Abstract
Carbonic anhydrase IX (CAIX) is a hypoxia-related protein considered as a predictor for oral squamous cell carcinoma (OSCC) biological behaviour. Nevertheless, this prognostic value is still yet to be validated. We aim to quantify prognostic significance of CAIX overexpression in OSCC by meta-analysis. We performed searches in MEDLINE, EMBASE, SCOPUS, WOS, WHO’S databases, CPCI, and OATD from inception to August 2019. Overall survival (OS), disease-free survival (DFS), locoregional control (LC), and disease-specific survival (DSS) were considered as outcomes of interest. Overall 18 studies were included. CAIX overexpression was associated with worse OS (hazard ratio [HR] = 1.45 95% confidence interval [CI] 1.17–1.80) and DFS (HR = 1.98 95% CI 1.18–3.32). To the contrary, it was neither associated with LC (HR = 1.01 95% CI 0.50–2.02) nor with DSS (HR = 1.35 95% CI 0.78–2.33). Heterogeneity was negligible in all analyses except for DSS. Small studies effect was not significant for OS and DFS. This study shows that immunohistochemical CAIX assessment is a useful OSCC prognostic biomarker.
1. Introduction
Hypoxia is one of the most complex conditions that cellular and extracellular matrix confront in order to preserve their homeostasisCitation1. Bearing the hallmarks of cancer in mind as described by Hanahan and Weinberg, hypoxic metabolic reprogramming elicits complex mechanisms which take place in the microenvironment of many solid tumoursCitation2,Citation3. Two of the key transcription factors that play major roles in this metabolic reprogramming are the hypoxia inducible factors (HIFs), namely HIF-1α and HIF-2αCitation4. Carbonic anhydrase IX (CAIX) is a HIF-1α-dependent protein that regulates cellular and extracellular pH homeostasis under hypoxia, playing a pivotal role in carcinogenesis and in the malignant transition of premalignant disordersCitation5–7. Several studies have evaluated the prognostic value of CAIX in different types of cancer, including oral squamous cell carcinoma (OSCC)Citation8,Citation9.
OSCC accounts for 95% of all oral malignant neoplasms and its global five-year survival rate ranges between 50 and 60%Citation10. The TNM cancer staging system is still considered to be of great prognostic value for this solid tumourCitation11. Nonetheless, this system cannot be used to accurately predict the biologic properties of OSSC, nor can it be used to provide guidance for treatment strategies from a molecular biology perspective. Analysing molecular alterations in order to detect specific abnormalities at a transcriptional, translational, and post-translational level has proven to be of particular interestCitation12,Citation13. In this line, immunohistochemistry (IHC) – the use of mono and polyclonal antibodies to determine the tissue distribution of an antigen – has an outstanding oncological impactCitation14. Nonetheless, the clinical translation of these IHC-based approaches in terms of decision-making, remains suboptimal for many solid tumoursCitation14,Citation15.
There is growing evidence which confirms the key role of CAIX in oral oncogenesis. Numerous publications have explored a possible relationship between CAIX expression and OSCC prognosis, as well as the rate of progression from oral potentially malignant disorders to OSCCCitation16–18.
The only meta-analysis carried out on this issue so far did not assess the prognostic value in subgroupsCitation19. It is known that the prognosis of head and neck cancers is different through tumour locations. It is then of paramount importance to carry out subgroup analysesCitation20.
We, therefore, decided to perform a systematic review and meta-analysis on the influence of CAIX expression on the long-term outcomes of patients suffering from OSCC.
2. Material and methods
2.1. Protocol and eligibility criteria
A systematic literature review was conducted in November 2019 and the protocol used adhered to the PRISMA guidelinesCitation21. The search question was formulated according to the PECO framework, and it read as follows: What is the prognostic value of tumoural CAIX immunohistochemical expression in patients with OSCC?
