Abstract
A series of amino acid–sulphonamide conjugates was prepared through benzotriazole mediated coupling reactions and characterised by 1H-NMR, 13C-NMR, MS, and FTIR spectroscopic techniques as well as elemental analysis. The carbonic anhydrase (CA, EC 4.2.1.1) inhibitory activity of the new compounds was determined against four human (h) isoforms, hCA I, hCA II, hCA VA, and hCA XII. Most of the synthesised compounds showed effective in vitro CA inhibitory properties. The new amino acid–sulphonamide conjugates showed potent inhibitory activity against hCA II, some of them at subnanomolar levels, exhibiting more effective inhibitory activity compared to the standard drug acetazolamide. Some of these sulphonamides were also found to be effective inhibitors of hCA I, hCA VA, and hCA XII, with activity from the low to high nanomolar range.
1. Introduction
The environmentally friendly synthesis of biologically active molecules is one of the most important issues in medicinal chemistryCitation1. Sulphonamides are one of the oldest antimicrobial compounds class, discovered in the 1930sCitation2 and they are present in many effective drugs, due to their excellent chemical and biological propertiesCitation3. Today, many sulphonamides are used for the treatment of urinary infections, burns, especially in combination with dihydrofolate reductase inhibitors such as trimethoprimCitation4. On the other hand, with the discovery of strong carbonic anhydrase (CA, EC 4.2.1.1) inhibition properties of primary sulphonamides and the relationship with various pathologies, including cancerCitation5–7, studies in this field have gained a different dimension. However, the widespread use of sulphonamide antibacterials also leads to the development of bacterial resistance. For this reason, the development of novel classes of antimicrobial compounds with less side effect and improved selectivity is urgently needed. Therefore, synthesis of new sulphonamide derivatives is an active research topic for organic and pharmaceutical chemistsCitation8,Citation9. Both sulphonamidesCitation10–12 and amino acidsCitation13–15 have been reported to have various biological activities, but there are few reports of their successful combination in one molecule as a hybrid drug candidateCitation16–18. Due to their antimicrobial and CA inhibitory properties, both primary and secondary sulphonamide derivatives are under investigation to determine compounds possessing the highest activity with possibly few side-effectsCitation19,Citation20. For this reason, many studies on sulphonamide derivatives have been done in recent yearsCitation3,Citation8,Citation9,Citation12,Citation18,Citation21. Moreover, our group previously observed the CA inhibitory properties of some primary sulphonamide derivatives incorporating dipeptide and amino acid moieties, which inhibited some pharmacologically relevant CA isoforms at nanomolar levelsCitation16–18. Following our synthetic and CA inhibition screening work on dipeptide and N-protected amino acid–sulphonamide conjugates, we synthesised and explored the inhibitory activity of new N-protected amino acid–sulphonamide conjugates against hCA I, II, VA, and XII enzymes, in the search of more effective and isoform-selective CA inhibitors (CAIs).
2. Material and methods
2.1. Chemistry
The starting materials and reagents used in the reactions were supplied commercially by Aldrich, Acros, Merck, AFG Bioscience, Bachem, Alfa Aesar, or Fluorochem (all from Milan, Italy). Nuclear magnetic resonance (1H-NMR, 13C-NMR) spectra were recorded using a Bruker Advance III 400 or 300 MHz spectrometers in DMSO-d6. Chemical shifts are reported in parts per million (ppm) and the coupling constants (J) are expressed in Hertz (Hz). The assignment of exchangeable protons (OH and NH) was confirmed by the addition of D2O. Positive or negative-ion electrospray ionisation (ESI) mass spectra were recorded on a double-focusing Finnigan MAT 95 instrument with BE geometry. Analytical thin-layer chromatography (TLC) was carried out on Merck silica gel F-254 plates. All microwave-assisted reactions were carried out in a microwave oven system manufactured by Milestone (Milestone Start S Microwave Labstation for Synthesis). Infra-red spectra were recorded with ATR equipment in the range 4000–650 cm−1 on a Perkin Elmer Spectrum one FTIR spectrophotometer. Elemental analyses were performed with a LECO CHNS-932 elemental analyser. Melting points were recorded using an Electrothermal-9200 melting point apparatus and are uncorrected. All starting N-protected dipeptides were prepared according to literature proceduresCitation17,Citation18,Citation22–24. The compounds 5, 10, and 18 are commercially available in the Scifinder database, but since no information is available, information on syntheses and characterisations is also provided (Spectral data are presented in Supplementary Material).
2.2. General procedure for the synthesis of amino acid–sulphonamide conjugates, 1–12
N-protected aminoacylbenzotriazole (1.0 equiv.) and 4-(2-aminoethyl)benzenesulphonamide (1.0 equiv.) were subjected to microwave irradiation (100 W, 70 °C) in DCM (5 ml) for 30 min. After completion of the reaction, all volatiles were removed by rotae vaporation and the obtained crude product was crystallised from ethanol.
2.2.1. Benzyl (R)-(4-methyl-1-oxo-1-((4-sulphamoylphenethyl)amino)pentan-2-yl)carbamate (1)
White solid (75%); mp 174–175 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.02 (t, 1H, NHCH2CH2, J = 4.5 Hz), 7.74 (d, 2H, Ar-H, J = 6.0 Hz), 7.40–7.30 (m, 10H, OCONHCH + Ar-H + NH2), 5.09–4.98 (m, 2H, CH2OCO), 4.01–3.93 (m, 1H, OCONHCH), 3.35–3.27 (m, 2H, NHCH2CH2), 2.79 (t, 2H, NHCH2CH2, J = 7.5 Hz), 1.56–1.24 (m, 3H, CHCH2CH(CH3)2), 0.84 (t, 6H, CH(CH3)2, J = 6.0 Hz).
13C NMR (75 MHz, DMSO-d6): δ 172.2 (CONHCH2CH2), 155.9 (CH2OCO), 143.6, 142.0, 137.1, 129.1, 128.3, 127.7, 127.7, 125.6 (Ar-C), 65.3 (CH2OCO), 53.2 (OCONHCH), 40.9 (CHCH2CH(CH3)2), 34.7 (CONHCH2CH2), 24.2 (CHCH2CH(CH3)2), 22.9 and 21.5 (CHCH2CH(CH3)2). ν(C–O)carbamate: 1651 cm−1, ν(C–O)amide: 1671 cm−1, ν(N-H)amine: 3304, 3676 cm−1. Anal. calculated for C22H29N3O5S: C, 59.04; H, 6.53; N, 9.39; S, 7.16. Found: C, 58.73; H, 6.19; N, 9.34; S, 7.09. HRMS m/z for C22H29N3O5S [M + H]+ calcd. 448.2, found 448.3; [M + Na]+ calcd. 470.2, found 470.3; [M + HCOO] − calcd. 492.2 found 492.3.
2.2.2. Benzyl (R)-(4-(methylthio)-1-oxo-1-((4-sulphamoylphenethyl)amino)butan-2-yl)carbamate (2)
White solid (88%); mp 173–174 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.04 (t, 1H, NHCH2CH2, J = 4.5 Hz), 7.75 (d, 2H, Ar-H, J = 9.0 Hz), 7.48 (d, 1H, OCONHCH, J = 9.0 Hz), 7.41–7.31 (m, 9H, Ar-H + NH2), 5.09–4.98 (m, 2H, CH2OCO), 4.07–4.00 (m, 1H, OCONHCH), 3.36–3.27 (m, 2H, NHCH2CH2), 2.79 (t, 2H, NHCH2CH2, J = 6.0 Hz), 2.45–2.34 (m, 2H, CHCH2CH2SCH3), 2.02 (s, 3H, CHCH2CH2SCH3), 1.86–1.70 (m, 2H, CHCH2CH2SCH3).
13C-NMR (100 MHz, DMSO-d6): δ 171.4 (CONHCH2CH2), 156.0 (CH2OCO), 143.6, 142.0, 137.0, 129.1, 128.3, 127.8, 127.7, 125.6 (Ar-C), 65.4 (CH2OCO), 53.9 (OCONHCH), 34.7 (CONHCH2CH2), 31.5 (CHCH2CH2SCH3), 29.7 (CHCH2CH2SCH3), 14.5 (CHCH2CH2SCH3). ν(C–O)carbamate: 1648 cm−1, ν(C–O)amide: 1677 cm−1, ν(N-H)amine: 3312 cm−1. Anal. calculated for C21H27N3O5S2: C, 54.18; H, 5.85; N, 9.03; S, 13.77. Found: C, 53.44; H, 5.85; N, 9.22; S, 13.06. HRMS m/z for C21H27N3O5S2 [M + H]+ calcd. 466.2 found 466.3; [M + Na]+ calcd. 488.1, found 488.3; [M + HCOO]− calcd. 510.1 found 510.2.
