h and 3i, respectively). However, the aminopropylamino substituted compound 3b was found more active than its analogue aminoethylamino quinazoline 3c against the 3D7 strain (IC50=0.62 µM for 3b versus IC50=1.06 for 3c). The substitution of the amino-alkylamino side chains at position 3 of the benzyl nucleus in these quinazoline series was not found detrimental in comparison with the substitution in position 4 against both of the P. falciparum strains.
In vitro antileishmanial activity against promastigote forms
In order to better understand the biological profile of our new heterocycles 1–3, some complementary antiparasitic analyses were also performed. Notably, P. falciparum belongs to the coccidian protozoan parasite family. Therefore, in vitro activity against flagellate protozoan parasite L. donovani was evaluated (). The reference drugs amphotericin B and pentamidine had IC50 values of 0.10 and 5.50 µM, respectively, against L. donovani. Unfortunately, none of our novel compounds showed any antileishmanial activity in vitro (all IC50 values ≥ 10 µM).
In vitro activity against Trypanosoma brucei brucei
These newly synthesised nitrogen heterocyclic derivatives 1–3 were evaluated against T. brucei brucei. Pentamidine, suramine, fexinidazole, and eflornithine were also used here as reference compounds. The screening data are presented in . All quinolines, isoquinolines, and quinazolines 1–3 were active against T. brucei brucei with IC50 values ranging from 0.27 to 2.39 µM; and most of them showed an antiparasitic activity around the µM value. Among them the quinazoline 3e was found as the less active compound (IC50 =2.39 µM), while the best activity against the T. brucei brucei strain was observed with 6-methoxy substituted quinazoline 3h with an IC50 of 0.27 µM. Surprisingly, as all these new tested nitrogen heterocycles 1–3 showed similar range of antiparasitic activities against T. brucei brucei, no relevant structure-activity relationships (SAR) could be deduced among these new derivatives. However, the quinoline 1g which is disubstituted with C3 morpholinopropyl chains on position 4 of its benzyl moieties, exhibited better antitrypanosomal activity than its C2 analogue (compound 1h), and was found four-time more active with IC50 of 0.38 versus 1.59 µM. In addition, the structural analogous 1a and 1b, bearing, respectively (dimethylaminobutyl)aminomethyl and (dimethylaminopropyl)aminomethyl side chains at position 4 of their phenyls, were found slightly more active than the (dimethylaminoethyl)aminomethyl compound 1c (IC50 =0.84, 0.82, and 1.48 µM for 1a, 1b, and 1c, respectively). In comparison, when we replaced the dimethylamino function by a methylpiperazine moiety (compounds 1d–f), quinoline 1f bearing a C2 side chains at position 4 of the phenyls showed an increase in the antitrypanosomal activity; i.e. IC50=1.22 µM for 1d and 1.78 µM for 1e versus 0.99 µM for 1f). In the isoquinoline series (compounds 2), derivatives 2a and 2b (IC50 of 0.64 and 0.35 µM) disubstituted with a C4 or C3 dimethylaminoalkyl chains on position 4 of the benzyl moieties, exhibited better antitrypanosomal activity than their methylpiperazinealkyl C4 or C3 analogues (compounds 2c and 2d) for which IC50 were noticed at 1.10 and 1.28 µM, respectively. Interestingly, a similar behaviour was also observed with their 6- and 7-methoxy substituted isoquinoline analogous (compounds 2e–h and 2i–l, respectively) for which the substitution of the dimethylamino terminal amine on the polyaminoalkyl side chains by a methylpiperazine moiety led to a decrease in the antitrypanosomal activity (IC50=0.52–0.55 µM for 2e–f versus 0.81–1.00 µM for 2g–h, and IC50=0.59–0.64 µM for 2i–j versus 0.99–1.34 µM for 2k–l). The influence of the length of the carbon chain in the polyaminoalkyl side-chain for the 6-methoxy substituted quinazolines 3h–j seems also to be detrimental: a shorter alkyl chain (C4 to C2) led to a decreased antitrypanosomal activity (IC50 =0.27 µM for 3h versus 1.04 and 0.44 µM for 3j and 3i, respectively).