2.2. Sources
Electronic searches were carried out in MEDLINE via PubMed, EMBASE via OVID, Web of Science, Scopus, the WHO five regional bibliographic databases (AIM, LILACS, IMEMR, IMSEAR, and WPRIM), and the Conference Proceedings Citation Index databases. For Medline, the following algorithm was used both in the Medical Subject Heading and in the free text words: (“CAIX”) OR (“ca9”) OR (“carbonic anhydrase IX”) OR (“carbonic anhydrase 9”) OR (“carbonic anhydrase-IX”) OR (“carbonic anhydrase-9”) OR (“CA-IX”) OR (“ca-9”) OR (“G250”) AND (“carcinoma, squamous cell” OR “carcinoma” AND “squamous” AND (“cell”) OR “squamous cell carcinoma”) OR (“mouth neoplasm”). The aforementioned syntax was conveniently adapted for each database. All of the databases were searched from inception to August 2019. This process was complemented by a manual search in a series of peer-reviewed journals with related content. Potentially relevant articles that any of the authors were familiar with, as well as reference lists from the retrieved articles, were also comprehensively checked. In these searches, no language restrictions were applied.
2.3. Study selection and data extraction process
The study eligibility criteria were applied independently by two trained reviewers (A.I.L.P. and M.P.S.). Any discrepancies were resolved by consensus of all participating authors.
Criteria for eligibility for retrieved studies in the qualitative/quantitative analysis were as follows: i) original research articles published in any language; ii) assessing CAIX expression in biopsies from patients with OSCC using IHC methods; iii) analysing the association between CAIX overexpression with any of the following long-term outcomes: overall survival (OS), disease-free survival (DFS), locoregional control (LC), and disease-specific Survival (DSS). The exclusion criteria were as follows: i) case reports, editorials, or letters; in vitro or animal-based studies; ii) insufficient statistical data to estimate predefined outcomes; iii) studies evaluating CAIX protein-related genes or miRNAs; iv) studies with duplicated cohorts.
In the first round, the title and abstract of the retrieved articles and studies which met the inclusion criteria were read and any texts which presented insufficient data in order for a clear decision to be made were assessed following a full-text protocol. Subsequently all of the studies which were considered eligible were fully examined in a second round and the final decision as to whether or not they were to be included was made. This form included the following items: first author, year of publication, country and continent where the study was conducted, sample size, recruitment period, tumour subsite, treatment modality, follow-up period, cut-off value for CAIX IHC positivity, immunostaining pattern (nuclear/cytoplasmic), hazard ratios (HRs) for long-term outcomes, and adjustment variables.
2.4. Quality assessment, data synthesis, and analysis
Quality was independently assessed by two authors (O.A.C. and C.M.C.P.) by means of a variation of the criteria formulated in the Reporting Recommendations for Tumour Marker Prognostic Studies (REMARK) guidelines for prognostic studies and the Standards for Reporting of Diagnostic Accuracy (STARD) developed by Troiano et alCitation22. This variation included six dimensions which evaluated:
Samples: i) Cohort (retrospective or prospective) study with a well-defined study population; ii) Medical treatment applied to the patients was explained. Authors have explained if all patients have received the same treatment or not.
Clinical data of the cohort: The basic clinical data such as age, gender, clinical stage, and histopathological grade was provided.
IHC: Well-described staining protocol or referred to original paper.
Prognosis: The analysed survival endpoints were well defined (e.g. OS and DFS).
Statistics: i) Cut-off point, which is used to divide the cases into risk groups was well described; ii) Estimated effect describing the relationship between the evaluated biomarker and the outcome was provided; (iii) Adequate statistical analysis (e.g. Cox regression modelling) was performed to adjust the estimation of the effect of the biomarker for known prognostic factors.
Classical prognostic factor: The prognostic value of other classical prognostic factors and its relationship with the studied factor was reported.
Each parameter could be identified by one of three attributes (i.e. adequate [A], inadequate [I], or non-evaluable [N/A]. Each item scored as adequate adds one point to overall quality assessment for each study. A score sheet was prepared for each included study and quality scoring was independently undertaken by aforementioned author. In the event of disagreement, the scores were discussed until a consensus was reached. Studies were categorised as high quality when the overall score was >4.
