Abstract
Tubulin polymerisation inhibitors exhibited an important role in the treatment of patients with prostate cancer. Herein, we reported the medicinal chemistry efforts leading to a new series of benzothiazoles by a bioisosterism approach. Biological testing revealed that compound 12a could significantly inhibit in vitro tubulin polymerisation of a concentration dependent manner, with an IC50 value of 2.87 μM. Immunofluorescence and EBI competition assay investigated that compound 12a effectively inhibited tubulin polymerisation and directly bound to the colchicine-binding site of β-tubulin in PC3 cells. Docking analysis showed that 12a formed hydrogen bonds with residues Tyr357, Ala247 and Val353 of tubulin. Importantly, it displayed the promising antiproliferative ability against C42B, LNCAP, 22RV1 and PC3 cells with IC50 values of 2.81 μM, 4.31 μM, 2.13 μM and 2.04 μM, respectively. In summary, compound 12a was a novel colchicine site tubulin polymerisation inhibitor with potential to treat prostate cancer.
Introduction
Prostate cancer is the second most commonly diagnosed cancer and the sixth leading cause of cancer death among men worldwide, with an estimated 1276000 new cancer cases and 359000 deaths in 2018Citation1–3. Microtubules play an important role in various cellular processes, including spindle formation, cellular shape maintenance, and intracellular transportationCitation4–6. Microtubule has been an attractive target for chemotherapeutic agents to treat prostate cancer Citation7. Anticancer agents targeting tubulin bind at four binding sites: taxanes, vinca alkaloids, colchicine and laulimalide sitesCitation8,Citation9. Although some tubulin inhibitors that bind to vinca alkaloids and taxanes sites have been approved by Food and Drug Administration (FDA), there are no FDA approved colchicine site tubulin inhibitors currently. Therefore, it is necessary to develop novel tubulin polymerisation inhibitors targeting the colchicine binding site to treat prostate cancer.
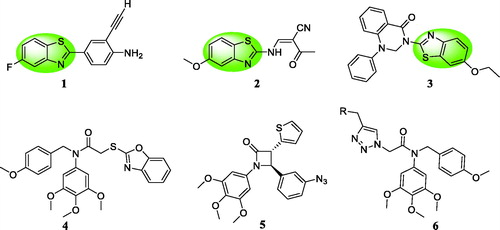
Recently, benzothiazole scaffold has been an attractive subject due to its potently anticancer activity in medicinal chemistryCitation10–12. 2–(4-Aminophenyl) benzothiazole analogue 1 () exhibited the antiproliferative activity against MCF-7 and MDA-468 cellsCitation13,Citation14. Benzothiazole 2 induced apoptosis and inhibited the growth of HL60 and U937 cellsCitation15. Benzothiazole linked a phenylpyridopyrimidinone 3 showed the significant cytotoxicity against human cervical cancer cell line ME-180 with an IC50 value of 4.01 μMCitation16. Based on these findings, benzothiazole was a promising scaffold to design anticancer agents. On the other hand, our group also reported three series of colchicine site tubulin inhibitors: (1) compound 4 could change the membrane potential of the mitochondria against MGC-803 cellsCitation17; (2) β-lactam containing a 3,4,5-trimethoxyphenyl unit 5 exhibited the well antiproliferative activity against MGC-803 cells in vitro and in vivoCitation18; (3) compound 6 could inhibit MGC803 cell growth and colony formation, induce G2/M phase arrest and promote apoptosisCitation19.
Figure 1. Benzothiazole analogues 1∼3 and colchicine site tubulin inhibitors 4∼6 as anticancer agents.
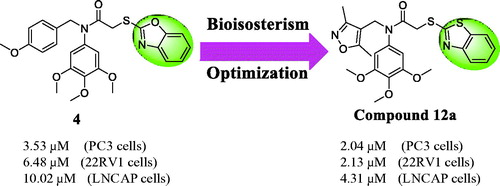
Bioisosterism is a useful strategy in rational drug design to improve pharmacodynamic or pharmacokinetic properties of lead compounds Citation20. In order to optimise the reported colchicine site tubulin inhibitor 4 and discovery more potently anticancer agentsCitation21–25, benzothiazole was used as a bioisostere of the benzoxazole in the lead structure. Herein, we synthesised a variety of benzothiazole derivatives based on the lead compound 4 by the bioisosterism approach. In addition, the preliminary structure activity relationship (SAR) was explored. The rational design of benzothiazole analogues was described in . Compared with the reported compound 4, compound 12a showed the better antiproliferative activity against prostate cancer cells.
Materials and methods
Chemistry
General procedure for preparation of compounds 9a∼9k and 12a∼12d
Imine intermediates 7a∼7k and 10a∼10d were synthesised according to our reported referencesCitation17,Citation19. A solution of intermediates 7a∼7k and 10a∼10d (3 mmol) in dichloromethane (20 ml) was reacted with 2-chloroacetyl chloride (6 mmol) at room temperature. After the mixture was stirred for 3 h, the reaction mixture was diluted with water. The organic layer was washed by aqueous NaHCO3, water, and brine, dried over anhydrous Na2SO4. The system was concentrated to provide the crude products 8a∼8k and 11a∼11d without purification.
Benzo[d]thiazole-2-thiol (1 mmol) and sodium hydroxide (2 mmol) were added to the solution of crude products 8a∼8k and 11a∼11d (1 mmol) in acetone (5 ml). After stirring at 60 °C for 5 h, the reaction mixture was concentrated to remove acetone. Then, the system was treated with a solution of ethyl acetate (30 ml) and H2O (30 ml). The organic layer was washed with aqueous Na2CO3 solution, water, and brine. The organic system was concentrated to obtain the residue, which was purified by column chromatography with n-hexane/ethyl acetate (10:1) to afford the target compounds 9a∼9k and 12a∼12d.