2.2.3. Benzyl (R)-(3-methyl-1-oxo-1-((4-sulphamoylphenethyl)amino)butan-2-yl)carbamate (3)
White solid (95%); mp 185–186 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.05 (t, 1H, NHCH2CH2, J = 4.5 Hz), 7.74 (d, 2H, Ar-H, J = 9.0 Hz), 7.41–7.23 (m, 10H, Ar-H + OCONHCH + NH2), 5.09–4.99 (m, 2H, CH2OCO), 3.78 (t, 1H, OCONHCH, J = 7.5 Hz), 3.41–3.26 (m, 2H, NHCH2CH2), 2.79 (t, 2H, NHCH2CH2, J = 6.0 Hz), 1.94–1.83 (m, 1H, CH(CH3)2), 0.79 (d, 6H, CH(CH3)2, J = 6.0 Hz).
13C NMR (75 MHz, DMSO-d6): δ 171.1 (CONHCH2CH2), 156.1 (CH2OCO), 143.6, 142.0, 137.1, 129.1, 128.3, 127.7, 127.6, 125.6 (Ar-C), 65.3 (CH2OCO), 60.3 (OCONHCH), 39.6 (CONHCH2CH2), 34.7 (CONHCH2CH2), 30.1 (CH(CH3)2), 19.8 and 18.1 (CH(CH3)2). ν(C–O)carbamate: 1643 cm−1, ν(C–O)amide: 1690 cm−1, ν(N-H)amine: 3307, 3676 cm−1. Anal. calculated for C21H27N3O5S: C, 58.18; H, 6.28; N, 9.69; S, 7.40. Found: C, 57.75; H, 5.88; N, 9.65; S, 7.09. HRMS m/z for C21H27N3O5S [M + H]+ calcd. 434.2, found 434.3; [M + Na]+ calcd. 456.2, found 456.3; [M + HCOO]− calcd. 478.2, found 478.3.
2.2.4. Benzyl (S)-(1-oxo-3-(phenylthio)-1-((4-sulphamoylphenethyl)amino)propan-2-yl)carbamate (4)
White solid (85%); mp 117–118 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.25 (t, 2H, NHCH2CH2, J = 6.0 Hz), 7.75 (d, 2H, Ar-H, J = 6.0 Hz), 7.67 (d, 1H, OCONHCH, J = 9.0 Hz), 7.41–7.21 (m, 14H, Ar-H + NH2), 5.11–5.00 (m, 2H, CH2OCO), 4.18–4.11 (m, 1H, OCONHCH), 3.35–3.26 (s, 3H, CH2SPh + NHCH2CH2), 3.11–3.04 (m, 1H, CH2SPh), 2.79 (t, 2H, NHCH2CH2, J = 6.0 Hz).
13C NMR (75 MHz, DMSO-d6): δ 169.8 (CONHCH2CH2), 155.9 (CH2OCO), 143.6, 142.0, 136.9, 135.7, 129.2, 129.1, 128.3, 128.3, 127.8, 127.7, 125.9, 125.6 (Ar-C), 65.6 (CH2OCO), 54.2 (OCONHCH), 40.5 (NHCH2CH2), 34.9 (CH2SPh), 34.6 (CONHCH2CH2). ν(C–O)carbamate: 1652 cm−1, ν(C–O)amide: 1712 cm−1, ν(N-H)amine: 3318, 3676 cm−1. Anal. calculated for C25H27N3O5S2: C, 58.46; H, 5.30; N, 8.18; S, 12.48. Found: C, 57.34; H, 5.13; N, 8.13; S, 11.87. HRMS m/z for C25H27N3O5S2 [M + H]+ calcd. 514.2, found 514.3; [M + Na]+ calcd. 536.2, found 456.3; [M + HCOO]− calcd. 558.2, found 558.2.
2.2.5. tert-Butyl (R)-(1-oxo-1-((4-sulphamoylphenethyl)amino)propan-2-yl)carbamate (5)
White solid (90%); mp 165–166 °C; 1H-NMR (300 MHz, DMSO-d6): δ 7.88 (t, 1H, NHCH2CH2, J = 4.5 Hz), 7.74 (d, 2H, Ar-H, J = 9.0 Hz), 7.39 (d, 2H, Ar-H, J = 9.0 Hz), 7.30 (s, 2H, NH2), 6.85 (d, 1H, OCONHCH, J = 9.0 Hz), 3.95–3.85 (m, 1H, OCONHCH), 3.38–3.25 (m, 2H, NHCH2CH2), 2.78 (t, 2H, NHCH2CH2, J = 6.0 Hz), 1.38 (s, 9H, OC(CH3)3), 1.12 (d, 3H, CHCH3, J = 9.0 Hz).
13C NMR (75 MHz, DMSO-d6): δ 172.7 (CONHCH2CH2), 155.0 ((CH3)3COCO), 143.6, 142.0, 129.1, 125.6 (Ar-C), 78.0 ((CH3)3COCO), 49.7 (OCONHCH), 34.7 (CONHCH2CH2), 28.2 (OC(CH3)3), 18.3 (CHCH3). ν(C–O)carbamate: 1664 cm−1, ν(C–O)amide: 1685 cm−1, ν(N-H)amine: 3373 cm−1. Anal. calculated for C16H25N3O5S: C, 51.74; H, 6.78; N, 11.31; S, 8.63. Found: C, 51.65; H, 6.67; N, 11.25; S, 8.26. HRMS m/z for C16H25N3O5S [M + Na]+ calcd. 394.2, found 394.2; [M + HCOO]− calcd. 416.2, found 416.1; [M-H]− calcd. 370.2, found 370.1.
2.2.6. (9H-fluoren-9-yl)methyl (2-oxo-2-((4-sulphamoylphenethyl)amino)ethyl)carbamate (6)
White solid (91%); mp 134–135 °C; 1H-NMR (300 MHz, DMSO-d6): δ 7.97 (t, 1H, NHCH2CH2, J = 4.5 Hz), 7.90 (d, 2H, Ar-H, J = 9.0 Hz), 7.74 (t, 4H, Ar-H, J = 7.5 Hz), 7.54 (t, 1H, OCONHCH2, J = 6.0 Hz), 7.45–7.32 (m, 8H, Ar-H + NH2), 4.32–4.24 (m, 3H, CHCH2OCO), 3.58 (d, 2H, OCONHCH2, J = 6.0 Hz), 3.34–3.29 (m, 2H, NHCH2CH2), 2.80 (t, 2H, NHCH2CH2, J = 7.5 Hz).
13C NMR (75 MHz, DMSO-d6): δ 169.0 (CONHCH2CH2), 156.5 (CH2OCO), 143.8, 143.6, 142.0, 140.7, 129.1, 127.6, 127.1, 125.7, 125.2, 120.1 (Ar-C), 65.7 (CH2OCO), 46.6 (CHCH2OCO), 43.4 (OCONHCH2), 34.8 (CONHCH2CH2). ν(C–O)carbamate: 1661 cm−1, ν(C–O)amide: 1700 cm−1, ν(N-H)amine: 3312 cm−1. Anal. calculated for C25H25N3O5S: C, 62.62; H, 5.25; N, 8.76; S, 6.69. Found: C, 61.66; H, 5.29; N, 8.75; S, 6.06. HRMS m/z for C25H25N3O5S [M + H]+ calcd. 480.2, found 480.3; [M + NH4]+ calcd. 497.2, found 497.3; [M + HCOO]− calcd. 524,2 found 524.2.
2.2.7. (9H-fluoren-9-yl)methyl (S)-(1-oxo-3-phenyl-1-((4-sulphamoylphenethyl)amino)propan-2-yl)carbamate (7)
White solid (93%); mp 174–175 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.15 (t, 1H, NHCH2CH2, J = 6.0 Hz), 7.89 (d, 2H, Ar-H, J = 9.0 Hz), 7.75 (t, 2H, Ar-H, J = 6.0 Hz), 7.68–7.63 (m, 3H, Ar-H + OCONHCH), 7.74–7.17 (m, 13H, Ar-H + NH2), 4.23–4.14 (m, 4H, CHCH2OCONHCH), 3.33–3.26 (m, 2H, NHCH2CH2), 2.96–2.90 (m, 1H, CHCH2Ph), 2.82–2.75 (m, 3H, CHCH2Ph + NHCH2CH2).
