ABTRACT
In this paper, a new series of isatin-sulphonamide based derivatives were designed, synthesised and evaluated as caspase inhibitors. The compounds containing 1-(pyrrolidinyl)sulphonyl and 2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl substitution at C5 position of isatin core exhibited better results compared to unsubstituted derivatives. According to the results of caspase inhibitory activity, compound 20d showed moderate inhibitory activity against caspase-3 and −7 in vitro compared to Ac-DEVD-CHO (IC50 = 0.016 ± 0.002 μM). Among the studied compounds, some active inhibitors with IC50s in the range of 2.33–116.91 μM were identified. The activity of compound 20d was rationalised by the molecular modelling studies exhibiting the additional van der Waals interaction of N-phenylacetamide substitution along with efficacious T-shaped π-π and pi-cation interactions. The introduction of compound 20d with good caspase inhibitory activity will help researchers to find more potent agents.
Introduction
Caspases, cysteinyl aspartate-specific proteases, are a family of signalling molecules playing a key role in apoptosis. Apoptosis is a physiological suicide process which gives an opportunity to dismantle unwanted cells population during animal development and tissue homeostasisCitation1. Morphological changes such as DNA strand breaks along with nuclear membrane damage occur as a result of some biochemical events during apoptosisCitation2. Two intrinsic and extrinsic pathways are responsible for initiating the apoptosis process. Binding of certain protein to the death receptor activates caspase-8 and subsequently triggers apoptosis by promoting effector caspases (–3, −6, −7). It should be noted that caspase enzymes are classified as initiator (caspase-2, caspase-8, caspase-9, and caspase-10) and effector (caspase-3, caspase-6, and caspase-7) which are exploited in re sponse to proapoptotic signalsCitation3,Citation4. Caspase-3, activated by the upstream caspase-8 and caspase-9, is considered as a crucial mediator of apoptotic cell death in mammals by which more than 500 cellular substrates are cleaved to execute the apoptosis programmeCitation5,Citation6. Regarding the close relationship between apoptosis and the wide range of disease, caspase inhibitors are capable of opening new paths to treat several diseases involving immunodeficiency, Alzheimer’s, Parkinson’s, Huntington’s diseases, ischaemia, brain trauma, and amyotrophic lateral sclerosisCitation7. Taking the obtained data from the X-ray structure of caspase-3 into account, four main binding sites (S1–S4) are determined in which the binding to the S2 and S3-pockets are responsible for inhibitory activity and selectivity of caspase-3, respectivelyCitation8–10. This knowledge along with the importance of this family clearly helps medicinal chemists to design new specific inhibitors of caspase enzymesCitation11–19.
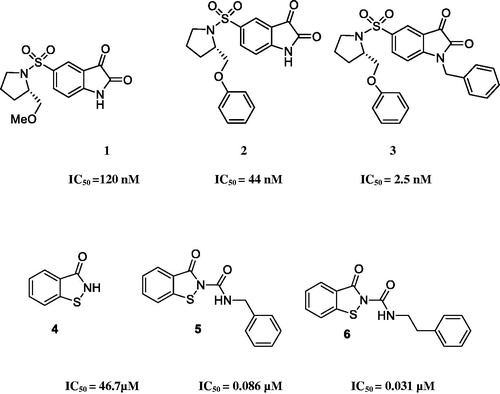
Isatin sulphonamides are introduced as a new class of potent and selective non-peptide caspase-3 and −7 inhibitors. Previously, various isatin sulphonamide derivatives were prepared and evaluated as caspase-3 inhibitorsCitation20–25. The studies indicated the connection between carbonyl group of isatin ring and cysteine thiol in the binding site of the enzyme. 5-Pyrrolidinyl sulphonyl isatins are evidently found effective in inhibition of the caspase-3 and −7 in vitro. The selectivity of 5-pyrrolidinyl sulphonyl isatins is referred to the interaction of pyrrolidine ring with S2 subsite of enzyme without the interaction with S1 subsite of caspase-3Citation26. The side-chains, attached to pyrrolidine meaning methoxymethyl or phenoxymethyl groups, occupy the S3 pocket. In this regard, many studies have been focussed on the synthesis of several modified isatin derivatives (1), relying on the structure-activity relationship (SAR) studies (). Interestingly, it was observed that good IC50 values in nanomolar ranges are obtained when hydrophobic groups are attached to the N-1 position of structure 1 (). Furthermore, the amide moiety is also found necessary in producing various potent inhibitorsCitation27–29. Considering the above mentioned findings about the importance of isatin sulphonamide derivatives, especially as caspase-3 inhibitors and following our ongoing projects on the design and synthesis of biologically active agentsCitation30–37, we synthesised isatin based compounds containing N-aryl acetamide and N-prop-2-yn-1-yl as caspase-3 and −7 inhibitors through the structural modification of compound 1.
Results and discussion
Chemistry
First of all, the N-alkylated isatin derivatives (11a–k) were obtained in 60–85% yields from the alkylation reaction of isatin 10 with propargyl bromide or 2-chloro-N-arylacetamide derivativesCitation38–40, synthesised from the reaction of chloroacetyl choride and aromatic amines (Part A, Scheme 1)Citation41.
Scheme 1. (A) Synthesis route for A series. Reagents and conditions. a: CH2Cl2, Et3N; b: NaH, DMF (B) Synthesis route for B series. Reagents and conditions. a: ClSO3H; b: pyrrolidine or 16, Et3N, DMF, c: acetic acid; d: 9 or propargyl bromide, NaH, DMF, 0 ˚C; e: p-toluenesulfonyl chloride, pyridine; f: phenol, NaH, THF; g: TFA, CH2Cl2.
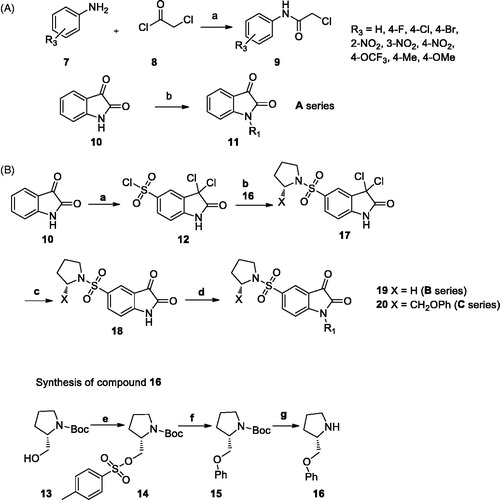
The synthesis of N-alkylated substituted 5-[1-(pyrrolidinyl)sulphonyl] isatin derivatives was started from heating isatin 10 in chlorosulfonic acid at 60 °C which is followed by amination with pyrrolidine or 2-phenoxymethyl pyrrolidine in dimethyl formamide (DMF)Citation42. The subsequent hydrolysis in acetic acid and addition of 2-chloro-N-arylacetamides 9 or propargyl bromide led to compounds 19a–k (64–85%) and 20a–k (47–65%) in good yields (Part B, Scheme 1).
In this paper, 33 compounds are synthesised and their structures are deduced by IR, 1H, 13 C NMR, mass spectroscopy, and elemental analysis. For example, the IR spectrum of these three series showed the stretching bands, related to C=O bonds of ketone and amide functional groups at nearly 1700 and 1670 cm−1, respectively. The mass spectrum of each compound displayed the molecular ion (M+) peak, which is consistent with a 1:1 adduct, formed by the substitution at NH of isatin and loss of chlorine and bromine atom of propargyl bromide or 2-chloro-N-arylacetamide derivatives. The 1H-NMR spectrum of compounds exhibited the characteristic signals at δ 4.3–4.6 and 8.2–8.8 ppm related to NCH2 and NH, respectively. The characteristic signals related to pyrrolidine and isatin moiety at aliphatic and aromatic region confirmed the structures of final compounds. The 1H-decoupled 13 C-NMR spectrum of compounds showed characteristic signals at related aliphatic and aromatic regions which are in agreement with the proposed structure.