2-(Benzo[d]thiazol-2-ylthio)-N-(4-fluorobenzyl)-N-(3,4,5-trimethoxyphenyl)acetamide (9a)
Yield: 52%, white solid, m.p:1 4 3 ∼ 144 °C. 1H NMR (400 MHz, CDCl3) δ 7.67 (d, J = 7.9 Hz, 1H), 7.60 (d, J = 8.1 Hz, 1H), 7.37–7.29 (m, 1H), 7.24 – 7.21 (m, 1H), 7.15 (dd, J = 8.5, 5.5 Hz, 2H), 6.87 (t, J = 8.7 Hz, 2H), 6.27 (s, 2H), 4.79 (s, 2H), 3.98 (s, 2H), 3.75 (s, 3H), 3.65 (s, 6H). 13 C NMR (100 MHz, CDCl3) δ 166.04, 164.53, 152.70, 137.06, 135.82, 134.54, 132.04, 129.95, 129.87, 124.95, 123.37, 120.18, 114.33, 114.12, 104.75, 104.72, 59.91, 55.18, 51.88, 35.49. HRMS (ESI+): m/z [M + H]+ calcd for C25H24FN2O4S2: 499.1162; found: 499.1167.
2-(Benzo[d]thiazol-2-ylthio)-N-(3-chlorobenzyl)-N-(3,4,5-trimethoxyphenyl)acetamide (9 b)
Yield: 69%, white solid, m.p:1 0 5 ∼ 106 °C. 1H NMR (400 MHz, CDCl3) δ 7.72–7.53 (m, 2H), 7.34–7.27 (m, 1H), 7.24–7.20 (m, 1H), 7.17 (d, J = 1.4 Hz, 2H), 7.12 (dd, J = 9.6, 5.3 Hz, 2H), 6.29 (s, 2H), 4.79 (s, 2H), 4.00 (s, 2H), 3.76 (s, 3H), 3.66 (s, 6H). 13 C NMR (100 MHz, CDCl3) δ 166.18, 164.45, 152.77, 151.80, 138.14, 137.18, 135.77, 134.54, 133.17, 128.76, 128.17, 126.81, 126.37, 124.98, 123.37, 120.29, 120.10, 104.73, 59.92, 55.21, 52.12, 35.47. HRMS (ESI+): m/z [M + H]+ calcd for C25H24ClN2O4S2: 515.0866; found: 515.0870.
2-(Benzo[d]thiazol-2-ylthio)-N-(4-chlorobenzyl)-N-(3,4,5-trimethoxyphenyl)acetamide (9c)
Yield: 27%, white solid, m.p:1 0 7 ∼ 109 °C. 1H NMR (400 MHz, CDCl3) δ 7.67 (d, J = 7.9 Hz, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.37–7.29 (m, 1H), 7.23 (dd, J = 11.1, 4.1 Hz, 1H), 7.19 – 7.05 (m, 4H), 6.29 (s, 2H), 4.79 (s, 2H), 3.98 (s, 2H), 3.75 (s, 3H), 3.66 (s, 6H). 13 C NMR (100 MHz, CDCl3) δ 166.13, 164.48, 152.74, 151.77, 137.10, 135.84, 134.70, 134.55, 132.50, 129.58, 127.56, 124.98, 123.39, 120.18, 120.14, 104.70, 59.92, 55.22, 52.02, 35.42. HRMS (ESI+): m/z [M + H]+ calcd for C25H24ClN2O4S2: 515.0866; found: 515.0869.
2-(Benzo[d]thiazol-2-ylthio)-N-(4-bromobenzyl)-N-(3,4,5-trimethoxyphenyl)acetamide (9d)
Yield: 82%, white solid, m.p:1 3 0 ∼ 131 °C. 1H NMR (400 MHz, CDCl3) δ 7.70–7.63 (m, 1H), 7.56 (d, J = 7.8 Hz, 1H), 7.37–7.28 (m, 3H), 7.25–7.20 (m, 1H), 7.07 (d, J = 8.4 Hz, 2H), 6.29 (s, 2H), 4.77 (s, 2H), 3.97 (s, 2H), 3.75 (s, 3H), 3.65 (s, 6H). 13 C NMR (100 MHz, DMSO-d6) δ 161.86, 160.43, 148.52, 147.34, 132.89, 131.59, 130.97, 130.21, 126.30, 125.71, 120.82, 119.23, 116.40, 115.92, 100.48, 55.70, 51.01, 47.87, 31.26. HRMS (ESI+): m/z [M + H]+ calcd for C25H24BrN2O4S2: 559.0361; found: 559.0368.
2-(Benzo[d]thiazol-2-ylthio)-N-(4-methylbenzyl)-N-(3,4,5-trimethoxyphenyl)acetamide (9e)
Yield: 43%, white solid, m.p:1 4 4 ∼ 146 °C. 1H NMR (400 MHz, CDCl3) δ 7.64 (dd, J = 16.8, 8.0 Hz, 2H), 7.33–7.28 (m, 1H), 7.26–7.20 (m, 1H), 7.06 (d, J = 8.0 Hz, 2H), 6.99 (d, J = 7.9 Hz, 2H), 6.26 (s, 2H), 4.78 (s, 2H), 4.00 (s, 2H), 3.75 (s, 3H), 3.63 (s, 6H), 2.25 (s, 3H). 13 C NMR (100 MHz, CDCl3) δ 165.94, 164.76, 152.62, 151.75, 137.02, 136.23, 135.97, 134.49, 133.12, 128.19, 128.04, 124.93, 123.35, 120.30, 120.07, 104.89, 59.90, 55.17, 52.37, 35.70, 20.11. HRMS (ESI+): m/z [M + H]+ calcd for C26H27N2O4S2: 495.1412; found: 495.1417.
2-(Benzo[d]thiazol-2-ylthio)-N-benzyl-N-(3,4,5-trimethoxyphenyl)acetamide (9f)
Yield: 69%, white solid, m.p:1 5 0 ∼ 152 °C. 1H NMR (400 MHz, CDCl3) δ 7.65 (dd, J = 14.2, 8.0 Hz, 2H), 7.34–7.27 (m, 1H), 7.24–7.15 (m, 6H), 6.25 (s, 2H), 4.83 (s, 2H), 4.00 (s, 2H), 3.74 (s, 3H), 3.62 (s, 6H). 13 C NMR (100 MHz, CDCl3) δ 166.00, 164.62, 152.62, 151.85, 136.99, 136.18, 135.89, 134.55, 128.19, 127.42, 126.59, 124.93, 123.33, 120.28, 120.09, 104.80, 59.90, 55.15, 52.58, 35.62. HRMS (ESI+): m/z [M + H]+ calcd for C26H27N2O5S2: 511.1361; found: 511.1367.
