Abstract
A novel 2-thiopyrimidine/chalcone hybrid was designed, synthesised, and evaluated for their cytotoxic activities against three different cell lines, K-562, MCF-7, and HT-29. The most active cytotoxic derivatives were 9d, 9f, 9n, and 9p (IC50=0.77–1.74 µM, against K-562 cell line), 9a and 9r (IC50=1.37–3.56 µM against MCF-7 cell line), and 9a, 9l, and 9n (IC50=2.10 and 2.37 µM against HT-29 cell line). Compounds 9a, 9d, 9f, 9n, and 9r were further evaluated for their cytotoxicity against normal fibroblast cell line WI38. Moreover, STAT3 and STAT5a inhibitory activities were determined for the most active derivatives 9a, 9d, 9f, 9n, and 9r. Dual inhibitory activity was observed in compound 9n (IC50=113.31 and 50.75 µM, against STAT3 and STAT5a, respectively). Prediction of physicochemical properties, drug likeness score, pharmacokinetic and toxic properties was detected.
Graphical Abstract
Introduction
One of the main causes of mortality all over the world is cancerCitation1,Citation2. The highest prevalence for cancer death is being for stomach, breast, prostatic, lung, and colonCitation3.
The most common female cancer around the world is breast cancer. It represents for 16% of all female cancers and 18.2% of all cancer death causes including both males and femalesCitation4.
On the other hand, about two million new cases are diagnosed every year for colorectal cancer. Thus, making it as one of the most common causes of cancer-related deathCitation5,Citation6.
Another common cause of cancer death is leukaemia, cancer in blood-forming cells of the bone marrow, which is chemoresistantCitation7–11. Although, treatment of cancer using chemotherapeutic agents is still used for several cancer types including breast, colon and leukaemia cancers, high toxicity level of chemotherapeutic drugs limit their useCitation12.
A critical signalling intermediate in cancer cells, specially leukaemia, breast and colon cancer cells is called signal transducer and activator of transcription (STAT) protein familyCitation13–17.
They are cytoplasmic transcription factors. STAT family consists of seven members, STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6. STAT2, STAT4, and STAT6 are responsible for regulation of immune response. While, STAT1, STAT3, and STAT5 can control cell cycle (cyclin D1, D2, c-Myc), cell survival (Bcl-xl, Bcl2, Mcl-1), and angiogenesis (HIF1α, VEGFR) through regulation of gene expressionCitation18,Citation19.
STAT can be activated either by receptor tyrosin kinases like JAKs, PDGFR, EGFR, and FLT3, or through non-receptor tyrosin kinases, Src, Brk, and Bcr-Abl. Also, activation of STAT may be from activation of cytokines (IL-6), growth factors or negative feedback mechanismsCitation20–24.
Phosphorylation of STATs transforms them to active form causing their homo- or heterodimerisation then migration to the nucleus to control gene expression. Over activation of STAT level can lead to tumorigenesisCitation20.
Several studies have demonstrated that blocking STAT3 or STAT5 signalling pathway led to apoptosis in tumour cells. While, normal cells were able to survive even under a very low concentration of STAT3 or STAT5 and also capable of growing using other mechanismsCitation21,Citation25.
Therefore, development of new anti-cancer agents with less toxicity and overcoming chemotherapeutic drug resistance can be achieved by: (1) using drugs that target two or three activators of STAT3Citation24 or (2) combined targeting of STAT3 and STAT5Citation8.
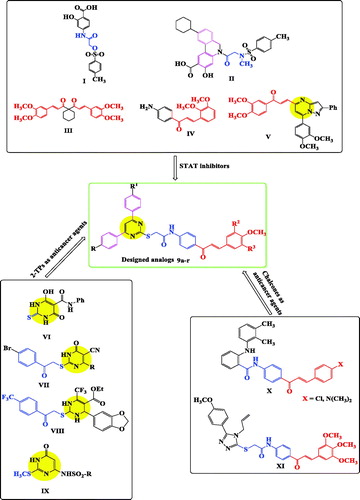
It was found that a potent STAT3 inhibitor, S31-201 (I, ), could inhibit proliferation of hepatocellular and breast carcinoma in miceCitation16.
Moreover, compound S3I-201.1066 (II, ), containing sulphonamide group could inhibit STAT3 function in both breast and myeloma cancer cells (EC50=10 and 16 µM, respectively)Citation26. Another compound, curcumine analogue, FLLL32 (III, ), showed potent inhibitory activity in many human cancer cell lines such as breast, colorectal, melanoma, and myeloma by preventing STAT3 dimerisation and downstream functioningCitation20,Citation27.
Moreover, treatment with chalcone IV () caused significant decrease in STAT3 level in leukaemia HL-60 cell lineCitation28.
Pyrazolo[1,5-a]pyrimidine/chalcone hybrid V () showed promising anti-proliferative activity by down regulation of STAT3 in MDA-MB-231 cellsCitation29.
Additionally, NCI library identified two compounds (BP-1108 and BP-1075) as the most potent STAT5 in K562 leukaemia cell lines through down regulation of STATs-defendant genesCitation30.
In medicinal chemistry, a very well-known heterocycle is pyrimidine. It takes its importance from its presence in thymidine, cytosine and uracil bases, the building blocks of DNA and RNA nucleic acidsCitation31,Citation32.
2-Thiopyrimidines (2-TPs), also named as 2-mercaptopyrimidines, are one of the most important class of pyrimidines.
They attract the biochemists attention due to their wide range of applications in preparation of cardiotonic drugs, antitubercular and anti-inflammatory agentsCitation33,Citation34.
Moreover, 2-TPs were evaluated for their anticancer activityCitation33. They were reported to have potent antitumor activity against leukaemia, colon and breast cell lines such as compounds VI–IX ()Citation35–38.
Synthesis of 2-TPs derivatives could be achieved from reaction of chalcone derivatives with thioureaCitation39. As chalcones constitute an important group of natural products, their biological activities were arisen from their chemical structure, α,β-unsaturated carbonyl groupCitation40.
Many synthesised chalcones were reported to have potent in vitro anticancer activity against human colon carcinoma, non-small cell lung carcinoma, and breast cancerCitation41–44.
Thus, both chloro- and dimethylamino-derivatives of compound X (), showed cytotoxic activity against human leukaemia cells with CC50=2.17 and 2.06 μM, respectivelyCitation40. While, compound XI () was apoptosis inducer in A549 cellsCitation45.
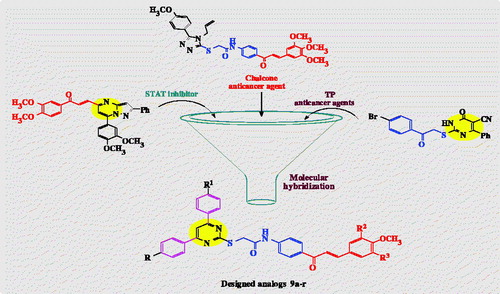
In light of the above facts, and as a part of our previously published anticancer research articlesCitation46,Citation47, our scope in this research was to design and synthesised a new series of 2-TP/chalcone hybrids (), through molecular hybridisation, by merging:
(i) 2-Thiopyrimidine scaffold, such as in compounds (VI–IX), (ii) chalcone part from compounds (III–V, X, XI), (iii) choosing substituents on phenyl rings of pyrimidine C-4, pyrimidine C-6, and chalcone as in compounds (I–V), and (iv) amide linkage to mimic that in compounds (I, II, VI, X, XI). The cytotoxic activities of the synthesised derivatives were evaluated against leukaemia (K-562), breast (MCF-7), and colon (HT-29) cancer cell lines. Inhibitory activities of the most potent bioactive molecules against STAT3 and STAT5a were measured, aiming at finding more effective anticancer therapeutics.
Experimental
Chemistry
Melting points were measured on the Griffin apparatus and were uncorrected. Determination of IR spectra was achieved using Shimadzu IR-435 spectrophotometer with KBr discs and values were obtained in cm−1. 1H NMR and 13C NMR were recorded on Bruker instrument at 400 MHz for 1H NMR and 100 MHz for 13C NMR spectrophotometer (Faculty of Pharmacy, Mansoura University, Mansoura, Egypt), in DMSO-d6 (as a solvent), D2O using TMS as an internal standard and chemical shifts (δ) were expressed in parts per million (ppm) compared to internal standard, TMS (δ = 0 ppm). Coupling constant (J) values were expressed in Hertz (Hz). Signal splitting patterns were designated as follows: s, singlet; d, doublet, t, triplet; q, quartette; m, multiplet. The electron impact (EI) mass spectra were carried out using Hewlett Packard 5988 spectrometer (Palo Alto, CA) at Faculty of Science, Cairo University, Giza, Egypt. Microanalysis was calculated for C, H, N on Perkin-Elmer 2400 at the Microanalytical centre, Faculty of Science, Cairo University, Egypt and was within ±0.4% of theoretical values. The progress of the reaction and purity of products were monitored by thin layer chromatography (TLC), pre-coated plastic sheets, 0.2 mm silica gel with UV indicator (Macherey-Nagel, Düren, Germany). All used reagents and solvents were purchased from the Aldrich Chemical Company (Milwaukee, WI).
General method for preparation of compounds 4a–f
A mixture of the appropriate chalcone derivative 3a–f (0.01 mol), thiourea (0.76 g, 0.01 mol), and KOH (0.11 g, 0.02 mol) in absolute ethanol (20 ml) was heated under reflux temperature for 12 h. The resulting solution was evaporated to dryness or (a precipitate in case of 3c and 3f). The obtained residue was solubilised in water, filtered and dried. The crude product was crystallised from ethanol/DMF (8:2) to get compounds 4a–f.
4-(4-Methoxyphenyl)-6-p-tolylpyrimidine-2-thiol (4a)
Yield 82%; yellow powder; (ethanol 95%); mp 149–151 °C; IR (cm−1): 3413 (NH), 3192 (CH aromatic), 2958 (CH aliphatic); 1H NMR (400 MHz, DMSO-d6) δ 2.50 (s, 3H, CH3), 4.43 (s, 3H, OCH3), 7.24–7.59 (m, 4H, p-methoxyphenyl H-3, H-5, p-tolyl H-3, H-5), 7.79–7.91 (m, 4H, pyrimidine H-5, p-methoxyphenyl H-2, H-6, NH, D2O exchangeable), 8.21 (d, J= 8.4 Hz, 2H, p-tolyl H-2, H-6); 13C NMR (100 MHz, DMSO-d6) δ 21.2 (CH3), 55.6 (OCH3), 101.0 (pyrimidine C-5), 114.7 (p-methoxyphenyl C-3, C-5), 126.1 (p-tolyl C-2, C-6), 128.1 (p-tolyl C-3, C-5), 129.4 (p-methoxyphenyl C-2, C-6), 130.9 (p-methoxyphenyl C-1), 134.5 (p-tolyl C-1), 136.7 (p-tolyl C-4), 159.2 (p-methoxyphenyl C-4), 164.6 (pyrimidine C-4) 172.9 (pyrimidine C-6), 175.2 (pyrimidine C-2); EIMS (m/z): 309.00 (M + 1, 31.75%), 308.00 (M+, 74.33%), 307.00 (100%); Anal. Calcd. for C18H16N2OS (308.40): C, 70.10; H, 5.23; N, 9.08. Found: C, 70.31; H, 5.07; N, 8.88.
4-(4-Methoxyphenyl)-6-(4-nitrophenyl)pyrimidine-2-thiol (4b)
Yield 75%; yellow crystals; mp 63–65 °CCitation39.