Biological activity
The inhibitory activities of newly synthesised 2–(2,3-dioxoindolin-1-yl)-N-substituted phenyl acetamide, 1-(prop-2-yn-1-yl)indoline-2,3-dione and two series of compounds containing 1-(pyrrolidinyl)sulphonyl and 2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl substitution at C5 position of isatin core (B, C, ) against caspase-3 and −7 were evaluated by using the acetyl-DEVD-AMC fluorogenic substrate assay. The results are expressed as inhibition percentage and IC50 values in . We used Ac-DEVD-CHO as the positive control.
Table 1. Structures of compounds 11a–k, 19a–k, and 20a–k displaying inhibitory effects on caspase-3 and -7.
As can be seen in , those compounds containing no substituent at C5 position of isatin core (R2 = H) are weak inhibitors compared to the positive control. All amounts are provided as inhibition percentage at 20 μg/ml. Among this series, the best and weakest activity was observed in 11c and 11f with inhibition percentage of 71% and 5%, respectively. The presence of 2-(phenoxymethyl)pyrrolidine functionality on isatin core led to the more active compounds against caspase-3 and −7 than that of substituted ones with pyrrolidin-1-yl sulphonyl moiety.
In compounds 20a–k, the comparison between the para substituted derivatives revealed that the electron-donating substituents (20j, 20k) exhibited the lowest enzymatic inhibition. The most active compound was the 4-chlorophenylacetamide containing derivative, meaning 20d against caspase-3 and −7. Compounds 20a–c and 20f have also appreciable IC50 values and can be regarded as moderated caspase-3 and −7 inhibitors in comparison to Ac-DEVD-CHO (IC50 = 0.016 ± 0.002 μM). In compounds 19a–k and 20 a–k, the least electronegative and more bulky atom, bromine, had clear negative effect on inhibitory potency of the compound compared to fluorine and chlorine containing derivatives. As previously reported, compounds with a selectivity index greater than 1.5 are considered as selective inhibitors of caspase-3, so, compounds 19a, 19d, 19e, 20c, 20d, and 20e exhibited this selectivity. Regarding the significant activity and selectivity of compound 20d, this compound could be studied for further modification to develop novel hit compounds.
Docking study
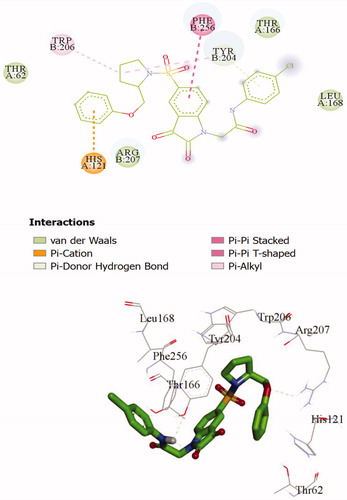
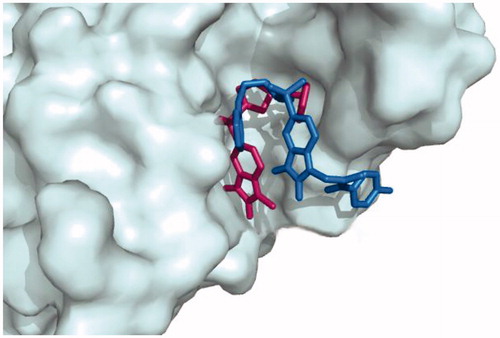
To investigate the binding mode of these potent inhibitors, molecular docking computations were performed using Autodock Tools (ver.1.5.6) programmeCitation43. Compound 20d was docked into the active site of caspase-3 crystallographic structure (PDB ID: 1GFW), retrieved from protein data bank (http://www.rcsb.org/pdb/home/home.do) (). The phenyl ring of phenoxymethyl group formed pi-cation interaction with HIS:121. The isatin core formed T-shaped π-π interactions with His 121 and Tyr 204. His 121 formed a carbon hydrogen bond in isatin sulphonamide crystal ligand. A Pi-alkyl interaction is formed between the oxygen of sulphonyl group and Trp 206 and Tyr 204. The carbonyl moiety interacted through π-sulfur with Cys 163 in compound 20d and through π-hydrogen bond in isatin sulphonamide. The π-π stacked interaction is formed between isatin core and Phe 256 in isatin sulphonamide and compound 20d. Moreover, N-phenylacetamide substitution provided enough length for more efficient interactions, like an additional van der Waals interaction between LeuA 168 and ThrA 166 and phenyl moiety. , presentesd the comparision between the type of interaction and involved amino acid residues of the most active compound, 20d, and isatin sulphonamide. These interactions along with distances are schematically presented in . Superimposition of the binding pose of 20d and natural ligand at the 1GFW active site is shown in . The binding interaction energy of compound 20d is −4.04 kcal/mol, which stated that this compound is less potent than statin sulphonamide (-5.44 kcal/mol) towards caspase-3.
Figure 4. 2 D representations of 20d (A) and isatin sulphonamide (B) interactions with caspase-3 active site.
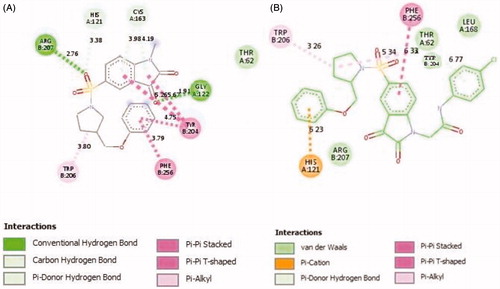
Table 2. The interactions of compound 20d and natural ligand in 1GFW at the active site.
Conclusion
A series of novel isatin-sulphonamide derivatives were designed, synthesised and evaluated for their caspase-3 and −7 inhibitory activity. The results showed that most of the synthesised compounds exhibited moderate inhibitory activity against caspase-3 and −7. The results revealed that 4-chloro phenylacetamide derivative 20d exhibited the best profile of inhibitory activity on caspase-3 with IC50 value of 2.33 µM. The docking studies showed the perfect binding of compound 20d to the active site of caspase-3 enzyme. The prepared product 20d in the present study may be subjected to further optimisation to find more effective agent as caspase-3 inhibitor.
Experimental
Chemistry
5-[1-(Pyrrolidinyl)sulphonyl] isatin derivatives 18 were prepared by the reaction of isatin 7, chlorosulfonic acid, pyrrolidine or 2-phenoxymethyl pyrrolidine 16 (Scheme 1). 2-Chloro-N-phenylacetamide derivatives 9Citation38–40 and 2-phenoxymethyl pyrrolidine 16Citation22 used in the synthesis of target products were conveniently prepared based on the previously reported procedure.
Other starting materials, chemical reagents, and solvents used in this study were commercially available (from Merck and Aldrich Chemicals) and were used without further purification. TLC was conducted on silica gel 250 micron. Melting points were determined on a Kofler hot stage apparatus and are uncorrected. The IR spectra were run on a Shimadzu 470 spectrophotometer (potassium bromide disks). Mass spectra were recorded on an Agilent Technologies (HP) 5973 mass spectrometer operating at an ionisation potential of 70 eV. The NMR spectra were recorded on a Varian unity 500 spectrometer, and the chemical shifts (δ) are reported in parts per million (ppm) relative to tetramethylsilane (TMS) as an internal standard.