2-(Benzo[d]thiazol-2-ylthio)-N-(3-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl)acetamide (9 g)
Yield: 69%, white solid, m.p:1 2 3 ∼ 124 °C. 1H NMR (400 MHz, CDCl3) δ 7.64 (dd, J = 14.7, 8.0 Hz, 2H), 7.36–7.26 (m, 1H), 7.24–7.19 (m, 1H), 7.10 (t, J = 8.1 Hz, 1H), 6.74 (dd, J = 6.0, 3.2 Hz, 3H), 6.28 (s, 2H), 4.80 (s, 2H), 4.01 (s, 2H), 3.75 (s, 3H), 3.63 (d, J = 7.2 Hz, 9H). 13 C NMR (100 MHz, CDCl3) δ 166.02, 164.57, 158.68, 152.63, 151.85, 137.68, 137.04, 135.93, 134.52, 128.36, 124.95, 123.34, 120.51, 120.32, 120.05, 113.49, 112.38, 104.82, 59.90, 55.18, 54.17, 52.58, 35.6. HRMS (ESI+): m/z [M + H]+ calcd for C26H27N2O5S2: 511.1361; found: 511.1367.
2-(Benzo[d]thiazol-2-ylthio)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl)acetamide (9 h)
Yield: 52%, white solid, m.p:1 2 5 ∼ 126 °C. 1H NMR (400 MHz, CDCl3) δ 7.63 (dd, J = 18.4, 7.9 Hz, 2H), 7.39–7.25 (m, 1H), 7.24–7.15 (m, 1H), 7.09 (d, J = 8.6 Hz, 2H), 6.71 (d, J = 8.6 Hz, 2H), 6.25 (s, 2H), 4.76 (s, 2H), 3.98 (s, 2H), 3.75 (s, 3H), 3.70 (s, 3H), 3.64 (s, 6H). 13 C NMR (100 MHz, CDCl3) δ 165.83, 164.65, 158.08, 152.60, 151.81, 136.93, 135.93, 134.52, 129.57, 128.35, 124.89, 123.31, 120.27, 120.08, 112.70, 104.83, 59.90, 55.17, 54.24, 51.99, 35.66. HRMS (ESI+): m/z [M + H]+ calcd for C26H27N2O5S2: 511.1361; found: 511.1366.
2-(Benzo[d]thiazol-2-ylthio)-N-(4-methoxybenzyl)-N-(p-tolyl)acetamide (9i)
Yield: 78%, yellow liquid. 1H NMR (400 MHz, CDCl3) δ 7.66 (t, J = 8.1 Hz, 2H), 7.31 (t, J = 7.7 Hz, 1H), 7.21–7.17 (m, 1H), 7.05 (dd, J = 8.2, 3.6 Hz, 4H), 6.91 (d, J = 8.1 Hz, 2H), 6.69 (d, J = 8.4 Hz, 2H), 4.76 (s, 2H), 3.92 (d, J = 1.0 Hz, 2H), 3.70 (s, 3H), 2.23 (s, 3H). 13 C NMR (100 MHz, CDCl3) δ 165.97, 157.93, 137.75, 137.45, 129.33, 129.29, 128.20, 127.22, 124.89, 123.26, 120.39, 120.00, 112.70, 54.18, 52.12, 35.97, 20.07. HRMS (ESI+): m/z [M + H]+ calcd for C24H23N2O2S2: 435.1201; found: 435.1207.
2-(Benzo[d]thiazol-2-ylthio)-N-(3,4-dichlorophenyl)-N-(4-methoxybenzyl)acetamide (9j)
Yield: 63%, yellow liquid. 1H NMR (400 MHz, CDCl3) δ 7.66 (t, J = 6.1 Hz, 2H), 7.37–7.29 (m, 2H), 7.27–7.20 (m, 2H), 7.02 (d, J = 8.4 Hz, 2H), 6.89 (dd, J = 8.4, 2.0 Hz, 1H), 6.69 (d, J = 8.4 Hz, 2H), 4.76 (s, 2H), 3.94 (s, 2H), 3.71 (s, 3H). 13 C NMR (100 MHz, CDCl3) δ 165.73, 164.19, 158.19, 151.46, 139.70, 134.46, 132.48, 131.99, 130.24, 129.64, 129.22, 127.34, 127.22, 125.03, 123.44, 120.37, 120.10, 112.94, 54.21, 52.13, 35.30. HRMS (ESI+): m/z [M + H]+ calcd for C23H19Cl2N2O2S2: 489.0265; found: 489.0269.
2-(Benzo[d]thiazol-2-ylthio)-N-(4-methoxybenzyl)-N-phenylacetamide (9k)
Yield: 68%, white solid, m.p: 109–110 °C. 1H NMR (400 MHz, CDCl3) δ 7.66 (t, J = 8.6 Hz, 2H), 7.29 (dt, J = 15.1, 7.7 Hz, 4H), 7.21 (d, J = 7.6 Hz, 1H), 7.09–7.01 (m, 4H), 6.68 (d, J = 8.6 Hz, 2H), 4.79 (s, 2H), 3.92 (s, 2H), 3.69 (s, 3H). 13 C NMR (100 MHz, CDCl3) δ 165.80, 164.67, 157.97, 151.79, 140.41, 134.50, 129.28, 128.76, 128.05, 127.52, 124.87, 123.26, 120.43, 120.02, 112.73, 54.18, 52.13, 36.01. HRMS (ESI+): m/z [M + H]+ calcd for C23H21N2O2S2: 421.1044; found: 421.1049.