4-[4-(2-Chloroethoxy)phenyl]-6-(4-methoxyphenyl)pyrimidine-2-thiol (4c)
Yield 69%; yellow powder; (ethanol 95%); mp 282–284 °C; IR (cm−1): 3436 (NH), 3192 (CH aromatic), 2927 (CH aliphatic); 1H NMR (400 MHz, DMSO-d6) δ 3.83–4.37 (m, 7H, OCH3, OCH2, CH2Cl), 6.96–7.10 (m, 7H, p-chloroethoxyphenyl H-2, H-3, H-5, H-6, p-methoxyphenyl H-3, H-5, NH, D2O exchangeable), 8.22–8.30 (m, 3H, p-methoxyphenyl H-2, H-6, pyrimidine H-5); 13C NMR (100 MHz, DMSO-d6) δ 42.9 (CH2Cl), 55.8 (OCH3), 68.1 (OCH2), 105.0 (pyrimidine C-5), 114.8 (p-methoxyphenyl C-3, C-5), 115.4 (p-chloroethoxyphenyl C-3, C-5), 121.8 (p-chlorophenyl C-2, C-6), 127.9 (p-methoxyphenyl C-1), 128.5 (p-methoxyphenyl C-2, C-6), 129.4 (p-chloroethoxyphenyl C-2, C-6), 130.0 (p-chloroethoxyphenyl C-1), 160.6 (p-methoxyphenyl C-4), 161.7 (p-chloroethoxyphenyl C-4), 162.5 (pyrimidine C-4) 176.1 (pyrimidine C-6), 179.3 (pyrimidine C-2); Anal. Calcd. for C19H17ClN2O2S (372.87): C, 61.20; H, 4.60; N, 7.51. Found: C, 61.41; H, 4.57; N, 7.75.
4-(4-Chlorophenyl)-6-p-tolylpyrimidine-2-thiol (4d)
Yield 91%; yellow crystals; mp 180–182 °CCitation47.
4-(4-Chlorophenyl)-6-(4-nitrophenyl)pyrimidine-2-thiol (4e)
Yield 65%; yellow powder; (ethanol 95%); mp 263–265 °C; IR (cm−1): 3434 (NH), 3064 (CH aromatic); 1H NMR (400 MHz, DMSO-d6) δ 7.60 (d, J= 8.4 Hz, 2H, p-chlorophenyl H-3, H-5), 7.96 (s, 1H, NH, D2O exchangeable), 8.33–8.34 (m, 4H, p-chlorophenyl H-2, H-6, p-nitrophenyl H-2, H-6), 8.54 (d, J= 8.4 Hz, 2H, p-nitrophenyl H-3, H-5), 8.66 (s, 1H, pyrimidine H-5); 13C NMR (100 MHz, DMSO-d6) δ 101.3 (pyrimidine C-5), 123.6 (p-chlorophenyl C-2, C-6), 128.0 (p-nitrophenyl C-3, C-5), 128.9 (p-chlorophenyl C-1), 129.3 (p-chlorophenyl C-3, C-5), 129.5 (p-nitrophenyl C-2, C-6), 133.5 (p-chlorophenyl C-4), 139.2 (p-nitrophenyl C-1), 150.2 (p-nitrophenyl C-4), 164.6 (pyrimidine C-4) 176.3 (pyrimidine C-6), 180.4 (pyrimidine C-2); Anal. Calcd. for C16H10ClN3O2S (343.79): C, 55.90; H, 2.93; N, 12.22. Found: C, 60.12; H, 2.87; N, 12.46.
4-[4-(2-Chloroethoxy)phenyl]-6-(4-chlorophenyl)pyrimidine-2-thiol (4f)
Yield 62%; yellow powder; (ethanol 95%); mp 242–244 °C; IR (cm−1): 3417 (NH), 3066 (CH aromatic), 2927 (CH aliphatic); 1H NMR (400 MHz, DMSO-d6) δ 3.98 (t, J= 8.4 Hz, 2H, CH2Cl), 4.36 (t, J= 8.4 Hz, 2H, OCH2), 7.07–7.12 (m, 4H, p-chloroethoxyphenyl H-3, H-5, p-chlorophenyl H-2, H-6), 7.31 (s, 1H, NH, D2O exchangeable), 7.60 (d, J= 8.4 Hz, 2H, p-chlorophenyl H-3, H-5), 7.96 (s, 1H, pyrimidine H-5), 8.31 (d, J= 8.4 Hz, 2H, p-chloroethoxyphenyl H-2, H-6); 13C NMR (100 MHz, DMSO-d6) δ 43.4 (CH2Cl), 68.5 (OCH2), 103.9 (pyrimidine C-5), 115.3 (p-chloroethoxyphenyl C-3, C-5), 120.2 (p-chlorophenyl C-2, C-6), 128.5 (p-chlorophenyl C-1), 129.4 (p-chloroethoxyphenyl C-2, C-6), 129.5 (p-chlorophenyl C-3, C-5), 130.7 (p-chloroethoxyphenyl C-1), 136.6 (p-chlorophenyl C-4), 161.7 (p-chloroethoxyphenyl C-4), 162.8 (pyrimidine C-4) 176.3 (pyrimidine C-6), 179.0 (pyrimidine C-2); EIMS (m/z): 376.95 (M + 1, 16.83%), 375.90 (M+, 19.31%), 55.10 (100%); Anal. Calcd. for C18H14ClN2OS (377.29): C, 57.30; H, 3.74; N, 7.42. Found: C, 57.41; H, 3.57; N, 7.68.
General method for preparation of compounds 9a–r
A mixture of pyrimidine derivatives 4a–f (0.01 mol), acetyl chloride derivatives 8a–c (0.01 mol), and catalytic amount of TEA is stirred in acetonitrile (20 ml) for 24 h. The solution was evaporated to dryness. The obtained residue was solubilised in ice cold water and neutralised with conc. HCl. The obtained solid was filtered, dried and crystallised from ethanol/DMF (8:2).
(ZE)-2-[4-(4-Methoxyphenyl)-6-p-tolylpyrimidin-2-ylthio]-N-{4-[3-(4 methoxyphenyl)acryloyl]phenyl}acetamide (9a)
Yield 82%; yellow powder; mp 226–228 °C; IR (cm−1): 3257 (NH), 3039 (CH aromatic), 2925 (CH aliphatic), 1795, 1663 (2C=O); 1H NMR (400 MHz, DMSO-d6) δ 1.86 (s, 3H, CH3), 4.41 (s, 3H, OCH3), 4.43 (s, 3H, OCH3), 4.84 (s, 2H, CH2), 6.67 (d, J= 8.4 Hz, 2H, p-methoxyphenylacroyl H-3, H-5), 7.10 (d, J= 7.6 Hz, 2H, p-methoxyphenyl H-3, H-5), 7.24 (d, J= 8.4 Hz, 2H, p-methoxyphenylacroyl H-2, H-6), 7.33 (d, J= 6.0 Hz, 2H, p-tolyl H-3, H-5), 7.44–7.74 (m, 7H, COCH=CH, pyrimidine H-5, p-methoxyphenyl H-2, H-6, p-tolyl H-2, H-6), 7.91–7.94 (m, 4H, phenyl H-2, H-3, H-5, H-6), 10.84 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 22.3 (CH3), 36.2 (CH2), 56.4 (2OCH3), 113.7 (p-methoxyphenylacroyl C-3, C-5), 118.8 (p-methoxyphenyl C-3, C-5), 120.1 (COCH=CH), 121.7 (phenyl C-2, C-6), 127.5 (p-methoxyphenylacroyl C-1), 127.6 (p-tolyl C-2, C-6), 129.0 (p-methoxyphenyl C-2, C-6), 129.1 (p-methoxyphenyl C-1), 130.2 (p-tolyl C-3, C-5), 130.8 (p-methoxyphenylacroyl C-2, C-6), 131.4 (phenyl C-3, C-5), 132.0 (p-tolyl C-4), 133.5 (phenyl C-4), 136.9 (p-tolyl C-1), 144.3 (phenyl C-1), 154.4 (COCH=CH), 155.1 (p-methoxyphenylacroyl C-4), 155.4 (p-methoxyphenyl C-4), 164.1 (pyrimidine C-6), 167.8 (pyrimidine C-4), 172.3 (pyrimidine C-2), 173.0 (CONH), 190.0 (CO); Anal. Calcd. for C36H31N3O4S (601.20): C, 71.86; H, 5.19; N, 6.98. Found: C, 71.67; H, 5.07; N, 6.93.
(ZE)-N-{4-[3-(3,4-Dimethoxyphenyl)acryloyl]phenyl}-2-[4-(4-methoxyphenyl)-6-p-tolylpyrimidin-2-ylthio]acetamide (9b)
Yield 65%; yellow powder; mp 165–167 °C; IR (cm−1): 3324 (NH), 3061 (CH aromatic), 2925 (CH aliphatic), 1663 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 2.36 (s, 3H, CH3), 3.81 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 4.23 (s, 2H, CH2), 6.97 (d, J= 8.4 Hz, 2H, p-methoxyphenyl H-3, H-5), 7.03 (d, J= 8.0 Hz, 1H, dimethoxyphenyl H-5), 7.28 (d, J= 8.0 Hz, 1H, dimethoxyphenyl H-6), 7.37 (d, J= 12.0 Hz, 1H, COCH=CH), 7.54 (s, 1H, dimethoxyphenyl H-2), 7.69 (d, J= 12.0 Hz, 1H, COCH=CH), 7.83–7.87 (m, 3H, p-tolyl H-3, H-5, pyridine H-5), 8.18–8.23 (m, 6H, phenyl H-2, H-6, p-methoxyphenyl H-2, H-6, p-tolyl H-2, H-6), 8.31 (d, J= 8.4 Hz, 2H, phenyl H-3, H-5), 10.84 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 21.4 (CH3), 36.4 (CH2), 55.8 (OCH3), 56.0 (OCH3), 56.1 (OCH3), 107.4 (pyrimidine C-5), 111.0 (dimethoxyphenyl C-2), 111.9 (dimethoxyphenyl C-5), 114.6 (dimethoxyphenyl C-6), 118.8 (p-methoxyphenyl C-3, C-5), 119.8 (COCH=CH), 124.4 (phenyl C-2, C-6), 127.7 (p-tolyl C-2, C-6), 128.0 (p-methoxyphenyl C-2, C-6), 129.6 (p-methoxyphenyl C-1), 130.3 (p-tolyl C-3, C-5), 130.4 (p-tolyl C-1), 133.0 (dimethoxyphenyl C-1), 133.6 (phenyl C-3, C-5), 141.7 (p-tolyl C-4), 143.9 (phenyl C-4), 144.4 (phenyl C-1), 149.4 (dimethoxyphenyl C-3), 150.0 (dimethoxyphenyl C-4), 151.6 (COCH=CH), 162.3 (p-methoxyphenyl C-4), 164.2 (pyrimidine C-6), 164.3 (pyrimidine C-4), 167.7 (pyrimidine C-2), 170.8 (CONH), 187.8 (CO); Anal. Calcd. for C37H33N3O5S (631.21): C, 70.34; H, 5.27; N, 6.65. Found: C, 70.54; H, 5.07; N, 6.71.
(ZE)-2-[4-(4-Methoxyphenyl)-6-p-tolylpyrimidin-2-ylthio]-N-{4-[3-(3,4,5-trimethoxyphenyl)acryloyl]phenyl}acetamide (9c)
Yield 56%; yellow powder; mp 195–197 °C; IR (cm−1): 3290 (NH), 2998 (CH aromatic), 2927 (CH aliphatic), 1665 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 2.36 (s, 3H, CH3), 3.72 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 3.87 (s, 6H, 2OCH3), 4.24 (s, 2H, CH2), 6.95 (s, 2H, trimethoxyphenyl H-2, H-6), 6.98–7.28 (m, 4H, p-methoxyphenyl H-3, H-5, p-tolyl H-3, H-5), 7.69 (d, J= 12.0 Hz, 1H, COCH=CH), 7.87–8.19 (m, 3H, COCH=CH, p-tolyl H-2, H-6), 8.22–8.31 (m, 7H, p-methoxyphenyl H-2, H-6, pyrimidine H-5, phenyl H-2, H-3, H-5, H-6), 10.86 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 21.4 (CH3), 36.4 (CH2), 55.8 (OCH3), 56.5 (2OCH3), 60.6 (OCH3), 106.8 (trimethoxyphenyl C-2, C-6), 107.4 (pyrimidine C-5), 114.5 (p-methoxyphenyl C-3, C-5), 118.8 (phenyl C-2, C-6), 121.5 (COCH=CH), 126.9 (p-tolyl C-2, C-6), 127.7 (trimethoxyphenyl C-1), 128.5 (p-methoxyphenyl C-1), 129.6 (p-methoxyphenyl C-2, C-6), 130.4 (p-tolyl C-3, C-5), 131.0 (p-tolyl C-4), 132.8 (phenyl C-3, C-5), 133.5 (p-tolyl C-1), 140.0 (phenyl C-4), 141.7 (trimethoxyphenyl C-4), 144.0 (phenyl C-1), 144.4 (COCH=CH), 153.5 (trimethoxyphenyl C-3, C-5), 162.3 (p-methoxyphenyl C-4), 164.2 (pyrimidine C-6), 164.3 (pyrimidine C-4), 167.7 (pyrimidine C-2), 170.7 (CONH), 187.9 (CO); EIMS (m/z): 662.05 (M + 1, 5.50%), 661.05 (M+, 12.94%), 322.05 (100%); Anal. Calcd. for C38H35N3O6S (661.22): C, 68.97; H, 5.33; N, 6.35. Found: C, 68.78; H, 5.17; N, 6.24.