Cytotoxicity and selectivity index
In order to assess selectivity of action, the cytotoxicities of these new synthesised antiparasitic heterocyclic compounds 1–3 were evaluated in vitro in the human cell line HepG2, which is a commonly used human-derived hepatocarcinoma cell line. These cells express many hepatocyte-specific metabolic enzymes. The aim of this assay using HepG2 cells was to evaluate the impact of metabolic activation of the tested compounds on cell viability. The cytotoxic concentrations 50% (CC50) were determined, and selectivity indexes (SIs), defined as the ratios of cytotoxic to antiparasitic activities (SI = CC50/IC50) were calculated. The results of cytotoxicity assays and the associated SI values are presented in . Most of these “quinoline-like” derivatives that were found active against the different parasites showed significant cytotoxicity against the HepG2 cells with CC50 values ranging from 0.48 to 29.57 µM. Concerning the W2 strain, the calculated SIs were between 0.11 and 31.46. For the CQ sensitive strain 3D7, the SIs were noticed from 0.12 to 96.88. Analyses of SI values led us to identify the quinoline compound 1c as a promising compound with a SI of 96.88 for the 3D7 strain. In addition, the quinazoline 3g also had interesting SI value of 27.20 for the CQ sensitive strain 3D7. Against the T. brucei brucei strain, quinazolines 3h and 3m had SIs of 43.22 and 33.51, respectively. These SI values could indicate that these new nitrogen heterocyclic derivatives warrant further investigation into their potential use as antiparasitic drugs.
Table 3. Selectivity indexes of compounds 1–3.
FRET-melting experiments
As the telomeres of the parasites P. falciparum and Trypanosoma could be potential targets of this kind of nitrogen heterocyclic compounds, we have also investigated stabilisation of the P. falciparum telomeric or T. brucei brucei chromosomic G-quadruplexes by our best bioactive compounds 1–3 through a FRET melting assays. We used a FRET melting assay to determine the degree to which the new quinoline, isoquinoline and quinazoline derivatives stabilise the G-quadruplexes formed by oligonucleotides with P. falciparum or T. brucei brucei as well as human telomeric sequences. For this purpose, we used two fluorescently labelled P. falciparum telomeric and one T. brucei brucei chromosomic sequences (FPf1T, FPf8T, and FtrypBT) and one human telomeric sequence (F21T).
To probe the G4 selectivity of our selected ligands 1–3 over duplex DNA, a FRET melting assay was performed using a duplex control sequence, FdxT. For comparison, we evaluated reference G4 ligand PhenDC3 and the antimalarial reference drugs CQ and MQ. To enable comparison of selectivities, we calculated the difference (ΔTm) between the Tm of the G-quadruplex formed by FPf1T, FPf8T, FtrypBT (FEBR1T), F21T, or FdxT in the presence or absence of each selected compound. These ΔTm values are presented in . For these selected compounds, the ΔTm values ranged from 0.1 to 27.3 °C at 2 µM ligand concentration. The best ligands which stabilise all the four G-quadruplexes sequences were compounds 2e–f, 3h, and 3m (). 7-Methoxy-1,3-bis{4-[(4-dimethylaminobutyl)aminomethyl]phenyl}isoquinoline 2e strongly stabilised all the four G-quadruplex sequences with ΔTm values ranged from 22.2 to 27.3 °C. These nitrogen heterocyclic compounds 2e–f, 3h, and 3m which exhibited a strong stabilisation profile were all substituted by a methoxy function on the heterocyclic moiety and dimethylaminobutylamino or dimethylaminopropylamino side chains at position 4 of the benzyl rings. Among the tested compounds, derivatives 2e–f and 3h displayed a better stabilisation profile for both the P. falciparum telomeric sequences than the human telomeric G-quadruplex. Moreover, it could be noticed that all the selected quinoline derivatives 1 scarcely stabilised the G-quadruplex, whereas all the isoquinolines 2 strongly stabilised the protozoal and human G-quadruplex. In addition, the quinazoline ligands 3 showed low to moderate ranges of stabilisation on the different telomeric G-quadruplex. These last results could also confirm the importance of the position of the nitrogen atom in the isoquinoline and quinazoline heterocycles. Concerning data noticed for the stabilisation of the T. brucei brucei non-telomeric G-quadruplexes (FtrypBT), the results showed the same profile as those obtained for the P. falciparum telomeric sequences (FPf1T, FPf8T) with slightly lower ΔTm values. FRET assays showed there was no selectivity to duplex DNA sequence.