2-(Benzo[d]thiazol-2-ylthio)-N-((3,5-dimethylisoxazol-4-yl)methyl)-N-(3,4,5-trimethoxyphenyl)acetamide (12a)
Yield: 83%, white solid, m.p:1 3 7 ∼ 138 °C. 1H NMR (400 MHz, CDCl3) δ 7.67 (d, J = 7.9 Hz, 1H), 7.57 (d, J = 8.1 Hz, 1H), 7.39–7.28 (m, 1H), 7.26–7.21 (m, 1H), 6.31 (s, 2H), 4.65 (s, 2H), 3.92 (s, 2H), 3.76 (s, 3H), 3.69 (s, 6H), 1.95 (s, 3H), 1.90 (s, 3H). 13 C NMR (100 MHz, CDCl3) δ 167.43, 165.70, 164.36, 158.95, 153.02, 151.69, 137.51, 134.80, 134.47, 125.14, 123.46, 120.16, 120.05, 109.21, 105.09, 60.05, 55.39, 39.84, 35.19, 9.74, 8.91. HRMS (ESI+): m/z [M + H]+ calcd for C24H26N3O5S2: 500.1314; found: 500.1317.
2-(Benzo[d]thiazol-2-ylthio)-N-(pyridin-4-ylmethyl)-N-(3,4,5-trimethoxyphenyl)acetamide (12 b)
Yield: 65%, white solid, m.p:1 2 9 ∼ 130 °C. 1H NMR (400 MHz, CDCl3) δ 8.43 (d, J = 5.5 Hz, 2H), 7.69 (d, J = 7.7 Hz, 1H), 7.63 (d, J = 8.1 Hz, 1H), 7.38–7.31 (m, 1H), 7.25 (d, J = 7.2 Hz, 1H), 7.20 (dd, J = 6.5, 4.5 Hz, 2H), 6.39 (s, 2H), 4.85 (s, 2H), 4.02 (s, 2H), 3.75 (s, 3H), 3.67 (s, 6H). 13 C NMR (100 MHz, CDCl3) δ 166.59, 164.51, 152.92, 151.71, 148.28, 145.96, 137.29, 135.91, 134.61, 125.13, 123.54, 122.71, 120.31, 119.97, 104.41, 59.92, 55.27, 52.00, 35.15. HRMS (ESI+): m/z [M + H]+ calcd for C24H24N3O4S2: 482.1208; found: 482.1213.
2-(Benzo[d]thiazol-2-ylthio)-N-((6-chloropyridin-3-yl)methyl)-N-(3,4,5-trimethoxyphenyl)acetamide (12c)
Yield: 73%, white solid, m.p:1 4 5 ∼ 146 °C. 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 2.2 Hz, 1H), 7.73–7.60 (m, 2H), 7.52 (d, J = 8.1 Hz, 1H), 7.38–7.30 (m, 1H), 7.23 (dd, J = 11.3, 4.0 Hz, 1H), 7.12 (d, J = 8.2 Hz, 1H), 6.34 (s, 2H), 4.80 (s, 2H), 3.96 (s, 2H), 3.76 (s, 3H), 3.69 (s, 6H). 13 C NMR (100 MHz, CDCl3) δ 166.47, 164.30, 153.00, 151.66, 149.88, 148.93, 138.94, 137.36, 135.60, 134.53, 130.76, 125.06, 123.47, 123.28, 120.20, 120.05, 104.57, 59.94, 55.31, 49.59, 35.13. HRMS (ESI+): m/z [M + H]+ calcd for C24H23ClN3O4S2: 516.0819; found: 516.0826.
2-(Benzo[d]thiazol-2-ylthio)-N-(naphthalen-2-ylmethyl)-N-(3,4,5-trimethoxyphenyl)acetamide (12d)
Yield: 52%, white solid, m.p:1 1 8 ∼ 119 °C. 1H NMR (400 MHz, CDCl3) δ 7.76–7.71 (m, 1H), 7.65 (dt, J = 10.7, 5.0 Hz, 3H), 7.55 (d, J = 8.5 Hz, 2H), 7.42–7.33 (m, 3H), 7.24–7.17 (m, 2H), 6.26 (s, 2H), 4.99 (s, 2H), 4.02 (s, 2H), 3.73 (s, 3H), 3.54 (s, 6H). 13 C NMR (100 MHz, CDCl3) δ 166.13, 164.56, 152.63, 151.81, 137.00, 135.89, 134.53, 133.61, 132.16, 131.79, 127.21, 127.19, 126.79, 126.58, 126.12, 125.12, 124.97, 124.91, 123.32, 120.29, 120.07, 104.81, 59.88, 55.11, 52.75, 35.63. HRMS (ESI+): m/z [M + H]+ calcd for C29H27N2O4S2: 531.1412; found: 531.1415.
Biology
Cell culture and MTT assay
PC3, C42B, 22RV1, and LNCAP cell lines were cultured in an atmosphere containing 5% CO2 at 37 °C, with RPMI-1640 medium with 10% foetal bovine serum, 100 U/ml penicillin and 0.1 mg/ml streptomycin. Cells were seeded at a density of 1500 per well in 96-well plates for 72 h. Then, 20 μL MTT (thiazolyl blue tetrazolium bromide) solution was added to each well, and incubated for 4 h at 37 °C. 150 μL DMSO was added to each well to dissolve the formazan after removing the liquid, the absorbance was determined at 570 nm.
In vitro tubulin polymerisation assay
Tubulin (5.6 mg/ml) was resuspended in PEM buffer (containing 80 mM PIPES, 1 mM ATP, 1 mM EGTA, 10.2% glycerol, 0.5 mM MgCl2) and then was preincubated with compound 12a, colchicine or vehicle DMSO on ice. The reaction was monitored by a spectrophotometer in absorbance at 420 nm (excitation wavelength is 340 nm).
Immunostaining and microscopy
PC3 cells were seeded on the slices and incubated overnight. Then, cells were treated with different concentrations of 12a. After 48 h, slices were fixed by 4% paraformaldehyde for 15 min after washed by PBS for 3 times. 0.5% Triton-X-100 was added and shaked for 20 min. 0.1% BSA was used to block for 30 min and then removed. The slices were added β-tubulin antibody (1:100) and incubated overnight. Then slices were washed by PBST 3 times, bind with secondary antibody with FITC signal (1:500) in a dark. DAPI was used to stain for 3 min and then removed. After that, images were captured by Laser scanning confocal microscope (Nikon, Japan).
