Abstract
Sulphonamides are biologically important compounds with low toxicity, many bioactivities and cost-effectiveness. Eight sulphonamide derivatives were synthesised and characterised by FT-IR, 13C NMR, 1H NMR, LC-MS and elemental analysis. Their inhibitory effect on AChE, and carbonic anhydrase I and II enzyme activities was investigated. Their antioxidant activity was determined using different bioanalytical assays such as radical scavenging tests with ABTS•+, and DPPH•+ as well as metal-reducing abilities with CUPRAC, and FRAP assays. All compounds showed satisfactory enzyme inhibitory potency in nanomolar concentrations against AChE and CA isoforms with KI values ranging from 10.14 ± 0.03 to 100.58 ± 1.90 nM. Amine group containing derivatives showed high metal reduction activity and about 70% ABTS radical scavenging activity. Due to their antioxidant activity and AChE inhibition, these novel compounds may be considered as leads for investigations in neurodegenerative diseases.
1. Introduction
Free radicals are molecules with unpaired electrons resulting from biochemical redox reactions that occur during cell metabolismCitation1,Citation2. The free radicals, which cause oxidation, affect important biomolecules such as lipids, proteins, DNA and carbohydrates, and cause the disruption of their structureCitation3,Citation4. Among the reactive oxygen species (ROS) produced in biological systems, free radicals such as hydroxyl radical (OH•), nitric oxide (NO) and peroxyl radical (RCOO•) are the most important factors in the induction of oxidative stressCitation5. Cells can normally reduce moderate oxidative stress through the antioxidant defence system. However, when oxidative stress reaches high levels, oxidative damage may occur in the cell if adaptation to oxidation products cannot be achieved. The association of oxidative damage of all biomolecules including protein, DNA and lipids with many diseases such as diabetes mellitus, cardiovascular, neurodegenerative and cancer increased the interest in antioxidant studiesCitation6–9. It is known that enzymatic and non-enzymatic antioxidant systems play an important role in the elimination of free radicals and metabolic products and in the prevention of various diseases and maintenance of normal cellular physiologyCitation10,Citation11. Antioxidants continue to attract attention because of their importance in the prevention and treatment of diseases such as coronary heart and neurodegenerative. The antioxidants can be defined as substances that prevent or delay the oxidation of a substance, although it is less common than an oxidisable substance in the same medium. Phenolic compounds, which have an important role in antioxidant activity, remove free radicals and prevent tissue damage due to their chemical structure containing a hydroxyl group (–OH) directly linked to an aromatic hydrocarbon ringCitation12,Citation13. It is known that the increase in the activity of many metabolically important enzymes may cause increased oxidative stress. Therefore, it is of great importance to design and develop new inhibitors for such enzymes. Pharmacological inhibitors of mitochondrial carbonic anhydrase have been reported to be useful in protecting against oxidative stress, which is an important cause in the development of many diseasesCitation14. Furthermore, the increase in the activity of the neurotransmitter acetylcholine hydrolysing AChE plays a role in the formation of β-amyloid (Aβ) deposited in extracellular toxic plaques in the brains of Alzheimer’s patientsCitation15,Citation16.
Many known synthetic antioxidants are most commonly used in food additives and pharmaceutical additives to prevent oxidation. In recent years, many side effects of synthetic antioxidants have raised concerns. In this case, there is a worldwide trend towards the use of safe antioxidants. Therefore, it is important to find new sources for the synthesis of safer and inexpensive antioxidantsCitation17–19.
Some sulphonamide derivatives were screened for their antioxidant activity, which showed good resultsCitation20–23. Sulphonamides chemically containing sulfamoyl (–SO2NH–) group are derivatives of amide. The first sulphonamide drug identified in 1932 was the prontosil and used as antibacterial agent and since then sulphonamides are most widely used in the world among groups of anti-infectives. Sulphonamides are a biologically significant group of compounds due to well absorption orally and excrete in urine, thus sulphonamides have less toxicity, increased reactivity and are cost-effective moleculesCitation24–26. Today, sulphonamides are widely used as antimicrobialCitation27,Citation28, anti-inflammatoryCitation29,Citation30, anticancerCitation31–36 and anti-viral agents as well as HIV protease inhibitorCitation37, anti-obesityCitation38, anti-thyroidCitation39, and also act as a potent Carbonic anhydrase hCA inhibitorsCitation40,Citation41.
There is considerable interest in the chemistry of Schiff base compounds by virtue of having applications in biological, industrial, pharmaceutical and many other fields of scienceCitation42. Sulphonamide Schiff base derivatives could be obtained by condensation of sulphonamide compounds with at least –NH2 group and aldehyde and this could led to biologically active compoundsCitation43. Schiff base derivatives obtained from sulfo drugs drug have drawn attention due to their biological propertiesCitation44.
Here we report the synthesis of compounds having sulphonamide and Schiff base moieties. By keeping in mind, the immense biological significance of sulphonamides and Schiff bases, novel derivatives were synthesised. The inhibitory effect of newly synthesised compounds on hCA I, hCA II, and AChE enzyme activities were investigated and then antioxidant activity was determined using radical scavenging tests with ABTS•+, and DPPH•+ and metal-reducing abilities with CUPRAC, and FRAP assays.
2. Methods and materials
2.1. General
All the chemicals were obtained from commercial suppliers (Merck, Sigma-Aldrich) and were used as received. Diethyl ether, chloroform, tetrahydrofuran (THF), Dichloromethane (DCM), dimethylformamide (DMF), methanol and ethanol were used as solvents. Reagents used were 3-Aminobenzenesulfonamide, 4-Aminobenzenesulfonamide, 3-Bromo-2-hydroxybenzaldehyde and 3-Chloro-2-hydroxybenzaldehyde, acetic acid and formic acid. Potassium ferrocyanide, trichloroacetic acid (TCA), iron III chloride, ammonium acetate, neocuproine, 1,1-Diphenyl-2-picrylhydrazine and 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid was used for antioxidant studies.
2.2. Instrumentation and measurements
Elemental analyses were carried out using a LECO CHNS-932 Elemental Analyser. FT-IR spectra were recorded on a Perkin Elmer Spectrum Two FT-IR Spectrometer in the region 400–4000 cm−1. A Shimadzu UV-1208 UV-Vis Spectrophotometer was used for the absorption spectra measurements of range 200–1100 nm in DMF at room temperature. NMR spectra were recorded on an Agilent 400 MR (1H NMR 400 MHz and 13C NMR 100 MHz) in DMSO-d6, with TMS as an internal standard, at 25 °C. Melting points of compounds were measured in open capillary tubes using an SMP3, Stuart Scientific Melting Point Apparatus. A Shimadzu LC MS 8040 Model Spectrometer was used for recording mass spectra. The course of reactions and product purities were assessed using TLC plates (Merck Silica Gel 60 F254).
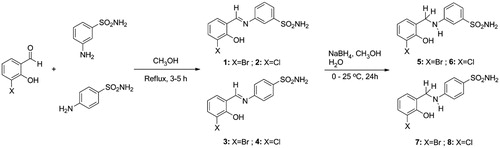
2.3. General procedure for the synthesis of imine derivatives (1–4)
To synthesise imino-derivatives, aromatic aldehyde derivatives (10 mmol) were dissolved in methanol (30 ml) and these solutions were added dropwise to the relevant sulphonamide solutions (10 mmol) dissolved in methanol (30 ml). A catalytic amount of formic acid was added, and the reaction stirred for 3–5 h under reflux. Reactions were monitored through IR spectroscopy and TLC, after completion the solvent was evaporated. The obtained solid was washed with ice-cold ethanol. Then, the obtained products were recrystallized from methanol/ethanol and dried under vacuum to give the corresponding products.