13C NMR (75 MHz, DMSO-d6): δ 171.4 (CONHCH2CH2), 155.7 (CH2OCO), 143.8, 143.7, 143.6, 142.0, 140.6, 138.2, 129.9, 129.2, 128.0, 127.6, 127.0, 126.2, 125.6, 125.4, 125.3, 120.1 (Ar-C), 65.6 (CH2OCO), 56.2 (CHCH2Ph), 46.5 (CHCH2OCO), 37.6 (CHCH2Ph), 34.7 (CONHCH2CH2). ν(C–O)carbamate: 1645 cm−1, ν(C–O)amide: 1683 cm−1, ν(N-H)amine: 3318 cm−1. Anal. calculated for C32H31N3O5S: C, 67.47; H, 5.49; N, 7.38; S, 5.63. Found: C, 66.24; H, 5.74; N, 7.47; S, 5.15. HRMS m/z for C32H31N3O5S [M + H]+ calcd. 592.2, found 592.4; [M + Na]+ calcd. 570.2, found 570.4; [M + HCOO]− calcd. 614.2, found 614.3.
2.2.8. Benzyl (R)-(3-(1H-indol-3-yl)-1-oxo-1-((4-sulphamoylphenethyl)amino)propan-2-yl)carbamate (8)
White solid (88%); mp 194–195 °C; 1H-NMR (400 MHz, DMSO-d6): δ 10.82 (s, 1H, Indole-NH), 8.14 (t, 2H, NHCH2CH2, J = 4.0 Hz), 7.74 (d, 2H, Ar-H, J = 8.0 Hz), 7.63 (d, 1H, OCONHCH, J = 8.0 Hz), 7.41–7.27 (m, 11H, Ar-H), 7.15–6.97 (m, 3H, Ar-H + NH2), 4.97 (s, 2H, CH2OCO), 4.26–4.20 (m, 1H, OCONHCH), 3.37–3.24 (m, 2H, NHCH2CH2), 3.07–3.03 (m, 1H, (3-Indolyl)CH2CH), 2.93–2.87 (m, 1H, (3-Indolyl)CH2CH), 2.73 (t, 2H, NHCH2CH2, J = 6.0 Hz).
13C NMR (100 MHz, DMSO-d6): δ 172.3 (CONHCH2CH2), 156.3 (CH2OCO), 144.2, 142.5, 137.5, 136.5, 129.6, 128.8, 128.2, 128.0, 127.7, 126.1, 124.3, 121.3, 119.0, 118.7, 111.8, 110.7 (Ar-C), 65.7 (CH2OCO), 56.1 (OCONHCH), 35.2 (CONHCH2CH2), 28.4 ((3-Indolyl)CH2CH). ν(C–O)carbamate: 1639 cm−1, ν(C–O)amide: 1687 cm−1, ν(N-H)amine: 3286, 3430, 3681 cm−1. Anal. calculated for C27H28N4O5S: C, 62.29; H, 5.42; N, 10.76; S, 6.16. Found: C, 62.92; H, 5.41; N, 10.84; S, 5.58. HRMS m/z for C27H28N4O5S [M + H]+ calcd. 521.2, found 521.3; [M-H]− calcd. 519.2, found 519.2.
2.2.9. tert-Butyl (S)-(4-(methylthio)-1-oxo-1-((4-sulphamoylphenethyl)amino)butan-2-yl)carbamate (9)
White solid (89%); mp 172–173 °C; 1H-NMR (400 MHz, DMSO-d6): δ 7.94 (t, 1H, NHCH2CH2, J = 4.0 Hz), 7.74 (d, 2H, Ar-H, J = 8.0 Hz), 7.39 (d, 2H, Ar-H, J = 8.0 Hz), 7.30 (s, 2H, NH2), 6.93 (d, 1H, OCONHCH, J = 8.0 Hz), 3.98–3.93 (m, 1H, OCONHCH), 3.34–3.26 (m, 2H, NHCH2CH2), 2.79 (t, 2H, NHCH2CH2, J = 6.0 Hz), 2.46–2.34 (m, 2H, CHCH2CH2SCH3), 2.02 (s, 3H, CHCH2CH2SCH3), 1.85–1.67 (m, 2H, CHCH2CH2SCH3), 1.39 (s, 9H, OC(CH3)3).
13C NMR (100 MHz, DMSO-d6): δ 172.2 (CONHCH2CH2), 155.8 ((CH3)3COCO), 144.2, 142.5, 129.62, 126.1 (Ar-C), 78.6 ((CH3)3COCO), 54.0 (OCONHCH), 40.3 (CONHCH2CH2), 35.2 (CONHCH2CH2), 32.2 (CHCH2CH2SCH3), 30.2 (CHCH2CH2SCH3), 28.7 (OC(CH3)3), 15.1 (CHCH2CH2SCH3). ν(C–O)carbamate: 1651 cm−1, ν(C–O)amide: 1680 cm−1, ν(N-H)amine: 3250, 3317, 3337 cm−1. Anal. calculated for C18H29N3O5S2: C, 50.10; H, 6.77; N, 9.74; S, 14.86. Found: C, 49.25; H, 7.04; N, 9.89; S, 15.29. HRMS m/z for C18H29N3O5S [M + Na]+ calcd. 454.1, found 454.3; [M + HCOO]− calcd. 476.2, found 476.2.
2.2.10. tert-Butyl (R)-(3-methyl-1-oxo-1-((4-sulphamoylphenethyl)amino)butan-2-yl)carbamate (10)
White solid (90%); mp 160–161 °C; 1H-NMR (400 MHz, DMSO-d6): δ 8.02 (t, 1H, NHCH2CH2, J = 6.0 Hz), 7.79 (d, 2H, Ar-H, J = 12.0 Hz), 7.39 (d, 2H, Ar-H, J = 8.0 Hz), 7.35 (s, 2H, NH2), 6.66 (d, 1H, OCONHCH, J = 12.0 Hz), 3.76 (t, 1H, OCONHCH, J = 8.0 Hz), 3.41–3.30 (m, 2H, NHCH2CH2), 2.84 (t, 2H, NHCH2CH2, J = 8.0 Hz), 1.94–1.86 (m, 1H, CH(CH3)2), 1.45 (s, 9H, OC(CH3)3), 0.83 (d, 6H, CH(CH3)2, J = 4.0 Hz).
13C NMR (100 MHz, DMSO-d6): δ 171.8 (CONHCH2CH2), 155.9 ((CH3)3COCO), 144.1, 142.5, 129.6, 126.1 (Ar-C), 78.4 ((CH3)3COCO), 60.3 (OCONHCH), 40.1 (CONHCH2CH2), 35.2 (CONHCH2CH2), 30.8 (CH(CH3)2), 28.7 (OC(CH3)3), 19.7 and 18.6 (CH(CH3)2). ν(C–O)carbamate: 1650 cm−1, ν(C–O)amide: 1679 cm−1, ν(N-H)amine: 3340, 3681 cm−1. Anal. calculated for C18H29N3O5S: C, 54.12; H, 7.32; N, 10.52; S, 8.02. Found: C, 53.80; H, 7.48; N, 10.92; S, 6.81. HRMS m/z for C18H29N3O5S [M + Na]+ calcd. 422.2, found 422.3; [M + HCOO]− calcd. 444.2, found 444.2.
2.2.11. tert-Butyl ((2R,3R)-3-methyl-1-oxo-1-((4-sulphamoylphenethyl)amino)pentan-2-yl)carbamate (11)
White solid (91%); mp 191–192 °C; 1H-NMR (400 MHz, DMSO-d6): δ 7.98 (t, 1H, NHCH2CH2, J = 4.0 Hz), 7.74 (d, 2H, Ar-H, J = 8.0 Hz), 7.40 (d, 2H, Ar-H, J = 8.0 Hz), 7.28 (s, 2H, NH2), 6.63 (d, 1H, OCONHCH, J = 8.0 Hz), 3.75 (t, 1H, OCONHCH, J = 8.0 Hz), 3.39–3.26 (m, 2H, NHCH2CH2), 2.79 (t, 2H, NHCH2CH2, J = 6.0 Hz), 1.63–1.61 (m, 1H, CHCH(CH3)CH2CH3), 1.39 (s, 10H, OC(CH3)3 + CHCH(CH3)CH2CH3), 1.10–1.00 (m, 1H, CHCH(CH3)CH2CH3), 0.79 (t, 3H, CHCH(CH3)CH2CH3, J = 8.0 Hz), 0.74 (d, 3H, CHCH(CH3)CH2CH3, J = 8.0 Hz).