Table 4. FRET-melting values for the selected compounds 1–3with FPf1T, FPf8T, FtryBT, F21T, and FdxT in K+ conditions at 2 μM.
Classification of bioactive ligands
The selected compounds 1–3 were classified through HAC in two clusters (), defining consistent compound types in relation to P. falciparum variables (FPf1T, FPf8T, F21T, and IC50 against the 3D7 strain). Both clusters included ten compounds and were clearly separated. The red cluster was distinguished by an increase in F21T, FPf1T, FPf8T, and 3D7 values, defining a compound class mainly characterised by a high affinity for P. falciparum and human telomeric G-quadruplexes and with a scarce antimalarial activity. On the other hand, the blue cluster was characterised by a decrease in FRET-melting and IC50 values, mainly defining a compound class with lower affinity for the employed G-quadruplex sequences and higher antimalarial activity. Consequently, these results suggest that the ability of our compounds to target P. falciparum telomeric G-quadruplex sequences is not a desirable property for antimalarial activity.
These selected bioactive compounds were classified in two consistent clusters also in relation to T. brucei brucei variables (FtryBT, F21T, and IC50 against T. brucei brucei strain) (). In sharp contrast with P. falciparum (), the red cluster was characterised by the most active molecules showing also a higher selectivity for the tested G-quadruplex sequences contrarily to the blue ones. The latter classification highlights a positive correlation between the anti-trypanosomal activity and the ability of these compounds to stabilise telomeric G-quadruplexes.
Conclusions
In this report, we described the design, the synthesis, the antiprotozoal activities, and the in vitro cytotoxicity towards human cells of a novel series of 2,4-bis[(substituted-aminomethyl)phenyl]quinoline, 1,3-bis[(substituted-aminomethyl)phenyl]isoquinoline and 2,4-bis[(substituted-aminomethyl)phenyl]quinazoline derivatives. These new “quinoline-like” derivatives were tested for their in vitro antiparasitic activity towards the CQ-sensitive 3D7 and CQ-resistant W2 P. falciparum strains, the promastigote form of L. donovani, and a T. brucei brucei strain. Among these new synthesised nitrogen heterocyclic molecules, a few of them were identified as potential in vitro antiplasmodial leads with IC50 ranging from 0.032 to 0.23 µM on the W2 and 3D7 strains of P. falciparum. The 2,4-bis[(substituted-aminomethyl)phenyl]quinoline 1c was identified as the most potent antimalarial candidate with a ratio of cytotoxic to antiparasitic activities of 97 against the P. falciparum CQ-sensitive strain 3D7. In general, the quinoline and quinazoline derivatives 1 and 3 were found more active against both Plasmodium strains than their isoquinoline analogues 2. Moreover, introduction of a methoxy substituent on the heterocyclic moieties generally did not led to an increase of the antimalarial activity. Unfortunately, none of our compounds showed activity against the promastigote forms of L. donovani. Moreover, the antiprotozoal activity spectrum of our new synthesised derivatives using a T. brucei brucei strain revealed IC50 values ranging from 0.27 to 2.39 µM, which warrant further investigations. The 2,4-bis[(substituted-aminomethyl)phenyl]quinazoline 3 h was also identified as the most potent trypanosomal candidate with SI of 43 on Trypanosoma brucei brucei strain. In addition, the in vitro cytotoxicity of these new heterocyclic compounds was assessed on the human HepG2 cell line. Structure-activity relationships of these new synthetic compounds are here also discussed, as well as their relative ability of targeting P. falciparum or Trypanosoma telomeres as an hypothetical mechanism of action. Thus, as the telomeres of the parasites could constitute interesting targets, we have also investigated the possibility of targeting Plasmodium telomeres or Trypanosoma chromosomes by stabilising the Plasmodium or Trypanosoma G-quadruplexes sequences through FRET melting assays with our best bioactive compounds. Concerning the stabilisation of the parasitic G-quadruplex, the isoquinoline derivatives 2 seem to better stabilise the protozoal and human G-4 structures in comparison with their quinoline and quinazoline homologues 1 and 3.