2.3.1. 3-((3-Bromo-2-hydroxybenzylidene)amino)benzenesulfonamide (1)
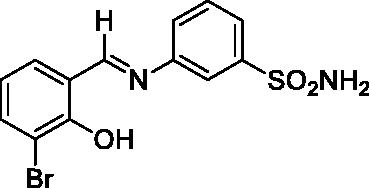
Yield: % 85; Colour: Orange; Melting Point: 206–208°C; Anal. Calcd for C13H11BrN2O3S (355.21 g/mol) (%): C, 43.96; H, 3.12; N, 7.89; S, 9.03, Found (%): C, 43.88; H, 3.08; N, 7.99; S, 8.93. FT-IR (U-ATR, υmax/cm−1): 3296, 3225 (NH2), 3130–3360 (O–H⋯N broad), 3085, 3056 (Ar-H), 1611 (–C=N–), 1333 (asymmetric), 1149 (symmetric) (S=O). 1H-NMR (DMSO-d6, TMS, 400 MHz, δ ppm): 14.05 (1H, s, Ar-OH), 9.05 (1H, s, –CH=N–), 7.44 (2H, s, –SO2NH2), 7.87 (1H, s, Ar-H), 7.77–7.64 (4H, m, Ar-H), 7.01–6.89 (2H, m, Ar-H). 13C-NMR (DMSO-d6, TMS, 100 MHz, δ ppm): 165.38 (–C=N–), 157.63 (Ar-C-OH), 147.68 (Ar-C-N), 145.86 (Ar-C–SO2NH2), 137.23, 133.69, 131.28, 125.71, 124.80, 121.55, 120.33, 118.99, 110.42 (Aromatic Carbons). LC-MS Mass (m/z): Monoisotopic Mass: 353.97; [M + H]+: 355.00
2.3.2. 3-((3-Chloro-2-hydroxybenzylidene)amino)benzenesulfonamide (2)
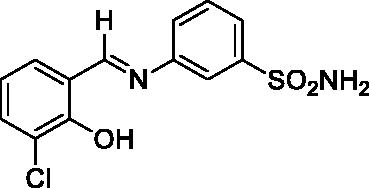
Yield: % 85; Colour: Dark yellow; Melting Point: 205–207°C; Anal. Calcd for C13H11ClN2O3S (310.76 g/mol) (%): C, 50.24; H, 3.57; N, 9.01; S, 10.32, Found (%): C, 50.18; H, 3.52; N, 9.12; S, 10.21. FT-IR (U-ATR, υmax/cm−1): 3359, 3266 (NH2), 3115–3410 (O–H⋯N broad), 3085, 3061 (Ar-H), 1617 (–C=N–), 1282 (asymmetric), 1139 (symmetric) (S=O). 1H-NMR (DMSO-d6, TMS, 400 MHz, δ ppm): 13.89 (1H, s, Ar-OH), 9.07 (1H, s, –CH=N–), 7.44 (2H, s, –SO2NH2), 7.87 (1H, s, Ar-H), 7.78–7.76 (1H, d, J = 8, Ar-H), 7.72–7.64 (3H, m, Ar-H), 7.60–7.58 (1H, d, J = 8, Ar-H), 7.02–6.98 (1H, t, J = 8, Ar-H).13C-NMR (DMSO-d6, TMS, 100 MHz, δ ppm): 165.46 (–C=N–), 156.69 (Ar-C-OH), 147.82 (Ar-C-N), 145.84 (Ar-C–SO2NH2), 134.49, 132.94, 131.31, 130.28, 125.67, 124.91, 121.02, 120.46, 119.43 (Other Aromatic Carbons). LC-MS Mass (m/z): Monoisotopic Mass: 310.02; [M + H]+: 311.00
2.3.3. 4-((3-Bromo-2-hydroxybenzylidene)amino)benzenesulfonamide (3)
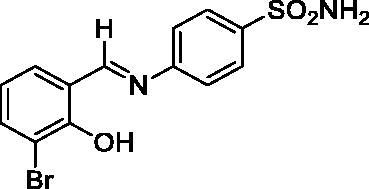
Yield: % 85; Colour: Red; Melting Point: 199–201 °C; Anal. Calcd for C13H11BrN2O3S (355.21 g/mol) (%):C, 43.96; H, 3.12; N, 7.89; S, 9.03, Found (%): C, 43.89; H, 3.08; N, 7.96; S, 8.97. FT-IR (U-ATR, υmax/cm−1): 3303, 3238 (NH2), 3140–3420 (O-H⋯N broad), 3064, 3038 (Ar-C-H), 1616 (–C=N–), 1327 (asymmetric), 1158 (symmetric) (S=O). 1H-NMR (DMSO-d6, TMS, 400 MHz, δ ppm): 14.06 (1H, s, Ar-OH), 9.04 (1H, s, -CH=N–), 7.42 (2H, s, –SO2NH2), 7.89–7.87 (2H, d, J = 8, Ar-H), 7.76–7,74(1H, d, J = 8, Ar-H), 7.68–7.66 (1H, d, J = 8, Ar-H), 7.63–7.61 (2H, d, J = 8, Ar-H), 6.97–6.93 (1H, t, J = 8, Ar-H). 13C-NMR (DMSO-d6, TMS, 100 MHz, δ ppm): 165.88 (–C=N–), 157.78 (Ar-C-OH), 149.96 (Ar-C-N), 142.96 (Ar-C–SO2NH2), 137.13, 133.28, 127.55, 123.03, 121.84, 120.19, 110.49 (Other Aromatic Carbons). LC-MS Mass (m/z): Monoisotopic Mass: 353.97; [M + H]+: 355.00
2.3.4. 4-((3-Chloro-2-hydroxybenzylidene)amino)benzenesulfonamide (4)
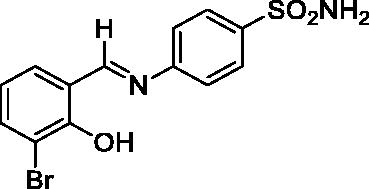
Yield: % 85; Colour: Red; Melting Point: 207–209 °C; Anal. Calcd for C13H11ClN2O3S (310.76 g/mol) (%): C, 50.24; H, 3.57; N, 9.01; S, 10.32, Found (%): C, 50.18; H, 3.52; N, 9.10; S, 10.24. FT-IR (U-ATR, υmax/cm−1): 3311, 3230 (NH2), 3140–3400 (O-H⋯N broad), 3064, 3032 (Ar-C-H), 1616 (–C=N–), 1330 (asymmetric), 1162 (symmetric) (S=O). 1H-NMR (DMSO-d6, TMS, 400 MHz, δ ppm): 13.89 (1H, s, Ar-OH), 9.04 (1H, s, -CH=N–), 7.41 (2H, s, –SO2NH2), 7.90–7.88 (2H, d, J = 8, Ar-H), 7.63–7.58 (4H, m, Ar-H) , 7.02–6.98 (1H, t, J = 8, Ar-H). 13C-NMR (DMSO-d6, TMS, 100 MHz, δ ppm): 165.85 (–C=N–), 156.83 (Ar-C-OH), 150.09 (Ar-C-N), 142.96 (Ar-C–SO2NH2), 134.52, 131.96, 127.42, 122.99, 121.92, 120.64, 119.40 (Other Aromatic Carbons). LC-MS Mass (m/z): Monoisotopic Mass: 310.02; [M + H]+: 311.00
2.4. General procedure for the synthesis of amine derivatives (5–8)
To synthesise amino-derivatives (5–8), sodium borohydride (NaBH4) (70 mmol) was added in small portions to imino-compounds (1–4) (10 mmol) dissolved in methanol (60 ml) at 0 °C, over 1 h. The mixture was left under stirring for 24 more hours at room temperature and reductions were monitored through IR spectroscopy and TLC. After the reduction was complete, half of the solvent in the reaction mixture was evaporated and the remaining mixture was poured on ice than the precipitate was filtered and extracted with DCM and chloroform. The solvent was then evaporated and the obtained products were recrystallized from ethanol/methanol (30/70) and dried under vacuum.