General procedure for the N-alkylation of isatin, 5-[1-(pyrrolidinyl)sulphonyl] isatins, 5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)isatin
Sodium hydride (0.25 mmol) was added to the stirred solution of isatin 10 or intermediate 18 (0.25 mmole) in DMF (3 ml), and the reaction was continued for 15 min at 0 °C. Corresponding N-phenylacetamides 9 or propargyl bromide (0.25 mmol) was added and the reaction was continued for one hour. TLC was used to find reaction completion time. Water (20 ml) was added to the reaction mixture and extracted with ethyl acetate. Resulted crude product was purified over flash column chromatography (mobile phase: ethyl acetate: hexane 20:80) to yield pure products 11a–k.
1-(Prop-2-yn-1-yl)indoline-2,3-dione (11a)Citation44: White solid; Yield: 85%; m.p. 158–160 °C; IR (KBr, cm−1): 1718 (C=OKetone), 1678 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 2.90 (s, 1H, CHAcetylene), 4.36 (s, 2H, CH2), 7.22 (d, J = 8.5 Hz, 1H, H7), 7.35 (d, J = 8.5 Hz, 1H), 7.47 (t, J = 7.85 Hz, 1H), 7.52 (t, J = 7.85 Hz, 1H); 13 C NMR (125 MHz, DMSO-d6): 37.0, 75.1, 82.0, 126.2, 126.6, 128.3, 135.1, 135.1, 145.6, 150.1, 163.3, 181.1; Anal. Calcd. For C11H7NO2: C, 71.35; H, 3.81; N, 7.56; Found: C, 71.07; H, 3.58; N, 7.84.
2–(2,3-Dioxoindolin-1-yl)-N-phenylacetamide (11b): White solid; Yield 78%; m.p. 174–176 °C; IR (KBr, cm−1): 3348 (NH), 1710 (C=OKetone), 1680 (C=OAmide), 1660 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 4.64 (s, 2H, CH2), 7.07 (d, J = 7.2 Hz, 1H), 7.14 (t, J = 7.2 Hz, 1H), 7.19 (t, J = 7.9 Hz, 1H), 7.32 (t, J = 7.9 Hz, 2H), 7.49 (d, J = 7.9 Hz, 2H), 7.70–7.62 (m, 2H),7.94 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 43.9, 110.2, 117.9, 121.2, 125.7, 127.6, 132.7, 134.8, 145.7, 148.5, 151.6, 162.6, 166.3, 182.1; Anal. Calcd. For C16H12N2O3: C, 68.56; H, 4.32; N, 9.99; Found: C, 68.27; H, 4.04; N, 10.17; MS (m/z, %): 280 (M+, 41) 146 (100), 134 (25), 90 (57), 77 (33), 55 (76).
2–(2,3-Dioxoindolin-1-yl)-N-(4-fluorophenyl)acetamide (11c): White solid; Yield 70%; m.p. 199–201 °C; IR (KBr, cm−1): 3340 (NH), 1700 (C=OKetone), 1688 (C=OAmide), 1665 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 4.64 (s, 2H, CH2), 6.89 (d, J = 7.5 Hz, 2H), 6.95 (d, J = 7.0 Hz, 1H), 6.99 (d, J = 7.5 Hz, 2H), 7.14 (t, J = 7.0 Hz, 1H), 7.58–7.63 (m, 2H), 8.05 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 46.6, 110.6, 121.3, 125.6, 127.2, 128.3, 130.5, 134.5, 148.1, 150.9, 158.3 (JC-F = 250 Hz), 162.6, 167.0, 181.8; Anal. Calcd. For C16H11FN2O3: C, 64.43; H, 3.72; N, 9.39; Found: C, 64.67; H, 3.44; N, 9.55; MS (m/z, %): 298 (M+, 63) 146 (100), 152 (32), 96 (48), 57 (40).
N-(4-Chlorophenyl)-2–(2,3-dioxoindolin-1-yl)acetamide (11d): White solid; Yield: 72%; m.p. 177–179 °C; IR (KBr, cm−1): 3356 (NH), 1708 (C=OKetone), 1682 (C=OAmide), 1655 (C=OAmide); 1H-NMR (500 MHz, CDCl3): 4.38 (s, 2H, CH2), 6.99 (d, J = 8.0 Hz, 1H), 7.21 (d, J = 6.9 Hz, 2H), 7.24 (t, J = 8.0 Hz, 1H), 7.29 (d, J = 6.9 Hz, 2H), 7.61 (t, J = 8.0 Hz, 2H), 8.01 (s, 1H, NH). 13 C-NMR (125 MHz, CDCl3): 43.8, 110.9, 117.7, 124.5, 125.6, 128.7, 137.3 (2 C), 138.7, 150.1, 158.5, 165.7, 182.4. Anal. Calcd. For C16H11ClN2O3: C, 61.06; H, 3.52; N, 8.90; Found: C, 61.37; H, 3.74; N, 9.05; MS (m/z, %): 316 (M + 2+, 39), 314 (M+, 14) 168 (35), 146 (100), 152 (52), 112 (64), 90 (28), 56 (40).
N-(4-Bromophenyl)-2–(2,3-dioxoindolin-1-yl)acetamide (11e): White solid; Yield: 66%; m.p. 179–181 °C; IR (KBr, cm−1): 3330 (NH), 1698 (C=OKetone), 1670 (C=OAmide), 1656 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 4.49 (s, 2H, CH2), 6.90 (d, J = 7.0 Hz, 1H), 6.90 (d, J = 7.0 Hz, 1H), 7.08 (d, J = 7.5 Hz, 2H), 7.16–7.21 (m, 1H), 7.54–7.56 (m, 2H), 7.64 (d, J = 7.9 Hz, 2H), 8.00 (s, 1H, NH). 13 C-NMR (125 MHz, DMSO-d6): 45.0, 110.6, 115.4, 117.2, 118.3, 124.9, 126.8, 135.8, 138.0, 149.8, 150.4, 158.3, 163.3, 182.1, Anal. Calcd. For C16H11BrN2O3: C, 53.50; H, 3.09; N, 7.80; Found: C, 53.87; H, 3.44; N, 7.57; MS (m/z, %): 359 (M + 2+, 26), 357 (M+, 24), 211 (47), 154 (69), 146 (100), 90 (36), 56 (50).
2–(2,3-Dioxoindolin-1-yl)-N-(2-nitrophenyl)acetamide (11f): White solid; Yield: 77%; m.p. 186–188 °C; IR (KBr, cm−1): 3340 (NH), 1725 (C=O Ketone), 1685 (C=O Amide), 1665 (C=OAmide); 1H-NMR (500 MHz, CDCl3): 4.58 (s, 2H, CH2), 7.00 (d, J = 7.0 Hz, 1H), 7.11 (t, J = 7.0 Hz, 1H), 7.27–7.33 (m, 2H), 7.37 (d, J = 8.0 Hz, 1H), 7.58 (t, J = 7.0 Hz, 1H), 7.80 (t, J = 8.0 Hz, 1H), 7.92 (d, J = 8.0 Hz, 1H), 8.50 (s, 1H, NH). 13 C-NMR (125 MHz, CDCl3): 45.8, 123.6, 127.3, 129.4, 129.9, 129.9, 132.3, 134.8, 135.2, 136.4, 139.9, 145.6, 146.5, 151.0, 161.2, 168.4, 179.3. Anal. Calcd. For C16H11N3O5: C, 59.08; H, 3.41; N, 12.92; Found: C, 59.37; H, 3.14; N, 13.17; MS (m/z, %): 325 (M+, 48), 179 (51), 146 (100), 123 (44), 92 (29), 57 (42).