EBI competition assay
6-Well plates were seeded with PC3 cells for 24 h. Then, cells were incubated with compound 12a, colchicine or DMSO for 2 h and afterward treated with EBI (100 mM) for 2 h. Then, the cells were harvested and lysed. Cell extracts were used for Western blotting analysis.
Western blot analysis
Cellular protein lysates were denatured in loading buffer at 100 °C prior to adding into SDS-PAGE. Proteins were transferred to nitrocellulose membranes, probed with indicated antibodies, and visualised by an enhanced chemiluminescence detection system. The western blotting bands were semi-quantified using Image J and adjusted for loading control.
DAPI staining
Cells were seeded on a 6-well plate (2 × 105/well) and treated with the test compound after the incubation for 24 h. Then, cells were stained by DAPI (4′,6-diamidino-2-phenylindole) in the dark for 30 min. The images of cells were observed in a fluorescence microscope (Nikon, Japan).
Molecular modelling
Molecular modelling was performed using the autodock 4 software from the Scripps Research Institute. Molecules were first generated using Pymol software. For the receptor preparation, the PDB code (1SA0) was downloaded from the Protein Data Bank. Dockings were initially performed using a blind docking method on a lower resolution docking. This was followed by a series of resolving steps to attempt to fine tune the compound. The final results were analysed by Pymol software.
Results and discussion
Synthesis of the target analogues 9a∼9k and 12a∼12d
The synthetic route of benzothiazoles containing the 3, 4, 5-trimethoxyphenyl fragment was shown in Scheme 1. Aniline derivatives and various benzyl chlorides were subjected to nucleophilic substitution reaction to afford imines 7a∼7k and 10a∼10d in the presence of potassium carbonate. The imine intermediates were reacted with chloroacetyl chloride to obtain the amide analogues 8a∼8k and 11a∼11d in the dichloromethane system. The target analogues 9a∼9k and 12a∼12d were easily obtained at the reflux condition with benzo[d]thiazole-2-thiol in the presence of sodium hydroxide. The chemical structures of all benzothiazole derivatives 9a∼9k and 12a∼12d were characterised using spectral methods, and all spectral data corroborated the structures.
Antiproliferative activity of novel benzothiazole derivatives
To discovery novel anti-prostate cancer agents and tubulin polymerisation inhibitors, we evaluated the antiproliferative activity of all benzothiazole derivatives 9a∼9k and 12a∼12d against a prostate cancer cell line 22RV1 using the 3–(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. Based on previous references, Citation5-fluorouracil (5-Fu) and was usually selected as a reference drug in the MTT assay to evaluate the antiproliferative activity of synthetic benzothiazole derivativesCitation26,Citation27. Therefore, 5-Fu was used as the control drug in this work. In addition, compound 4 was also the control drug to compare the antiproliferative activity.
From the antiproliferative results of benzothiazole derivatives in , benzothiazoles without the 3, 4, 5-trimethoxyphenyl fragment (9i∼9k) displayed very weak activity against 22RV1 cells with IC50 values of >20 μM. However, benzothiazole-trimethoxyphenyl hybrids 9a∼9h showed moderate to potent activity with IC50 values from 5.24 μM to 19.07 μM against 22RV1 cells. Based on this findings, it revealed that 3, 4, 5-trimethoxyphenyl fragment exhibited an important role for the antiproliferative activity of these benzothiazole derivatives.
Table 1. Antiproliferative activity of the synthetic derivatives 9a∼9k against 22RV1 cells.
To obtain potently antiproliferative compounds, trimethoxyphenyl fragment as B ring was reserved and the molecular deversity of different substituent groups attaching on the A ring was explored. Among them, compound 9 h showed the most potent activity with an IC50 value of 5.24 μM against 22RV1 cells. We found that the substitution on the A ring was important for the activity showing an over 3-fold activity loss, when the p-Br group was replaced with the m-Cl group (compounds 9d vs. 9 b). In addition, compounds 9 g∼9h with an electron-donating methoxy group on the A ring had a more potent inhibitory effect (7.37 and 5.24 μM, respectively) against 22RV1 cells than compounds 9 b∼9c (IC50 >12 μM) with an electron-withdrawing group. From the biological data of compounds 9a∼9h, we can conclude that the substituents on the A ring had a remarkable effect on their cytotoxic activity.
To determine whether the aromatic ring or heterocycles have an effect on the activity, compounds with a 3, 5-dimethylisoxazole ring (12a), a pyridine ring (12 b), a 2-chloropyridine ring (12c), and a naphthalene ring (12d) were synthesised and their antiproliferative activity results were shown in . Replacement of the pyridine scaffold of compound 12 b with a 2-chloropyridine fragment (12c) or a naphthalene unit (12d) led to a loss of the activity. However, changing the pyridine ring of compound 12 b to a 3, 5-dimethylisoxazole ring of compound 12a led to an improvement of the activity against 22RV1 cells. All these results indicated that 3, 5-dimethylisoxazole displayed the better inhibitory activity than other selected heterocycles and phenyl ring.
Table 2. Antiproliferative activity of the synthetic derivatives 12a∼12d against 22RV1 cells.