2.4.1. 3-((3-Bromo-2-hydroxybenzyl)amino)benzenesulfonamide (5)
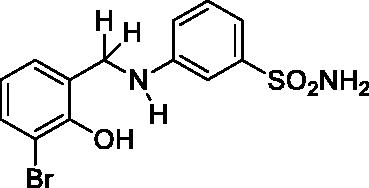
Yield: % 60; Colour: White; Melting Point: 123–124 °C; Anal. Calcd for C13H13BrN2O3S (357.22 g/mol) (%): C, 43.71; H, 3.67; N, 7.84; S, 8.98, Found (%): C, 43.63; H, 3.58; N, 7.93; S, 8.91. FT-IR (U-ATR, υmax/cm−1): 3355, 3335 (NH, NH2), 3255 (OH), 3114, 3066 (Ar-H), 2840–2985 (Aliphatic –C–H), 1611 (–C=N–) (disappeared), 1330, 1315 (asymmetric), 1155 (symmetric) (S=O). 1H-NMR (DMSO-d6, TMS, 400 MHz, δ ppm): 3.00–5.50 (2H, broad, Ar-OH and N-H), 7.32–7.30 (1H, d, J = 8, Ar-H), 7.18–7.14 (1H, t, J = 8, Ar-H), 7.10–7.08 (1H, d, J = 8, Ar-H), 7.18–7.10 (2H, broad, –SO2NH2), 7.02 (1H, s, Ar-H), 6.93–6.91 (1H, t, J = 8, Ar-H), 6.67–6.65 (1H, t, J = 8, Ar-H), 6.62–6.59 (1H, t, J = 8, Ar-H), 4.29 (2H, s, Ar-CH2). 13C-NMR (DMSO-d6, TMS, 100 MHz, δ ppm): 153.59 (Ar-C-OH), 149.26 (Ar-C-N), 145.13, 131.79, 130.25, 129.23, 128.03, 120.14, 114.52, 113.62, 112.43, 109.74 (Other Aromatic Carbons), 42.81 (Ar-CH2-N). LC-MS Mass (m/z): Monoisotopic Mass: 355.98; [M + H]+: 357.00
2.4.2. 3-((3-Chloro-2-hydroxybenzyl)amino)benzenesulfonamide (6)
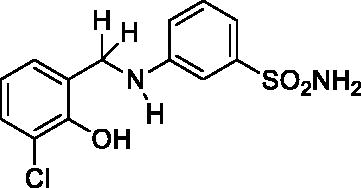
Yield: % 60; Colour: White; Melting Point: 121–122 °C; Anal. Calcd for C13H13ClN2O3S (312.77 g/mol) (%): C, 49.92; H, 4.19; N, 8.96; S, 10.25, Found (%): C, 50.01; H, 4.12; N, 9.01; S, 10.16. FT-IR (U-ATR, υmax/cm−1): 3344, 3324 (NH, NH2), 3253 (OH), 3111, 3078 (Ar-C-H), 2860–2995 (Aliphatic –C–H), 1617 (–C=N–) (disappeared), 1325, 1315 (asymmetric), 1153, 1138 (symmetric) (S=O). 1H-NMR (DMSO-d6, TMS, 400 MHz, δ ppm): 3.00–5.00 (2H, broad, Ar-OH and N-H), 7.22–7.16 (4H, m, Ar-H and –SO2NH2), 7.11–7.09 (1H, d, J = 8, Ar-H), 7.02 (1H, s, Ar-H), 6.96–6.94 (1H, t, J = 8, Ar-H), 6.78–6.74 (1H, t, J = 8, Ar-H), 6.68–6.66 (1H, t, J = 8, Ar-H), 4.27 (2H, s, Ar-CH2). 13C-NMR (DMSO-d6, TMS, 100 MHz, δ ppm): 151.03(Ar-C-OH), 149.20 (Ar-C-N), 145.17, 130.28, 129.00, 127.46, 121.10, 119.83, 114.52, 113.34, 112.31, 109.62 (Other Aromatic Carbons), 42.18 (Ar-CH2-N). LC-MS Mass (m/z): Monoisotopic Mass: 312.03; [M + H]+: 313.00
2.4.3. 4-((3-Bromo-2-hydroxybenzyl)amino)benzenesulfonamide (7)
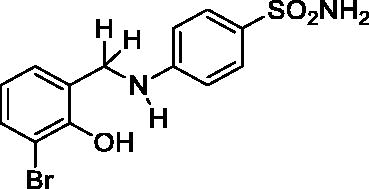
Yield: % 60; Colour: White; Melting Point: 170–172 °C; Anal. Calcd for C13H13BrN2O3S (357.22 g/mol) (%):C, 43.71; H, 3.67; N, 7.84; S, 8.98, Found (%): C, 43.62; H, 3.58; N, 7.93; S, 8.93. FT-IR (U-ATR, υmax/cm−1): 3368, 3348 (NH, NH2), 3254 (OH), 3116, 3066 (Ar-C-H), 2840–2985 (Aliphatic –C–H), 1616 (–C=N–) (disappeared), 1338 (asymmetric), 1135 (symmetric) (S=O). 1H-NMR (DMSO-d6, TMS, 400 MHz, δ ppm): 3.50–5.00 (2H, broad, Ar-OH and N-H), 6.87 (2H, s, –SO2NH2), 7.45–7.43 (2H, d, J = 8, Ar-H), 7.32–7.30 (1H, d, J = 8, Ar-H), 7.07–7.05 (1H, d, J = 8, Ar-H), 6.61–6.56 (3H, m, Ar-H), 4.32 (2H, s, Ar-CH2). 13C-NMR (DMSO-d6, TMS, 100 MHz, δ ppm): 153.97 (Ar-C-OH), 151.65 (Ar-C-N), 131.76,.130.48, 129.06, 128.32, 127.15, 119.84, 112.65, 111.12 (Other Aromatic Carbons), 42.47 (Ar-CH2-N). LC-MS Mass (m/z): Monoisotopic Mass: 355.98; [M + H]+: 357.00
2.4.4. 4-((3-Chloro-2-hydroxybenzyl)amino)benzenesulfonamide (8)
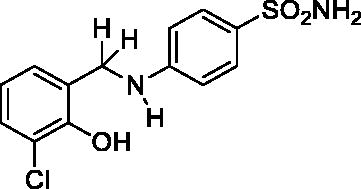
Yield: % 60; Colour: White; Melting Point: 180–182 °C; Anal. Calcd for C13H13ClN2O3S (312.77 g/mol) (%): C, 49.92; H, 4.19; N, 8.96; S, 10.25, Found (%):C, 50.01; H, 4.10; N, 9.01; S, 10.16. FT-IR (U-ATR, υmax/cm−1): 3362 3347 (NH, NH2), 3254 (OH), 3125, 3075 (Ar-C-H), 2880–2960 (Aliphatic -C-H), 1616 (–C=N–) (disappeared), 1308 (asymmetric), 1135 (symmetric) (S=O). 1H-NMR (DMSO-d6, TMS, 400 MHz, δ ppm): 3.50–5.00 (2H, broad, Ar-OH and N-H), 6.88 (2H, s, –SO2NH2), 7.45–7.43 (2H, d, J = 8, Ar-H), 7.16–7.14 (1H, d, J = 8, Ar-H), 7.03–7.01 (1H, d, J = 8, Ar-H), 6.66–6.62 (1H, t, J = 8, Ar-H), 6.58–6.56 (2H, d, J = 8, Ar-H), 4.30 (2H, s, Ar-CH2). 13C-NMR (DMSO-d6, TMS, 100 MHz, δ ppm): 153.02 (Ar-C-OH), 151.71 (Ar-C-N), 130.40,129.01, 128.31, 127.18, 121.83, 119.17, 111.70, 111.08 (Other Aromatic Carbons), 42.20 (Ar-CH2-N). LC-MS Mass (m/z): Monoisotopic Mass: 312.03; [M + H]+: 313.00.
2.5. Evaluation of synthesised compounds bioactivity
2.5.1. Metal reducing antioxidant power assay as CUPRAC and FRAP
The power by metal-reducing of novel synthesised sulphonamide derivatives were determined by modified Oyaizu methodCitation45,Citation46. Different amounts of derivatives in 1 ml of ethanol were mixed with 0.5 ml of phosphate buffer (0.2 M, pH 6.6) and 0.5 ml of 1% potassium ferricyanide (K3Fe(CN)6). After the mixtures were incubated at 50 °C for 20 min, trichloroacetic acid (0.5 ml, 10%) was added to each mixture and centrifuged (at 1,008 G for 10 min.). The upper layers of the resulting solutions (0.5 ml) were first mixed with distilled water (0.5 ml) and then FeCl3 (0.1 ml, 0.1%) in the given order. absorbances were measured at 700 nm. The high absorbance of the reaction in the mixture shows that the reducing power is increased.
The reduction capacity for cupric ions (Cu2+) was determined by Cupric Ions Reducing Assay (CUPRAC) assay as previously describedCitation47,Citation48. A volume of 0.25 ml neocuproine (7.5 mM) in ethanol, 0.25 ml NH4Ac (1 M) and 0.25 ml CuCl2 (0.01 M) was mixed with sample at different amounts and standards.
2.5.2. DPPH• and ABTS•+ free radical scavenging activity
The free radical scavenging activity of standard antioxidants and synthesised sulphonamide derivatives was measured by DPPH using the Blois methodCitation49,Citation50 0.1 mM solution of DPPH• in ethanol was prepared and 1 ml of this solution was added to 3 ml of the samples solution in ethanol at different concentrations. These solutions were vortexed thoroughly and kept in the darkness for 30 min. The absorbance values of this final mixture were measured at 517 nm. The reduced absorbance of the reaction mixture indicates higher free radical scavenging activity.