2–(2,3-Dioxoindolin-1-yl)-N-(3-nitrophenyl)acetamide (11g): White solid; Yield: 75%; m.p. 191–192 °C; IR (KBr, cm−1): 3348 (NH), 1725 (C=OKetone), 1680 (C=OAmide), 1658 (C=OAmide); 1H-NMR (500 MHz, CDCl3): 4.49 (s, 2H, CH2), 7.08–7.11 (m, 1H), 7.24 (d, J = 8.0 Hz, 1H), 7.32–7.34 (m, 1H), 7.56–7.58 (m, 1H), 7.64 (d, J = 7.0 Hz, 1H), 7.83 (t, J = 7.0 Hz, 1H), 8.00 (s, 1H), 8.18 (d, J = 7.5 Hz, 1H), 8.39 (s, 1H, NH). 13 C-NMR (125 MHz, CDCl3): 45.60, 124.1, 127.8, 127.9, 130.0, 130.2, 132.2, 135.4, 135.9, 139.4, 140.3, 146.0, 146.9, 160.4, 168.6, 179.6. Anal. Calcd. For C16H11N3O5: C, 59.08; H, 3.41; N, 12.92; Found: C, 59.35; H, 3.73; N, 13.19.
2–(2,3-Dioxoindolin-1-yl)-N-(4-nitrophenyl)acetamide (11h): White solid; Yield: 69%; m.p. 179–181 °C; IR (KBr, cm−1): 3338 (NH), 1718 (C=OKetone), 1680 (C=O Amide), 1665 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 4.49 (s, 2H, CH2), 7.13 (d, J = 7.2 Hz, 1H), 7.19–7.24 (m, 1H), 7.38–7.41 (m, 2H), 7.66–7.69 (m, 1H), 7.85–7.88 (m, 1H), 8.00–8.04 (m, 2H), 8.37 (s, 1H, NH). 13 C-NMR (125 MHz, DMSO-d6): 46.1, 124.6, 127.3, 128.1, 129.1, 131.5, 135.6, 139.1, 145.4, 145.7, 150.4, 161.6, 167.0, 178.4. Anal. Calcd. For C16H11N3O5: C, 59.08; H, 3.41; N, 12.92; Found: C, 59.31; H, 3.79; N, 13.11.
2–(2,3-Dioxoindolin-1-yl)-N-(4-(trifluoromethoxy)phenyl)acetamide (11i): White solid; Yield: 75%; m.p. 209– 211 °C; IR (KBr, cm−1): 3348 (NH), 1721 (C=OKetone), 1680 (C=OAmide), 1665 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 4.52 (s, 2H, CH2), 7.23–7.27 (m, 3H), 7.11 (d, J = 7.5 Hz, 1H), 7.38 (d, J = 8.5 Hz, 2H), 7.57 (t, J = 7.5 Hz, 1H), 7.93 (d, J = 7.5 Hz, 1H), 8.35 (s, 1H, NH). 13 C-NMR (125 MHz, DMSO-d6): 45.2, 116.0, 121.6, 124.5, 126.5, 128.3, 128.6, 131.7, 134.2, 135.4, 138.9, 149.2, 161.9, 166.7, 178.9. Anal. Calcd. For C17H11F3N2O4: C, 56.05; H, 3.04; N, 7.69; Found: C, 55.91; H, 3.42; N, 7.84; MS (m/z, %): 364 (M+, 26), 218 (M+, 18), 162 (44), 146 (100), 90 (51), 56 (32).
2–(2,3-Dioxoindolin-1-yl)-N-(p-tolyl)acetamide (11j): White solid; Yield: 60%; m.p. 196–198 °C; IR (KBr, cm−1): 3354 (NH), 1728 (C=OKetone), 1688 (C=O Amide), 1659 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 2.34 (s, 3H), 4.59 (s, 2H, CH2), 6.97 (d, J = 7.6 Hz, 1H), 7.21 (d, J = 8.0 Hz, 2H), 7.27 (d, J = 7.6 Hz, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.71 (d, J = 7.8 Hz, 1H), 7.88 (t, J = 7.8 Hz, 1H), 8.37 (s, 1H, NH). Anal. Calcd. For C17H14N2O3: C, 69.38; H, 4.79; N, 9.52; Found: C, 69.51; H, 4.92; N, 9.66; MS (m/z, %): 294 (M+, 42), 148 (34), 146 (100), 91 (44), 56 (68).
2–(2,3-Dioxoindolin-1-yl)-N-(4-methoxyphenyl)acetamide (11k): White solid; Yield: 68%; m.p. 201–203 °C; IR (KBr, cm−1): 3354 (NH), 1728(C=OKetone), 1688 (C=OAmide), 1659 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 3.70 (s, 3H, OMe), 4.47 (s, 2H, CH2), 6.88 (d, J = 8.0 Hz, 2H), 7.08–7.11 (m, 1H), 7.20–7.25 (m, 3H), 7.55–7.57 (m, 1H), 7.91–7.92 (m, 1H), 8.22 (s, 1H, NH). 13 C-NMR (125 MHz, DMSO-d6): 45.9, 55.0, 113.8, 116.1, 121.6, 124.4, 126.5, 128.4, 134.2, 149.0, 149.2, 161.9 (2 C), 167.6, 178.4. Anal. Calcd. For C17H14N2O4: C, 65.80; H, 4.55; N, 9.03; Found: C, 65.55; H, 4.28; N, 9.33; MS (m/z, %): 310 (M+, 26), 164 (18), 108 (44), 146 (100), 92 (51), 58 (32).
1-(Prop-2-yn-1-yl)-5-(pyrrolidin-1-ylsulfonyl)indoline-2,3-dione (19a): White solid; Yield: 82%; m.p. 203–205 °C; IR (KBr, cm−1): 3345 (NH), 1710 (C=OKetone), 1680 (C=OAmide), 1660 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.63–1.70 (m, 4H, CH2-Pyrrole), 2.98–3.04 (m, 4H, CH2-Pyrrole), 3.24 (s, 1H, CH), 4.73 (s, 2H, CH2), 7.13 (d, J = 8.5 Hz, 1H), 7.82 (d, J = 8.5 Hz, 1H), 8.08 (s, 1H); 13 C-NMR (125 MHz, DMSO-d6): 25.9, 47.2, 61.9, 71.9, 73.8, 112.0, 126.5, 128.0, 136.6, 152.5, 161.5, 166.6, 179.8; Anal. Calcd. For C15H14N2O4S: C, 56.59; H, 4.43; N, 8.80; Found: C, 56.77; H, 4.11; N, 8.57; MS (m/z, %): 318 (M+, 42), 279 (26), 208 (51), 146 (100), 71 (22).