The differences in the levels of CAIX staining were categorised as high and low, according to the cut-off value which was chosen by the authors of the studies. HRs and 95% confidence intervals (CIs) were used as the measure of association in order to estimate the impact of CAIX expression on the aforementioned long-term outcomes (OS, DFS, LC, and DSS). Multivariate or univariate HRs values were used but, when available, the formers were chosen. When data on the HRs could not be directly traced, these were calculated using the approximation methods described by Parmar et al.Citation23 and Tierney et al.Citation24. Lastly, when access was provided to full databases, the HRs were directly extracted.
Pooled analyses were obtained using both fixed-effect models (i.e. Mantel–Haenszel method) and random-effect models (i.e. DerSimonian and Laird method), but when substantial heterogeneity was detected, we based our assessment only on random-effects models. A subgroup analysis on the basis of several variables was planned (i.e. quality score, ethnic variations, tumour subsite, CAIX antibody, cut-off point, and type of covariate adjustment). The heterogeneity was assessed using the proportion of the total variance due to the variance between studies (Ri)Citation25. Large values (>0.75) indicate a large amount of heterogeneity, values between 0.4 and 0.75 suggest a moderate amount, whereas small values (<0.4) indicate a low amount of heterogeneity. Funnel plots were used to visually assess publication bias and the Egger’s test was used in order to conduct a more formal analysis. Stata version 14.1 (Stata Corp, College Station, TX, US) and HEpiMA version 2.1.3 (Corunna, Galicia, Spain) were employedCitation26.
3. Results
3.1. Characteristics of the included studies
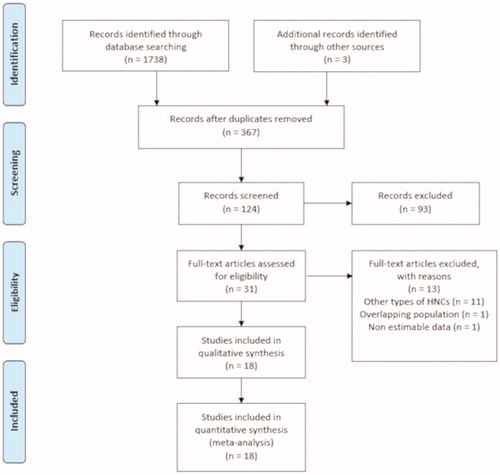
Out of the 1741 publications which were initially retrieved, 18 studies met our inclusion criteria and were included in the meta-analysis, as depicted in . The studies reported on a total of 1616 OSCC-affected patientsCitation27–44.
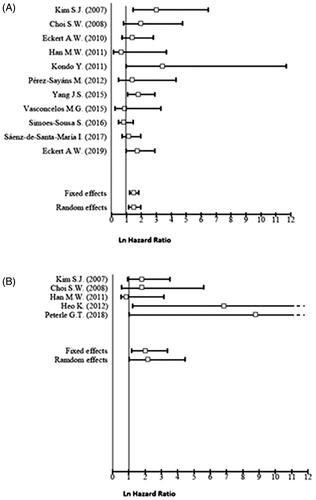
Figure 2. Forest plot for the association of higher CAIX expression with overall survival (A), disease‐free survival (B).
The data was collected in a period spanning from 1987 to 2015Citation27–44 while the year of publication ranged from 2008 to 2019. The anatomic location of the tumours was mainly divided into exclusively tongueCitation27,Citation28,Citation30,Citation31,Citation37,Citation39,Citation42, or mixed subsite tumours Citation29,Citation32–36,Citation38,Citation40,Citation41. The population sample ranged from 21 in Roh et al. studyCitation30 to 271 in Yang et al. studyCitation38. The studies were carried out in Asia, Europe, and America. Half of the studies were carried out in Asia (specifically in ChinaCitation38, JapanCitation27,Citation33, and South KoreaCitation28–30,Citation32,Citation35,Citation37) and the other half in BrazilCitation39,Citation43, CanadaCitation34, GermanyCitation31,Citation44, PortugalCitation40, SpainCitation36,Citation40,Citation42, and the USCitation41. The retrieved data are summarised in . The individual HRs for each selected long-term outcome with its respective adjustment is reflected in . The authors had full access to three full databasesCitation36,Citation40,Citation42. An article-based doctoral dissertation retrieved via LILACS needed to be fully assessed for its HR estimationCitation39.