(ZE)-2-[4-(4-Methoxyphenyl)-6-(4-nitrophenyl)pyrimidin-2-ylthio]-N-{4-[3-(4-methoxyphenyl)acryloyl]phenyl}acetamide (9d)
Yield 57%; yellow powder; mp 183–185 °C; IR (cm−1): 3407 (NH), 3063 (CH aromatic), 2930 (CH aliphatic), 1664 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.83 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 4.24 (s, 2H, CH2), 6.98 (d, J= 8.8 Hz, 2H, p-methoxyphenylacryloyl H-3, H-5), 7.03 (d, J= 8.8 Hz, 2H, p-methoxyphenyl H-3, H-5), 7.69 (d, J= 15.6 Hz, 1H, COCH=CH), 7.80–7.86 (m, 5H, COCH=CH, p-methoxyphenylacryloyl H-2, H-6, p-methoxyphenyl H-2, H-6), 8.18 (d, J= 8.8 Hz, 2H, phenyl H-2, H-6), 8.27 (d, J= 8.8 Hz, 2H, phenyl H-3, H-5), 8.33 (d, J= 8.8 Hz, 2H, p-nitrophenyl H-2, H-6), 8.40 (s, 1H, pyrimidine H-5), 8.56 (d, J= 8.8 Hz, 2H, p-nitrophenyl H-3, H-5), 10.86 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 36.4 (CH2), 55.8 (OCH3), 55.9 (OCH3), 109.1 (pyrimidine C-5), 114.7 (p-methoxyphenylacryloyl C-3, C-5), 114.8 (p-methoxyphenyl C-3, C-5), 118.8 (phenyl C-2, C-6), 119.8 (p-nitrophenyl C-3, C-5), 124.2 (COCH=CH), 127.8 (p-nitrophenyl C-2, C-6), 128.2 (p-methoxyphenyl C-2, C-6), 129.1 (p-methoxyphenylacryloyl C-1), 129.8 (p-methoxyphenyl C-1), 130.0 (p-methoxyphenylacryloyl C-2, C-6), 130.3 (phenyl C-3, C-5), 131.1 (p-nitrophenyl C-1), 133.1 (phenyl C-4), 142.4 (phenyl C-1), 143.8 (COCH=CH), 149.3 (p-nitrophenyl C-4), 161.7 (p-methoxyphenylacryloyl C-4), 162.2 (p-methoxyphenyl C-4), 162.6 (pyrimidine C-6), 164.9 (pyrimidine C-4), 167.6 (pyrimidine C-2), 171.3 (CONH), 187.8 (CO); Anal. Calcd. for C35H28N4O6S (632.17): C, 66.44; H, 4.46; N, 8.86. Found: C, 66.42; H, 4.39; N, 8.74.
(ZE)-N-{4-[3-(3,4-Dimethoxyphenyl)acryloyl]phenyl}-2-[4-(4-methoxyphenyl)-6-(4-nitrophenyl)pyrimidin-2-ylthio]-acetamide (9e)
Yield 54%; yellow powder; mp 225–227 °C; IR (cm−1): 3431 (NH), 3039 (CH aromatic), 2924 (CH aliphatic), 1656 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.83 (s, 3H, OCH3), 3.87 (s, 6H, 2OCH3), 4.27 (s, 2H, CH2), 6.99 (d, J= 8.4 Hz, 2H, p-methoxyphenyl H-3, H-5), 7.03 (d, J= 8.8 Hz, 1H, dimethoxyphenyl H-5), 7.39 (d, J= 8.8 Hz, 1H, dimethoxyphenyl H-6), 7.55 (s, 1H, dimethoxyphenyl H-2), 7.68 (d, J= 15.2 Hz, 1H, COCH=CH), 7.82–7.88 (m, 3H, COCH=CH, p-methoxyphenyl H-2, H-6), 8.20 (d, J= 8.8 Hz, 2H, phenyl H-2, H-6), 8.28 (d, J= 8.8 Hz, 2H, phenyl H-3, H-5), 8.34 (d, J= 8.8 Hz, 2H, p-nitrophenyl H-2, H-6), 8.41 (s, 1H, pyrimidine H-5), 8.57 (d, J= 8.8 Hz, 2H, p-nitrophenyl H-3, H-5), 10.87 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 36.4 (CH2), 55.9 (OCH3), 56.0 (OCH3), 56.2 (OCH3), 109.1 (pyrimidine C-5), 111.0 (dimethoxyphenyl C-2), 112.0 (dimethoxyphenyl C-5), 114.7 (p-methoxyphenyl C-3, C-5), 118.8 (dimethoxyphenyl C-6), 119.8 (phenyl C-2, C-6), 124.2 (p-nitrophenyl C-3, C-5), 124.4 (COCH=CH), 128.0 (p-nitrophenyl C-2, C-6), 128.2 (p-methoxyphenyl C-2, C-6), 129.1 (dimethoxyphenyl C-1), 129.8 (p-methoxyphenyl C-1), 130.3 (phenyl C-3, C-5), 132.0 (phenyl C-4), 142.4 (phenyl C-1), 143.8 (p-nitrophenyl C-1), 144.4 (COCH=CH), 149.4 (dimethoxyphenyl C-4), 149.4 (dimethoxyphenyl C-3), 153.5 (p-nitrophenyl C-4), 162.2 (p-methoxyphenyl C-4), 162.6 (pyrimidine C-6), 165.0 (pyrimidine C-4), 167.6 (pyrimidine C-2), 171.3 (CONH), 187.8 (CO); Anal. Calcd. for C36H30N4O7S (662.18): C, 65.24; H, 4.56; N, 8.45. Found: C, 65.35; H, 4.71; N, 8.24.
(ZE)-2-[4-(4-Methoxyphenyl)-6-(4-nitrophenyl)pyrimidin-2-ylthio]-N-{4-[3-(3,4,5-trimethoxyphenyl)acryloyl]phenyl}acetamide (9f)
Yield 69%; yellow powder; mp 254–256 °C; IR (cm−1): 3265 (NH), 3103 (CH aromatic), 2933 (CH aliphatic), 1663 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.71 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 3.87 (s, 6H, 2OCH3), 4.27 (s, 2H, CH2), 6.98 (d, J= 8.0 Hz, 2H, p-methoxyphenyl H-3, H-5), 7.23 (s, 2H, trimethoxyphenyl H-2, H-6), 7.69 (d, J= 12.0 Hz, 1H, COCH=CH), 7.86–7.92 (m, 3H, COCH=CH, p-methoxyphenyl H-2, H-6), 8.21 (d, J= 8.0 Hz, 2H, phenyl H-2, H-6), 8.27 (d, J= 8.0 Hz, 2H, phenyl H-3, H-5), 8.33 (d, J= 8.0 Hz, 2H, p-nitrophenyl H-2, H-6), 8.40 (s, 1H, pyrimidin H-5), 8.56 (d, J= 8.0 Hz, 2H, p-nitrophenyl H-3, H-5), 10.89 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 36.4 (CH2), 55.8 (OCH3), 56.5 (2OCH3), 60.6 (OCH3), 106.8 (trimethoxyphenyl C-2, C-6), 109.0 (pyrimidine C-5), 114.6 (p-methoxyphenyl C-3, C-5), 118.8 (phenyl C-2, C-6), 121.4 (COCH=CH), 124.2 (p-nitrophenyl C-3, C-5), 128.1 (trimethoxyphenyl C-1), 129.1 (p-nitrophenyl C-2, C-6), 129.8 (p-methoxyphenyl C-2, C-6), 130.4 (phenyl C-3, C-5), 130.7 (p-methoxyphenyl C-1), 132.9 (phenyl C-4), 140.0 (trimethoxyphenyl C-4), 142.3 (p-nitrophenyl C-1), 144.3 (phenyl C-1), 144.4 (COCH=CH), 149.3 (p-nitrophenyl C-4), 153.5 (trimethoxyphenyl C-3, C-5), 162.2 (p-methoxyphenyl C-4), 162.6 (pyrimidine C-6), 164.9 (pyrimidine C-4), 167.6 (pyrimidine C-2), 171.3 (CONH), 187.9 (CO); EIMS (m/z): 693.00 (M + 1, 0.93%), 692.00 (M+, 1.33%), 55.10 (100%); Anal. Calcd. for C37H32N4O8S (692.19): C, 64.15; H, 4.66; N, 8.09. Found: C, 63.98; H, 4.57; N, 7.89.
(ZE)-2-{4-[4-(2-Chloroethoxy)phenyl]-6-(4-methoxyphenyl)pyrimidin-2-ylthio}-N-{4-[3-(4-methoxyphenyl)acryloyl]phenyl}acetamide (9g)
Yield 52%; yellow powder; mp 280–282 °C; IR (cm−1): 3431 (NH), 3039 (CH aromatic), 2935 (CH aliphatic), 1598 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.70 (s, 3H, OCH3), 3.83–3.85 (m, 5H, OCH3 and CH2Cl), 4.21–4.23 (m, 4H, OCH2 and CH2), 7.02–7.16 (m, 6H, p-methoxyphenyl H-3, H-5, p-methoxyphenylacryloyl H-3, H-5 and p-chloroethoxyphenyl H-3, H-5), 7.71–7.96 (m, 9H, p-methoxyphenyl H-2, H-6, p-methoxyphenylacryloyl H-2, H-6, p-chloroethoxyphenyl H-2, H-6, phenyl H-2, H-6 and COCH=CH), 8.16 (d, J= 8.4 Hz, 2H, phenyl H-3, H-5), 8.18–8.20 (m, 2H, pyrimidin H-5 and COCH=CH), 10.89 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 36.2 (CH2), 40.6 (CH2Cl), 55.4 (OCH3), 55.8 (OCH3), 68.9 (OCH2), 107.8 (pyrimidine C-5), 114.1 (p-methoxyphenylacroyl C-3, C-5), 114.7 (p-methoxyphenyl C-3, C-5), 114.8 (p-chloroethoxyphenyl C-3, C-5), 121.3 (COCH=CH), 122.1 (phenyl C-2, C-6), 127.4 (p-chloroethoxyphenyl C-1), 127.5 (p-methoxyphenylacroyl C-1), 128.3 (p-chloroethoxyphenyl C-2, C-6), 129.5 (p-methoxyphenyl C-2, C-6), 130.3 (p-methoxyphenylacroyl C-2, C-6), 131.1 (phenyl C-3, C-5), 133.5 (phenyl C-4), 144.0 (phenyl C-1), 144.3 (p-methoxyphenyl C-1), 145.4 (COCH=CH), 159.3 (p-chloroethoxyphenyl C-4), 159.8 (p-methoxyphenylacroyl C-4), 160.6 (p-methoxyphenyl C-4), 162.8 (pyrimidine C-6), 164.9 (pyrimidine C-4), 168.6 (pyrimidine C-2), 172.3 (CONH), 189.9 (CO); Anal. Calcd. for C37H32ClN3O5S (665.18): C, 66.71; H, 4.84; N, 6.31. Found: C, 66.98; H, 4.57; N, 6.28.