13C NMR (100 MHz, DMSO-d6): δ 171.9 (CONHCH2CH2), 155.8 ((CH3)3COCO), 144.1, 142.6, 129.6, 126.1 (Ar-C), 78.4 ((CH3)3COCO), 59.3 (OCONHCH), 40.1 (CONHCH2CH2), 36.9 (CHCH(CH3)CH2CH3), 35.2 (CONHCH2CH2), 28.7 (OC(CH3)3), 24.8 (CHCH(CH3)CH2CH3), 15.8 (CHCH(CH3)CH2CH3), 11.4 (CHCH(CH3)CH2CH3). ν(C–O)carbamate: 1651 cm−1, ν(C–O)amide: 1679 cm−1, ν(N-H)amine: 3286, 3312, 3340 cm−1. Anal. calculated for C19H31N3O5S: C, 55.19; H, 7.56; N, 10.16; S, 7.75. Found: C, 55.13; H, 7.33; N, 10.27; S, 7.01. HRMS m/z for C19H31N3O5S [M + Na]+ calcd. 436.2, found 436.3; [M-H]− calcd. 412.2, found 412.2; [M + HCOO]−calcd. 458.2, found 458.2.
2.2.12. Benzyl (R)-(1,4-dioxo-1,4-bis((4-sulphamoylphenethyl)amino)butan-2-yl)carbamate (12)
White solid (73%); mp 249–250 °C; 1H-NMR (400 MHz, DMSO-d6): δ 8.02 (t, 1H, NHCH2CH2, J = 6.0 Hz), 7.96 (t, 1H, NHCH2CH2, J = 6.0 Hz), 7.75 (d, 4H, Ar-H, J = 8.0 Hz), 7.40–7.32 (m, 14H, OCONHCH + Ar-H + NH2), 5.09–5.00 (m, 2H, CH2OCO), 4.35–4.30 (m, 1H, OCONHCH), 3.29 (bs, 4H, NHCH2CH2 + NHCH2CH2), 2.80–2.74 (m, 4H, NHCH2CH2 + NHCH2CH2), 2.49–2.33 (m, 2H, CHCH2CO).
13C NMR (100 MHz, DMSO-d6): δ 171.6 (CONHCH2CH2), 169.8 (CONHCH2CH2), 156.2 (CH2OCO), 144.2, 142.5, 137.4, 129.7, 129.6, 128.8, 128.3, 128.2, 126.2, 126.1 (Ar-C), 66.0 (CH2OCO), 52.3 (OCONHCH), 40.5 (CONHCH2CH2), 40.4 (CONHCH2CH2), 38.1 (CHCH2CO), 35.3 (NHCH2CH2), 35.2 (NHCH2CH2). ν(C–O)carbamate: 1634 cm−1, ν(C–O)amide: 1684 cm−1, ν(N-H)amine: 3311 cm−1. Anal. calculated for C28H33N5O8S2: C, 53.24; H, 5.27; N, 11.09; S, 10.15. Found: C, 52.99; H, 5.46; N, 10.73; S, 9.91. HRMS m/z for C28H33N5O8S2 [M + H]+ calcd. 632.2, found 632.4; [M-H]− calcd. 630.2, found 630.3.
2.3. General procedure for the synthesis of amino acid–sulphonamide conjugates, 13–24
N-protected aminoacylbenzotriazole (1.0 equiv.), (4-sulphamoylphenyl)methanaminium chloride (1.0 equiv.), and Et3N (2.5 equiv.) were subjected to microwave irradiation (100 W, 70 °C) in DCM (5 ml) for 30 min. After completion of the reaction (monitoring TLC plate), all volatiles were removed by rotavapour and the obtained crude product was crystallised from ethanol.
2.3.1. Benzyl (R)-(4-methyl-1-oxo-1-((4-sulphamoylbenzyl)amino)pentan-2-yl)carbamate (13)
White solid (74%); mp 173–174 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.60 (t, 1H, CONHCH2, J = 6.0 Hz), 7.77 (d, 2H, Ar-H, J = 9.0 Hz), 7.52 (d, 1H, OCONHCH, J = 9.0 Hz), 7.43–7.31 (m, 9H, Ar-H + NH2), 5.05 (d, 2H, CH2OCO), 4.34 (d, 2H, CONHCH2, J = 3.0 Hz), 4.11–4.03 (m, 1H, OCONHCH), 1.67–1.39 (m, 3H, CHCH2CH(CH3)2), 0.90–0.85 (m, 6H, CH(CH3)2).
13C NMR (75 MHz, DMSO-d6): δ 172.6 (CONHCH2), 156.0 (CH2OCO), 143.6, 142.5, 137.0, 128.3, 127.8, 127.7, 127.3, 125.6 (Ar-C), 65.4 (CH2OCO), 53.2 (OCONHCH), 41.7 (CONHCH2), 40.6 (CHCH2CH(CH3)2), 24.2 (CHCH2CH(CH3)2), 22.9 and 21.4 (CHCH2CH(CH3)2). ν(C–O)carbamate: 1659 cm−1, ν(C–O)amide: 1683 cm−1, ν(N-H)amine: 3304 cm−1. Anal. calculated for C21H27N3O5S: C, 58.18; H, 6.28; N, 9.69; S, 7.40. Found: C, 57.56; H, 5.88; N, 9.64; S, 7.15. HRMS m/z for C21H27N3O5S [M + H]+ calcd. 434.2, found 434.3; [M + Na]+ calcd. 466.2, found 466.3; [M + HCOO]− calcd. 478.2, found 478.2.
2.3.2. Benzyl (R)-(4-(methylthio)-1-oxo-1-((4-sulphamoylbenzyl)amino)butan-2-yl)carbamate (14)
White solid (80%); mp 167–168 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.59 (t, 1H, CONHCH2, J = 6.0 Hz), 7.78 (d, 2H, Ar-H, J = 9.0 Hz), 7.58 (d, 1H, OCONHCH, J = 6.0 Hz), 7.44–7.33 (m, 9H, Ar-H + NH2), 5.10–5.01 (m, 2H, CH2OCO), 4.35 (d, 2H, CONHCH2, J = 6.0 Hz), 4.18–4.11 (m, 1H, OCONHCH), 2.51–2.40 (m, 2H, CHCH2CH2SCH3), 2.03 (s, 3H, CHCH2CH2SCH3), 1.94–1.80 (m, 2H, CHCH2CH2SCH3).
13C-NMR (75 MHz, DMSO-d6): δ 171.7 (CONHCH2), 156.1 (CH2OCO), 143.6, 142.5, 136.9, 128.3, 127.8, 127.7, 127.3, 125.6 (Ar-C), 65.5 (CH2OCO), 54.0 (OCONHCH), 41.7 (CONHCH2), 31.3 (CHCH2CH2SCH3), 29.7 (CHCH2CH2SCH3), 14.6 (CHCH2CH2SCH3). ν(C–O)carbamate: 1646 cm−1, ν(C–O)amide: 1692 cm−1, ν(N-H)amine: 3331 cm−1, ν(N-H)amine: 3331 cm−1. Anal. calculated for C20H25N3O5S2: C, 53.20; H, 5.58; N, 9.31; S, 14.20. Found: C, 53.15; H, 5.39; N, 9.22; S, 13.30. HRMS m/z for C20H25N3O5S2 [M + H]+ calcd. 452.1, found 452.2; [M + HCOO]− calcd. 496.2, found 496.2.
2.3.3. Benzyl (R)-(3-methyl-1-oxo-1-((4-sulphamoylbenzyl)amino)butan-2-yl)carbamate (15)
White solid (89%); mp 141–142 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.58 (t, 1H, CONHCH2, J = 4.5 Hz), 7.77 (d, 2H, Ar-H, J = 9.0 Hz), 7.45–7.33 (m, 10H, Ar-H + OCONHCH + NH2), 5.06 (s, 2H, CH2OCO), 4.36 (d, 2H, CONHCH2, J = 3.0 Hz), 3.87 (t, 1H, OCONHCH, J = 6.0 Hz), 2.05–1.94 (m, 1H, CH(CH3)2), 0.86 (d, 6H, CH(CH3)2, J = 6.0 Hz).
13C NMR (75 MHz, DMSO-d6): δ 171.4 (CONHCH2), 156.2 (CH2OCO), 143.5, 142.6, 137.0, 128.3, 127.8, 127.7, 127.5, 125.6 (Ar-C), 65.4 (CH2OCO), 60.5 (OCONHCH), 41.7 (CONHCH2), 30.0 (CH(CH3)2), 19.2 and 18.2 (CH(CH3)2). ν(C–O)carbamate: 1649 cm−1, ν(C–O)amide: 1688 cm−1, ν(N-H)amine: 3287 cm−1. Anal. calculated for C20H25N3O5S: C, 57.26; H, 6.01; N, 10.02; S, 7.64. Found: C, 57.16; H, 6.22; N, 9.72; S, 7.11. HRMS m/z for C20H25N3O5S [M + H]+ calcd. 420.2, found 420.2; [M + Na]+ calcd. 442.1, found 442.3; [M + HCOO]−calcd. 464.2, found 464.2.