Compound 12a inhibited cell proliferation against prostate cancer
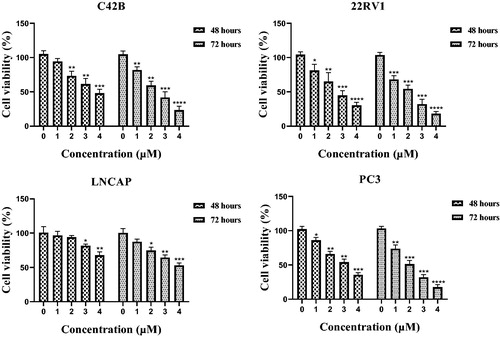
Among all benzothiazole derivatives 9a∼9k and 12a∼12d, 12a displayed the most potently antiproliferative activity with an IC50 value of 2.13 μM against 22RV1 cells. Compound 12a was further examined for possible cytotoxicity against RWPE-1 (normal human prostatic cell line). We found that compound 12a exhibited no cytotoxicity against RWPE-1 (>20 μM). The results indicated that compound 12a had good selectivity between the selected cancer cell line (22RV1) and a normal cell line (RWPE-1). To evaluate its antiproliferative activity against other prostate cancer cell lines, C42B, LNCAP and PC3 cells were selected to perform MTT assay. As shown in the , benzothiazole derivative 12a exhibited the well antiproliferative activity against C42B, LNCAP and PC3 cell lines with IC50 values of 2.81 ± 0.43 μM, 4.31 ± 0.27 μM and 2.04 ± 0.51 μM, respectively.
Compound 12a inhibited tubulin polymerisation by immunostaining assay
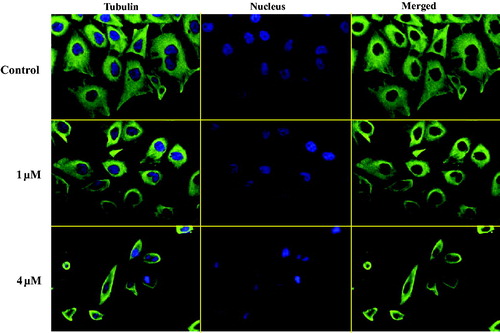
As the microtubule system played an important role in the maintenance of cell shape and basic cellular functions, an immunofluorescence staining assay was performed to study whether benzothiazole hybrid 12a could disrupt the microtubule dynamics in living cellsCitation28. As shown in , in the control group, the microtubule network in PC3 cells exhibited normal arrangement and organisation, characterised by regularly assembled, normal microtubules wrapped around the cell nucleus. Benzothiazole hybrid 12a at the concentration of 1 μM moderately depolymerised interphase microtubules whereas 12a at the higher concentration of 4 μM induced much stronger depolymerisation effects. These immunofluorescence experiments indicated that compound 12a inhibited tubulin polymerisation in a concentration dependent manner against PC3 cells.
Compound 12a inhibited tubulin polymerisation targeting the colchicine binding site
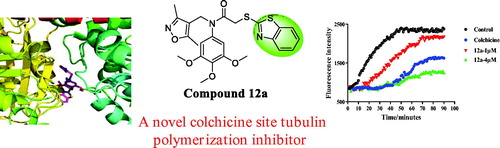
The in vitro tubulin polymerisation inhibition activity of compound 12a was evaluated because of its best antiproliferative activity results among all benzothiazole hybrids. When tubulin was incubated with the tested compound 12a, the increased tendency of the fluorescence intensity was obviously slowed down compared with the control. The IC50 value of compound 12a was 2.87 μM against tubulin (). It revealed thatbenzothiazole hybrid 12a was a novel tubulin polymerisation inhibitor.
Figure 5. (A) Tubulin polymerisation inhibitory activity of 12a. (B) EBI competition assay of 12a in PC3 cells.
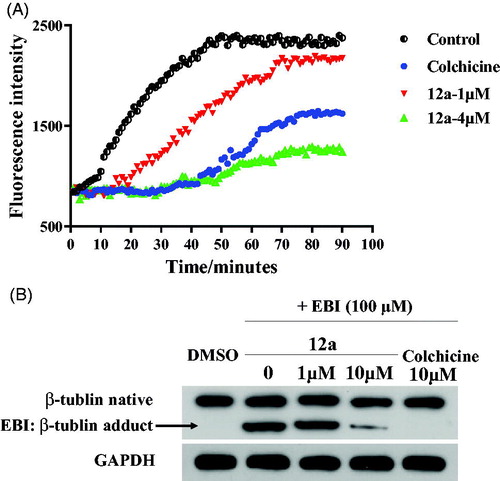
In order to evaluate whether benzothiazole hybrid 12a directly binds to tubulin at the colchicine binding site, we carried out N,N′-ethylenebis(iodoacetamide) (EBI) competition assay in PC3 cells according to previously published referencesCitation29,Citation30. EBI is an alkylating agent that has the property to specifically cross-link the Cys239 and the Cys354 residues of β-tubulin involved in the colchicine-binding siteCitation31. The occupancy of colchicine-binding site by tubulin polymerisation inhibitors could inhibit the formation of the EBI: β-tubulin adduct. Preincubation of compound 12a at 10 μM prevented the formation of the EBI: β-tubulin adduct, resulting in the decrease of the adduct band comparing with DMSO and EBI treatment. Thus, the assay () indicated that compound 12a directly bind to the colchicine-binding site of β-tubulin.
Compound 12a induced morphological changes and affected the expression of apoptosis-related proteins
As most tubulin destabilising agents displayed the ability to induce morphological changes and affect the expression of apoptosis-related proteinsCitation32, DAPI staining and western blotting experiments were performed to determine the effects of benzothiazole hybrid 12a. After 48 h incubation with compound 12a (0, 1 μM, 2 μM and 4 μM), characteristic apoptotic morphological changes were observed by fluorescence microscopy, including chromatin shrinkage and formation of apoptotic bodies (). The studies revealed that compound 12a induced morphological changes of prostate cancer (22RV1 cells and PC3 cells) possibly by inducing apoptosis.
Figure 6. (A) Morphological changes of prostate cancer cells with the treatment of 12a. (B) The expression levels of apoptosis-related proteins (Bcl-2 and Cleaved-parp). **p < 0.01 versus control, ***p < 0.001 versus control, ****p < 0.0001 versus control.