ABTS•+ scavenging activity assay was performed according to Re methodCitation51,Citation52. The process of ABTS•+ (2.0 mM) in water with potassium persulfate (K2S2O8) (2.45 mM) at room temperature in dark for 4 h. gave the ABTS cation radical. Dilution of ABTS•+ was applied with sodium phosphate buffer (Na3PO4) (0.1 mol/l, pH 7.4) to measure the absorbance at 734 nm. The reactions of ABTS•+ solution (1.0 ml) with samples solution in ethanol at different concentrations were performed. The inhibition was calculated at 734 nm for each concentrationCitation53. The DPPH• and ABTS•+ free radical scavenging ability was calculated as the following equation: Radical scavenging activity (%) = [(Ac – As)/Ac] × 100. Where, Ac is the absorbance value of DPPH• and ABTS•+ before sample addition, while as is the absorbance value after sample addition.
2.5.3. The activity of purified CA isoenzymes
According to our previous studies, the purity and presence of human (h) carbonic anhydrase isoforms purified using Sepharose-4B-L-tyrosine-sulfanilamide affinity chromatographyCitation54 was tested by SDS-PAGE techniqueCitation55,Citation56 with Laemmli’s (1970) procedureCitation57–59. In the inhibition studies, according to our previous studies, the esterase activities of hCA isoforms were determined by the p-nitrophenylacetate that used as a substrate converted by both isoforms to the p-nitrophenolate ion in this techniqueCitation60–62.
2.5.4. The activity of anticholinesterase
AChE activity isolated from electric eel (purchased) was performed by a modified version of the Ellman methodCitation63. The measurement of AChE activity was performed using Acetylthiocholine (ATChI) iodide as the substrates and 5,5-Dithiobis(2-nitrobenzoic) acid (DTNB)Citation64. The ATChI iodide as the substrates was monitored spectrophotometrically at 412 nm as in our previous assaysCitation65,Citation66.
2.5.5. In vitro inhibition study
The inhibition effects of synthesised derivatives were determined with least five different inhibitor concentrations on hCA isoenzymes and AChE. IC50 of synthesised compound was calculated from Activity (%)-[synthesised derivatives] graphs for each compound. The inhibition types and KI values were found by Lineweaver and Burk’s (1934) curvesCitation67,Citation68.
2.6. Computational study
Qikprop softwareCitation69, included in the Schrodinger Suite 2019–4, was used to foresee pharmaceutically relevant various ADMET, and drug-likeness parameters of the synthesised sulphonamide imine, and amine compounds (1–8), such as number of violations of Jorgensen’s rule of threeCitation70, and number of violations of Lipinski’s rule of fiveCitation71 that are significant in the novel drug discovery and development process.
The X-ray crystallographic structure of hCA I, II, and AChE co-crystallized with 3UF, V50, and E20 (PDB ID: 4WUP 1.75 ÅCitation72, 4HT0 1.60 ÅCitation73 and 4EY7 2.35 ÅCitation74, respectively) were accessed from the protein data bank (rcsb.org)Citation75. The proteins were prepared for the docking work utilising the Protein Preparation Wizard Citation76 in Maestro with default options (Schrodinger Suite 2019–4). The 3 D structures of the ligands (1–8) were drawn by using the ChemDraw software and were prepared using the LigPrep platformCitation77, considering their ionisation state at pH 7.0 ± 0.5Citation78 with Epic. The energy minimises of the receptors and ligands (1–8) were conducted using OPLS3e force field protocolCitation79. The docking grid was centred on the centre of mass of the co-crystallized ligands (3UF, V50 and E20) and was built using the Receptor Grid Generation toolCitation80. The molecular docking experiment was accomplished for all the synthesised agents against target receptors by using the Glide extra precision (XP) algorithmCitation81.
2.7. Statistical study
Analysis of the data, and drawing of graphs were realised using GraphPad Prism version 6 for Mac, GraphPad Software, La Jolla, CA. Also, the determination of KI constants was conducted using SigmaPlot version 12, from Systat Software, San Jose, CA. The results were exhibited as mean ± standard deviation (95% confidence intervals). Differences between data sets were considered as statistically significant when the p values was less than 0.05.
3. Results and discussion
3.1. Synthesis of sulphonamide imine (1–4) and amine (5–8) compounds
In this study, the sulphonamides containing imine group (1–4) were synthesised and then, the secondary amine sulphonamides (5–8) were obtained through reduction of the imine derivatives (1–4) according to literature methodsCitation82. 3-Aminobenzenesulfonamide and 4-aminobenzenesulfonamide were condensed with 3-bromo-2-hydroxybenzaldehyde and 3-chloro-2-hydroxybenzaldehyde in the presence of catalytic amounts of formic acid. In separate reactions, imine compounds synthesised as above were then reduced with NaBH4 to obtain the novel secondary amine sulphonamides. The synthesis of the imine (1–4) and amine (5–8) sulphonamide compounds are illustrated in Scheme 1. The synthesised imine (1–4) and amine (5–8) derivatives were obtained as solid products, stable at room temperature.
FT-IR, 1H NMR, 13C NMR, LC-MS-MS and elemental analysis were performed to know the exact nature of functional groups, the arrangement of protons and carbons, the molecular mass and fragmentation pattern and the percentage of the constituting elements respectively. In addition, their purity was investigated with these methods and it was determined that they did not contain any residue. The sulphonamide derivatives, imine (1–4) and amine (5–8) compounds, were in good agreement with calculated values. Data given in the experimental section are in complete agreement with those of previous studies for other such sulphonamide derivativesCitation42,Citation82–85.
3.1.1. Fourier transform infra-red spectroscopy (FT-IR) measurements
FT-IR spectra of starting materials, imine and amine compounds were obtained via U-ATR at range 400–4000 cm−1 and were used to give particular information on spectroscopic characterisation of the compounds. All sulphonamides showed characteristic vibrations at ranges of 3324–3368 cm−1 for -NH2, 3032–3116 cm−1 for aromatic C-H and 1135–1338 cm−1 for S=O. Due to the strong intramolecular interactions between –OH and –C=N– groups in the compound, O–H stretching peak could not be observed in the region expected (∼3300 cm−1). This band observed at 3100–3400 cm−1. As evidence of the reduction of imines (1–4) characteristic vibrations at ranges of 1611–1617 cm−1 for –C=N– weren’t observed in the reduced compounds (5–8). Also –N–H vibrations at ranges of 3324–3368 cm−1 and aliphatic –CH vibrations at ranges of 2840–2985 cm−1 were observed in the reduced compounds (5–8) whereas were not detected at imine compounds (1–4).
3.1.2. 1H and 13C NMR spectroscopic analyses
The NMR spectra of all compounds were recorded in DMSO-d6 with TMS as an internal standard and data given in the experimental section.
The 1H NMR spectra of all compounds exhibited singlets at δ 6.87–7.44 ppm for sulphonamide protons (–SO2NH2) and singlets for aromatic ring protons at δ 7.02–7.87 ppm. Imine compounds (1–4) gave singlets at δ 13.89–14.06 ppm which being attributed aromatic –OH protons. Singlets for aromatic ring protons were observed at δ 7.02–7.87 ppm besides singlets for imine proton (–CH=N) were observed at δ 9.04–9.07 which were unseen in the reduced compounds (5–8). Also, as another proof of reduction of the imine compounds (1–4), singlets were seen at δ 4.29–4.32 ppm, which were attributed to Ar-CH2 group in the reduced compounds (5–8). A broad signal was observed at amine compounds (5–8) in the region δ 4.29–4.32 ppm arising from –NH protons could admit another evidence for reduction of imine compounds (1–4). Doublet peaks were observed for aromatic proton (Ar-H) groups, both neighbouring single protons. Multiplet peaks were often observed and these represented the aromatic protons (Ar-H) of benzenesulfonamide ring and aromatic aldehyde ring for all derivatives.
The 13C NMR spectra of all compounds gave aromatic carbons in the region δ 109.62–145.17 ppm deriving from benzenesulfonamide ring and aromatic aldehyde ring. The imine carbon (–C=N–) at imine compounds (1–4) was observed in the region δ 165.38–165.88 ppm which unseen in the reduced compounds (5–8). A methylene carbon (Ar-CH2-N) at amine compounds (5–8) observed at δ 42.20–42.81 ppm could be a sign for reduction of imine compounds (1–4).