2.3.4. Benzyl (S)-(1-oxo-3-(phenylthio)-1-((4-sulphamoylbenzyl)amino)propan-2-yl)carbamate (16)
White solid (72%); mp 196–197 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.76 (t, 2H, CONHCH2, J = 6.0 Hz), 7.76 (d, 2H, Ar-H, J = 9.0 Hz), 7.44–7.19 (m, 15H, Ar-H + OCONHCH + NH2), 5.11–4.99 (m, 2H, CH2OCO), 4.35 (d, 2H, CONHCH2, J = 3.0 Hz), 4.26–4.19 (m, 1H, OCONHCH), 3.42–3.35 (m, 1H, CH2SPh), 3.17–3.10 (m, 1H, CH2SPh).
13C NMR (75 MHz, DMSO-d6): δ 170.1 (CONHCH2), 156.0 (CH2OCO), 143.3, 142.5, 136.84, 135.6, 129.1, 128.4, 128.3, 127.8, 127.7, 127.3, 126.0, 125.6 (Ar-C), 65.6 (CH2OCO), 54.3 (OCONHCH), 41.9 (CONHCH2), 34.8 (CH2SPh). ν(C–O)carbamate: 1648 cm−1, ν(C–O)amide:1673, 1689 cm−1, ν(N-H)amine: 3308, 3676 cm−1. Anal. calculated for C24H25N3O5S2: C, 57.70; H, 5.04; N, 8.41; S, 12.83. Found: C, 56.95; H, 4.94; N, 8.51; S, 11.79. HRMS m/z for C24H25N3O5S2 [M + H]+ calcd. 500.1, found 500.3; [M + HCOO]− calcd. 544.1, found 544.2.
2.3.5. tert-Butyl (2-oxo-2-((4-sulphamoylbenzyl)amino)ethyl)carbamate (17)
White solid (70%); mp 172–173 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.41 (t, 1H, CONHCH2, J = 6.0 Hz), 7.76 (d, 2H, Ar-H, J = 9.0 Hz), 7.43 (d, 2H, Ar-H, J = 9.0 Hz), 7.31 (s, 2H, NH2), 7.05 (d, 1H, OCONHCH2, J = 6.0 Hz), 4.35 (d, 2H, CONHCH2, J = 6.0 Hz), 3.58 (d, 2H, OCONHCH2 J = 6.0 Hz), 1.40 (s, 9H, OC(CH3)3).
13C NMR (75 MHz, DMSO-d6): δ 169.6 (CONHCH2), 155.8 ((CH3)3COCO), 143.6, 142.5, 127.4, 125.5 (Ar-C), 78.1 ((CH3)3COCO), 43.4 (OCONHCH2), 41.6 (CONHCH2), 28.2 OC(CH3)3). ν(C–O)carbamate: 1644 cm−1, ν(C–O)amide: 1680 cm−1, ν(N-H)amine: 3326 cm−1. Anal. calculated for C14H21N3O5S: C, 48.97; H, 6.16; N, 12.24; S, 9.34. Found: C, 48.62; H, 6.01; N, 12.11; S, 9.25. HRMS m/z for C14H21N3O5S [M + Na]+ calcd. 366.1, found 366.1; [M-H]− calcd. 342.1, found 342.1; [M + HCOO]− calcd. 388.1, found 388.1.
2.3.6. tert-Butyl (R)-(1-oxo-1-((4-sulphamoylbenzyl)amino)propan-2-yl)carbamate (18)
White solid (85%); mp 197–198 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.42 (t, 1H, CONHCH2, J = 6.0 Hz), 7.75 (d, 2H, Ar-H, J = 9.0 Hz), 7.42 (d, 2H, Ar-H, J = 9.0 Hz), 7.31 (s, 2H, NH2), 7.02 (d, 1H, OCONHCH, J = 9.0 Hz), 4.34 (d, 2H, CONHCH2 J = 6.0 Hz), 4.03–3.93 (m, 1H, OCONHCH), 1.40 (s, 9H, OC(CH3)3), 1.21 (d, 3H, CHCH3, J = 9.0 Hz).
13C NMR (75 MHz, DMSO-d6): δ 173.0 (CONHCH2), 155.2 ((CH3)3COCO), 143.7, 142.5, 127.1, 125.5 (Ar-C), 78.0 ((CH3)3COCO), 49.9 (OCONHCH), 41.6 (CONHCH2), 28.2 (OC(CH3)3), 17.9 (CHCH3). ν(C–O)carbamate: 1650 cm−1, ν(C–O)amide: 1682 cm−1, ν(N-H)amine: 3328, 3676 cm−1. Anal. calculated for C15H23N3O5S: C, 50.41; H, 6.49; N, 11.76; S, 8.97. Found: C, 49.97; H, 6.73; N, 11.66; S, 8.88. HRMS m/z for C15H23N3O5S [M + Na]+ calcd. 380.1, found 380.2; [M-H]− calcd. 356.1, found 356.1; [M + HCOO]− calcd. 402.1, found 402.1.
2.3.7. tert-Butyl (R)-(1-oxo-3-phenyl-1-((4-sulphamoylbenzyl)amino)propan-2-yl)carbamate (19)
White solid (95%); mp 198–199 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.55 (t, 1H, CONHCH2, J = 6.0 Hz), 7.72 (d, 2H, Ar-H, J = 9.0 Hz), 7.34–7.20 (m, 9H, Ar-H + NH2), 7.07 (d, 1H, OCONHCH, J = 9.0 Hz), 4.33 (d, 2H, CONHCH2, J = 6.0 Hz), 4.24–4.16 (m, 1H, OCONHCH), 3.00–2.93 (m, 1H, CHCH2Ph), 2.83–2.75 (m, 1H, CHCH2Ph), 1.33 (s, 9H, OC(CH3)3).
13C NMR (75 MHz, DMSO-d6): δ 171.8 (CONHCH2), 155.3 ((CH3)3COCO), 143.3, 142.7, 138.1, 129.2, 128.0, 127.2, 126.9, 125.5 (Ar-C), 78.0 ((CH3)3COCO), 56.0 (CHCH2Ph), 41.6 (CONHCH2), 37.3 (CHCH2Ph), 28.1 (OC(CH3)3). ν(C–O)carbamate: 1660 cm−1, ν(C–O)amide: 1677 cm−1, ν(N-H)amine: 3329, 3677 cm−1. Anal. calculated for C21H27N3O5S: C, 58.18; H, 6.28; N, 9.69; S, 7.40. Found: C, 57.87; H, 6.02; N, 9.60; S, 7.15. HRMS m/z for C21H27N3O5S [M + Na]+ calcd. 456.2, found 456.2; [M + HCOO]− calcd. 478.2, found 478.2.
2.3.8. (9H-fluoren-9-yl)methyl (S)-(1-oxo-3-phenyl-1-((4-sulphamoylbenzyl)amino)propan-2-yl)carbamate (20)
White solid (88%); mp 221–222 °C; 1H-NMR (300 MHz, DMSO-d6): δ 8.64 (t, 1H, CONHCH2, J = 6.0 Hz), 7.89 (d, 2H, Ar-H, J = 6.0 Hz), 7.74 (d, 2H, Ar-H, J = 9.0 Hz), 7.66 (t, 2H, Ar-H, J = 6.0 Hz), 7.44–7.21 (m, 14H, OCONHCH + Ar-H + NH2), 4.37–4.14 (m, 6H, CHCH2OCONHCHCONHCH2), 3.06–2.99 (m, 1H, CHCH2Ph), 2.89–2.81 (m, 1H, CHCH2Ph). 13C NMR (75 MHz, DMSO-d6): δ 171.5 (CONHCH2), 155.8 (CH2OCO), 143.7, 143.3, 142.5, 140.6, 138.0, 129.2, 128.1, 127.6, 127.3, 127.0, 126.3, 125.6, 125.3, 120.1 (Ar-C), 65.6 (CH2OCO), 56.3 (CHCH2Ph), 46.5 (CHCH2OCO), 41.9 (CONHCH2), 37.5 (CHCH2Ph). ν(C–O)carbamate: 1641 cm−1, ν(C–O)amide: 1686 cm−1, ν(N-H)amine: 3263 cm−1. Anal. calculated for C31H29N3O5S: C, 67.01; H, 5.26; N, 7.56; S, 5.77. Found: C, 51.40; C, 67.78; H, 5.41; N, 7.71; S, 5.06. HRMS m/z for C31H29N3O5S [M + Na]+ calcd. 556.2, found 556.3; [M + HCOO]−calcd. 600.2, found 600.2.