2–(2,3-Dioxo-5-(pyrrolidin-1-ylsulfonyl)indolin-1-yl)-N-phenylacetamide (19b): White solid; Yield: 85%; m.p. 238–240 °C; IR (KBr, cm−1): 3364 (NH), 1725 (C=OKetone), 1684 (C=OAmide), 1655 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.60– 1.67 (m, 4H, CH2-Pyrrole), 3.08–3.12 (m, 4H, CH2-Pyrrole), 4.49 (s, 2H, CH2), 6.92 (d, J = 7.5 Hz, 1H), 7.12 (d, J = 8.8 Hz, 2H), 7.36 (d, J = 8.0 Hz, 1H), 7.44 (t, J = 7.5 Hz, 1H), 7.64 (t, J = 8.0 Hz, 2H), 7.96 (s, 1H), 8.48 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 23.8, 47.8, 62.2, 123.7, 129.0, 129.2, 131.5, 134.4, 142.6, 143.2, 143.4, 145.4, 147.2, 162.3, 167.2, 182.3; Anal. Calcd. For C20H19N3O5S: C, 58.10; H, 4.63; N, 10.16; Found: C, 58.37; H, 4.91; N, 10.35; MS (m/z, %): 413 (M+, 39), 279 (43), 146 (100), 134 (57), 71 (29).
2–(2,3-Dioxo-5-(pyrrolidin-1-ylsulfonyl)indolin-1-yl)-N-(4-fluorophenyl)acetamide (19c): White solid; Yield: 80%; m.p. 191–193 °C; IR (KBr, cm−1): 3345 (NH), 1711 (C=OKetone), 1670 (C=OAmide), 1654 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.69–1.77 (m, 4H, CH2-Pyrrole) 2.98–3.11 (m, 4H, CH2-Pyrrole), 4.41 (s, 2H, CH2), 4.41 (s, 2H, CH2), 6.94 (d, J = 7.5 Hz, 1H), 7.14 (d, J = 8.8 Hz, 2H), 7.36 (d, J = 8.8 Hz, 2H), 7.42 (d, J = 7.5 Hz, 1H), 8.05 (s, 1H), 8.49 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 25.5, 48.0, 60.0, 110.5, 114.4 (JC-F = 6.75 Hz), 119.2, 126.8 (JC-F = 24.5 Hz), 128.1, 131.0, 135.6, 149.0, 151.6, 158.6, 162.0 (JC–F = 245 Hz), 165.6, 183.2; Anal. Calcd. For C20H18FN3O5S: C, 55.68; H, 4.21; N, 9.74; Found: C, 55.37; H, 4.51; N, 9.49; MS (m/z, %): 431 (M+, 27), 278 (39), 154 (51), 146 (100), 135 (57), 95 (31).
N-(4-Chlorophenyl)-2–(2,3-dioxo-5-(pyrrolidin-1-ylsulfonyl)indolin-1-yl)acetamide (19d): White solid; Yield: 71%; m.p. 300–302 °C; IR (KBr, cm−1): 3330 (NH), 1706 (C=OKetone), 1685 (C=OAmide), 1658 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.65–1.58 (m, 4H, CH2-Pyrrole). 2.98–3.05 (m, 4H, CH2-Pyrrole), 4.24 (s, 2H, CH2), 6.77 (d, J = 8.65 Hz, 1H), 7.38 (d, J = 8.0 Hz, 2H), 7.65 (d, J = 8.0 Hz, 2H), 7.72 (d, J = 8.65 Hz, 1H), 8.08 (s, 1H), 8.34 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 26.7, 47.5, 62.8, 111.2, 120.3, 125.9, 126.8, 128.4, 129.3, 129.6, 134.9, 144.5, 151.4, 158.4, 165.9, 182.8; Anal. Calcd. For C20H18ClN3O5S: C, 53.63; H, 4.05; N, 9.38; Found: C, 53.85; H, 3.78; N, 9.09; MS (m/z, %): 449 (M + 2+, 36), 447 (M+, 11), 276 (43), 168 (35), 146 (100), 134 (18), 110 (29).
N-(4-Bromophenyl)-2–(2,3-dioxo-5-(pyrrolidin-1-ylsulfonyl)indolin-1-yl)acetamide (19e): White solid; Yield: 68%; m.p. 290–292 °C; IR (KBr, cm−1): 3334 (NH), 1705 (C=OKetone), 1675 (C=OAmide), 1660 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.62–1.66 (m, 4H, CH2-Pyrrole) 3.00–3.07 (m, 4H, CH2-Pyrrole), 4.49 (s, 2H, CH2), 7.11 (d, J = 8.6 Hz, 1H), 7.49 (d, J = 8.5 Hz, 2H), 7.61 (d, J = 8.5 Hz, 2H), 7.78 (d, J = 7.5 Hz, 1H), 8.18 (s, 1H), 8.52 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 20.7, 43.8, 62.7, 113.3, 123.2, 125.9, 126.0, 127.0, 127.4, 128.5, 129.1, 130.7142.5, 160.8, 165.5, 181.8; Anal. Calcd. For C20H18BrN3O5S: C, 48.79; H, 3.69; N, 8.53; Found: C, 48.45; H, 3.46; N, 8.78; MS (m/z, %): 493 (M + 2+, 28), 491 (M+, 26), 278 (51), 211 (29), 154 (48), 146 (100), 133 (24).
2–(2,3-Dioxo-5-(pyrrolidin-1-ylsulfonyl)indolin-1-yl)-N-(2-nitrophenyl)acetamide (19f): White solid; Yield: 74%; m.p. 195–197 °C; IR (KBr, cm−1): 3345 (NH), 1725 (C=OKetone), 1685 (C=OAmide), 1660 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.61–1.66 (m, 4H, CH2-Pyrrole), 2.99–3.07 (m, 4H, CH2-Pyrrole), 4.49 (s, 2H, CH2), 7.12 (d, J = 7.0 Hz, 1H), 7.24 (t, J = 8.0 Hz, 1H), 7.52 (d, J = 8.6 Hz, 1H), 7.73 (t, J = 8.0 Hz, 1H), 8.01 (s, 1H), 8.12 (t, J = 7.0 Hz, 1H), 8.37 (d, J = 7.0 Hz, 1H), 8.66 (s, 1H, NH); Anal. Calcd. For C20H18N4O7S: C, 52.40; H, 3.96; N, 12.22; Found: C, 52.53; H, 4.09; N, 12.35; MS (m/z, %): 458 (M+, 41), 279 (37), 211 (40), 163 (27), 146 (100), 135 (19).
2–(2,3-Dioxo-5-(pyrrolidin-1-ylsulfonyl)indolin-1-yl)-N-(3-nitrophenyl)acetamide (19g): White solid; Yield 73%; m.p. 223–225 °C; IR (KBr, cm−1): 3340 (NH), 1720 (C=OKetone), 1680 (C=OAmide), 1660 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.58–1.65 (m, 4H, CH2-Pyrrole), 2.89–3.03 (m, 4H, CH2-Pyrrole), 4.38 (s, 2H, CH2), 7.12 (d, J = 7.5 Hz, 1H), 7.32 (t, J = 7.5 Hz, 1H), 7.71 (d, J = 7.5 Hz, 1H), 7.82 (d, J = 7.5 Hz, 1H), 8.01 (s, 1H), 8.17 (s, 1H), 8.20 (s, 1H), 8.54 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 25.1, 49.5, 61.3, 110.1, 122.8, 123.4, 126.1, 128.2, 129.0, 130.4, 132.1, 134.1, 135.0, 143.4, 150.9, 158.9, 167.6, 180.0; Anal. Calcd. For C20H18N4O7S: C, 52.40; H, 3.96; N, 12.22; Found: C, 52.78; H, 4.14; N, 11.90.