(ZE)-2-{4-[4-(2-Chloroethoxy)phenyl]-6-(4-methoxyphenyl)pyrimidin-2-ylthio}-N-{4-[3-(3,4-dimethoxyphenyl)acryloyl]phenyl}acetamide (9h)
Yield 52%; yellow powder; mp 135–137 °C; IR (cm−1): 3426 (NH), 3067 (CH aromatic), 2928 (CH aliphatic), 1657 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.70 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 3.96 (t, J= 7.2 Hz, 2H, CH2Cl), 4.23 (s, 2H, CH2), 4.34 (t, J= 7.2 Hz, 2H, OCH2), 6.82–7.12 (m, 7H, p-methoxyphenyl H-3, H-5, p-chloroethoxyphenyl H-3, H-5, dimethoxyphenyl H-2, H-5, H-6), 7.38 (d, J= 15.2 Hz, 1H, COCH=CH), 7.67–7.86 (m, 5H, COCH=CH, p-methoxyphenyl H-2, H-6, p-chloroethoxyphenyl H-2, H-6), 8.18–8.29 (m, 5H, phenyl H-2, H-6, phenyl H-3, H-5, pyrimidin H-5), 10.89 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 42.2 (CH2), 43.4 (CH2Cl), 55.8 (OCH3), 56.1 (OCH3), 56.2 (OCH3), 75.1 (OCH2), 109.0 (pyrimidine C-5), 111.5 (dimethoxyphenyl C-2), 111.9 (dimethoxyphenyl C-5), 114.5 (p-methoxyphenyl C-3, C-5), 115.1 (p-chloroethoxyphenyl C-3, C-5), 118.8 (phenyl C-2, C-6), 119.8 (COCH=CH), 124.4 (dimethoxyphenyl C-6), 127.3 (dimethoxyphenyl C-1), 127.4 (p-chloroethoxyphenyl C-1), 128.0 (p-chloroethoxyphenyl C-2, C-6), 128.7 (p-methoxyphenyl C-1), 129.6 (p-methoxyphenyl C-2, C-6), 130.3 (phenyl C-3, C-5), 131.3 (phenyl C-4), 143.9 (phenyl C-1), 144.4 (COCH=CH), 149.4 (dimethoxyphenyl C-4), 151.6 (dimethoxyphenyl C-3), 157.2 (p-chloroethoxyphenyl C-4), 161.2 (p-methoxyphenyl C-4), 162.2 (pyrimidine C-6), 164.3 (pyrimidine C-4), 167.6 (pyrimidine C-2), 170.7 (CONH), 187.6 (CO); Anal. Calcd. for C38H34ClN3O6S (695.19): C, 65.56; H, 4.92; N, 6.04. Found: C, 65.74; H, 5.07; N, 5.99.
(ZE)-2-{4-[4-(2-Chloroethoxy)phenyl]-6-(4-methoxyphenyl)pyrimidin-2-ylthio}-N-{4-[3-(3,4,5-trimethoxyphenyl)acryloyl]phenyl}acetamide (9i)
Yield 54%; yellow powder; mp 116–118 °C; IR (cm−1): 3417 (NH), 3039 (CH aromatic), 2934 (CH aliphatic), 1658 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.71 (s, 3H, OCH3), 3.72 (s, 3H, OCH3), 3.82 (t, J= 3.6 Hz, 2H, CH2Cl), 3.88 (s, 6H, 2OCH3), 4.23 (s, 2H, CH2), 4.34 (t, J= 3.6 Hz, 2H, OCH2,), 7.25 (s, 2H, trimethoxyphenyl H-2, H-6), 7.68–7.72 (m, 3H, p-chloroethoxyphenyl H-3, H-5 and COCH=CH), 7.81 (d, J= 8.8 Hz, 2H, p-methoxyphenyl H-3, H-5), 7.90 (d, J= 8.8 Hz, 2H, phenyl H-3, H-5), 7.95 (d, J= 8.2 Hz, p-chloroethoxyphenyl H-2, H-6) , 8.20–8.22 (m, 4H, phenyl H-2, H-6 and p-methoxyphenyl H-2, H-6), 8.29–8.31 (m, 2H, COCH=CH and pyrimidin H-5), 10.71 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 43.4 (CH2), 55.4 (CH2Cl), 55.8 (OCH3), 56.5 (2OCH3), 60.6 (OCH3), 68.6 (OCH2), 106.5 (trimethoxyphenyl C-2, C-6), 106.9 (pyrimidine C-5), 114.1 (p-methoxyphenyl C-3, C-5), 114.9 (p-chloroethoxyphenyl C-3, C-5), 118.9 (phenyl C-2, C-6), 119.8 (COCH=CH), 121.5 (trimethoxyphenyl C-1), 129.6 (p-chloroethoxyphenyl C-1), 129.7 (p-chloroethoxyphenyl C-2, C-6), 130.4 (phenyl C-3, C-5), 130.7 (p-methoxyphenyl C-1), 131.0 (p-methoxyphenyl C-2, C-6), 131.6 (phenyl C-4), 140.0 (trimethoxyphenyl C-4), 143.7 (phenyl C-1), 144.4 (COCH=CH), 153.5 (trimethoxyphenyl C-3, C-5), 160.6 (p-methoxyphenyl C-4), 161.7 (p-chloroethoxyphenyl C-4), 164.1 (pyrimidine C-6), 164.0 (pyrimidine C-4), 168.6 (pyrimidine C-2), 172.3 (CONH), 187.9 (CO); Anal. Calcd. for C39H36ClN3O7S (725.20): C, 64.50; H, 5.00; N, 5.79. Found: C, 64.38; H, 4.98; N, 5.53.
(ZE)-2-[4-(4-Chlorophenyl)-6-p-tolylpyrimidin-2-ylthio]-N-{4-[3-(4-methoxyphenyl)acryloyl]phenyl}acetamide (9j)
Yield 59%; yellow powder; mp 242–244 °C; IR (cm−1): 3256 (NH), 3038 (CH aromatic), 2917 (CH aliphatic), 1663 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 2.36 (s, 3H, CH3), 3.82 (s, 3H, OCH3), 4.24 (s, 2H, CH2), 7.02 (d, J= 8.8 Hz, 2H, methoxyphenyl H-3, H-5), 7.27 (d, J= 8.0 Hz, 2H, p-tolyl H-3, H-5), 7.52 (d, J= 8.8 Hz, 2H, p-chlorophenyl H-3, H-5), 7.69 (d, J= 11.6 Hz, 1H, COCH=CH), 7.82 (d, J= 11.6 Hz, 1H, COCH=CH), 7.83–7.85 (m, 4H, p-tolyl H-2, H-6, p-methoxyphenyl H-2, H-6), 8.16 (d, J= 8.8 Hz, 2H, phenyl H-2, H-6), 8.22 (d, J= 8.8 Hz, 2H, phenyl H-3, H-5), 8.30 (s, 1H, pyrimidine H-5), 8.34 (d, J= 8.8 Hz, 2H, p-chlorophenyl H-2, H-6), 10.84 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 21.4 (CH3), 36.4 (CH2), 55.8 (OCH3), 108.3 (pyrimidine C-5), 114.8 (p-methoxyphenyl C-3, C-5), 118.8 (phenyl C-2, C-6), 119.8 (COCH=CH), 127.8 (p-tolyl C-2, C-6), 129.3 (p-chlorophenyl C-2, C-6), 129.6 (p-chlorophenyl C-3, C-5), 129.9 (p-tolyl C-3, C-5), 130.3 (p-methoxyphenyl C-2, C-6), 131.1 (phenyl C-3, C-5), 133.0 (p-methoxyphenyl C-1), 133.3 (p-tolyl C-4), 135.1 (p-tolyl C-1), 136.6 (phenyl C-4), 142.0 (p-chlorophenyl C-1), 143.8 (phenyl C-1), 143.8 (p-chlorophenyl C-4), 148.0 (COCH=CH), 161.7 (p-methoxyphenyl C-4), 163.4 (pyrimidine C-6), 164.9 (pyrimidine C-4), 167.6 (pyrimidine C-2), 171.1 (CONH), 187.8 (CO); EIMS (m/z): 607.20 (M + 2, 1.54%), 606.15 (M + 1, 1.28%), 605.15 (M+, 2.65%), 57.10 (100%); Anal. Calcd. for C35H28ClN3O3S (605.15): C, 69.35; H, 4.66; N, 6.93. Found: C, 69.41; H, 4.87; N, 7.13.
(ZE)-2-[4-(4-Chlorophenyl)-6-p-tolylpyrimidin-2-ylthio]-N-{4-[3-(3,4-dimethoxyphenyl)acryloyl]phenyl}acetamide (9k)
Yield 57%; yellow powder; mp 144–146 °C; IR (cm−1): 3273 (NH), 3039 (CH aromatic), 2924 (CH aliphatic), 1665 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 2.35 (s, 3H, CH3), 3.85 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 4.24 (s, 2H, CH2), 7.01 (d, J= 8.4 Hz, 1H, dimethoxyphenyl H-5), 7.27 (d, J= 8.0 Hz, 2H, p-tolyl H-3, H-5), 7.37 (d, J= 8.4 Hz, 1H, dimethoxyphenyl H-6), 7.50 (d, J= 8.8 Hz, 2H, p-chlorophenyl H-3, H-5), 7.53 (s, 1H, dimethoxyphenyl H-2), 7.69 (d, J= 11.6 Hz, 1H, COCH=CH), 7.81 (d, J= 11.6 Hz, 1H, COCH=CH), 7.85 (d, J= 8.0 Hz, 2H, p-tolyl H-2, H-6), 8.18 (d, J= 8.0 Hz, 2H, phenyl H-2, H-6), 8.22 (d, J= 8.0 Hz, 2H, phenyl H-3, H-5), 8.28 (s, 1H, pyrimidine H-5), 8.34 (d, J= 8.8 Hz, 2H, p-chlorophenyl H-2, H-6), 10.91 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 21.4 (CH3), 36.2 (CH2), 56.1 (OCH3), 56.8 (OCH3), 108.3 (pyrimidine C-5), 111.0 (dimethoxyphenyl C-2), 111.9 (dimethoxyphenyl C-5), 118.8 (phenyl C-2, C-6), 119.6 (dimethoxyphenyl C-6), 119.8 (p-tolyl C-2, C-6), 124.3 (COCH=CH), 127.8 (p-chlorophenyl C-2, C-6), 128.0 (dimethoxyphenyl C-1), 129.3 (p-chlorophenyl C-3, C-5), 129.6 (p-tolyl C-3, C-5), 129.9 (phenyl C-3, C-5), 130.3 (p-tolyl C-4), 133.0 (p-tolyl C-1), 133.3 (p-chlorophenyl C-1), 134.0 (p-chlorophenyl C-4), 135.1 (phenyl C-4), 136.6 (phenyl C-1), 144.7 (COCH=CH), 149.4 (dimethoxyphenyl C-4), 151.6 (dimethoxyphenyl C-3), 163.4 (pyrimidine C-6), 164.9 (pyrimidine C-4), 167.6 (pyrimidine C-2), 171.1 (CONH), 187.9 (CO); Anal. Calcd. for C36H30ClN3O4S (635.16): C, 67.97; H, 4.75; N, 6.61. Found: C, 67.85; H, 4.58; N, 6.42.