3.1.3. Mass spectra (LC-MS-MS)
LC-MS was used to obtain the molecular masses of newly synthesised imine compounds (1–4) and amine compounds (5–8) and spectra were used as evidence for the formation of the proposed structures. Synthesised compounds support the suggested structures and molecular ions [M]+ of the derivatives were detected as single sharp peaks and observed as [M + H]+ ions. All spectra were in agreement with the molecular structures of the derivatives and the values of molecular weights supplied by mass spectrometer given in the experimental section.
3.2. Biological evaluation
CA isoenzymes (hCA I and hCA II isoenzymes), which play an important role in many biochemical and physiological processes in living organisms and have a higher catalytic rate in the cytosol of erythrocytes, play a key role in biosynthetic reactions such as ureagenesis and lipogenesisCitation86,Citation87. In recent years, it has been known that diuretics, antiglaucoma, antiobesity, antitumor and anticonvulsant agents have been used as hCA inhibitors in the treatment of many disease symptomsCitation88.
Specific inhibitors of hCA I, and II isoenzymes have been used for the treatment of several diseases in clinic such as glaucoma, duodenal and gastric ulcers, epilepsy, congestive heart failure, mountain sickness, and as diuretic agents. However, clinically used CAIs show side effects due to lack of isoenzyme selectivity of the compounds. In this study, it was determined the effect of novel sulphonamides for the inhibition of two physiologically relevant CAs, the cytosolic isoforms hCA I and II as well as AChE. As the reference inhibitor, acetazolamide (AZA) was utilised for hCA I, and II, and tacrine (TAC) was used for AChE.
hCA I was strongly inhibited by Schiff bases sulphonamides 1–8. The secondary amines group (compounds 5–8) showed a potent inhibitory effect, according to the imine group (compounds 1–4). These compounds demonstrated KI values in the low nanomolar range (KI values ranging from 32.1 ± 0.4 to 100.6 ± 1.9 nM). The order of the KI values of the sulphonamides was 8 (KI: 32.1 ± 0.4 nM) > 4 (KI: 40.1 ± 0.6 nM) > 5 (KI: 52.1 ± 0.8 nM) > 1 (KI: 59.7 ± 0.8 nM) > 7 (KI: 62.3 ± 1.3 nM) > 2 (KI: 66.6 ± 1.0 nM) > 3 (KI: 80.5 ± 1.5 nM) > 6 (KI: 100.6 ± 1.9 nM) (). Compound 8 showed the best inhibition profile against hCA I compared to that of a standard drug, AAZ (KI: 436.2 ± 12.2 nM, approx. 13.5-fold) (). On the other hand, the least effective compound was 6 with KI of 100.6 ± 1.9 nM.
Table 1. KI values of hCA I, II and AChE with derivatives 1–8, AAZ and TAC as standard inhibitors.
Table 2. Selectivity index values for KI values of the compounds 1–8.
The physiologically most abundant cytosolic isozyme hCA II was inhibited by many of the compounds in the range of KI value of 10.1 ± 0.1–79.3 ± 0.2 nM (). The order of the KI values of the inhibitory strength of the sulphonamides was as 7 (KI: 10.1 ± 0.1 nM) > 2 (KI: 13.8 ± 0.1 nM) > 1 (KI: 16.4 ± 0.8 nM) > 5 (KI: 19.8 ± 1.2 nM) > 3 (KI: 26.3 ± 0.1 nM) > 6 (KI: 29.1 ± 0.3 nM) > 8 (KI: 62.0 ± 0.2 nM) > 4 (KI: 79.3 ± 0.2 nM). hCA II was more weakly inhibited by Schiff bases sulphonamides 1–8 as compared to hCA I. Although, the secondary amines 5–8 were highly effective hCA II inhibitors with KIs in the range of 10.1 ± 0.1–62.0 ± 0.2 nM in comparison with AAZ, a clinical drug (KI: 93.5 ± 1.2 nM).
The disorder in the cholinergic system is caused by increased activity of AChE catalysing hydrolysis of neurotransmitter acetylcholine. The increase in activity increases the formation of amyloid protein and hydrolysis of acetylcholine, causing neurodegenerative diseases such as Alzheimer’s. Inhibition of AChE has been used in the treatment of some symptoms that may occur with excessive hydrolysis of ACh and increased amyloid proteinsCitation89. Many synthetic and natural substances in metabolism can affect the metabolic pathway by modifying enzyme activities at low concentrations. Inhibition of AChE by novel synthesis compounds is currently important to improve the effective treatment of ADCitation90.
Since sulphonamides were reported with their significant inhibitory potency on AChE enzyme, which is a well-known therapeutic target of Alzheimer’s disease, novel sulphonamides in this study were screened on AChE enzyme. The KI values of the compounds (1–8) were given in . All of the compounds showed the satisfactory inhibition profile in nanomolar concentrations against AChE which demonstrated KI values ranging from 21.0 ± 0.9 to 77.0 ± 9.3 nM when compared to TAC (KI: 109.8 ± 2.4 nM). The order of the KI constants of the inhibitory strength of the sulphonamides was as 2 (KI: 21.0 ± 0.9 nM) > 1 (KI: 24.3 ± 1.0 nM) > 6 (KI: 25.7 ± 1.0 nM) > 4 (KI: 27.9 ± 1.3 nM) > 8 (KI: 31.4 ± 1.2 nM) > 3 (KI: 34.9 ± 1.4 nM) > 5 (KI: 61.2 ± 4.0 nM) > 7 (KI: 77.0 ± 9.3 nM). In contrast to hCA I and hCA II, the secondary amine groups showed less inhibitory effect on AChE enzyme activity according to our results. While compound 2 had the best inhibition profile against AChE, the lowest effective compound was 7.
The free radical scavenging, metal chelating, metal-reducing capacity of a phenolic acid depends on the hydrogen atom or electron donation in the structure of the compound or the number and position (–OH) of hydroxyl groupsCitation91–93. Therefore, the structure and functional groups of the compounds are important for their bioactive properties.
In this study, the reducing power of synthesised compounds was investigated by FRAP and CUPRAC assays. An important parameter in the evaluation of antioxidant activity is the reduction of Cu+2/Fe3+ (ferricyanide) complex to Cu+1/Fe2+ form. As seen in , Fe3+ reducing ability of standard and synthesised compounds 1–8 decreased in the following order: trolox > BHA > BHT > 6 > 7 > 8 > 5 > 4 > 2 > 1 > 3. According to results obtained from CUPRAC assay, which is based on reduction of Cu2+ to Cu+1 by synthesised compounds (). The cupric ion (Cu2+) reducing power of standard and synthesised compounds 1–8 decreased in the following order: BHA > BHT > trolox > 7 > 5 > 6 > 8 > 3 > 2 > 4 > 1. The compounds 5–8 have a higher metal reduction capacity, while the compounds 1–4 have a lower metal reduction capacity. Among the synthesised compounds (1–8), the compounds that are reduced and contain secondary amine groups (5–8) have a higher reduction capacity compared to others (1–4). This may be because the reduced compounds (5–8) contain extra N-H bonds compared to normal Schiff bases (1–4).
Table 3. The radical scavenging and metal reduction activity of synthesised compounds (1–8).
DPPH•, ABTS•+, DMPD•+ and O2• scavenging assays are often used to determine the radical removal activities of synthesised or isolated pure compoundsCitation94. In this study, the radical scavenging ability of the synthesised compounds 1–8 was evaluated by ABTS•+ and DPPH• scavenging assays. The synthesised compounds exhibited radical scavenging activity in range from 7.3 to 78.4% for ABTS at concentration of 200 μg/mL, and in range from 1.9 to 18.7% for DPPH at concentration of 50 μg/mL. As shown in the , compound 1 to DPPH assay and compound 5–8 for ABTS assay have the ability to remove radicals close to some standards.
In this assay, the synthesised compounds (5, 6, 7, and 8), which are primary sulphonamides, showed ABTS radical scavenging activity because they contained both hydroxyl group and nitrogen bound hydrogen. These results clearly demonstrated significant free radical scavenging activity of the newly synthesised sulphonamide compounds 5–8, which have more electron donor properties than others.
3.3. Computational study
The ADMET properties, and some pharmacokinetic parameters of the new synthesised sulphonamide imine, and amine compounds (1–8) were estimated by using the QikProp module. The overall estimated values are summarised in . As a result, the ADMET study exhibited that these active derivatives (1–8) possess the drug-likeness criteria complied by both Jorgensen’s rule of three and Lipinski’s rule of five.
Table 4. ADMET–related parameters of the derivatives (1–8).