2.3.9. tert-Butyl (R)-(4-(methylthio)-1-oxo-1-((4-sulphamoylbenzyl)amino)butan-2-yl)carbamate (21)
White solid (85%); mp 142–143 °C; 1H-NMR (400 MHz, DMSO-d6): δ 8.48 (t, 1H, CONHCH2, J = 6.0 Hz), 7.75 (d, 2H, Ar-H, J = 8.0 Hz), 7.42 (d, 2H, Ar-H, J = 8.0 Hz), 7.32 (s, 2H, NH2), 7.09 (d, 1H, OCONHCH, J = 8.0 Hz), 4.35 (d, 2H, CONHCH2, J = 8.0 Hz), 4.06–4.01 (m, 1H, OCONHCH), 2.49–2.40 (m, 2H, CHCH2CH2SCH3), 2.04 (s, 3H, CHCH2CH2SCH3), 1.89–1.80 (m, 2H, CHCH2CH2SCH3), 1.41 (s, 9H, OC(CH3)3).
13C NMR (100 MHz, DMSO-d6): δ 172.6 (CONHCH2), 156.0 ((CH3)3COCO), 144.2, 143.0, 127.7, 126.0 (Ar-C), 78.7 ((CH3)3COCO), 54.2 (OCONHCH), 42.2 (CONHCH2), 31.8 (CHCH2CH2SCH3), 30.3 (CHCH2CH2SCH3), 28.7 (OC(CH3)3), 15.1 (CHCH2CH2SCH3). ν(C–O)carbamate: 1656 cm−1, ν(C–O)amide: 1695 cm−1, ν(N-H)amine: 3311, 3348 cm−1. Anal. calculated for C17H27N3O5S2: C, 48.90; H, 6.52; N, 10.06; S, 15.36. Found: C, C, 47.89; H, 6.78; N, 10.20; S, 14.53. HRMS m/z for C17H27N3O5S2 [M + Na]+ calcd. 440.1, found 440.2; [M-H]− calcd. 416.1, found 416.2; [M + HCOO]− calcd. 462.1, found 462.2.
2.3.10. tert-Butyl (R)-(3-methyl-1-oxo-1-((4-sulphamoylbenzyl)amino)butan-2-yl)carbamate (22)
White solid (87%); mp 163–164 °C; 1H-NMR (400 MHz, DMSO-d6): δ 8.49 (t, 1H, CONHCH2, J = 6.0 Hz), 7.75 (d, 2H, Ar-H, J = 8.0 Hz), 7.43 (d, 2H, Ar-H, J = 8.0 Hz), 7.32 (s, 2H, NH2), 6.80 (d, 1H, OCONHCH, J = 8.0 Hz), 4.35 (t, 2H, CONHCH2, J = 6.0 Hz), 3.77 (t, 1H, OCONHCH, J = 8.0 Hz), 1.99–1.91 (m, 1H, CH(CH3)2), 1.41 (s, 9H, OC(CH3)3), 0.85 (d, 6H, CH(CH3)2, J = 4.0 Hz).
13C NMR (100 MHz, DMSO-d6): δ 172.2 (CONHCH2), 156.1 ((CH3)3COCO), 144.1, 143.0, 127.9, 126.0 (Ar-C), 78.5 ((CH3)3COCO), 60.7 (OCONHCH), 42.1 (CONHCH2), 30.4 (CH(CH3)2), 28.7 (OC(CH3)3), 19.8 and 18.9 (CH(CH3)2). ν(C–O)carbamate: 1654 cm−1, ν(C–O)amide: 1684 cm−1, ν(N-H)amine: 3333 cm−1. Anal. calculated for C17H27N3O5S: C, 52.97; H, 7.06; N, 10.90; S, 8.32. Found: C, 52.58; H, 7.20; N, 10.91; S, 7.89. HRMS m/z for C17H27N3O5S [M + Na]+ calcd. 408.2, found 408.2; [M-H]− calcd. 384.2, found 384.2; [M + HCOO]− calcd. 430.2, found 430.2.
2.3.11. Benzyl (R)-(3-(1H-indol-3-yl)-1-oxo-1-((4-sulphamoylbenzyl)amino)propan-2-yl)carbamate (23)
White solid (91%); mp 184–185 °C; 1H-NMR (400 MHz, DMSO-d6): δ 10.86 (s, 1H, Indole-NH), 8.66 (t, 2H, CONHCH2, J = 6.0 Hz), 7.74 (d, 2H, Ar-H, J = 8.0 Hz), 7.66 (d, 1H, Ar-H, J = 8.0 Hz), 7.52 (d, 1H, Ar-H, J = 8.0 Hz), 7.38–7.28 (m, 10H, Ar-H + NH2), 7.18 (bs, 1H, Ar-H), 7.09 (t, 1H, Ar-H, J = 8.0 Hz), 6.99 (t, 1H, Ar-H, J = 6.0 Hz), 4.98 (s, 2H, CH2OCO), 4.37–4.32 (m, 3H, OCONHCHCONHCH2), 3.18–3.13 (m, 1H, (3-Indolyl)CH2CH), 3.00–2.94 (m, 1H, (3-Indolyl)CH2CH).
13C NMR (100 MHz, DMSO-d6): δ 172.5 (CONHCH2), 156.4 (CH2OCO), 144.0, 143.0, 137.5, 136.6, 128.8, 128.2, 128.0, 127.8, 127.7, 126.1, 124.4, 121.4, 119.0, 118.7, 111.8, 110.6 (Ar-C), 65.8 (CH2OCO), 56.2 (OCONHCH), 42.3 (CONHCH2), 28.3 ((3-Indolyl)CH2CH). ν(C–O)carbamate: 1656 cm−1, ν(C–O)amide: 1682 cm−1, ν(N-H)amine: 3311, 3681 cm−1. Anal. calculated for C26H26N4O5S: C, 61.65; H, 5.17; N, 11.06; S, 6.33. Found: C, 61.85; H, 5.20; N, 11.03; S, 5.28. HRMS m/z for C26H26N4O5S [M + H]+ calcd. 507.2, found 507.3; [M-H]− calcd. 505.2, found 505.2.
2.3.12. tert-Butyl ((2R,3R)-3-methyl-1-oxo-1-((4-sulphamoylbenzyl)amino)pentan-2-yl)carbamate (24)
White solid (80%); mp 180–181 °C; 1H-NMR (400 MHz, DMSO-d6): δ 8.49 (t, 1H, NHCH2CH2, J = 6.0 Hz), 7.74 (d, 2H, Ar-H, J = 8.0 Hz), 7.43 (d, 2H, Ar-H, J = 8.0 Hz), 7.32 (s, 2H, NH2), 6.81 (d, 1H, OCONHCH, J = 8.0 Hz), 4.35 (t, 2H, CONHCH2, J = 4.0 Hz), 3.81 (t, 1H, OCONHCH, J = 8.0 Hz), 1.73–1.69 (m, 1H, CHCH(CH3)CH2CH3), 1.41 (s, 10H, OC(CH3)3 + CHCH(CH3)CH2CH3), 1.18–1.05 (m, 1H, CHCH(CH3)CH2CH3), 0.84–0.80 (m, 6H, CHCH(CH3)CH2CH3).
13C NMR (100 MHz, DMSO-d6): δ 172.3 (CONHCH2), 156.0 ((CH3)3COCO), 144.1, 143.0, 127.8, 126.0 (Ar-C), 78.5 ((CH3)3COCO), 59.5 (OCONHCH), 42.1 (CONHCH2), 36.5 (CHCH(CH3)CH2CH3), 28.7 (OC(CH3)3), 25.0 (CHCH(CH3)CH2CH3), 15.9 (CHCH(CH3)CH2CH3), 11.4 (CHCH(CH3)CH2CH3). ν(C–O)carbamate: 1660 cm−1, ν(C–O)amide: 1678 cm−1, ν(N-H)amine: 3265, 3326, 3681 cm−1. Anal. calculated for C18H29N3O5S: C, 54.12; H, 7.32; N, 10.52; S, 8.02. Found: C, 53.77; H, 7.24; N, 10.75; S, 7.28. HRMS m/z for C18H29N3O5S [M + Na]+ calcd. 422.2, found 422.3; [M-H]− calcd. 398.2, found 398.1; [M + HCOO]− calcd. 444.2, found 444.2.