(ZE)-2-[4-(4-Chlorophenyl)-6-p-tolylpyrimidin-2-ylthio]-N-{4-[3-(3,4,5-trimethoxyphenyl)acryloyl]phenyl}acetamide (9l)
Yield 62%; yellow powder; mp 234–236 °C; IR (cm−1): 3280 (NH), 3088 (CH aromatic), 2927 (CH aliphatic), 1663 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 2.36 (s, 3H, CH3), 3.71 (s, 3H, OCH3), 3.86 (s, 6H, 2OCH3), 4.25 (s, 2H, CH2), 7.23 (s, 2H, trimethoxyphenyl H-2, H-6), 7.28 (d, J= 8.0 Hz, 2H, p-tolyl H-3, H-5), 7.52 (d, J= 8.4 Hz, 2H, p-chlorophenyl H-3, H-5), 7.69 (d, J= 12.8 Hz, 1H, COCH=CH), 7.87 (d, J= 8.0 Hz, 2H, p-tolyl H-2, H-6), 7.93 (d, J= 12.8 Hz, 1H, COCH=CH), 8.19–8.24 (m, 4H, phenyl H-2, H-3, H-5, H-6), 8.31 (s, 1H, pyrimidine H-5), 8.35 (d, J= 8.4 Hz, 2H, p-chlorophenyl H-2, H-6), 10.86 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 21.4 (CH3), 36.4 (CH2), 56.0 (2OCH3), 60.6 (OCH3), 106.8 (trimethoxyphenyl C-2, C-6), 108.3 (pyrimidine C-5), 118.8 (phenyl C-2, C-6), 121.5 (COCH=CH), 127.9 (p-tolyl C-2, C-6), 129.3 (trimethoxyphenyl C-1), 129.9 (p-chlorophenyl C-2, C-6), 130.4 (p-chlorophenyl C-3, C-5), 130.4 (p-tolyl C-3, C-5), 130.7 (phenyl C-3, C-5), 132.8 (p-tolyl C-4), 133.3 (p-tolyl C-1), 135.1 (phenyl C-4), 136.6 (p-chlorophenyl C-1), 140.0 (p-chlorophenyl C-4), 142.0 (trimethoxyphenyl C-4), 144.0 (phenyl C-1), 144.4 (COCH=CH), 153.5 (trimethoxyphenyl C-3, C-5), 163.4 (pyrimidine C-6), 164.9 (pyrimidine C-4), 167.6 (pyrimidine C-2), 171.1 (CONH), 187.8 (CO); Anal. Calcd. for C37H32ClN3O5S (665.18): C, 66.71; H, 4.84; N, 6.31. Found: C, 66.57; H, 4.76; N, 6.37.
(ZE)-2-[4-(4-Chlorophenyl)-6-(4-nitrophenyl)pyrimidin-2-ylthio]-N-{4-[3-(4-methoxyphenyl)acryloyl]phenyl}acetamide (9m)
Yield 55%; yellow powder; mp 190–192 °C; IR (cm−1): 3403 (NH), 3066 (CH aromatic), 2927 (CH aliphatic), 1658 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.83 (s, 3H, OCH3), 4.29 (s, 2H, CH2), 7.03 (d, J= 8.8 Hz, 2H, p-methoxyphenyl H-3, H-5), 7.55 (d, J= 8.8 Hz, 2H, p-chlorophenyl H-3, H-5), 7.72 (d, J= 11.6 Hz, 1H, COCH=CH), 7.77–7.93 (m, 5H, COCH=CH, p-methoxyphenyl H-2, H-6, phenyl H-2, H-6), 8.17 (d, J= 8.8 Hz, 2H, phenyl H-3, H-5), 8.28 (d, J= 8.8 Hz, 2H, p-nitrophenyl H-2, H-6), 8.40 (d, J= 8.8 Hz, 2H, p-chlorophenyl H-2, H-6), 8.50 (s, 1H, pyrimidine H-5), 8.58 (d, J= 8.8 Hz, 2H, p-nitrophenyl H-3, H-5), 10.89 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 38.8 (CH2), 55.8 (OCH3), 109.7 (pyrimidine C-5), 114.8 (p-methoxyphenyl C-3, C-5), 118.8 (phenyl C-2, C-6), 120.1 (COCH=CH), 123.2 (p-nitrophenyl C-3, C-5), 124.3 (p-nitrophenyl C-2, C-6), 125.8 (p-chlorophenyl C-2, C-6), 127.9 (p-methoxyphenyl C-1), 129.4 (p-chlorophenyl C-3, C-5), 129.4 (p-methoxyphenyl C-2, C-6), 133.5 (phenyl C-3, C-5), 133.8 (p-chlorophenyl C-1), 134.4 (p-chlorophenyl C-4), 134.5 (phenyl C-4), 141.9 (p-nitrophenyl C-1), 144.3 (phenyl C-1), 145.4 (COCH=CH), 149.2 (p-nitrophenyl C-4), 161.8 (p-methoxyphenyl C-4), 162.4 (pyrimidine C-6), 164.6 (pyrimidine C-4), 167.8 (pyrimidine C-2), 171.5 (CONH), 187.5 (CO); Anal. Calcd. for C34H25ClN4O5S (636.12): C, 64.10; H, 3.96; N, 8.79. Found: C, 64.24; H, 4.06; N, 8.47.
(ZE)-2-[4-(4-Chlorophenyl)-6-(4-nitrophenyl)pyrimidin-2-ylthio]-N-{4-[3-(3,4-dimethoxyphenyl)acryloyl]phenyl}acetamide (9n)
Yield 53%; yellow powder; mp 147–149 °C; IR (cm−1): 3256 (NH), 3079 (CH aromatic), 2922 (CH aliphatic), 1660 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.82 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 4.29 (s, 2H, CH2), 7.03 (d, J= 8.4 Hz, 1H, dimethoxyphenyl H-5), 7.39 (d, J= 8.4 Hz, 1H, dimethoxyphenyl H-6), 7.53–7.55 (m, 3H, p-chlorophenyl H-3, H-5, dimethoxyphenyl H-2), 7.70 (d, J= 15.6 Hz, 1H, COCH=CH), 7.82–7.87 (m, 3H, p-chlorophenyl H-2, H-6, COCH=CH), 8.19 (d, J= 8.8 Hz, 2H phenyl H-2, H-6), 8.27 (d, J= 8.8 Hz, 2H phenyl H-3, H-5), 8.38 (d, J= 8.4 Hz, 2H p-nitrophenyl H-2, H-6), 8.49 (s, 1H, pyrimidine H-5), 8.57 (d, J= 8.4 Hz, 2H, p-nitrophenyl H-3, H-5), 10.88 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 36.5 (CH2), 56.0 (OCH3), 56.1 (OCH3), 109.9 (pyrimidine C-5), 111.0 (dimethoxyphenyl C-2), 111.9 (dimethoxyphenyl C-5), 118.8 (phenyl C-2, C-6), 119.8 (dimethoxyphenyl C-6), 124.2 (p-nitrophenyl C-3, C-5), 124.3 (COCH=CH), 128.0 (p-nitrophenyl C-2, C-6), 129.2 (dimethoxyphenyl C-1), 129.4 (p-chlorophenyl C-2, C-6), 129.8 (p-chlorophenyl C-3, C-5), 130.3 (phenyl C-3, C-5), 133.1 (p-chlorophenyl C-1), 134.7 (p-chlorophenyl C-4), 137.0 (phenyl C-4), 142.1 (p-nitrophenyl C-1), 143.8 (phenyl C-1), 144.4 (COCH=CH), 149.4 (p-nitrophenyl C-4), 151.6 (dimethoxyphenyl C-4), 152.3 (dimethoxyphenyl C-3), 162.7 (pyrimidine C-6), 164.1 (pyrimidine C-4), 167.5 (pyrimidine C-2), 171.6 (CONH), 187.8 (CO); Anal. Calcd. for C35H27ClN4O6S (666.13): C, 63.01; H, 4.08; N, 8.40. Found: C, 62.89; H, 4.15; N, 8.37.
(ZE)-2-[4-(4-Chlorophenyl)-6-(4-nitrophenyl)pyrimidin-2-ylthio]-N-{4-[3-(3,4,5-trimethoxyphenyl)acryloyl]phenyl}acetamide (9o)
Yield 47%; yellow powder; mp 268–270 °C; IR (cm−1): 3371 (NH), 3059 (CH aromatic), 2935 (CH aliphatic), 1656 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.72 (s, 3H, OCH3), 3.87 (s, 6H, 2OCH3), 4.29 (s, 2H, CH2), 7.24 (s, 2H, trimethoxyphenyl H-2, H-6), 7.55 (d, J= 8.8 Hz, 2H, p-chlorophenyl H-3, H-5), 7.70 (d, J= 15.6 Hz, 1H, COCH=CH), 7.87 (d, J= 8.8 Hz, 2H, p-chlorophenyl H-2, H-6), 7.92 (d, J= 15.6 Hz, 1H, COCH=CH), 8.21 (d, J= 8.8 Hz, 2H, phenyl H-2, H-6), 8.28 (d, J= 8.8 Hz, 2H, phenyl H-3, H-5), 8.39 (d, J= 8.8 Hz, 2H, p-nitrophenyl H-2, H-6), 8.51 (s, 1H, pyrimidine H-5), 8.58 (d, J= 8.8 Hz, 2H, p-nitrophenyl H-3, H-5), 10.89 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 36.5 (CH2), 56.6 (2OCH3), 60.6 (OCH3), 106.9 (trimethoxyphenyl C-2, C-6), 110.0 (pyrimidine C-5), 118.8 (phenyl C-2, C-6), 121.5 (COCH=CH), 124.3 (p-nitrophenyl C-3, C-5), 129.3 (p-nitrophenyl C-2, C-6), 129.4 (p-chlorophenyl C-2, C-6), 129.8 (p-chlorophenyl C-3, C-5), 130.5 (trimethoxyphenyl C-1), 130.8 (phenyl C-3, C-5), 132.9 (p-chlorophenyl C-1), 134.8 (p-chlorophenyl C-4), 137.0 (phenyl C-4), 140.0 (trimethoxyphenyl C-4), 142.1 (phenyl C-1), 143.9 (p-nitrophenyl C-1), 144.4 (COCH=CH), 149.5 (p-nitrophenyl C-4), 153.5 (trimethoxyphenyl C-3, C-5), 162.8 (pyrimidine C-6), 164.2 (pyrimidine C-4), 167.5 (pyrimidine C-2), 171.6 (CONH), 187.8 (CO); EIMS (m/z): 696.20 (M+, 0.11%), 86.15 (100%); Anal. Calcd. for C36H29ClN4O7S (696.14): C, 62.02; H, 4.19; N, 8.04. Found: C, 61.98; H, 4.35; N, 7.98.
(ZE)-2-{4-[4-(2-Chloroethoxyphenyl)phenyl]-6-(4-chlorophenyl)pyrimidin-2-ylthio}-N-{4-[3-(4-methoxyphenyl)acryloyl]phenyl}acetamide (9p)
Yield 52%; yellow powder; mp 136–138 °C; IR (cm−1): 3181 (NH), 3043 (CH aromatic), 2927 (CH aliphatic), 1663 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.83 (s, 3H, OCH3), 3.97 (t, J= 6.9 Hz, 2H, CH2Cl), 4.25 (s, 2H, CH2), 4.32 (t, J= 6.9 Hz, 2H, OCH2), 6.90–7.14 (m, 4H, methoxyphenyl H-3, H-5, p-chloroethoxyphenyl H-3, H-5), 7.43–7.69 (m, 10H, COCH=CH, COCH=CH, phenyl H-2, H-6, p-methoxyphenyl H-2, H-6, p-chloroethoxyphenyl H-2, H-6, p-chlorophenyl H-3, H-5), 8.12–8.35 (m, 5H, phenyl H-3, H-5, pyrimidine H-5, p-chlorophenyl H-2, H-6), 10.88 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 36.2 (CH2), 43.4 (CH2Cl), 54.6 (OCH3), 55.8 (OCH2), 107.8 (pyrimidine C-5), 114.8 (p-methoxyphenyl C-3, C-5), 115.3 (p-chloroethoxyphenyl C-3, C-5), 118.8 (phenyl C-2, C-6), 121.8 (COCH=CH), 127.4 (p-chloroethoxyphenyl C-1), 128.5 (p-methoxyphenyl C-1), 128.6 (p-chlorophenyl C-2, C-6), 129.4 (p-chlorophenyl C-3, C-5), 129.6 (p-methoxyphenyl C-2, C-6), 129.6 (p-chloroethoxyphenyl C-2, C-6), 130.7 (p-chlorophenyl C-1), 132.7 (phenyl C-3, C-5), 133.9 (p-chlorophenyl C-4), 140.1 (phenyl C-4), 143.0 (phenyl C-1), 144.4 (COCH=CH), 159.4 (p-chloroethoxyphenyl C-4), 159.9 (p-methoxyphenyl C-4), 162.8 (pyrimidine C-6), 164.3 (pyrimidine C-4), 167.3 (pyrimidine C-2), 171.4 (CONH), 187.8 (CO); Anal. Calcd. for C36H29Cl2N3O4S (669.13): C, 64.48; H, 4.36; N, 6.27. Found: C, 64.36; H, 4.19; N, 6.35.