2–(2,3-Dioxo-5-(pyrrolidin-1-ylsulfonyl)indolin-1-yl)-N-(4-nitrophenyl)acetamide (19h): White solid; Yield: 69%; m.p. 216–218 °C; IR (KBr, cm−1): 3340 (NH), 1728 (C=OKetone), 1680 (C=OAmide), 1646 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.64–1.67 (m, 4H, CH2-Pyrrole), 2.94–2.98 (m, 4H, CH2-Pyrrole), 4.68 (s, 2H, CH2), 7.12 (d, J = 7.5 Hz, 1H), 7.46 (dd, J = 8.0, 3.5 Hz, 2H), 7.68 (d, J = 7.5 Hz, 1H), 7.90 (s, 1H), 8.21 (dd, J = 8.0 Hz, J = 3.5 Hz, 2H), 8.51 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 25.2, 48.1, 60.3, 111.3, 122.9, 123.4, 130.4, 132.1, 133.2, 134.1, 142.4, 143.3, 149.2, 160.1, 167.6, 183.4; Anal. Calcd. For C20H18N4O7S: C, 52.40; H, 3.96; N, 12.22; Found: C, 52.67; H, 4.23; N, 12.53.
2–(2,3-Dioxo-5-(pyrrolidin-1-ylsulfonyl)indolin-1-yl)-N-(4-(trifluoromethoxy)phenyl)acetamide (19i): White solid; Yield: 64%; m.p. 266–267 °C; IR (KBr, cm−1): 3340 (NH), 1710 (C=OKetone), 1678 (C=OAmide), 1656 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.65–1.70 (m, 4H, CH2-Pyrrole), 3.08–3.13- (m, 4H, CH2-Pyrrole), 4.66 (s, 2H, CH2), 6.85 (d, J = 7.0 Hz, 2H), 7.14 (d, J = 8.5 Hz, 1H), 7.20 (d, J = 7.0 Hz, 2H), 7.44 (d, J = 8.5 Hz, 1H), 8.04 (s, 1H), 8.54 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 25.8, 47.4, 62.5, 113.1, 123.8, 129.0, 129.2, 131.5, 134.4, 142.6, 143.2, 143.4, 147.2, 151.4, 160.2, 167.3, 180.9; Anal. Calcd. For C21H18F3N3O6S: C, 50.70; H, 3.65; N, 8.45; Found: C, 50.47; H, 3.81; N, 8.12; MS (m/z, %): 497 (M+, 51), 279 (41), 218 (23), 161 (29), 146 (100), 77 (22).
2–(2,3-Dioxo-5-(pyrrolidin-1-ylsulfonyl)indolin-1-yl)-N-(p-tolyl)acetamide (19j): White solid; Yield: 77%; m.p. 248–250 °C; IR (KBr, cm−1): 3356 (NH), 1716 (C=OKetone), 1688 (C=OAmide), 1653 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.64–1.69 (m, 4H, CH2-Pyrrole), 2.25 (s, 3H, CH3), 3.09–3.17 (m, 4H, CH2-Pyrrole), 4.64 (s, 2H, CH2), 7.12 (d, J = 7.85 Hz, 2H), 7.39 (d, J = 8.25 Hz, 1H), 7.43 (d, J = 7.85 Hz, 2H), 7.86 (s, 1H), 8.11 (d, J = 8.25 Hz, 1H), 8.47 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6):18.3, 24.9, 49.4, 59.9, 112.0, 119.2, 126.9, 127.5, 128.9, 129.4, 130.6, 131.6, 134.3, 148.7, 158.2, 165.1, 183.3; Anal. Calcd. For C21H21N3O5S: C, 59.00; H, 4.95; N, 9.83; Found: C, 59.37; H, 5.21; N, 10.12; MS (m/z, %): 427 (M+, 52), 278 (41), 150 (29), 146 (100), 134 (39), 57 (36).
2–(2,3-Dioxo-5-(pyrrolidin-1-ylsulfonyl)indolin-1-yl)-N-(4-methoxyphenyl)acetamide (19k): White solid; Yield: 73%; m.p. 194–196 °C; IR (KBr, cm−1): 3356 (NH), 1716 (C=OKetone), 1688 (C=OAmide), 1653 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.64–1.68 (m, 4H, CH2-Pyrrole), 2.82 (t, J = 6.0 Hz, 4H, CH2-Pyrrole), 3.94 (s, 3H, O-CH3), 4.19 (s, 2H, CH2), 6.85 (d, J = 8.0 Hz, 2H), 7.10 (d, J = 8.5 Hz, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.50 (d, J = 8.5 Hz, 1H), 8.12 (s, 1H), 8.46 (s, 1H, NH);13C-NMR (125 MHz, DMSO-d6): 25.1, 45.7, 55.3, 63.7, 110.3, 114.5, 122.4 (2 C), 123.4, 126.5, 129.7, 132.1, 134.0, 159.1, 160.2, 167.6, 181.1; Anal. Calcd. For C21H21N3O6S: C, 56.88; H, 4.77; N, 9.48; Found: C, 56.57; H, 4.31; N, 9.12; MS (m/z, %): 443 (M+, 52), 278 (41), 150 (29), 146 (100), 134 (39), 57 (36).
(S)-5-((2-(Phenoxymethyl)pyrrolidin-1-yl)sulphonyl)-1-(prop-2-yn-1-yl)indoline-2,3-dione (20a): White solid; Yield: 65%; m.p. 282–284 °C; IR (KBr, cm−1): 1725 (C=OKetone), 1680 (C=OAmide), 1658 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.61–1.67 (m, 4H, CH2-Pyrrole), 2.66–2.69 (m, 2H, CH2-Pyrrole), 3.38–3.42 (m, 2H, CHAcetylene,CHPyrrole), 4.00–4.09 (m, 2H, O-CH2), 4.61 (s, 2H, N-CH2), 6.93 (d, J = 7.2 Hz, 2H), 7.28 (t, J = 7.2 Hz, 3H), 7.42 (d, J = 7.7 Hz, 1H), 7.87 (s, 1H), 8.21 (d, J = 7.7 Hz, 1H); 13 C-NMR (125 MHz, DMSO-d6): 23.6, 26.0, 46.1, 58.5, 62.4, 72.0, 73.6, 118.6, 123.7, 124.4, 126.2, 128.5, 129.8, 138.5, 139.5, 151.9, 161.9, 164.0, 179.6; Anal. Calcd. For C22H20N2O5S: C, 62.25; H, 4.75; N, 6.60; Found: C, 62.51; H, 4.97; N, 6.32; MS (m/z, %): 424 (M+, 48), 385 (31), 248 (54), 208 (40), 176 (23), 146 (100), 107 (21), 77 (30).
(S)-2–(2,3-Dioxo-5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)indolin-1-yl)-N-phenylacetamide (20b): White solid; Yield: 56%; m.p. 238–240 °C; IR (KBr, cm−1): 1720 (C=OKetone), 1688 (C=OAmide), 1660 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.78–1.84 (m, 4H, CH2-Pyrrole), 2.62–2.65 (m, 2H, CH2-Pyrrole), 3.30–3.36 (m, 1H, CHChiral), 3.87 (d, J = 12.0 Hz, 1H, O-CH2-Diastropic), 4.14 (d, J = 12.0 Hz, 1H, O-CH2-Diastropic), 4.60 (s, 2H, N-CH2), 6.99–7.05 (m, 3H), 7.37–7.43 (m, 6H), 7.50 (d, J = 8.0 Hz, 2H), 8.02 (d, J = 8.0 Hz, 1H), 8.10 (s, 1H), 8.46 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 22.5, 27.6, 45.6, 58.5, 63.2, 117.7, 119.2, 120.9, 127.2, 128.4, 128.7, 128.9, 129.2, 129.5, 134.0, 135.2, 135.7, 139.8, 151.1, 161.3, 166.2, 179.7; Anal. Calcd. For C27H25N3O6S: C, 62.42; H, 4.85; N, 8.09; Found: C, 62.66; H, 4.97; N, 8.39; MS (m/z, %): 519 (M+, 52), 278 (41), 150 (29), 146 (100), 134 (39), 77 (36).