To understand the trends investigated for the monitored relative selectivity of synthesised novel sulphonamide imine, and amine agents (1–8), the molecular docking study was achieved for the most active selected among the compounds. The X-ray crystal structures of 4WUP and 4EY7 were available in the form of a homodimer chain, therefore, their chain A was chosen for in silico works. The 3UF (C8H11NO3S2, 4-[(2-hydroxyethyl)sulfanyl]benzenesulfonamide), V50 (C12H9F4N3O2S2, 4-[(4,6-dimethylpyrimidin-2-yl)thio] − 2,3,5,6-tetrafluorobenzenesulfonamide), and E20 (C24H29NO3, 1-benzyl-4-[(5,6-dimethoxy-1-indanon-2-yl)methyl]piperidine) were the co-crystallized ligand with 4WUP (for hCA I), 4HT0 (for hCA II), and 4EY7 (for AChE), respectively. The in silico molecular docking procedure for these receptors was validated by extracting the bound agents (3UF, V50 and E20) from the proteins and again redocking it on similar regions. The docking poses were superimposed, and RMSD values were calculated to be 1.09, 1.28 and 0.08 Å, respectively.
According to the literature, the native ligand (3UF) displays two major interactions, like H-bond interaction with His67, and Thr199 and the docking score was −5.01 kcal/mol, in the catalytic domain of 4WUP For the most active compound (8, KI 32.14 ± 0.39 nM) the docking score (−6.90 kcal/mol) was found low to the co-crystallized ligand. Compound 8 exhibited H-bond interactions with Gln92, and Thr199 ().
Figure 1. Interaction of the ligands with the key amino acids within the active site of hCA I (PDB ID: 4WUP). (A) Docking pose of the native ligand 3UF (4-[(2-hydroxyethyl)sulfanyl]benzenesulfonamide, PubChem CID: 4269754). (B) Docking pose of compound 8 (4-((3-chloro-2-hydroxybenzyl)amino)benzenesulfonamide).
![Figure 1. Interaction of the ligands with the key amino acids within the active site of hCA I (PDB ID: 4WUP). (A) Docking pose of the native ligand 3UF (4-[(2-hydroxyethyl)sulfanyl]benzenesulfonamide, PubChem CID: 4269754). (B) Docking pose of compound 8 (4-((3-chloro-2-hydroxybenzyl)amino)benzenesulfonamide).](/cms/asset/8a130b3b-e8c1-4d02-af0c-385981e1a9da/ienz_a_1746784_f0001_c.jpg)
The 4HT0 complexed with V50 shows an H-bond with Thr199. Moreover, V50 exhibits pi-pi stacking with His94. The docking score was −4.85 kcal/mol, although it was expected to be in the range of at least −5.00 to −6.00 kcal/mol. As shown in , compound 7 (KI 10.14 ± 0.03 nM, docking score −6.31 kcal/mol) formed an H-bond with the active site residue (Thr199), and pi-pi stacking with Phe131. Apart from this, this agent showed hydrophobic interactions with Val121, Phe131, Val135, Leu141, Val143, Leu198, Pro201, Pro202, Leu204, Val207 and Trp209.
Figure 2. Interaction of the ligands with the key amino acids within the active site of hCA II (PDB ID: 4HT0). (A) Docking pose of the native ligand V50 (4-[(4,6-dimethylpyrimidin-2-yl)thio]-2,3,5,6-tetrafluorobenzenesulfonamide, PubChem CID: 71299336). (B) Docking pose of compound 7 (4-((3-bromo-2-hydroxybenzyl)amino)benzenesulfonamide).
![Figure 2. Interaction of the ligands with the key amino acids within the active site of hCA II (PDB ID: 4HT0). (A) Docking pose of the native ligand V50 (4-[(4,6-dimethylpyrimidin-2-yl)thio]-2,3,5,6-tetrafluorobenzenesulfonamide, PubChem CID: 71299336). (B) Docking pose of compound 7 (4-((3-bromo-2-hydroxybenzyl)amino)benzenesulfonamide).](/cms/asset/92bac284-2ecc-44dd-8820-208e413c6224/ienz_a_1746784_f0002_c.jpg)
E20, which has previously considered to be the native ligand, and compound 2 (KI 20.98 ± 0.89 nM), which is the most active analogue of the synthesised agents, were analysed in terms of interactions with AChE. The docking positions into 4EY7 determined for the two inhibitors were like to that estimated for their other compounds and both E20 and agent 2 displayed the same interactions with Trp286. Moreover, the docking scores were −16.06 kcal/mol, and −9.26 kcal/mol, respectively. Within the 4EY7 active site, the hydroxy group on the aryl ring, and the amino moiety of the benzenesulfonamide group of compound 2 showed potential two H-bonds with Tyr124 and Ser293, respectively. Also, hydrophobic interaction was monitored between compound 2 and Tyr72, Tyr124, Trp286, Leu289, Val294, Phe295, Phe297, Tyr337, Phe338 and Tyr341. Apart from these, agent 2 also exhibited pi–pi stacking with Trp337 ().
Figure 3. Interaction of the ligands with the key amino acids within the active site of AChE (PDB ID: 4EY7). (A) Docking pose of the native ligand E20 (1-benzyl-4-[(5,6-dimethoxy-1-indanon-2-yl)methyl]piperidine, PubChem CID: 1150567). (B) Docking pose of compound 2 (3-((3-chloro-2-hydroxybenzylidene)amino)benzenesulfonamide).
![Figure 3. Interaction of the ligands with the key amino acids within the active site of AChE (PDB ID: 4EY7). (A) Docking pose of the native ligand E20 (1-benzyl-4-[(5,6-dimethoxy-1-indanon-2-yl)methyl]piperidine, PubChem CID: 1150567). (B) Docking pose of compound 2 (3-((3-chloro-2-hydroxybenzylidene)amino)benzenesulfonamide).](/cms/asset/046b0168-cc4a-49c8-88af-588793356734/ienz_a_1746784_f0003_c.jpg)
4. Conclusions
In this study, a series of eight sulphonamide Schiff bases (1–4) and their reduced counterparts (5–8) were synthesised by the condensation of two well-known sulphonamide derivatives (3-aminobenzenesulfonamide and 4-aminobenzenesulfonamide) with substituted aromatic aldehydes. The obtained novel compounds (1–8) were investigated as inhibitors of the cytosolic CA isozymes hCA I and hCA II, and cholinesterase (AChE) enzymes. The antioxidant activity of the compounds was also performed using different bioanalytical assays such as radical scavenging tests with ABTS•+, and DPPH•+ and metal-reducing abilities with CUPRAC, and FRAP assays. In general, all compounds showed great inhibition potency against hCA I with KI values ranging from 16.05 ± 0.47 to 29.66 ± 0.50 nM. Among the series, one of the most potent inhibition results was observed against hCA II isozyme with compound 7 (KI: 10.14 ± 0.03 nM). Another interesting finding from the current study is that all the synthesised compounds showed a better inhibition profile than Tacrine (KI: 109.75 ± 2.39) against AChE with KIs in between 20.98 ± 0.89 to 77.02 ± 9.34 nM. Also, the reduced derivatives (5–8) displayed high metal reduction activity and about 70% ABTS radical scavenging activity. As a result, since AChE inhibition is an important task in the treatment of Alzheimer’s disease, these compounds may have an interesting future for the development of new drugs to neurodegenerative disorders.
Acknowledgements
The authors thank Harran University, Scientific Research Council (HÜBAK, Projects number: 18168) for financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest 1982;47:412–26.
- Lunec J, Blake D. Oxygen free radicals: their relevance to disease processes. In Cohen RD, Lewis B, eds. The metabolic and molecular basis of acquired disease. London: Balliere Tindall; 1990:189–212.
- Budak H, Ceylan H, Kocpinar EF, et al. Expression of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in oxidative stress induced by long-term iron toxicity in rat liver. J Biochem Mol Toxicol 2014;28:217–23.
- Işık M, Beydemir Ş, Yılmaz A, et al. Oxidative stress and mRNA expression of acetylcholinesterase in the leukocytes of ischemic patients. Biomed Pharmacother 2017;87:561–7.
- Babior BM. Phagocytes and oxidative stress. Am J Med 2000;109:33–44.
- Sies H. Oxidative stress: from basic research to clinical application. Am J Med 1991;91:S31–S38.
- Nordberg J, Arner E. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biol Med 2001;31:1287–312.
- Işık M, Beydemir Ş. AChE mRNA expression as a possible novel biomarker for the diagnosis of coronary artery disease and Alzheimer’s disease, and its association with oxidative stress. Arch Physiol Biochem 2019;1–8.