(ZE)-2-{4-[4-(2-Chloroethoxyphenyl)phenyl]-6-(4-chlorophenyl)pyrimidin-2-ylthio}-N-{4-[3-(3,4-dimethoxyphenyl)acryloyl]phenyl}acetamide (9q)
Yield 49%; yellow powder; mp 210–212 °C; IR (cm−1): 3429 (NH), 3061 (CH aromatic), 2927 (CH aliphatic), 1663 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.72–3.82 (m, 5H, OCH3 and CH2Cl), 3.83–3.88 (m, 5H, OCH3 and OCH2), 4.34 (s, 2H, CH2), 6.98–7.15 (m, 4H, dimethoxyphenyl H-5, H-6, p-chloroethoxyphenyl H-3, H-5), 7.31–7.40 (m, 2H, dimethoxy H-2 and COCH=CH), 7.52–7.70 (m, 4H, p-chlorophenyl H-3, H-5 and p-chloroethoxyphenyl H-2, H-6), 7.75–7.88 (m, 4H, phenyl H-2, H-6 and p-chlorophenyl H-2, H-6), 7.95 (d, J= 8.8 Hz, 2H, phenyl H-3, H-5), 8.28 (d, J= 12.0 Hz, 1H, COCH=CH), 8.46 (s, 1H, pyrimidine H-5), 10.76 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 38.4 (CH2), 42.9 (CH2Cl), 56.1 (OCH3), 56.6 (OCH3), 68.6 (OCH2), 107.3 (pyrimidine C-5), 111.5 (dimethoxyphenyl C-2), 112.7 (dimethoxyphenyl C-5), 114.9 (p-chloroethoxyphenyl C-3, C-5), 121.1 (phenyl C-2, C-6), 121.3 (COCH=CH), 122.5 (dimethoxyphenyl C-6), 127.3 (dimethoxyphenyl C-1), 127.4 (p-chloroethoxyphenyl C-1), 128.1 (p-chloroethoxyphenyl C-2, C-6), 128.9 (p-chlorophenyl C-2, C-6), 129.3 (p-chlorophenyl C-3, C-5), 131.4 (phenyl C-3, C-5), 133.9 (p-chlorophenyl C-1), 134.3 (p-chlorophenyl C-4), 135.5 (phenyl C-4), 144.3 (phenyl C-1), 145.1 (COCH=CH), 149.3 (dimethoxyphenyl C-4), 149.7 (dimethoxyphenyl C-3), 151.1 (p-chloroethoxyphenyl C-4), 162.5 (pyrimidine C-6), 164.4 (pyrimidine C-4), 168.7 (pyrimidine C-2), 172.7 (CONH), 189.7 (CO); Anal. Calcd. for C37H31Cl2N3O5S (699.14): C, 63.43; H, 4.46; N, 6.00. Found: C, 63.33; H, 4.27; N, 6.11.
(ZE)-2-{4-[4-(2-Chloroethoxyphenyl)phenyl]-6-(4-chlorophenyl)pyrimidin-2-ylthio}-N-{4-[3-(3,4,5-trimethoxyphenyl)acryloyl]phenyl}acetamide (9r)
Yield 53%; yellow powder; mp 221–223 °C; IR (cm−1): 3421 (NH), 3061 (CH aromatic), 2931 (CH aliphatic), 1665 (broad, 2C=O); 1H NMR (400 MHz, DMSO-d6) δ 3.70 (s, 3H, OCH3), 3.85 (s, 6H, 2OCH3), 3.96 (t, J= 7.2 Hz, 2H, CH2Cl), 4.24 (s, 2H, CH2), 4.45 (t, J= 7.2 Hz, 2H, OCH2), 7.03–7.20 (m, 4H, trimethoxyphenyl H-2, H-6, chloroethoxyphenyl H-3, H-5), 7.29 (d, J= 12.0 Hz, 1H, COCH=CH), 7.51–7.60 (m, 4H, p-chlorophenyl H-3, H-5, chloroethoxyphenyl H-2, H-6), 7.87–7.96 (m, 3H, phenyl H-2, H-6, COCH=CH), 8.18–8.32 (m, 5H, phenyl H-3, H-5, p-chlorophenyl H-2, H-6, pyrimidine H-5), 10.91 (s, 1H, NH, D2O exchangeable); 13C NMR (100 MHz, DMSO-d6) δ 36.8 (CH2), 43.9 (CH2Cl), 55.5 (2OCH3), 57.8 (OCH2), 60.8 (OCH3), 106.7 (trimethoxyphenyl C-2, C-6), 109.4 (pyrimidine C-5), 114.9 (p-chloroethoxyphenyl C-3, C-5), 118.8 (phenyl C-2, C-6), 121.5 (COCH=CH), 126.4 (trimethoxyphenyl C-1), 127.4 (p-chloroethoxyphenyl C-1), 128.1 (p-chloroethoxyphenyl C-2, C-6), 129.5 (p-chlorophenyl C-2, C-6), 129.8 (p-chlorophenyl C-3, C-5), 132.7 (phenyl C-3, C-5), 135.4 (p-chlorophenyl C-1), 136.8 (p-chlorophenyl C-4), 140.3 (phenyl C-4), 141.7 (trimethoxyphenyl C-4), 143.0 (phenyl C-1), 144.4 (COCH=CH), 153.5 (trimethoxyphenyl C-3, C-5), 159.4 (p-chloroethoxyphenyl C-4), 162.2 (pyrimidine C-6), 164.1 (pyrimidine C-4), 167.7 (pyrimidine C-2), 171.7 (CONH), 187.7 (CO); EIMS (m/z): 728.10 (M-1, 0.28%), 58.10 (100%); Anal. Calcd. for C38H33Cl2N3O6S (729.15): C, 62.47; H, 4.55; N, 5.75. Found: C, 62.53; H, 4.76; N, 5.58.
Biological evaluations
Cytotoxic assay
To investigate cytotoxic activity of the final target compounds 9a–r, MTT assay was performed. Three different cell lines were used, leukaemia (K-562), breast (MCF-7) and colon (HT-29) cell lines. Cisplatin and erlotinib were the reference drugs used in this study. Half maximal concentration at which 50% of cells were viable was calculated as IC50 in μM, according to cytotoxic assay reported protocolCitation47.
STAT3/STAT5a assays
Both K-562 and MCF-7 cell lines were seeded overnight in plates, then 10 µM of test compounds (9a and 9r for MCF-7 cells; 9d, 9f, and 9n for K-562 cells) or reference drug pacritinib was added for 24 h. A nuclear extract kit was used to extract nuclear fractions from treated cells using the manufacture’s procedure. STAT3 and STAT5a activations were analysed using the collected nuclear extracts (20 µg) through TransAM STAT3 and STAT5a activation assay guided by the manufacture’s protocol. The obtained results were expressed in the form of mean ± SD. Each experiment was done in triplicate.
Biological properties
The target compounds 9a–r were drawn using ChemDraw Ultra 10.0. Biological properties and drug likeness were predicted using online computational tool MolinspirationCitation48.
Predicted pharmacokinetic and toxicity properties
Pharmacokinetic properties (absorption, distribution, metabolism, and excretion) through determination of human intestinal absorption (HIA), in vitro caco-2 cell permeability, in vitro Madin-Darby Canine Kidney (MDCK) cell permeability, plasma protein binding (PPB), blood–brain binding (BBB), skin permeability, p-glycoprotein (Pgp), and cytochrome p450 isoforms inhibition data, in addition to toxicity (Ames test, rodent carcinogenicity assay and hERG-inhibition) were evaluated through preADMET online serverCitation49.
Statistical analysis
Data obtained were expressed as means ± standard deviations (SDs). The results were considered significant when *p ˂ 0.05 or **p ˂ 0.005 using Student’s t-test was compared to reference drugs. The obtained values were representative of triplicate independent experiments.
Results and discussion
Chemistry
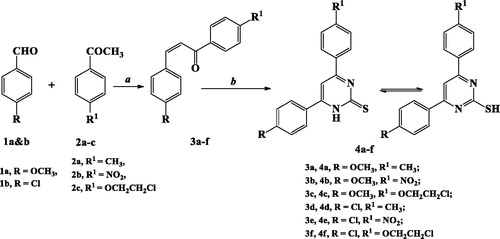
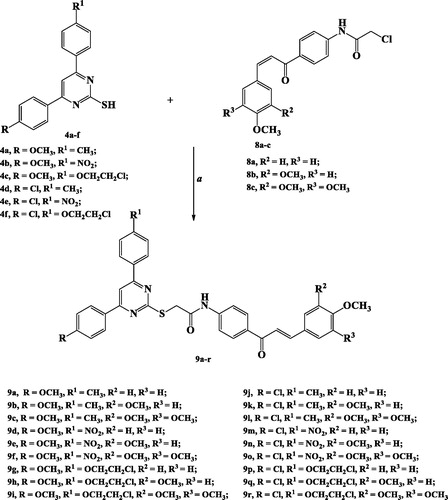
The target 2-TP/chalcone hybrids 9a–r were prepared from two synthesised starting materials 4a–f and 8a–c, as depicted in Schemes 1–3.
Heating under reflux condition chalcone derivatives 3a–f (synthesised from condensation of p-methoxy/chlorobenzaldehyde 1a&b with p-methyl/nitro/or ethoxychloroacetophenone 2a–c) and thiourea in presence of KOH afforded 2-TP derivatives 4a–f. The method was reported for compounds 4b and 4dCitation39,Citation50 (Scheme 1).
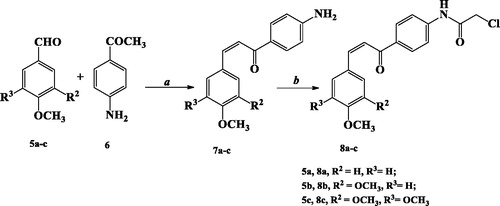
The other starting materials, chloroacetyl chalcone derivatives 8a–c were obtained by stirring p-aminochalcone derivatives 7a–c with chloroacetyl chloride, K2CO3 in chloroform at room temperatureCitation24 (Scheme 2).
S-Alkylation of 2-TPs 4a–f with acetylated chalcones 8a–c was achieved in acetonitrile using TEA as a base catalysis to obtain the target compounds 9a–r in 47–82% yield.
1H NMR and 13C NMR spectroscopic tools were used to confirm formation of the target derivatives 9a–r. Thus, 1H NMR spectra of compounds 9a–r displayed a singlet signal at δ 3.82–4.84 ppm attributed to (SCH2CO) protons. Additionally, protons of chalcone fragment appeared as two doublet signals at δ 7.29–7.72 ppm and 7.69–8.31 ppm with coupling constant J= 11.6–15.6 Hz. Furthermore, amide NH proton appeared as a singlet signal at δ 10.71–10.91 ppm.
13C NMR spectra of compounds 9a–r showed appearance of a peak at δ 36.21–43.44 ppm characterised to SCH2 carbon. Moreover, two carbonyl carbons at δ 170.71–173.07 ppm and 187.58–190.01 ppm related to (NHCO) and (C=O), respectively, were also appeared (Scheme 3).
Biological activity
Cytotoxic activity
All target compounds 9a–r were screened against three different cancer cell lines, leukaemia (K-562), breast (MCF-7), and colon (HT-29). MTT assay was used. Both cisplatin and erlotinib were used as the reference drugs. Cytotoxicity results are recorded in .
Table 1. Cytotoxicity results of pyrimidine/chalcone hybrids 9a–r against three different cancer cell lines.
Regarding cytotoxic activity of the test compounds against leukaemia (K-562) cell line, compounds 9d, 9f, 9n, and 9p were the most potent compounds with IC50 ranged from 0.77 to 1.74 μM if compared to cisplatin, the reference drug (IC50=2.31 μM). Their common feature was presence of one or more para substituted phenyl ring(s) with electron withdrawing group (NO2, Cl) at pyrimidine core.
Compounds 9b, 9e, 9g–j, 9m, and 9q exhibited potent inhibitory activity with IC50 values ranged from 3.17 to 9.71 μM, if compared to the second reference erlotinib (IC50: 9.85 μM). P-Methoxyacyloyl derivative 9k, with IC50 value = 9.95 μM, was nearly equal in potency to erlotinib. Compounds 9a, 9c, 9o, and 9r showed moderate inhibitory activity (IC50=10.67–18.40 μM). The lowest inhibitory activity was observed in compound 9l (IC50=42.60 μM), bearing p-chlorophenyl and p-tolyl rings at pyrimidine scaffold, beside, trimethoxyphenyl chalcone hybrid.