(S)-2–(2,3-Dioxo-5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)indolin-1-yl)-N-(4-fluorophenyl)acetamide (20c): White solid; Yield: 53%; m.p. 108–110 °C; IR (KBr, cm−1): 1725 (C=OKetone), 1690 (C=OAmide), 1655 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.78–1.84 (m, 4H, CH2-Pyrrole), 2.62–2.65 (m, 2H, CH2-Pyrrole), 3.43–3.46 (m, 1H, CHChiral), 3.73 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.10 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.58 (s, 2H, N-CH2), 6.95–7.02 (m, 3H), 7.27 (t, J = 7.0 Hz, 2H), 7.35 (d, J = 7.0 Hz, 2H), 7.41 (d, J = 7.0 Hz, 1H), 7.59 (t, J = 7.0 Hz, 2H), 8.02 (d, J = 8.0 Hz, 1H), 8.08 (s, 1H), 8.56 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 22.4, 27.6, 45.6, 57.3, 62.4, 117.5, 117.7, 119.2, 120.9, 127.2, 128.4, 130.1, 131.4, 131.6, 135.7, 136.9, 142.4, 151.5, 160.9, 161.6, 163.5, 181.2; Anal. Calcd. For C27H24FN3O6S: C, 60.33; H, 4.50; N, 7.82; Found: C, 60.05; H, 4.82; N, 7.55; MS (m/z, %): 537 (M+, 41), 385 (28), 152 (35), 240 (49), 146 (100), 97 (26), 77 (29).
(S)-N-(4-chlorophenyl)-2–(2,3-dioxo-5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)indolin-1-yl)acetamide (20d): White solid; Yield: 48%; m.p. 209–211 °C; IR (KBr, cm−1): 1718 (C=OKetone), 1700 (C=OAmide), 1670 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.78–1.84 (m, 4H, CH2-Pyrrole), 2.61–2.63 (m, 2H, CH2-Pyrrole), 3.44–3.48 (m, 1H, CHChiral), 3.99 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.18 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.58 (s, 2H, N-CH2), 7.03 (t, J = 7.0 Hz, 2H), 7.34 (d, J = 7.5 Hz, 1H), 7.36–7.41 (m, 3H), 7.48–7.55 (m, 4H), 8.03 (d, J = 8.0 Hz, 1H), 8.07 (s, 1H),8.58 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 22.5, 28.6, 46.6, 28.6, 62.4, 117.3, 119.2, 127.2, 128.3, 129.0, 129.2, 130.8, 131.0, 132.8, 134.2, 135.2, 135.7, 139.9, 152.0, 161.7, 166.5, 180.8; Anal. Calcd. For C27H24ClN3O6S: C, 58.53; H, 4.37; N, 7.58; Found: C, 58.73; H, 4.18; N, 7.29; MS (m/z, %): 554 (M+, 26), 385 (62), 240 (48), 168 (21), 146 (100), 112 (17), 77 (41), 58 (55).
(S)-N-(4-Bromophenyl)-2–(2,3-dioxo-5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)indolin-1-yl)acetamide (20e): White solid; Yield: 51%; m.p. 196–198 °C; IR (KBr, cm−1): 1722 (C=OKetone), 1680 (C=OAmide), 1660 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.78–1.83 (m, 4H, CH2-Pyrrole), 2.60–2.63 (m, 2H, CH2-Pyrrole), 3.40–3.45 (m, 1H, CHChiral), 4.13 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.28 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.68 (s, 2H, N-CH2), 7.03 (t, J = 7.0 Hz, 2H), 7.16–7.21 (m, 3H), 7.37–7.42 (m, 5H), 8.02 (d, J = 8.0 Hz, 1H), 8.14 (s, 1H), 8.56 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 22.6, 28.6, 46.6, 58.1, 62.4, 116.0, 117.5, 117.7, 119.0, 127.2, 128.7, 129.0, 129.6, 130.9, 135.2, 135.7, 139.0, 139.8, 150.0, 162.3, 167.8, 179.0; Anal. Calcd. For C27H24BrN3O6S: C, 54.19; H, 4.04; N, 7.02; Found: C, 54.34; H, 4.44; N, 7.38; MS (m/z, %): 600 (M + 2+, 37), 598 (M+, 35), 385 (41), 240 (29), 155 (42), 146 (100), 77 (36).
(S)-2–(2,3-Dioxo-5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)indolin-1-yl)-N-(2-nitrophenyl)acetamide (20f): White solid; Yield: 47%; m.p. 180–182 °C; IR (KBr, cm−1): 1720 (C=OKetone), 1685 (C=OAmide), 1654 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.79–1.83 (m, 4H, CH2-Pyrrole), 2.63–2.67 (m, 2H, CH2-Pyrrole), 3.38–3.41 (m, 1H, CHChiral), 3.86 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.17 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.68 (s, 2H, N-CH2), 6.99–7.04 (m, 3H), 7.28 (t, J = 8.0 Hz, 1H), 7.34 (t, J = 7.0 Hz, 2H), 7.38–7.41 (m, 2H), 7.77 (t, J = 8.0 Hz, 1H), 8.03 (d, J = 8.0 Hz, 1H), 8.07 (d, J = 7.5 Hz, 1H), 8.18 (s, 1H), 8.62 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 22.6, 28.6, 46.6, 58.1, 62.4, 116.0, 117.5, 117.9, 119.2, 120.9, 127.2, 128.3, 130.1, 131.4, 131.6, 135.2, 135.8, 140.0, 142.4, 143.6, 144.0, 161.1, 167.0, 179.3; Anal. Calcd. For C27H24N4O8S: C, 57.44; H, 4.28; N, 9.92; Found: C, 57.74; H, 4.49; N, 10.21; MS (m/z, %): 564 (M+, 48), 324 (39), 385 (53), 240 (27), 179 (33), 144 (100), 123 (61), 77 (25).
(S)-2–(2,3-Dioxo-5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)indolin-1-yl)-N-(3-nitrophenyl)acetamide (20g): White solid; Yield: 52%; m.p. 181–183 °C; IR (KBr, cm−1): 1725 (C=OKetone), 1686 (C=OAmide), 1656 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.78–1.87 (m, 4H, CH2-Pyrrole), 2.63–2.66 (m, 2H, CH2-Pyrrole), 3.49–3.52 (m, 1H, CHChiral), 3.77–3.80 (m, 1H, O-CH2-Diastropic), 3.97–4.01 (m, 1H, O-CH2-Diastropic), 4.58 (s, 2H, N-CH2), 7.00–7.05 (m, 3H), 7.36 (d, J = 7.5 Hz, 1H), 7.41 (d, J = 7.5 Hz, 1H), 7.42–7.44 (m, 1H), 7.45 (d, J = 7.0 Hz, 2H), 7.48 (t, J = 8.0 Hz, 1H), 8.03 (d, J = 8.0 Hz, 1H), 8.07 (d, J = 7.0 Hz, 1H), 8.18 (s, 1H), 8.71 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 22.52, 26.61, 47.25, 57.36, 62.40, 117.5, 117.8, 122.8, 127.2, 128.5, 129.9, 130.9 (2 C), 131.7, 131.9, 135.1, 136.2, 139.9, 143.8, 144.5, 151.9, 162.4, 166.7, 179.1; Anal. Calcd. For C27H24N4O8S: C, 57.44; H, 4.28; N, 9.92; Found: C, 57.71; H, 4.46; N, 10.17.