2.4. CA inhibition
An Applied Photophysics Stopped-Flow instrument has been used for assaying the CA catalysed CO2 hydration activity by using method of KhalifahCitation25. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM HEPES (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled–deionised water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were pre-incubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-square methods using PRISM (www.graphpad.com), and non-linear least squares methods, values representing the mean of at least three different determinations, as described earlier by usCitation26–30.
3. Results and discussion
3.1. Synthesis and characterisation of the new amino acid–sulphonamides conjugates
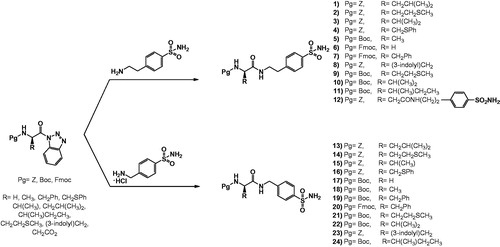
This work was designed to synthesise, characterise, and explore the potential CA inhibitory properties of N-protected amino acid–sulphonamide conjugates. The syntheses of novel homosulphonamide and 4-aminoethylsulphonamide–amino acid conjugates prepared in this study are shown in Scheme 1. Since N-acylbenzotriazoles have been successfully utilised in many acylation reaction for the preparation N-, O-, S-, and C- substituted amino acid or peptidesCitation31–40, we chose the benzotriazole-mediated methodology to synthesise the targeted amino acid–sulphonamide conjugates. Compounds 1–12 were prepared through a facile benzotriazole mediated acylation reactions in one step (Scheme 1) at 70 °C under microwave irradiation for 30 min, in dry dichloromethane as solvent, whereas compounds 13–24 were prepared using the same method except the presence of triethylamine in the reaction mixture, in order to remove hydrogen chloride from the homosulphonamide . HCl reagent.
All compounds were fully characterised by 1H, 13C NMR, MS, and FTIR (ATR) spectroscopy and elemental analyses. The analytical and spectral data of all the synthesised compounds were in full agreement with the proposed structures. The characteristic NH resonances of the sulphonamide part of the amino acid conjugates 1–12 were observed at 7.88–8.25 ppm region as triplet peak whereas compounds 13–24 were observed at 8.41–8.76 ppm region as triplet peak in the 1H NMR spectrum. NH2 resonances of sulphonamide part were observed generally in the aromatic region together with aromatic protons. These NH or NH2 protons were confirmed by D2O exchange. The singlet that peak around 5.00 ppm for compounds 1–4, 8, 12, 13–16, and 23 was assigned to the CH2 protons for benzyloxycarbonyl protected group whereas the upfield singlet that signals around 1.30 ppm was assigned to the tert-butyl protons of Boc-protected group for compounds 5, 9–11, 17–22, and 24. The 1H NMR spectra of compounds 13–24 revealed a doublet peak due to CH2 protons at 4.32–4.36 ppm. The multiplet peak around 4.20 ppm for compounds 6, 7, and 20, those containing Fmoc protecting group, were assigned to the CH and CH2 protons for Fmoc group. Carbonyl resonances of the amide carbonyls and carbamate carbonyl were observed downfield around 170 and 155 ppm, respectively. All other aliphatic and aromatic protons and carbons were observed in the expected regions (see Materials and methods). The molecular ion or appropriate positive or negative ion peaks were observed for all proposed structures of novel compounds in the mass spectra. The IR spectra of amino-sulphonamide conjugates, 1–24, showed characteristic amide carbonyl peaks around between 1671 and 1712 cm−1, whereas the carbamate carbonyl peaks around between 1634 and 1664 cm−1. All other spectral data were in accordance with the assumed structures.
3.2. Carbonic anhydrase inhibition
As can be seen from , some derivatives of 4-(2-aminoethyl) sulphonamide bearing leucine, methionine, valine, and glycine (1–3, 5, and 6) showed better inhibitory properties with KI values ranging from 9.6 to 73.5 nM against hCA I than standard sulphonamide compound AZA. Four homosulphonamide derivatives bearing leucine, methionine, valine, and isoleucine (13–15 and 24) showed better inhibitory properties with KI values ranging from 9.3 to 177.8 nM against hCA I than standard sulphonamide compound AZA. Among the sulphonamide derivatives, those carrying benzyloxycarbonyl (Z) protecting group generally showed a better inhibitory property against the hCA I compared to the corresponding deprotected derivatives. Most of the compounds exhibited inhibition constants KI values ranging from 0.5 to 8.9 nM against hCA II, being more effective inhibitors than the standard sulphonamide acteazolamide AZA. In parallel to the results in hCA I, 4-(2-aminoethyl)benzenesulphonamide derivatives (1–12) showed better inhibition than the corresponding homosulphonamide derivatives (13–24). Compounds 1 and 3 also showed a better inhibition against hCA VA compared to AAZ, while compounds 2, 4–6 showed a comparable inhibition to that of AAZ. Other compounds were found to be ineffective at the low nanomolar levels, being inhibitory only in the micromolar range. Two out of the twenty-four sulphonamide derivatives (compounds 8 and 21) showed strong inhibitory properties against hCA XII, being more effective than the standard drug. Among these compounds, 3, 4, 8, 17, and 19 also showed comparable inhibitory properties against hCA XII to that of AAZ. The results of show that alkyl and benzyloxycarbonyl protected groups contribute positively to sulphonamide conjugates inhibitory properties. This type of structure-activity relationship was observed for other structurally-related CAIs belonging to other chemotypes, when the tails of the amino acyl type or of other nature, effectively contributed to the inhibitory effects, due to favourable contacts made with the middle and external part of the active siteCitation41–51.
Table 1. Inhibition data of hCA I, hCA II, hCA VA, and hCA XII with compounds 1–24 and the standard sulphonamide inhibitor acetazolamide (AAZ) by a stopped flow CO2 hydrase assay.
4. Conclusions
In conclusion, novel sulphonamide derivatives containing amino acid moieties have been prepared in good to excellent yields, using a facile benzotriazole mediated reaction, by condensation of 4-amino-substituted sulphonamides with desired benzyloxycarbonyl protected amino acid derivatives. The CA inhibitory properties of the novel compounds were determined using a stopped flow instrument and four human CA isoforms with pharmacologic relevance. Most of the new sulphonamide–amino acid conjugates exhibited better inhibitory properties against hCA I and II than standard sulphonamide AAZ. Some compounds were also found to be efficient inhibitors against hCA VA and hCA XII, in nanomolar levels, with KI values ranging from 58.7 to 68.7 nM and from 3.9 to 8.8 nM, respectively.
Supplemental Material
Download PDF (2.2 MB)Disclosure statement
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Fukutake T, Wada K, Liu GC, et al. Striking effects of a titania support on the low-temperature activities of Ir catalysts for the dehydrogenative synthesis of benzimidazole and indole. Catal Today 2018;303:235–40.
- Tacic A, Nikolic V, Nikolic L, Savic I. Antimicrobial sulfonamide drugs. Adv Technol 2017;6:58–71.
- (a) Hu Z, Awakawa T, Ma Z, Abe I. Aminoacyl sulfonamide assembly in SB-203208 biosynthesis. Nat Commun 2019;10:184. (b) Scozzafava A, Owa T, Mastrolorenzo A, Supuran C. C. Anticancer and antiviral sulfonamides. Curr Med Chem 2003;10:925–53.
- (a) Capasso C, Supuran CT. Dihydropteroate synthase (sulfonamides) and dihydrofolate reductase inhibitors. In: Bonev BB, Brown NM, eds. Bacterial resistance to antibiotics – from molecules to man. London: John Wiley & Sons Ltd; 2020:163–72. (b) Capasso C, Supuran CT. Bacterial carbonic anhydrases. In Supuran CT, Capasso C, eds. Zinc enzyme inhibitors – enzymes from microorganisms. Vol. 22. New York (NY): Springer International Publishing; 2017:135–52.
- Supuran CT, Alterio V, Di Fiore A, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev 2018;38:1799–836.
- Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70.
- (a) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Rev Drug Discov 2008;7:168–81. (b) Supuran CT. The management of glaucoma and macular degeneration. Expert Opin Ther Pat 2019;29:745–7. (c) Supuran CT. Agents for the prevention and treatment of age-related macular degeneration and macular edema: a literature and patent review. Expert Opin Ther Pat 2019; 29:761–7.