Table 1. Characteristics of the included studies.
Table 2. Synthesis of data extracted from the included studies related to outcomes pooled in the meta‐analysis.
3.2. Quality assessment and pooled analysis of the included studies
The quality assessment which was performed in accordance with the REMARK guidelines is summarised in . According to these criteria ten studies showed a good quality, although eight were considered at high risk of bias. lists the pooled effect estimates for all 18 studies, for each of the selected long-term outcomes, as well as the analysis of its subgroups. A fixed-effects model was used to evaluate the pooled HR with 95% CI for the outcomes of OS, DFS, and LC, given that they displayed low heterogeneity. A random-effects model was used for DSS due to its high heterogeneity. A higher CAIX expression is associated with a statistically significant worse OS (HR = 1.45, 95% CI 1.17–1.80), and in the case of DFS, the pooled analysis reflected almost a two-fold increase in the hazard for this outcome (HR = 1.98, 95% CI 1.18–3.32). However, higher CAIX expression is apparently not related to LC (HR = 1.01, 95% CI 0.50–2.02), nor to DSS (HR = 1.35, 95% CI 0.78–2.33).
Table 3. Quality score according to the REMARK guidelines.
Table 4. Pooled hazard ratios and 95% confidence intervals.
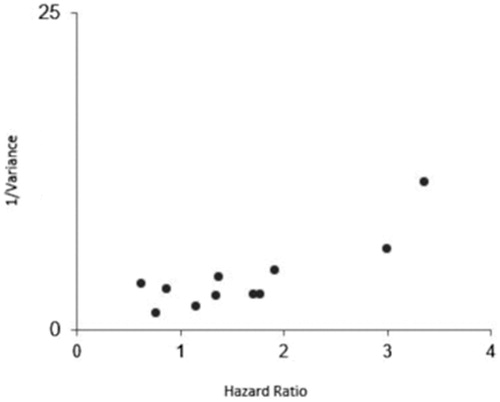
The funnel plot for OS () indicates a minimal skewness to the right that was not confirmed by the Egger’s test (PEgger = 0.59). In the case of DFS, this statistical test also indicates the absence of publication bias (PEgger = 0.29).
In the OS subgroup analysis, the statistically significant association was not preserved in the non-Asian (HR = 1.19, 95% CI 0.91–1.57), low quality (HR = 1.21, 95% CI 0.64–2.29), and tongue carcinoma subgroups (HR = 1.18, 95% CI 0.84–1.66). The analysis showed that the pooled HR was twice higher for the Asian group (HR = 2.01, 95% CI 1.42–2.86) when compared to the Non-Asian group. Furthermore, the use of M75 antibody and the choice of a 10% cut-off point were related with increased HR, [HR = 1.93, 95% CI 1.29–2.88], and HR =1.72, 95% CI 1.14–2.59), respectively. In the case of DFS, its statistical significance was preserved in the Asian group (HR = 1.81, 95% CI 1.06–3.09), mixed subsites (HR = 3.24, 95% CI 1.35–7.76), and use of other cut-off points than 10% for IHC subgroups (HR = 2.50 95% CI 0.91–6.89) ().
4. Discussion
Globally, this systematic review and meta-analysis show that CAIX overexpression is correlated with worse OS and DFS in OSCC patients, indicating that positivity for this test implies that the overall risk of dying increases by about 50%. The relation between CAIX expression and OS and DFS appears to confirm the prognostic value that is attributed to this marker on the basis of its relation with the HIF pathwayCitation9,Citation45. CAIX contributes to the tumour microenvironment by maintaining extracellular acidic pH and helping cancer cells grow and metastasise in several other solid tumoursCitation5,Citation6.
Our study showed a lower prognostic value for OS and DFS than that shown in Peridis et al.’s studyCitation19. Our study may be more reliable due to the accumulation of studies with high-quality scores and lower heterogeneity across studies. In addition, to our knowledge, our study is the first meta-analysis to measure this prognostic value exclusively in OSCC.