Supplemental Material
Download PDF (9.3 MB)Acknowledgements
We thank the DGA and ANR (projects ANR-12-ASTR-003). The authors also thank Philippe Grellier for supplying the 3D7 and W2 strains (Museum National d'Histoire Naturelle collection, Paris, France).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- World Health Organization. World malaria report 2018. Geneva, Switzerland: World Health Organization; 2018.
- Yeung S, Socheat D, Moorthy VS, et al. Artemisinin resistance on the Thai-Cambodian border. Lancet 2009;374:1418–19.
- Müller O, Sié A, Meissner P, et al. Artemisinin resistance on the Thai-Cambodian border. Lancet 2009;374:1419.
- Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014;505:50–5.
- World Health Organization. Artemisinin resistance and artemisinin-based combination therapy efficacy. Geneva, Switzerland: World Health Organization; 2018.
- World Health Organization. Guidelines for the treatment of malaria. 3rd ed. Geneva, Switzerland: World Health Organization; 2015.
- De D, Krogstad FM, Cogswell FB, et al. Aminoquinolines that circumvent resistance in Plasmodium falciparum in vitro. Am J Trop Med Hyg 1996;55:579–83.
- Ridley RG, Hofheinz W, Matile H, et al. 4-aminoquinoline analogs of chloroquine with shortened side chains retain activity against chloroquine-resistant Plasmodium falciparum. Antimicrob Agents Chemother 1996;40:1846–54.
- Rout S, Mahapatra RK. Plasmodium falciparum: multidrug resistance. Chem Biol Drug Des 2019;93:737–59.
- Ashley EA, Phyo AP. Drugs in development for malaria. Drugs 2018;78:861–79.
- Hu YQ, Gao C, Zhang S, et al. Quinoline hybrids and their antiplasmodial and antimalarial activities. Eur J Med Chem 2017;139:22–47.
- Hussaini SM. Therapeutic significance of quinolines: a patent review. Expert Opin Ther Pat 2016;26:1201–21.
- Parhizgar AR, Tahghighi A. Introducing new antimalarial analogues of chloroquine and amodiaquine: a narrative review. Iran J Med Sci 2017;42:115–28.
- Nqoro X, Tobeka N, Aderibigbe BA. Quinoline-based hybrid compounds with antimalarial activity. Molecules 2017;22:2268.
- Deshpande S, Kuppast B. 4-Aminoquinolines: an overview of antimalarial chemotherapy. Med Chem 2016;06:1–11.
- Kumar S, Singh RK, Patial B, et al. Recent advances in novel heterocyclic scaffolds for the treatment of drug-resistant malaria. J Enzyme Inhib Med Chem 2016;31:173–86.
- Manohar S, Tripathi M, Rawat DS. 4-aminoquinoline based molecular hybrids as antimalarials: an overview. Curr Top Med Chem 2014;14:1706–33.