- (a) Patel TS, Vanparia SF, Patel UH, et al. Novel 2,3-disubstituted quinazoline-4(3H)-one molecules derived from amino acid linked sulphonamide as a potent malarial antifolates for DHFR inhibition. Eur J Med Chem 2017;129:251–65. (b) Scozzafava A, Menabuoni L, Mincione F, Supuran CT. Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyamino-polycarboxylate tails and of their metal complexes possessing long-lasting, topical intraocular pressure-lowering properties. J Med Chem 2002;45:1466–76.
- Chinthakindi PK, Benediktsdottir A, Ibrahim A, et al. Synthesis of sulfonimidamide-based amino acid building blocks with orthogonal protecting groups. Eur J Org Chem 2019;2019:1045–57.
- Meleddu R, Distinto S, Cottiglia F, et al. Tuning the dual inhibition of carbonic anhydrase and cyclooxygenase by dihydrothiazole benzensulfonamides. ACS Med Chem Lett 2018;9:1045–50.
- Supuran CT. Carbon-versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors? J Enzyme Inhib Med Chem 2018;33:485–95.
- Bai Q, Tang J, Wang H. Functionalization of sulfonamide-containing peptides through late-stage palladium-catalyzed C(sp 3) –H arylation. Org Lett 2019;21:5858–61.
- Chiaramonte N, Romanelli MN, Teodori E, Supuran CT. Amino acids as building blocks for carbonic anhydrase inhibitors. Metabolites 2018;8:1–22.
- Moree WJ, Van Der Marel GA, Liskamp RJ. Synthesis of peptidosulfinamides and peptidosulfonamides: peptidomimetics containing the sulfinamide or sulfonamide. J Org Chem 1995;60:5157–69.
- Nocentini A, Supuran CT. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin Drug Discov 2019;14:1175–97.
- Küçükbay FZ, Küçükbay H, Tanc M, Supuran CT. Synthesis and carbonic anhydrase I, II, IV and XII inhibitory properties of N-protected amino acid – sulfonamide conjugates. J Enzyme Inhib Med Chem 2016;31:1476–83.
- Buğday N, Küçükbay FZ, Küçükbay H, et al. Synthesis of novel dipeptide sulfonamide conjugates with effective carbonic anhydrase I, II, IX, and XII inhibitory properties. Bioorg Chem 2018;81:311–8.
- Küçükbay H, Buğday N, Küçükbay FZ, et al. Bioorganic chemistry synthesis and carbonic anhydrase inhibitory properties of novel 4-(2-aminoethyl) benzenesulfonamide-dipeptide conjugates. Bioorg Chem 2019;83:414–23.
- Supuran CT, Capasso C. An overview of the bacterial carbonic anhydrases. Metabolites 2017;7:56.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- Sagar NR, Durgamma S, Srinivasulu C, et al. A unified approach to access N-acyl sulfonamide tethered peptide conjugates. ChemistrySelect 2019;4:6408–13.
- Küçükbay FZ, Buğday N, Küçükbay H, et al. Synthesis, characterization and carbonic anhydrase inhibitory activity of novel benzothiazole derivatives. J Enzyme Inh Med Chem 2016;31:1221–5.
- Buǧday N, Küçükbay FZ, Apohan E, et al. Synthesis and evaluation of novel benzimidazole conjugates incorporating amino acids and dipeptide moieties. Lett Org Chem 2017;14:198–206.
- Küçükbay FZ, Küçükbay H, Tanc M, Supuran CT. Synthesis and carbonic anhydrase inhibitory properties of amino acid – coumarin/quinolinone conjugates incorporating glycine, alanine and phenylalanine moieties. J Enzyme Inhib Med Chem 2016;31:1198–202.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- Mincione F, Starnotti M, Menabuoni L, et al. Carbonic anhydrase inhibitors: 4-Sulfamoyl-benzenecarboxamides and 4-chloro-3-sulfamoyl-benzenecarboxamides with strong topical antiglaucoma properties. Bioorganic Med Chem Lett 2001;11:1787–91.
- Del Prete S, Vullo D, De Luca V, et al. Biochemical characterization of recombinant β-carbonic anhydrase (PgiCAb) identified in the genome of the oral pathogenic bacterium Porphyromonas gingivalis. J Enzyme Inhib Med Chem 2015;30:366–70.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorganic Med Chem 2007;15:4336–50.
- Maresca A, Vullo D, Scozzafava A, et al. Inhibition of the β-class carbonic anhydrases from Mycobacterium tuberculosis with carboxylic acids. J Enzyme Inhib Med Chem 2013;28:392–6.
- Scozzafava A, Passaponti M, Supuran CT, Gülçin I. Carbonic anhydrase inhibitors: guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX and XII). J Enzyme Inhib Med Chem 2015;30:586–91.
- Katritzky AR, Angrish P, Narindoshvili T. Chiral O-(Z-alpha-aminoacyl) sugars: convenient building blocks for glycopeptide libraries. Bioconjug Chem 2007;18:2202–3.
- Katritzky AR, El-Nachef C, Bajaj K, et al. Efficient syntheses of thiadiazole peptides. J Org Chem 2010;75:6009–11.
- Katritzky AR, Avan I, Tala SR, Steel PJ. Benzotriazole-mediated syntheses of depsipeptides and oligoesters. J Org Chem 2011;76:4884–93.
- Panda SS, Ibrahim MA, Küçükbay H, et al. Synthesis and antimalarial bioassay of quinine - peptide conjugates. Chem Biol Drug Des 2013;82:361–6.
- Ibrahim MA, Panda SS, Oliferenko AA, et al. Macrocyclic peptidomimetics with antimicrobial activity: synthesis, bioassay, and molecular modeling studies. Org Biomol Chem 2015;13:9492–503.
- Katritzky AR, Suzuki K, Singh SK. Highly diastereoselective peptide chain extensions of unprotected amino acids with N-(Z-α-aminoacyl)benzotriazoles. Synthesis 2004;5:2645–52.
- Bajaj K, Panda SS, El-Nachef C, Katritzky AR. Syntheses of chiral N-(protected) tri- and tetrapeptide conjugates. Chem Biol Drug Des 2012;80:17–26.
- Panda SS, Hall CD, Scriven E, Katritzky AR. Aminoacyl benzotriazolides: versatile reagents for the preparation of peptides and their mimetics and conjugates. Aldrichimica Act 2013;46:43–58.
- Elagawany M, Ibrahim MA, Panda SS. One-pot synthesis of bi- and tricyclic heterocyclic compounds using benzotriazole chemistry. Tetrahedron Lett 2016;57:4910–3.
- Albers T, Watkins D, Gameiro AF, et al. Benzotriazole-based strategies toward peptidomimetics, conjugates, and other peptide derivatives In: Monbaliu JCM, ed. The chemistry of benzotriazole derivatives. Vol. 43: Cham: Springer International Publishing; 2016:95–141.
- Köhler K, Hillebrecht A, Schulze Wischeler J, et al. Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem Int Ed Engl 2007;46:7697–9.
- Akocak S, Lolak N, Bua S, Supuran CT. Discovery of novel 1, 3-diaryltriazene sulfonamides as carbonic anhydrase I, II, VII, and IX inhibitors. J Enzyme Inhib Med Chem 2018;33:1575–80.
- De Simone G, Supuran CT. (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29.
- Supuran CT, Ilies MA, Scozzafava A. Carbonic anhydrase inhibitors. Part 29. Interaction of isozymes I, II and IV with benzolamide-like derivatives. Eur J Med Chem 1998;33:739–52.
- Tars K, Vullo D, Kazaks A, et al. Sulfocoumarins (1,2-benzoxathiine 2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J Med Chem 2013;56:293–300.
- Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors: perfluoroalkyl/aryl-substituted derivatives of aromatic/heterocyclic sulfonamides as topical intraocular pressure-lowering agents with prolonged duration of action. J Med Chem 2000;43:4542–51.
- Sentürk M, Gülçin I, Daştan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11.
- Scozzafava A, Briganti F, Mincione G, et al. Carbonic anhydrase inhibitors: synthesis of water-soluble, aminoacyl/dipeptidyl sulfonamides possessing long-lasting intraocular pressure-lowering properties via the topical route. J Med Chem 1999;42:3690–700.
- Sarikaya SBÖ, Topal F, Şentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62.
- Supuran CT, Clare BW. Carbonic anhydrase inhibitors. Part 57. Quantum chemical QSAR of a group of 1,3,4-thiadiazole and 1,3,4-thiadiazoline disulfonamides with carbonic anhydrase inhibitory properties. Eur J Med Chem 1999;34:41–50.
- Supuran CT, Nicolae A, Popescu A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;31:431–8.