- Türkeş C, Demir Y, Beydemir Ş. Anti-diabetic properties of calcium channel blockers: Inhibition effects on aldose reductase enzyme activity. Appl Biochem Biotechnol 2019;189:318–29.
- Matés JM, Pérez-Gómez C, De Castro IN. Antioxidant enzymes and human diseases. Clin Biochem 1999;32:595–603.
- Variji A, Shokri Y, Fallahpour S, et al. The combined utility of myeloperoxidase (mpo) and paraoxonase 1 (pon1) as two important HDL-associated enzymes in coronary artery disease: which has a stronger predictive role? Atherosclerosis 2019;280:7–13.
- Gülçin İ. Antioxidant and antiradical activities of l-carnitine. Life Sci 2006;78:803–11.
- Necip A, Işık M. Bioactivities of Hypericum perforatum L and Equisetum arvense L fractions obtained with different solvents. Int J Life Sci Biotech 2019;2:221–30.
- Shah GN, Morofuji Y, Banks WA, et al. High glucose-induced mitochondrial respiration and reactive oxygen species in mouse cerebral pericytes is reversed by pharmacological inhibition of mitochondrial carbonic anhydrases: implications for cerebral microvascular disease in diabetes. Biochem Biophys Res Commun 2013;440:354–8.
- Bartolini M, Bertucci C, Cavrini V, Andrisano V. Β-amyloid aggregation induced by human acetylcholinesterase: inhibition studies. Biochem Pharm 2003;65:407–16.
- Işık M. The binding mechanisms and inhibitory effect of intravenous anesthetics on ache in vitro and in vivo: Kinetic analysis and molecular docking. Neurochem Res 2019;44:2147–55.
- Gülçin İ. Antioxidant activity of caffeic acid (3, 4-dihydroxycinnamic acid). Toxicol 2006;217:213–20.
- Berchtold NC, Cotman CW. Evolution in the conceptualization of dementia and alzheimer’s disease: Greco-roman period to the 1960s. Neurobiol Aging 1998;19:173–89.
- Choudhary MI. Bioactive natural products as a potential source of new pharmacophores. A theory of memory. Pure App Chem 2001;73:555–60.
- Doungsoongnuen S, Worachartcheewan A, Pingaew R, et al. Investigation on biological activities of anthranilic acid sulfonamide analogs. Excli J 2011;10:155.
- Abbas A, Murtaza S, Tahir MN, et al. Synthesis, antioxidant, enzyme inhibition and DNA binding studies of novel n-benzylated derivatives of sulfonamide. J Mol Struct 2016;1117:269–75.
- Chandrasekhar M, Prasad GS, Venkataramaiah C, et al. Synthesis, spectral characterization, docking studies and biological activity of urea, thiourea, sulfonamide and carbamate derivatives of imatinib intermediate. Mol Diversity 2019;23:723–16.
- Siddique M, Saeed AB, Ahmad S, Dogar NA. Synthesis and biological evaluation of hydrazide based sulfonamides. J Sci Innovative Res 2013;2:627–33.
- Patrick GL. Quantitative structure-activity relationships. An introduction to medicinal chemistry. 2nd ed. New York: Oxford University Press; 2001:258–88.
- Deng Y, Li B, Zhang T. Bacteria that make a meal of sulfonamide antibiotics: Blind spots and emerging opportunities. Environ Sci Technol 2018;52:3854–68.
- Khan KM, Ahmad I, Afzal S. Synthesis and biological studies of some new n-substituted derivatives of n-(1, 3-benzodioxol-5-yl)-4-methylbenzenesulfonamide. J Chem Soc Pakistan 2015;37:150–6.
- Genç Y, Özkanca R, Bekdemir Y. Antimicrobial activity of some sulfonamide derivatives on clinical isolates of Staphylococus aureus. Ann Clin Microbiol Antimicrob 2008;7:17.
- Capasso C, Supuran CT. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin Ther Targets 2015;19:1689–704.
- Borne RF, Peden RL, Waters IW, et al. Anti-inflammatory activity of para-substituted n-benzenesulfonyl derivatives of anthranilic acid. J Pharm Sci 1974;63:615–7.
- Keche AP, Hatnapure GD, Tale RH, et al. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg Med Chem Lett 2012;22:3445–8.
- Durgun M, Turkmen H, Zengin G, et al. Synthesis, characterization, in vitro cytotoxicity and antimicrobial investigation and evaluation of physicochemical properties of novel 4-(2-methylacetamide) benzenesulfonamide derivatives. Bioorg Chem 2017;70:163–72.
- Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Anticancer and antiviral sulfonamides. Curr Med Chem 2003;10:925–53.
- (a) Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70. (b) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88.
- De Simone G, Vitale RM, Di Fiore A, et al. Carbonic anhydrase inhibitors: hypoxia-activatable sulfonamides incorporating disulfide bonds that target the tumor-associated isoform IX. J Med Chem 2006;49:5544–51.
- (a) Nocentini A, Supuran CT. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: a patent review (2008-2018). Expert Opin Ther Pat 2018;28:729–40. (b) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- Koyuncu I, Gonel A, Durgun M, et al. Assessment of the antiproliferative and apoptotic roles of sulfonamide carbonic anhydrase ix inhibitors in hela cancer cell line. J Enzyme Inhib Med Chem 2019;34:75–86.
- de Clercq E. New developments in anti-hiv chemotherapy. Curr Med Chem 2001;8:1543–72.
- Moreno-Díaz H, Villalobos-Molina R, Ortiz-Andrade R, et al. Antidiabetic activity of n-(6-substituted-1, 3-benzothiazol-2-yl) benzenesulfonamides. Bioorg Med Chem Lett 2008;18:2871–7.
- Xanthopoulos D, Kritsi E, Supuran CT, et al. Discovery of HIV type 1 aspartic protease hit compounds through combined computational approaches. Chem Med Chem 2016;11:1646–52.
- Mann T, Keilin D. Sulfanilamide as a specific inhibitor of carbonic anhydrase. Nature 1940;146:164–5.
- (a) Nocentini A, Supuran CT. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin Drug Discov 2019;14:1175–97. (b) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- (a) Kausar N, Muratza S, Raza MA, et al. Sulfonamide hybrid schiff bases of anthranilic acid: synthesis, characterization and their biological potential. J Mol Struct 2019;1185:8–20. (b) Carradori S, De Monte C, D’Ascenzio M, et al. Salen and tetrahydrosalen derivatives act as effective inhibitors of the tumor-associated carbonic anhydrase XII—A new scaffold for designing isoform-selective inhibitors. Bioorg Med Chem Lett 2013;23:6759–63.
- Hirayama N, Taga J, Oshima S, Honjo T. Sulfonamide-type di-Schiff base ligands as chelate extraction reagents for divalent metal cations. Anal Chim Acta 2002;466:295–301.
- Khan F, Khan S, Athar A. Synthesis, spectral characterization and antibacterial study of a schiff base metal complexes derived from n-[(e)-(5-chloro-2-hydroxyphenyl) methylidene]-4-nitrobenzenesulfonamide. Am Eur J Agric Environ Sci 2015;15:216–20.
- Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of Browning reaction. Jpn J Nutr 1986;44:307–15.
- Elmastaş M, Gülçin İ, Beydemir Ş, et al. A study on the in vitro antioxidant activity of juniper (Juniperus communis L.) fruit extracts. Anal Lett 2006;39:47–65.
- Apak R, Güçlü K, Özyürek M, et al. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nut 2006;57:292–304.
- Isik M, Korkmaz M, Bursal E, et al. Determination of antioxidant properties of Gypsophila bitlisensis bark. Int J Pharmacol 2015;11:366–71.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199–200.
- Işık M, Dikici E, Tohma H, et al. Antioxidant activity and total phenolic/flavonoid contents of Phlomis pungens L. 2017.
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radical Biol Med 1999;26:1231–7.
- Topal F, Nar M, Gocer H, et al. Antioxidant activity of taxifolin: an activity–structure relationship. J Enzyme Inhib Med Chem 2016;31:674–83.
- Topal M, Gocer H, Topal F, et al. Antioxidant, antiradical, and anticholinergic properties of cynarin purified from the illyrian thistle (Onopordum illyricum L.). J Enzyme Inhib Med Chem 2016;31:266–75.
- Sağlık BN, Çevik UA, Osmaniye D, et al. Synthesis, molecular docking analysis and carbonic anhydrase i-ii inhibitory evaluation of new sulfonamide derivatives. Bioorg Chem 2019;91:103–53.