- O’Neill PM, Ward SA, Berry NG, et al. A medicinal chemistry perspective on 4-aminoquinoline antimalarial drugs. Curr Top Med Chem 2006;6:479–507.
- Malmquist NA, Moss TA, Mecheri S, et al. Small-molecule histone methyltransferase inhibitors display rapid antimalarial activity against all blood stage forms in Plasmodium falciparum. Proc Natl Acad Sci USA 2012;109:16708–13.
- Fröhlich T, Tsogoeva SB. In Vivo and in vitro optimization of screening antimalarial hits toward lead molecules for preclinical development. J Med Chem 2016;59:9668–71.
- Gilson PR, Tan C, Jarman KE, et al. Optimization of 2-anilino 4-amino substituted quinazolines into potent antimalarial agents with oral in vivo activity. J Med Chem 2017;60:1171–88.
- Baragaña B, Norcross NR, Wilson C, et al. Discovery of a quinoline-4-carboxamide derivative with a novel mechanism of action, multistage antimalarial activity, and potent in vivo efficacy. J Med Chem 2016;59:9672–85.
- Lubin AS, Rueda-Zubiaurre A, Matthews H, et al. Development of a photo-cross-linkable diaminoquinazoline inhibitor for target identification in Plasmodium falciparum. ACS Infect Dis 2018;13:523–30.
- Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet 2018;392:951–70.
- Alvar J, Canavate C, Gutierrez-Solar B, et al. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev 1997;10:298–319.
- World Health Organization. WHO Technical report series n°975, research priorities for chagas disease, human African trypanosomiasis and leishmaniasis. Geneva, Switzerland: World Health Organization; 2012: 116.
- World Health Organization. WHO interim guidelines for the treatment of gambiense human African trypanosomiasis. Geneva, Switzerland: World Health Organization; 2019.
- Guillon J, Grellier P, Labaied M, et al. Synthesis, antimalarial activity, and molecular modeling of new pyrrolo[1,2-a]quinoxalines, bispyrrolo[1,2-a]quinoxalines, bispyrido[3,2-e]pyrrolo[1,2-a]pyrazines, and bispyrrolo[1,2-a]thieno[3,2-e]pyrazines. J Med Chem 2004;47:1997–2009.
- Guillon J, Forfar I, Mamani-Matsuda M, et al. Synthesis, analytical behaviour and biological evaluation of new 4-substituted pyrrolo[1,2-a]quinoxalines as antileishmanial agents. Bioorg Med Chem 2007;15:194–210.
- Guillon J, Forfar I, Desplat V, et al. Synthesis of new 4-(E)-alkenylpyrrolo[1,2-a]quinoxalines as antileishmanial agents by Suzuki-Miyaura cross-coupling reactions. J Enzyme Inhib Med Chem 2007;22:541–9.
- Guillon J, Moreau S, Mouray E, et al. New ferrocenic pyrrolo[1,2-a]quinoxaline derivatives: synthesis, and in vitro antimalarial activity. Bioorg Med Chem 2008;16:9133–44.
- Guillon J, Mouray E, Moreau S, et al. New ferrocenic pyrrolo[1,2-a]quinoxaline derivatives: synthesis, and in vitro antimalarial activity-Part II. Eur J Med Chem 2011;46:2310–26.
- Ronga L, Del Favero M, Cohen A, et al. Design, synthesis and biological evaluation of novel 4-alkapolyenylpyrrolo[1,2-a]quinoxalines as antileishmanial agents–part III. Eur J Med Chem 2014;81:378–93.
- Guillon J, Cohen A, Gueddouda NM, et al. Design, synthesis and antimalarial activity of novel bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine derivatives. J Enzyme Inhib Med Chem 2017;32:547–63.
- Guillon J, Cohen A, Das RN, et al. Design, synthesis, and antiprotozoal evaluation of new 2,9-bis[(substituted-aminomethyl)phenyl]-1,10-phenanthroline derivatives. Chem Biol Drug Des 2018;91:974–95.