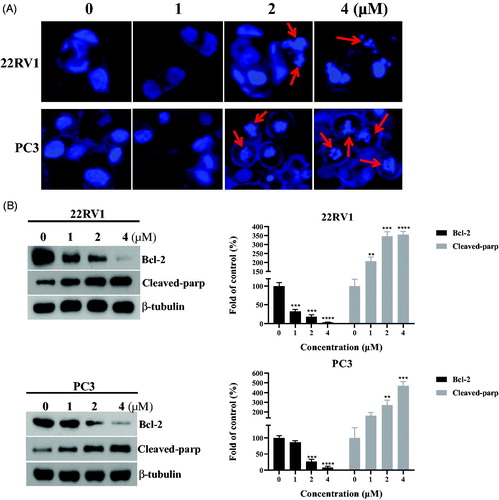
Bcl-2 family proteins are key regulators of apoptosis and overexpression of the prominent pro-survival Bcl-2 family members like Bcl-2 is a common feature responsible for deregulation of apoptosis in prostate cancer cellsCitation33. In order to investigate the apoptosis effects of compound 12a, the expression of apoptosis-related proteins (Bcl-2 and Cleaved-parp) were tested. As shown in the , the expression level of Bcl-2 was decreased and the expression level of Cleaved-parp was increased in a concentration-dependent manner against prostate cancer (22RV1 cells and PC3 cells).
Molecular modelling analysis of compound 12a
In the current work, trimethoxyphenyl-benzothiazole hybrid 12a has been identified as a novel colchicine site tubulin polymerisation inhibitor by the immunostaining, in vitro tubulin polymerisation assay and EBI competition assay. Furthermore, molecular docking methodologies were also used to explore any molecular interaction exist between 3,4,5-trimethoxyphenyl-benzothiazole hybrid 12a and residues lies in the active site cativity of tubulin. We have used Autodock as an automated tool to perform docking and selected PDB code 1SA0.
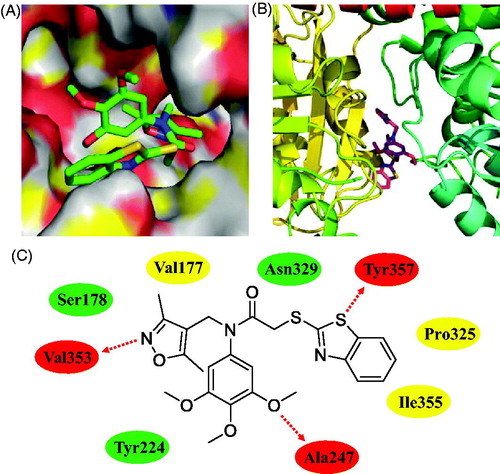
As shown in , benzo[d]thiazole, 3,4,5-trimethoxyphenyl ring, and isoxazole unit formed three hydrogen bonds with residues Tyr357, Ala247, and Val353, respectively. This finding illustrated the importance of 3,4,5-trimethoxyphenyl scaffold for tubulin polymerisation inhibitors. 3,4,5-Trimethoxyphenyl-benzothiazole hybrid 12a formed hydrophilic interactions with the residues of Ser178, Asn329 and Tyr224. In addition, 12a also formed hydrophobic interactions with the residues of Pro325, Val177 and Ile355. All these results suggested that hybrid 12a as a novel tubulin polymerisation inhibitor could display the potently antiproliferative activity.
Figure 7. Molecular docking studies of compound 12a. (A) Compound 12a bound to β-tubulin (surface). (B) The complex formed between tubulin and compound 12a. (C) Hydrogen bonds (red ellipse), hydrophilic interactions (green ellipse) and hydrophobic interactions (yellow ellipse) observed between compound 12a and residues.
Conclusions
A series of benzothiazole derivatives were designed, synthesised and evaluated for their antiproliferative activity against prostate cancer. Among them, compound 12a possessed the potently antiproliferative ability against C42B, LNCAP, 22RV1 and PC3 cell lines with IC50 values of 2.81 μM, 4.31 μM, 2.13 μM and 2.04 μM, respectively. Molecular mechanisms illustrated that compound 12a could induce morphological changes and affect the expression levels of apoptosis-related proteins (Bcl-2 and Cleaved-parp) against prostate cancer cells. From the in vitro tubulin polymerisation inhibition assay and EBI competition assay, compound 12a was a novel colchicine site tubulin polymerisation inhibitor. Taken together, compound 12a might be a lead candidate for its further development in treatment of prostate cancer.
Acknowledgements
The authors gratefully thank the support from Zhengzhou university.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Culp MB, Soerjomataram I, Efstathiou JA, et al. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol 2020;77:38–52.
- Xu YH, Deng JL, Wang G, et al. Long non-coding RNAs in prostate cancer: Functional roles and clinical implications. Cancer Lett 2019;464:37–55.
- Vasquez JL, Lai Y, Annamalai T, et al. Inhibition of base excision repair by natamycin suppresses prostate cancer cell proliferation. Biochimie 2020;168:241–50.
- Tripathi A, Durrant D, Lee RM, et al. Hydropathic analysis and biological evaluation of stilbene derivatives as colchicine site microtubule inhibitors with anti-leukemic activity. J Enzyme Inhib Med Chem 2009;24:1237–44.
- Ahmed RI, Osman EEA, Awadallah FM, et al. Design, synthesis and molecular docking of novel diarylcyclohexenone and diarylindazole derivatives as tubulin polymerization inhibitors. J Enzyme Inhib Med Chem 2017;32:176–88.
- Wang G, Liu W, Gong Z, et al. Synthesis, biological evaluation, and molecular modelling of new naphthalene-chalcone derivatives as potential anticancer agents on MCF-7 breast cancer cells by targeting tubulin colchicine binding site. J Enzyme Inhib Med Chem 2020;35:139–44.
- Arnst KE, Wang Y, Hwang DJ, et al. A potent, metabolically stable tubulin inhibitor targets the colchicine binding site and overcomes taxane resistance. Cancer Res 2018;78:265–77.
- Li W, Sun H, Xu F, et al. Synthesis, molecular properties prediction and biological evaluation of indole-vinyl sulfone derivatives as novel tubulin polymerization inhibitors targeting the colchicine binding site. Bioorg Chem 2019;85:49–59.
- Bukhari SNA, Kumar GB, Revankar HM, et al. Development of combretastatins as potent tubulin polymerization inhibitors. Bioorg Chem 2017;72:130–47.