- Söyüt H, Beydemir S, Türkeş C. Inhibition effects of gemcitabine hydrochloride, acyclovir, and 5-fluorouracil on human serum paraoxonase-1 (hpon1): in vitro. Open J Biochem 2014;1:15.
- Akbaba Y, Türkeş C, Polat L. Synthesis and paroxonase activities of novel bromophenols. J Enzyme Inhib Med Chem 2013;28:1073–79.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970;227:680.
- Türkeş C, Söyüt H, Beydemir Ş. Effect of calcium channel blockers on paraoxonase-1 (pon1) activity and oxidative stress. Pharm Rep 2014;66:74–80.
- Türkeş C, Söyüt H. Beydemir Ş. Human serum paraoxonase-1 (hpon1): In vitro inhibition effects of moxifloxacin hydrochloride, levofloxacin hemihidrate, cefepime hydrochloride, cefotaxime sodium and ceftizoxime sodium. J Enzyme Inhib Med Chem 2015;30:622–28.
- Gündoğdu S, Türkeş C, Arslan M, et al. New isoindole 1, 3-dione substituted sulfonamides as potent inhibitors of carbonic anhydrase and acetylcholinesterase: design, synthesis, and biological evaluation. ChemistrySelect 2019;4:13347–55.
- Aslan HE, Demir Y, Özaslan MS, et al. The behavior of some chalcones on acetylcholinesterase and carbonic anhydrase activity. Drug Chem Toxicol 2019;42:634–40.
- Kaya ED, Erğun B, Demir Y, et al. The in vitro impacts of some plant extracts on carbonic anhydrase i, ii and paraoxonase-1. Hacettepe J Biol Chem 2019;47:51–9.
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharm 1961;7:88–95.
- Topal F. Inhibition profiles of voriconazole against acetylcholinesterase, α-glycosidase, and human carbonic anhydrase i and ii isoenzymes. J Biochem Mol Toxicol 2019;33:e22385.
- Işık M, Demir Y, Durgun M, et al. Molecular docking and investigation of 4-(benzylideneamino)-and 4-(benzylamino)-benzenesulfonamide derivatives as potent AChe inhibitors. Chem Papers 2019;1–11.
- Taslimi P, Kandemir FM, Demir Y, et al. The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: pharmacological evaluation of some metabolic enzyme activities. J Biochem Mol Toxicol 2019;33:e22313.
- Demir Y, Işık M, Gülçin İ, Beydemir Ş. Ş. Phenolic compounds inhibit the aldose reductase enzyme from the sheep kidney. J Biochem Mol Toxicol 2017;31:e21936.
- Türkeş C, Söyüt H, Beydemir Ş. In vitro inhibitory effects of palonosetron hydrochloride, bevacizumab and cyclophosphamide on purified paraoxonase-i (hpon1) from human serum. Environ Toxicol Pharm 2016;42:252–57.
- Işık M, Beydemir Ş, Demir Y. Benzenesulfonamide derivatives containing imine and amine groups: Inhibition on human paraoxonase and molecular docking studies. Int J Biol Macromol 2020 [in press].
- Jorgensen WL, Duffy EM. Prediction of drug solubility from Monte Carlo simulations. Bioorg Med Chem Lett 2000;10:1155–58.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Del Rev 1997;23:3–25.
- (a) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist?. J Enzyme Inhib Med Chem 2016; 31:345–60. (b) Supuran CT. Carbonic anhydrases and metabolism. Metabolites 2018;8:25. (c) Supuran CT. The management of glaucoma and macular degeneration. Expert Opin Ther Pat. 2019;29:745–47.
- (a) De Simone G, Supuran CT, (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29. (b) Supuran CT, Carbon- versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors? J Enzyme Inhib Med Chem 2018; 33:485–95. (c) Tars K, Vullo D, Kazaks A, et al. Sulfocoumarins (1,2-benzoxathiine-2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J Med Chem 2013;56:293–300.
- Cheung J, Rudolph MJ, Burshteyn F, et al. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem 2012;55:10282–86.
- Türkeş C. Inhibition effects of phenolic compounds on human serum paraoxonase-1 enzyme. J Inst Sci Tech 2019;9:1013–22.
- Beydemir Ş, Türkeş C, Yalçın A. Gadolinium-based contrast agents: in vitro paraoxonase 1 inhibition, in silico studies. Drug Chem Toxicol 2019;1–10.
- Türkeş C, Beydemir Ş, Küfrevioğlu Öİ. In vitro and in silico studies on the toxic effects of antibacterial drugs as human serum paraoxonase 1 inhibitor. Chem Select 2019;4:9731–36.
- Türkeş C, Arslan M, Demir Y, et al. Synthesis, biological evaluation and in silico studies of novel n-substituted phthalazine sulfonamide compounds as potent carbonic anhydrase and acetylcholinesterase inhibitors. Bioorg Chem 2019;89:103004.
- Türkeş C. Investigation of potential paraoxonase-i inhibitors by kinetic and molecular docking studies: chemotherapeutic drugs. Protein Pept Lett 2019;26:392–402.
- Türkeş C. A potential risk factor for paraoxonase 1: In silico and in‐vitro analysis of the biological activity of proton‐pump inhibitors. J Pharm Pharmacol 2019;71:1553–64.
- Türkeş C, Beydemir Ş. Inhibition of human serum paraoxonase-i with antimycotic drugs: in vitro and in silico studies. Appl Biochem Biotechnol 2020;190:252–69.
- Durgun M, Turkmen H, Ceruso M, Supuran CT. Synthesis of 4-sulfamoylphenyl-benzylamine derivatives with inhibitory activity against human carbonic anhydrase isoforms I, II, IX and XII. Bioorg Med Chem 2016;24:982–88.
- Sarikaya B, Ceruso M, Carta F, Supuran CT. Inhibition of carbonic anhydrase isoforms i, ii, ix and xii with novel schiff bases: Identification of selective inhibitors for the tumor-associated isoforms over the cytosolic ones. Bioorg Med Chem 2014;22:5883–90.
- Ceylan Ü, Durgun M, Türkmen H, et al. Theoretical and experimental investigation of 4-[(2-hydroxy-3-methylbenzylidene) amino] benzenesulfonamide: structural and spectroscopic properties, nbo, nlo and npa analysis. J Mol Struct 2015;1089:222–32.
- Durgun M, Turkmen H, Ceruso M, Supuran CT. Synthesis of schiff base derivatives of 4-(2-aminoethyl)-benzenesulfonamide with inhibitory activity against carbonic anhydrase isoforms i, ii, ix and xii. Bioorg Med Chem Lett 2015;25:2377–81.
- Gulçin İ, Abbasova M, Taslimi P, et al. Synthesis and biological evaluation of aminomethyl and alkoxymethyl derivatives as carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase inhibitors. J Enzyme Inhib Med Chem 2017;32:1174–82.
- Huyut Z, Beydemir Ş, Gülçin İ. Inhibitory effects of some phenolic compounds on the activities of carbonic anhydrase: from in vivo to ex vivo. J Enzyme Inhib Med Chem 2016;31:1234–40.
- (a) Genç H, Kalin R, Köksal Z, et al. Discovery of potent carbonic anhydrase and acetylcholinesterase inhibitors: 2-aminoindan β-lactam derivatives. Int J Mol Sci 2016;17:1736. (b) Köhler K, Hillebrecht A, Schulze Wischeler J, et al. Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem Int Ed Engl 2007;46:7697–99.
- Dastan T, Kocyigit UM, Durna Dastan S, et al. Investigation of acetylcholinesterase and mammalian DNA topoisomerases, carbonic anhydrase inhibition profiles, and cytotoxic activity of novel bis (α‐aminoalkyl) phosphinic acid derivatives against human breast cancer. J Biochem Mol Toxicol 2017;31:e21971.
- Taslimi P, Akıncıoglu H, Gülçin İ. Synephrine and phenylephrine act as α‐amylase, α‐glycosidase, acetylcholinesterase, butyrylcholinesterase, and carbonic anhydrase enzymes inhibitors. J Biochem Mol Toxicol 2017;31:e21973.
- Afanas’ ev IB, Dcrozhko AI, Brodskii AV, et al. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol 1989;38:1763–69.
- Amarowicz R, Pegg RB, Rahimi-Moghaddam P, et al. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem 2004;84:551–62.
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol Med 1996;20:933–56.
- Gülçin I. Measurement of antioxidant ability of melatonin and serotonin by the dmpd and cuprac methods as trolox equivalent. J Enzyme Inhib Med Chem 2008;23:871–76.