Concerning MCF-7 cell line, the most active derivative was 9r (IC50=1.37 μM) compared to the reference drug cisplatin (IC50=6.62 μM). It is characterised by presence of p-chlorophenyl ring and p-chloroethoxyphenyl ring at pyrimidine core together with trimethoxy chalcone part.
Other compounds exerted excellent activity were 9a, 9c, 9f, 9j, 9m, and 9o (IC50=3.56–6.26 μM). Additionally, compound 9q was nearly equipotent to the reference drug cisplatin (IC50=6.81 μM). Derivatives 9g and 9h with IC50=10.40 and 7.70 μM, respectively, were more potent than erlotinib (IC50=10.64 μM).
Moderate activity was observed in compounds 9d, 9e, 9i, 9l, 9n, and 9p (IC50=11.47–18.74 μM).
Compounds 9b and 9k showed weak inhibitory activity with IC50 values equal to 22.45 and 28.65 μM, respectively. Both of them have dimethoxyphenyl ring on chalcone part, and p-tolyl ring at pyrimidine core.
By inspecting cytotoxicity results of HT-29 cell line, compounds 9a, 9l, and 9n showed IC50 (2.10–2.37 μM) near in potency to the reference drug cisplatin (IC50=1.12 μM). Compounds 9c, 9e, 9j, 9k, 9m, 9o, 9q, and 9r showed significant activity with IC50 values between 3.62 and 8.70 μM, compared to erlotinib (IC50=9.20 μM). While, rest of the compounds exhibited weak inhibitory activity (IC50=9.41–25.92 μM). Results showed that no effect was observed regarding substituents on the two hybrid structures pyrimidine and chalcone.
Finally, dual cytotoxic activity was observed for compound 9f (against K-562 and MCF-7 cell lines), and for compound 9a (on MCF-7 and HT-29 cell lines) and 9n (against K-562 and HT-29 cell lines).
Cytotoxicity against normal cell line (WI38)
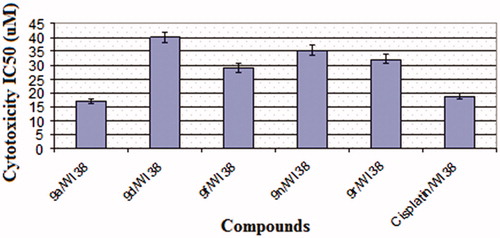
To know cytotoxicity of the most active compounds, they were tested against normal human fibroblast cell line (WI38) and IC50 values are represented as in . Cisplatin was used as a reference drug. All the test compounds showed higher IC50 values (29.19–40.13 µM) than the reference drug (18.86 µM) except compound 9a which exerted cytotoxic activity (IC50=17.09 µM) slightly less than cisplatin.
STAT3 and STAT5a inhibitory activity determination
The most active compounds in cytotoxic assay against leukaemia cell line K-562 and human breast adenocarcinoma cells MCF-7 were further tested as inhibitors for STAT3 and STAT5a enzymes. Pacritinib, an inhibitor for both STAT3 and STAT5aCitation49 was used in this study as a reference drug.
The results are listed in . They indicated that the test compounds showed inhibitory activity against both STAT3 and STAT5a. Compounds 9d, 9n, and 9r were the most active against STAT3. Additionally, compound 9n was the most effective as STAT5a inhibitor. Compounds 9a, 9f, and 9r had also strong inhibitory activity against STAT5a. Dual inhibitory activity against STAT3 and STAT5a was observed mainly in compound 9n. For this compound, both phenyl rings on pyrimidine core were para-substituted with electron withdrawing groups (NO2 and Cl), beside presence of disubstituted methoxyphenyl ring at chalcone hybrid.
Table 2. STAT3 and STAT5a inhibitory activity of compounds 9a, 9d, 9f, 9n, 9r and reference drug pacritinib.
Biological properties
Molinspiration was used to predict bioactivity scores for all the target compounds 9a–r. The obtained results are recorded in . It was found that most of the prepared compounds had bioactivity values in the range −0.5 to 0.00. This revealed that the designed pyrimidine/chalcone derivatives might be involved in moderate interactions with G-protein-coupled receptors (GPCRs) and protease inhibitors. However, the bioactivity prediction was not in the standard range against other receptors such as ion channel modulator, kinases and nuclear receptor ligand.
Table 3. Biological properties, prediction, and drug likeness of the target compounds.
Drug likeness is a complex balance between various molecular properties like, molecule size, hydrogen bonding characters, electronic distribution and hydrophobicityCitation51. The results in showed that all the final target compounds had positive predictable score values which stranded for good drug likeness behaviour, especially compound 9r as represented in .
Predicted pharmacokinetic and toxicity properties
Prediction of the major pharmacokinetic parameters such as absorption, distribution, metabolism, and excretion, in addition to toxicological properties, such as mutagenicity, carcinogenicity, and cardiac toxicity was estimated using Pharmacokinetics/PreADMET Toxicity PredictorCitation52 ().
Table 4. Pharmacokinetic properties assessment of the target synthesised compounds 9a–r.
Absorption refers to the process by which the drug can go to the systemic circulation through the organs of the body. Several routes for absorption such as oral absorption (human intestinal absorption, HIA), skin permeability (SP, logKp), and permeability through certain cells such as Caco2 (derived from human colon adenocarcinoma cells) and MDCK cells were measured.
By inspecting results recorded in , it was found that compounds 9a–r showed good intestinal absorption all above 97.25% (permissible limit: 70–100%abs). Skin permeability was found to be slightly less than acceptable range (−2.5 logKp). Moreover, moderate permeability through in vitro Caco2 cells ranged from 54.34 to 28.65 nm/sc were observed. While, low values were detected for in vitro MDCK cells.
The second property is the distribution, through which the transformation of the molecules from one tissue or organ to another can be predicted. Blood–brain barrier (BBB) and PPB were two distribution parameters used in this study. BBB permits the diffusion of hydrophobic and small molecules to the brain. It is an important predictor for central nervous system (CNS) drug discovery. Moreover, the measured of percentage of a molecule bound to plasma protein (%PPB) was also helpful in prediction of distribution for the novel target compounds.
Results showed that all the test compounds displayed strong PPB value (90.94–99.63%) indicating prolonged half-lives and limited brain penetration. Consequently, BBB (unbound brain-to-plasma ratio) was low in most compounds except in nitrophenyl containing derivatives 9d–9f and 9m–9o (0.37–0.57) which was medium and around the acceptable range to be CNS active compounds (>0.4).
Metabolism, the biotransformation or chemical modification of exogenous compounds to increase their water solubility by increasing their hydrophobicity facilitating their excretion can be predicted either in phase I or phase II. Cytochrome P450 isoforms, calculate the ability of the test compounds to be inhibitor to drug metabolising enzymes such as CYP2C19, CYP2C9, CYP2D6, CYP3A4, and CYP1A2. Moreover, glycoprotein (P-gp) inhibition measured to predict excretion property of the target compounds.
The test compounds showed good inhibitory behaviour for CYP2C9 and CYP3A4 and did not show inhibition behaviour for CYP2C19 and CYP3A4. All compounds had inhibitory effect on P-gp.
Prediction of toxicological behaviour of the test compounds was obtained by measuring AMES test (to predict mutagenicity of the compounds), carcino-Mouse/Rate (to test carcinogenicity of the compounds), and hERG-inhibition (to check cardiac toxicity of the target synthesised molecules) (). Half of test compounds showed non-mutagenic behaviour in AMES test. All compounds had negative carcinogenic effect in mouse and rats, in addition to medium risk as cardiotoxic agents. From the predicted ADMET properties of the novel synthesised compounds, it was justified that they may have good characters as lead compounds.
Table 5. Toxicity assessment of the target synthesised compounds 9a–r.
Structure–activity relationship of target compounds
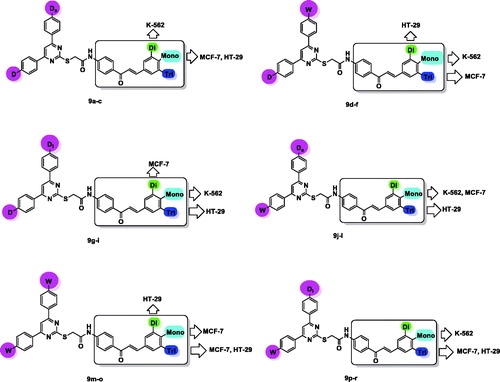
Structure–activity relationship (SAR) study for the target compounds 9a–r focussed on two important scaffolds, pyrimidine and chalcone. There was a relationship between presence of small electron donating group such as –CH3, (Ds), large electron donating group such as –OCH2CH2Cl, (Dl) or electron withdrawing group, –NO2, (W) in para position of phenyl ring at pyrimidine C-4 and electron donating group, –OCH3, (D) or electron withdrawing group, –Cl, (W) in para position of phenyl ring at pyrimidine C-6 with that of (mono-, di-, or tri-)methoxyphenyl ring of chalcone part, and between cytotoxic activities on the three different tested cell lines K-562, MCF-7, and HT-29, as represented in .
In compounds 9a–c, pyrimidine core carried p-tolyl group and p-methoxyphenyl group at C-4 and C-6, respectively. Cytotoxic activity against K-562 cell line was maximised in compound 9b bearing dimethoxyphenyl chalcone moiety then decreased in 9c and 9a (trimethoxyphenyl and methoxyphenyl chalcones, respectively).
The order of reactivity was altered when evaluated against MCF-7 or HT-29 cell lines, where 9a>9c>9b.
For compounds 9d–f, replacement of p-tolyl group at pyrimidine C-4 with p-nitrophenyl group and keeping p-methoxyphenyl group at pyrimidine C-6 constant, led to variation in cytotoxic activity. Thus, compound 9d (with p-methoxyphenyl chalcone part) was the most potent against K-562 cell line, than 9f (trimethoxyphenyl chalcone analogue) and finally 9e (dimethoxyphenyl chalcone analogue). While against MCF-7, the order was 9f>9d>9e. For HT-29, 9e was the most potent than 9f and at last 9d.
Regarding compounds 9g–i, they characterised by bearing electron donating groups at para position of two phenyl rings at pyrimidine C-4 and C-6; however, presence of large sized electron donating group such as –OCH2CH2Cl at phenyl ring of pyrimidine C-4 led to increase its lipophilic character.
The order of cytotoxic activity against K-562 was found to be 9g (methoxyphenyl chalcone)>9h (dimethoxyphenyl chalcone)>9i (trimethoxyphenyl chalcone), and for MCF-7 cell line was, 9h>9g>9i. While converted to be 9i>9g>9h in case of HT-29 cell line.
In 9j–l derivatives, electron withdrawing group (–Cl) was introduced to para position of phenyl ring at pyrimidine C-6, while pyrimidine C-4 carried small sized electron donating group (–CH3) at para position of its phenyl ring.
The most active compound was 9j (methoxyphenyl chalcone) in both K-562 and MCF-7 cell lines, while 9l (trimethoxyphenyl chalcone) was the most potent against HT-29 cell line.
Compounds 9m–o, p-tolyl ring were replaced with p-nitrophenyl ring at pyrimidine C-4, while p-chlorophenyl ring at pyrimidine C-6 was kept constant.
The most potent derivatives against K-562 were dimethoxyphenyl chalcone 9n, than methoxyphenyl chalcone derivative 9m. While, trimethoxyphenyl chalcone derivative 9o and methoxyphenyl chalcone analogue 9m showed nearly equal potency against MCF-7. For HT-29, 9n was the most potent than 9o and finally 9m.
In compounds 9p–r, incorporation of p-chloroethoxyphenyl group at pyrimidine C-4, while keeping p-chlorophenyl group at pyrimidine C-6 constant, resulted in changing order of cytotoxic activity against K-562 to be 9p (methoxyphenyl chalcone)>9q (dimethoxyphenyl chalcone), and still the least potent compound was 9r (trimethoxyphenyl chalcone). However, for MCF-7 and HT-29, it was observed that 9r was the most potent than 9q and finally 9p.