- Diao PC, Lin WY, Jian XE, et al. Discovery of novel pyrimidine-based benzothiazole derivatives as potent cyclin-dependent kinase 2 inhibitors with anticancer activity. Eur J Med Chem 2019;179:196–207.
- Aouad MR, Almehmadi MA, Rezki N, et al. Design, click synthesis, anticancer screening and docking studies of novel benzothiazole-1,2,3-triazoles appended with some bioactive benzofused heterocycles. J Mol Struct 2019;1188:153–64.
- El-Damasy AK, Lee JH, Seo SH, et al. Design and synthesis of new potent anticancer benzothiazole amides and ureas featuring pyridylamide moiety and possessing dual B-RafV600E and C-Raf kinase inhibitory activities. Eur J Med Chem 2016;115:201–16.
- Hutchinson I, Chua MS, Browne HL, et al. Antitumor Benzothiazoles. 14. Synthesis and in vitro biological properties of fluorinated 2-(4-aminophenyl)benzothiazoles. J Med Chem 2001;44:1446–55.
- Hutchinson I, Bradshaw TD, Matthews CS, et al. Antitumour benzothiazoles. Part 20:††For Part 19 of the series, see ref 1. 3′-Cyano and 3′-Alkynyl-Substituted 2-(4′-Aminophenyl)benzothiazoles as new potent and selective analogues. Bioorg Med Chem Lett 2003;13:471–4.
- Repicky A, Jantova S, Cipak L. Apoptosis induced by 2-acetyl-3-(6-methoxybenzothiazo)-2-yl-amino-acrylonitrile in human leukemia cells involves ROS-mitochondrial mediated death signaling and activation of p38 MAPK. Cancer Lett 2009;277:55–63.
- Kamal Ä, Ashraf M, Vishnu Vardhan MVPS, et al. Synthesis and anticancer potential of benzothiazole linked phenylpyridopyrimidinones and their diones as mitochondrial apoptotic inducers. Bioorg Med Chem Lett 2014;24:147–51.
- Fu DJ, Yang JJ, Li P, et al. Bioactive heterocycles containing a 3,4,5-trimethoxyphenyl fragment exerting potent antiproliferative activity through microtubule destabilization. Eur J Med Chem 2018;157:50–61.
- Fu DJ, Fu L, Liu YC, et al. Structure-activity relationship studies of β-lactam-azide analogues as orally active antitumor agents targeting the tubulin colchicine site. Sci Rep 2017;7:12788.
- Fu DJ, Li P, Wu BW, et al. Molecular diversity of trimethoxyphenyl-1,2,3-triazole hybrids as novel colchicine site tubulin polymerization inhibitors. Eur J Med Chem 2019;165:309–22.
- Arabi AA. Routes to drug design via bioisosterism of carboxyl and sulfonamide groups. Future Med Chem 2017;9:2167–2180.
- Fu DJ, Zhang SY, Liu YC, et al. Design, synthesis and antiproliferative activity studies of 1,2,3-triazole-chalcones. MedChemComm 2016;7:1664–71.
- Fu DJ, Zhao RH, Li JH, et al. Molecular diversity of phenothiazines: design and synthesis of phenothiazine–dithiocarbamate hybrids as potential cell cycle blockers. Mol Divers 2017;21:933–42.
- Fu DJ, Liu JF, Zhao RH, et al. Design and antiproliferative evaluation of novel sulfanilamide derivatives as potential tubulin polymerization inhibitors. Molecules 2017;22:1470.
- Fu DJ, Zhang L, Song J, et al. Design and synthesis of formononetin-dithiocarbamate hybrids that inhibit growth and migration of PC-3cells via MAPK/Wnt signaling pathways. Eur J Med Chem 2017;127:87–99.
- Fu DJ, Song J, Hou YH, et al. Discovery of 5,6-diaryl-1,2,4-triazines hybrids as potential apoptosis inducers. Eur J Med Chem 2017;138:1076–88.
- Gurdal EE, Durmaz I, Cetin-Atalay R, et al. Cytotoxic activities of some benzothiazole-piperazine derivatives. J Enzyme Inhib Med Chem 2015;30:649–54.
- Cindrić M, Jambon S, Harej A, et al. Novel amidino substituted benzimidazole and benzothiazole benzo[b]thieno-2-carboxamides exert strong antiproliferative and DNA binding properties. Eur J Med Chem 2017;136:468–79.
- Yan J, Chen J, Zhang S, et al. Synthesis, evaluation, and mechanism study of novel indole-chalcone derivatives exerting effective antitumor activity through microtubule destabilization in vitro and in vivo. J Med Chem 2016;59:5264–83.
- Cao D, Liu Y, Yan W, et al. Design, synthesis, and evaluation of in vitro and in vivo anticancer activity of 4-substituted coumarins: a novel class of potent tubulin polymerization inhibitors. J Med Chem 2016;59:5721–39.
- Greene TF, Wang S, Greene LM, et al. Synthesis and biochemical evaluation of 3-phenoxy-1,4-diarylazetidin-2-ones as tubulin-targeting antitumor agents. J Med Chem 2016;59:90–113.
- Luduena RF, Roach MC. Tubulin sulfhydryl groups as probes and targets for antimitotic and antimicrotubule agents. Pharmacol Ther 1991;49:133–52.
- Subba Rao AV, Swapna K, Shaik SP, et al. Synthesis and biological evaluation of cis-restricted triazole/tetrazole mimics of combretastatin-benzothiazole hybrids as tubulin polymerization inhibitors and apoptosis inducers. Bioorg Med Chem 2017;25:977–99.
- Bray K, Chen HY, Karp CM, et al. Bcl-2 modulation to activate apoptosis in prostate cancer. Mol Cancer Res 2009;7:1487–96.

![Scheme 1. Reagents and conditions: (a) K2CO3, aniline derivatives or various benzyl chlorides, acetone, reflux; (b) Chloroacetyl chloride, dichloromethane, rt; (c) NaOH, benzo[d]thiazole-2-thiol, acetone, reflux.](/cms/asset/27153cfb-dfb2-4b2b-81d4-da48cf469b40/ienz_a_1753721_sch0001_c.jpg)