The relative consistency of the results across subgroups reinforces the plausibility of the findings. First, publication bias is a highly unlikely explanation for the present results given the findings of the asymmetry tests of the funnel plot for both OS and DFS (). It is worth mentioning that we observed that a relevant part of the studies included in our meta-analysis did not provide HRs estimates adjusted for relevant cofounders, although subgroup analysis demonstrated that even after the use of fully adjusted models for multiple established OSCC risk factors the OS remained with the same magnitude. Also, the hypoxic nature of these tumours is highly influenced by the crosstalk with other molecular pathways such cell cycle or angiogenesis, and in our review several reports took these factors into accountCitation9.
Second, the heterogeneity of the studies included in the present meta-analysis was generally small especially in relation to LC, DFS, and OS (). On the contrary, there was high heterogeneity in DSS. We relate this finding to the fact that survival parameters were undefined in some studies, largely due to the lack of international consensus on the definitions of long-term outcomes. In the OS subgroup analysis, CAIX overexpression also had a particularly negative impact on the OS of Asian patients. These differences could be linked to genetic and lifestyle variabilitiesCitation10. This study also identified that variations at the level of staining protocol also resulted in significant variations in survival endpoints. In the case of the DFS subgroup analysis, a stronger association in the OSCC mixed subsites subgroup, when compared exclusively with the tongue carcinoma subgroup, was observed. Prognostic OSCC studies have classically described tongue carcinomas as those tumours which carry the worst prognosis due to the lymphatic richness and the more pronounced diagnostic delayCitation9–12. We relate this finding to the possible existence of residual confounding given that some studies pooled data regarding unspecified oral cancer subsites.
This study dealt with the IHC-based CAIX expression in OSCC tissue as a prognostic but not a predictive biomarker. Nonetheless, recent studies have shed light on its promising value as a predictor of the malignant transformation of some orally potentially malignant disorders, such as oral leukoplakiaCitation16. CAIX as a target is of particular interest in oncology as there are a number of CAIX inhibitors availableCitation7. The interference of the HIF pathway with these inhibitors in OSCC has been poorly explored, nonetheless, according to the recent studies carried out by our group, several CAIX inhibitors which were synthesised on phenolic bis mannich bases and sulphonamides showed promising in vitro cytotoxicities in several OSCC cell linesCitation46–49.
The results from this systematic review and meta-analysis are supported by strong evidence. However, when interpreting the main results from this meta-analysis, some of the limitations of the individual studies cannot be ignored. Some authors recorded ambiguity in the distinction between OS and DSS. In addition, some authors did not directly report HR values in the survival analysis and this had to be approximated. In addition, adjustment for multiple established OSCC risk factors varied widely in the included studies. Despite this, we believe that these results are reliable and more widely applicable. However, further immunohistochemical reports are needed in order to validate this biomarker.
5. Conclusions
In view of the results obtained, we believe that IHC assessment of CAIX expression may be useful as a prognostic biomarker for OSCC, especially in the case of OS and DFS. These results open up the possibility of using this hypoxia-related protein in the prognosis of OSCC, and in its prevention and early control. Future studies with larger sample sizes and well-designed inclusion criteria are warranted in order to assess the role of CAIX IHC-based expression in determining the prognosis of OSCC.
Author Contributions
To qualify for authorship, we indicate the contribution of each author to this manuscript: Lorenzo-Pouso, A.I.: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, revising it critically for important intellectual content and final approval of the version to be published. Perez-Sayáns, M.; Chamorro-Petronacci C.M.: conception and design, acquisition of data, analysis and interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published. Gallas-Torreira M.; Álvarez-Calderón O.: revising the article critically for important intellectual content and final approval of the version to be published. Takkouche, B: analysis and interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published. Supuran, C.T.; García-García, A.: conception and design, revising the article critically for important intellectual content and final approval of the version to be published.
Acknowledgements
C.M. Chamorro-Petronacci is the recipient of a fellowship from the Foundation Health Research Institute of Santiago de Compostela (FIDIS). Furthermore, we would like to thank Prof. M.D Chiara (Institute of Sanitary Research of Asturias, Central Hospital of Asturias, University of Oviedo, Oviedo, Spain) for providing additional information about her study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004;4:437–47.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74.
- Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 2014;14:430–9.
- Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 1999;59:5830–5.
- Pastoreková S, Závadová Z, Kostál M, et al. A novel quasi-viral agent, MaTu, is a two-component system. Virology 1992;187:620–6.
- Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 2000;60:7075–83.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Eckert AW, Kappler M, Schubert J, Taubert H. Correlation of expression of hypoxia-related proteins with prognosis in oral squamous cell carcinoma patients. Oral Maxillofac Surg 2012;16:189–96.
- Pérez-Sayáns M, Supuran CT, Pastorekova S, et al. The role of carbonic anhydrase IX in hypoxia control in OSCC. J Oral Pathol Med 2013;42:1–8.
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009;45:309–16.
- Lydiatt WM, Patel SG, O'Sullivan B, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:122–37.
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011;11:9–22.
- Yap T, Celentano A, Seers C, et al. Molecular diagnostics in oral cancer and oral potentially malignant disorders-A clinician’s guide. J Oral Pathol Med 2020;49:1–8.
- Zhu S, Schuerch C, Hunt J. Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors. Arch Pathol Lab Med 2015;139:55–66.
- Rivera C, Oliveira AK, Costa RAP, et al. Prognostic biomarkers in oral squamous cell carcinoma: a systematic review. Oral Oncol 2017;72:38–47.
- Zhang X, Kim KY, Zheng Z, et al. Nomogram for risk prediction of malignant transformation in oral leukoplakia patients using combined biomarkers. Oral Oncol 2017;72:132–9.
- Pérez-Sayáns M, Suárez-Peñaranda JM, Torres-López M, et al. The use of CA-IX as a diagnostic method for oral leukoplakia. Biotech Histochem 2015;90:124–31.
- Zhang X, Han S, Han HY, et al. Risk prediction for malignant conversion of oral epithelial dysplasia by hypoxia related protein expression. Pathology 2013;45:478–83.
- Peridis S, Pilgrim G, Athanasopoulos I, Parpounas K. Carbonic anhydrase-9 expression in head and neck cancer: a meta-analysis. Eur Arch Otorhinolaryngol 2011;268:661–70.
- Wong DT, Todd R, Tsuji T, Donoff RB. Molecular biology of human oral cancer. Crit Rev Oral Biol Med 1996;7:319–28.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700.
- Troiano G, Caponio VCA, Zhurakivska K, et al. High PD-L1 expression in the tumour cells did not correlate with poor prognosis of patients suffering for oral squamous cells carcinoma: a meta-analysis of the literature. Cell Prolif 2019;52:e12537.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform metaanalyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16.
- Takkouche B, Khudyakov P, Costa-Bouzas J, Spiegelman D. Confidence intervals for heterogeneity measures in meta-analysis. Am J Epidemiol 2013;178:993–1004.
- Costa-Bouzas J, Takkouche B, Cadarso-Suárez C, Spiegelman D. HEpiMA: software for the identification of heterogeneity in meta-analysis. Comput Methods Programs Biomed 2001;64:101–7.
- Sakata K, Someya M, Nagakura H, et al. Brachytherapy for oral tongue cancer: an analysis of treatment results with various biological markers. Jpn J Clin Oncol 2008;38:402–7.
- Kim SJ, Shin HJ, Jung KY, Baek SK, et al. Prognostic value of carbonic anhydrase IX and Ki-67 expression in squamous cell carcinoma of the tongue. Jpn J Clin Oncol 2007;37:812–9.
- Choi SW, Kim JY, Park JY, et al. Expression of carbonic anhydrase IX is associated with postoperative recurrence and poor prognosis in surgically treated oral squamous cell carcinoma. Hum Pathol 2008;39:1317–22.
- Roh JL, Cho KJ, Kwon GY, et al. The prognostic value of hypoxia markers in T2-staged oral tongue cancer. Oral Oncol 2009;45:63–8.
- Eckert AW, Lautner MH, Schütze A, et al. Co-expression of Hif1alpha and CAIX is associated with poor prognosis in oral squamous cell carcinoma patients. J Oral Pathol Med 2010;39:313–7.