Conclusions
A novel series of 2-TP/chalcone hybrids 9a–r was designed to be as anticancer agents. They were synthesised and identified using different spectroscopic techniques. Their cytotoxic activities against three different cancerous cell lines, K-562, MCF-7, and HT-29 were evaluated. The synthesised compounds showed strong to moderate cytotoxic activities especially against K-562 and MCF-7 cell lines. The highest cytotoxic activity against K-562 cell line was observed in compounds 9d, 9f, 9n, and 9p with IC50 values in the range of 0.77–1.74 µM, compared to the reference drug, cisplatin (IC50=2.31 µM). For cytotoxic activity against MCF-7 cell line, compounds 9a, 9c, 9f, 9j, 9m, 9o, and 9r exhibited the highest activities with IC50 values of 1.37–6.26 µM (cisplatin IC50=6.62 µM). While, moderate cytotoxic activity was noticed for test compounds against colon HT-29 cell line. The most potent derivatives between them were 9a, 9l, and 9n (IC50=2.10–2.37 µM), if compared with cisplatin (IC50=1.12 µM).
The most active derivatives 9a, 9d, 9f, 9n, and 9r (either against K-562 and/or MCF-7 cell lines) were selected for further evaluation against human normal fibroblast cells (WI38). All of them had IC50 values (29.19–40.13 µM) higher than that of the reference cisplatin (IC50=18.86 µM), except 9a analogue (IC50=17.09 µM) which was slightly less than cisplatin.
Moreover, STAT3 and STAT5a inhibitory activities were determined for the five later compounds. Compounds 9d and 9n showed remarkable inhibitory activity against STAT3, while, compounds 9a, 9f, 9n, and 9r were the most effective at inhibiting STAT5a. Dual inhibitory activity at STAT3 and STAT5a was observed in compound 9n which beared p-nitrophenyl and p-chlorophenyl rings at pyrimidine core in addition to dimethoxyphenyl at chalcone part. On the other hand, physicochemical properties, drug likeness scores, pharmacokinetics and toxicity properties were predicted for all the synthesised compounds 9a–r.
Supplemental Material
Download PDF (3.9 MB)Acknowledgements
The authors thank Dr. Essam Rashwan, head of the confirmatory diagnostic unit VACSERA-Egypt for evaluation of anticancer activity and enzyme assays.
Disclosure statement
The authors declare that there is no any conflict of interest.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics. CA Cancer J Clin 2015;65:87–108.
- Rao YK, Fang SH, Tzeng YM. Differential effects of synthesized 2′-oxygenated chalcone derivatives: modulation of human cell cycle phase distribution. Bioorg Med Chem 2004;12:2679–86.
- Roco A, Quinones L, Acevedo C, et al. Situacion del cancer en Chile 2000–2010. Cuad Med Social 2013;53:83–94.
- Rajak H, Deshmukh R, Veerasamy R, et al. Novel semicarbazones based 25-disubstituted-1,3,4-oxadiazoles: one more step towards establishing four binding site pharmacophoric model hypothesis for anticonvulsant activity. Bioorg Med Chem Lett 2010;20:4168–72.
- Marmol I, Sanchez-De-Diego C, Dieste AP, et al. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci 2017;18:E197.
- Stewart B, Wild C. World cancer report 2014. World Health Organization; 2014.
- Sawyers CL, Denny CT, Witte ON. Leukemia and the disruption of normal hematopoiesis. Cell 1991;64:337–50.
- Gleixner KV, Schneeweiss M, Eisenwort G, et al. Combined targeting of STAT3 and STAT5: a novel approach to overcome drug resistance in chronic myeloid leukemia. Haematologica 2017;102:1519–29.
- Pallis M, Turzanski J, Higashi Y, Russell N. P-glycoprotein in acute myeloid leukaemia: therapeutic implications of its association with both a multidrug-resistant and an apoptosis-resistant phenotype. Leuk Lymphoma 2002;43:1221–8.
- Ghiaur G, Wroblewski M, Loges S. Acute myelogenous leukemia and its microenvironment: a molecular conversation. Semin Hematol 2015;52:200–6.
- Bosc C, Selak MA, Sarry J-E. Resistance is futile: targeting mitochondrial energetics and metabolism to overcome drug resistance in cancer treatment. Cell Metab 2017;26:705–7.
- Coolbrandt A, Van den Heede K, Vanhove E, et al. Immediate versus delayed self-reporting of symptoms and side effects during chemotherapy: does timing matter? Eur J Oncol Nurs 2011;15:130–6.
- McMurray JS, Ren Z, David C, et al. Inhibitors of signal transducer and activator of transcription 3. US 0010428 A1; 2007.
- Mandal PK, Gao F, Lu Z, et al. Potent and selective phosphopeptide mimetic prodrugs targeted to the src homology 2 (SH2) domain of signal transducer and activator of transcription 3. J Med Chem 2011;54:3549–63.
- Turkson J, Gunning PT. 2-(9h-Purin-9-yl) acetic acid analogues as inhibitors of STAT proteins: University of Central Florida Research Foundation, University of Toronto Mississauga. Patent WO 163424 A2; 2011.
- Siddiquee K, Zhang S, Guida WC, et al. Selective chemical probe inhibitor of STAT3, identified through structure-based virtual screening, induces antitumour activity. Proc Natl Acad Sci USA 2007;104:7391–6.
- Segatto I, Baldassarre G, Belletti B. STAT3 in breast cancer onset and progression: a matter of time and context. Int J Mol Sci 2018;19:2818–27.
- Furqan M, Mukhi N, Lee B, et al. Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res 2013;1:5–15.
- Ping-Shan L, David AR, Ahmed MA, et al. A STAT inhibitor patent review: progress since 2011. Expert Opin Ther Patents 2015;25:12–37.
- Furqan M, Akinleye A, Mukhi N, et al. STAT inhibitors for cancer therapy. J Hematol Oncol 2013;6:90.
- Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res 2002;8:945–54.
- Weaver AM, Silva CM. Signal transducer and activator of transcription 5b: a new target of breast tumor kinase/protein tyrosine kinase 6. Breast Cancer Res 2007;9:R79–89.
- Jatiani SS, Baker SJ, Silverman LR, et al. JAK/STAT pathways in cytokine signaling and myelo-proliferative disorders: approaches for targeted therapies. Genes Cancer 2011;1:979–93.
- Fathi MAA, Abd El-Hafeez AA, Abdelhamid D, et al. 1,3,4-Oxadiazole/chalcone hybrids: design, synthesis, and inhibition of leukemia cell growth and EGFR, Src, IL-6 and STAT3 activities. Bioorg Chem 2019;84:150–63.
- Yu H, Jove R. The STATs of cancer – new molecular targets come of age. Nat Rev Cancer 2004;4:97–105.
- Zhang X, Yue P, Fletcher S, et al. A novel small-molecule disrupts STAT3 SH2 domain-phosphotyrosine interactions and STAT3-dependent tumor processes. Biochem Pharmacol 2010;79:1398–409.
- Li PK, Li C, Lin J, et al. Curcumin analogs as dual JAK2/STAT3 Inhibitors and methods of making and using the same. The Ohio State University Research Foundation. US 0053208 A1; 2012.
- Novilla A, Mustofa M, Astuti I, et al. Cytotoxic activity of methoxy-4′-amino chalcone derivatives against leukemia cell lines. Mol Cell Biomed Sci 2019;3:34–41.
- Bagul C, Rao GK, Makani VKK, et al. Synthesis and biological evaluation of chalcone linked pyrazolo[1,5-a]pyrimidines as potential anticancer agents. Med Chem Commun 2017;8:1810–6.
- Page BDG, Khoury H, Laister RC, et al. Small molecule STAT5-SH2 domain inhibitors exhibit potent antileukemia activity. J Med Chem 2012;55:1047–55.
- Ahmed NM, Mohamed MS. Synthesis and biological value of thiouracils and fused thiouracils (a review). J Adv Pharm Res 2017;1:75–88.
- Prachayasittikul S, Worachartcheewan A, Nantasenamat C, et al. Synthesis and structure activity relationship of 2-thiopyrimidine-4-one analogs as antimicrobial and anticancer agents. Eur J Med Chem 2011;46:738–42.
- Sondhi SM, Goyal RN, Lahoti AM, et al. Synthesis and biological evaluation of 2-thiopyrimidine derivatives. Bioorg Med Chem 2005;13:3185–95.
- Yuh-Wen H, Maw CS. Thioxopyrimidine in heterocyclic synthesis I: synthesis of some novel 6-(heteroatom-substituted)-(thio)pyrimidine derivatives. J Chem 2013;1:1–15.
- Angelo R, Andre S, Silvia S, et al. Synthesis and antiproliferative activity of basic thioanalogues of merbarone. Bioorg Med Chem 2003;11:2575–89.
- Chen H, Tsalkova T, Mei FC, et al. 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg Med Chem Lett 2012;22:4038–43.
- Oluropo CA, Olugbeminiyi OF, Adamson SF, et al. Synthesis and in-vitro cytotoxicity evaluation of some fluorinated hexahydropyrimidine derivatives. Bioorg Med Chem Lett 2011;21:989–92.
- Mosaad SM, Samir MA, Omar AF, et al. Synthesis of new pyrimidine derivatives and their antiproliferative activity against selected human cancer cell lines. Res Chem Intermed 2015;41:1789–801.
- Narwal S, Kumar S, Verma PK. Design, synthesis and antimicrobial evaluation of pyrimidin-2-ol/thiol/amine analogues. Chem Cent J 2017;11:52–61.
- Al-Hazam HA, Al-Shamkani ZA, Al-Masoudia NA, et al. New chalcones and thiopyrimidine analogues derived from mefenamic acid: microwave-assisted synthesis, anti-HIV activity and cytotoxicity as antileukemic agents. Z Naturforsch 2017;72:1–8.
- Al-Masoudi NA, Kadhim RA, Abdul-Rida NA, et al. New biaryl-chalcone derivatives of pregnenolone via Suzuki–Miyaura cross-coupling reaction. Synthesis, CYP17 hydroxylase inhibition activity, QSAR, and molecular docking study. Steroids 2015;101:43–50.
- Amor EC, Villasenor IM, Antemano R, et al. Cytotoxic C-methylated chalcones from Syzygium samarangense. Pharm Biol 2007;45:777–83.
- Zhang EH, Wang RF, Guo SZ, et al. An update on antitumor activity of naturally occurring chalcones. J Evid Based Complement Altern Med 2013;2013:1–22.
- Peng F, Meng CW, Zhou QM, et al. Cytotoxic evaluation against breast cancer cells of isoliquiritigenin analogues from Spatholobus suberectus and their synthetic derivatives. J Nat Prod 2016;79:248–51.
- Ahmed FF, Abd El-Hafeez AA, Abbas SH, et al. New 1,2,4-triazole-chalcone hybrids induce caspase-3 dependent apoptosis in A549 human lung adenocarcinoma cells. Eur J Med Chem 2018;151:705–22.
- Lamie PF, Philoppes JN. Design and synthesis of three series of novel antitumor – azo derivatives. Med Chem Res 2017;26:1228–40.
- Philoppes JN, Lamie PF. Design and synthesis of new benzoxazole/benzothiazole-phthalimide hybrids as antitumor-apoptotic agents. Bioorg Chem 2019;89:102978–91.
- Molinspiration Cheminformatics. Nova ulica, SK-900 26 Slovensky Grob, Slovak Republic. Available from: http://www.molinspiration.com/.
- PreADMET is a web-based application for predicting ADME data and building drug-like library using in silico method. Available from: https://preadmet.bmdrc.kr/.
- Stefani HA, Oliveira CB, Almeida RB, et al. Dihydropyrimidin-(2H)-ones obtained by ultrasound irradiation: a new class of potential antioxidant agents. Eur J Med Chem 2006;41:513–8.
- Walters WP, Ajay , Murcko MA. Recognizing molecules with drug-like properties. Curr Opin Chem Biol 1999;3:384–7.
- Derenzini E, Younes A. Targeting the JAK-STAT pathway in lymphoma: a focus on pacritinib. Expert Opin Investig Drugs 2013;22:775–85.