- Calvo EP, Wasserman M. G-Quadruplex ligands: potent inhibitors of telomerase activity and cell proliferation in Plasmodium falciparum. Mol Biochem Parasitol 2016;207:33–8.
- Tidwell R, Boykin D, Ismail M, et al. Dicationic compounds which selectively recognize G-quadruplex DNA, Patent EP-1792613-A2, 2007.
- Leeder WM, Hummel NF, Göringer HU. Multiple G-quartet structures in pre-edited mRNAs suggest evolutionary driving force for RNA editing in trypanosomes. Sci Rep 2016;6:29810.
- Lombraña R, Álvarez A, Fernández-Justel JM, et al. Transcriptionally driven DNA replication program of the human parasite leishmania major. Cell Rep 2016;16:1774–86.
- Bottius E, Bakhsis N, Scherf A. Plasmodium falciparum telomerase: de novo telomere addition to telomeric and nontelomeric sequences and role in chromosome healing. Mol Cell Biol 1998;18:919–25.
- Raj DK, Das BR, Dash AP, et al. Identification of telomerase activity in gametocytes of Plasmodium falciparum. Biochem Biophys Res Commun 2003;309:685–8.
- De Cian A, Grellier P, Mouray E, et al. Plasmodium telomeric sequences: structure, stability and quadruplex targeting by small compounds. ChemBioChem 2008;9:2730–9.
- Desjardins RE, Canfield CJ, Haynes JD, et al. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother 1979;16:710–18.
- Bennett TN, Paguio M, Gligorijevic B, et al. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob Agents Chemother 2004;48:1807–10.
- Bacon DJ, Latour C, Lucas C, et al. Comparison of a SYBR green I-based assay with a histidine-rich protein II enzyme-linked immunosorbent assay for in vitro antimalarial drug efficacy testing and application to clinical isolates. Antimicrob Agents Chemother 2007;51:1172–8.
- Kaddouri H, Nakache S, Houzé S, et al. Assessment of the drug susceptibility of Plasmodium falciparum clinical isolates from africa by using a Plasmodium lactate dehydrogenase immunodetection assay and an inhibitory maximum effect model for precise measurement of the 50-percent inhibitory concentration. Antimicrob Agents Chemother 2006;50:3343–9.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
- Emami SA, Zamanai Taghizadeh Rabe S, Ahi A, et al. Inhibitory activity of eleven artemisia species from Iran against Leishmania major parasites. Iran J Basic Med Sci 2012;15:807–11.
- Räz B, Iten M, Grether-Bühler Y, et al. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop 1997;68:139–47.
- Baltz T, Baltz D, Giroud C, et al. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J 1985;4:1273–7.
- De Cian A, Guittat L, Kaiser M, et al. Fluorescence-based melting assays for studying quadruplex ligands. Methods 2007;42:183–95.
- Belmonte-Reche E, Martínez-García M, Guédin A, et al. G-quadruplex identification in the genome of protozoan parasites points to naphthalene diimide ligands as new antiparasitic agents. J Med Chem 2018;61:1231–40.
- Le S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw 2008;25:1–18.
- Zeileis A, Hothorn T. Diagnostic checking in regression relationships. R News 2002;2:7–10.
- R Core Team. R: a language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria: R Core Team; 2013.
- Achelle S, Rodrı́guez-López J, Robin-le Guen F. Synthesis and photophysical studies of a series of quinazoline chromophores. J Org Chem 2014;79:7564–71.
- Jun JV, Petersson EJ, Chenoweth DM. Rational design and facile synthesis of a highly tunable quinoline-based fluorescent small-molecule scaffold for live cell imaging. J Am Chem Soc 2018;140:9486–93.
- Kang D, Kim H, Shin C. Compound for organic optoelectric device, organic light emitting device containing same, and display device containing said organic light emitting device, Patent EP-2842954, 2015.