- Han MW, Lee HJ, Cho KJ, et al. Role of FDG-PET as a biological marker for predicting the hypoxic status of tongue cancer. Head Neck 2012;34:1395–402.
- Kondo Y, Yoshikawa K, Omura Y, et al. Clinicopathological significance of carbonic anhydrase 9, glucose transporter-1, Ki-67 and p53 expression in oral squamous cell carcinoma. Oncol Rep 2011;25:1227–33.
- Brockton NT, Klimowicz AC, Bose P, et al. High stromal carbonic anhydrase IX expression is associated with nodal metastasis and decreased survival in patients with surgically-treated oral cavity squamous cell carcinoma. Oral Oncol 2012;48:615–22.
- Heo K, Kim YH, Sung HJ, et al. Hypoxia-induced up-regulation of apelin is associated with a poor prognosis in oral squamous cell carcinoma patients. Oral Oncol 2012;48:500–6.
- Pérez-Sayáns M, Suárez-Peñaranda JM, Pilar GD, et al. Expression of CA-IX is associated with advanced stage tumors and poor survival in oral squamous cell carcinoma patients. J Oral Pathol Med 2012;41:667–74.
- Hwa JS, Kwon OJ, Park JJ, Woo SH, et al. The prognostic value of immunohistochemical markers for oral tongue squamous cell carcinoma. Eur Arch Otorhinolaryngol 2015;272:2953–9.
- Yang JS, Lin CW, Chuang CY, et al. Carbonic anhydrase IX overexpression regulates the migration and progression in oral squamous cell carcinoma. Tumour Biol 2015;36:9517–24.
- Vasconcelos MG, Vasconcelos RG, Pereira de Oliveira DH, et al. Distribution of hypoxia-inducible factor-1α and glucose transporter-1 in human tongue cancers. J Oral Maxillofac Surg 2015;73:1753–60.
- Simões-Sousa S, Granja S, Pinheiro C, et al. Prognostic significance of monocarboxylate transporter expression in oral cavity tumors. Cell Cycle 2016;15:1865–73.
- Brockton NT, Lohavanichbutr P, Enwere EK, et al. Impact of tumoral carbonic anhydrase IX and Ki-67 expression on survival in oral squamous cell carcinoma patients. Oncol Lett 2017;14:5434–42.
- Sáenz-de-Santa-María I, Bernardo-Castiñeira C, Secades P, et al. Clinically relevant HIF-1α-dependent metabolic reprogramming in oropharyngeal squamous cell carcinomas includes coordinated activation of CAIX and the miR-210/ISCU signaling axis, but not MCT1 and MCT4 upregulation. Oncotarget 2017;8:13730–46.
- Peterle GT, Maia LL, Trivilin LO, et al. PAI-1, CAIX, and VEGFA expressions as prognosis markers in oral squamous cell carcinoma. J Oral Pathol Med 2018;47:566–74.
- Eckert AW, Horter S, Bethmann D, et al. Investigation of the prognostic role of carbonic anhydrase 9 (CAIX) of the cellular mRNA/protein level or soluble CAIX protein in patients with oral squamous cell carcinoma. Int J Mol Sci 2019;20:375.
- Svastová E, Hulíková A, Rafajová M, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 2004;577:439–45.
- Inci Gul H, Yamali C, Tugce Yasa A, et al. Carbonic anhydrase inhibition and cytotoxicity studies of Mannich base derivatives of thymol. J Enzyme Inhib Med Chem 2016;31:1375–80.
- Yamali C, Gul HI, Sakagami H, Supuran CT. Synthesis and bioactivities of halogen bearing phenolic chalcones and their corresponding bis Mannich bases. J Enzyme Inhib Med Chem 2016;31(Supp 4):125–31.
- Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70.
- Yamali C, Gul HI, Ece A, et al. Synthesis, biological evaluation and in silico modelling studies of 1,3,5-trisubstituted pyrazoles carrying benzenesulfonamide as potential anticancer agents and selective cancer-associated hCA IX isoenzyme inhibitors. Bioorg Chem 2019;92:103222.