(S)-2–(2,3-Dioxo-5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)indolin-1-yl)-N-(4-nitrophenyl)acetamide (20h): White solid; Yield: 51%; m.p. 162–164 °C; IR (KBr, cm−1): 1718 (C=OKetone), 1688 (C=OAmide), 1655 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.78–1.82 (m, 4H, CH2-Pyrrole), 2.59–2.63 (m, 2H, CH2-Pyrrole), 3.44–3.47 (m, 1H, CHChiral), 3.86–3.89 (m, 1H, O-CH2-Diastropic), 4.06–4.10 (m, 1H, O-CH2-Diastropic), 4.59 (s, 2H, N-CH2), 6.98–7.02 (m, 3H), 7.35–7.53 (m, 5H), 8.13–8.15 (m, 1H), 8.15–8.18 (m, 2H), 8.20 (s, 1H), 8.62 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 22.5, 27.0, 45.5, 57.1, 61.3, 117.5, 117.8, 127.2, 128.2, 129.9, 130.9, 131.7, 131.9, 135.1, 135.7, 139.9, 142.5, 144.1, 150.7, 161.9, 166.8, 180.0; Anal. Calcd. For C27H24N4O8S: C, 57.44; H, 4.28; N, 9.92; Found: C, 57.77; H, 4.52; N, 9.66.
(S)-2–(2,3-Dioxo-5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)indolin-1-yl)-N-(4-(trifluoromethoxy)phenyl)acetamide (20i): White solid; Yield: 56%; m.p. 218–220 °C; IR (KBr, cm−1): 1724 (C=OKetone), 1690 (C=OAmide), 1668 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.80–1.84 (m, 4H, CH2-Pyrrole), 2.60–2.68 (m, 2H, CH2-Pyrrole), 3.70–3.73 (m, 1H, CHChiral), 3.90 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.14 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.57 (s, 2H, N-CH2), 7.03 (t, J = 7.5 Hz, 2H), 7.32 (t, J = 7.5 Hz, 1H), 7.43–7.47 (m, 3H), 7.53 (d, J = 7.0 Hz, 1H), 7.59 (d, J = 8.0 Hz, 2H), 7.72 (d, J = 8.0 Hz, 1H), 7.92 (d, J = 7.0 Hz, 1H), 8.10 (s, 1H), 8.90 (s, 1H, NH);13C-NMR (125 MHz, DMSO-d6): 22.5, 27.8, 44.8, 57.2, 61.4, 117.6, 119.1, 120.8, 125.3, 127.2, 127.7, 128.9, 129.3, 131.0, 133.5, 135.3, 136.2 (2 C), 144.7, 150.8, 162.0, 166.5, 179.0; Anal. Calcd. For C28H24F3N3O7S: C, 55.72; H, 4.01; N, 6.96; Found: C, 55.89; H, 4.25; N, 7.15; MS (m/z, %): 603 (M+, 60), 442 (54), 385 (29), 240 (52), 161 (42), 146 (100), 93 (17), 77 (29).
(S)-2–(2,3-Dioxo-5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)indolin-1-yl)-N-(p-tolyl)acetamide (20j): White solid; Yield: 48%; m.p. 209–211 °C; IR (KBr, cm−1): 1723 (C=OKetone), 1677 (C=OAmide), 1649 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.76–1.81 (m, 4H, CH2-Pyrrole), 2.09 (s, 3H), 2.59–2.62 (m, 2H, CH2-Pyrrole), 3.44–3.48 (m, 1H, CHChiral), 3.79 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.10 (d, J = 13.0 Hz, 1H, O-CH2-Diastropic), 4.51 (s, 2H, N-CH2), 7.01–7.04 (m, 3H), 7.13 (d, J = 7.0 Hz, 1H), 7.23 (d, J = 7.5 Hz, 2H), 7.34 (t, J = 7.5 Hz, 2H), 7.40 (d, J = 7.0 Hz, 2H), 8.12 (d, J = 7.5 Hz, 1H), 8.19 (s, 1H), 8.50 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 18.6, 24.5, 28.2, 45.5, 57.9, 61.4, 117.8, 119.2, 120.7, 126.5, 127.2, 128.2, 129.4, 130.8, 131.5, 135.1, 135.7, 137.0, 139.9, 151.5, 162.6, 166.9, 179.8; Anal. Calcd. For C28H27N3O6S: C, 63.03; H, 5.10; N, 7.87; Found: C, 63.38; H, 5.35; N, 8.13; MS (m/z, %): 533 (M+, 52), 385 (42), 240 (38), 148 (100), 93 (37), 77 (49).
(S)-2–(2,3-Dioxo-5-((2-(phenoxymethyl)pyrrolidin-1-yl)sulphonyl)indolin-1-yl)-N-(4-methoxyphenyl)acetamide (20k): White solid; Yield: 48%; m.p. 204–206 °C; IR (KBr, cm−1): 1720 (C=OKetone), 1680 (C=OAmide), 1656 (C=OAmide); 1H-NMR (500 MHz, DMSO-d6): 1.78–1.83 (m, 4H, CH2-Pyrrole), 2.60–2.62 (m, 2H, CH2-Pyrrole), 3.46–3.50 (m, 1H, CHChiral), 3.62 (s, 3H), 4.14–4.21 (m, 2H, O-CH2), 4.64 (s, 2H, N-CH2), 6.90 (d, J = 7.5 Hz, 2H), 7.00–7.03 (m, 3H), 7.27–7.31 (m, 2H), 7.41 (d, J = 7.8 Hz, 1H), 7.54 (d, J = 7.5 Hz, 2H), 8.08 (d, J = 7.8 Hz, 1H), 8.18 (s, 1H), 8.61 (s, 1H, NH); 13 C-NMR (125 MHz, DMSO-d6): 22.5, 28.5, 46.6, 55.1, 58.3, 62.6, 109.5, 110.1, 119.3, 121.0, 127.2, 128.4, 130.2, 131.4, 131.5, 135.2, 135.8, 140.0, 142.6, 152.1, 158.5, 161.5, 163.4,179.3; Anal. Calcd. For C28H27N3O7S: C, 61.19; H, 4.95; N, 7.65; Found: C, 61.38; H, 5.12; N, 7.82; MS (m/z, %): 549 (M+, 40), 385 (21), 164 (37), 146 (100), 108 (42), 77 (61).
Caspase-3 and -7 inhibition assay
The activity assay of caspase-3 was performed in a system of 50 µL containing 150 mM NaCl, 1 mM EDTA, 2 mM DTT, 50 mM HEPES pH 7.4, 10 µM Ac-DEVD-AMC (Bachem Bioscience, Philadelphia, PA, USA) and 2 nM caspase-3 in the 1 µL DMSO. Caspase-3 was incubated with synthesised compounds in 384-well plates for 10 min. The %inhibition of target compounds was measured at 20 µg/ml. The enzymatic activity of the caspase-3 was measured based on production of a fluorogenic substrate, 7-amino-4-methyl coumarin, which was monitored for 10 min and detected using an EnVision (PerkinElmer, Wellesley, MA, USA) at λex = 360 nm and λem= 460 nm. The initial rate of hydrolysis was determined using the early linear region of the enzymatic reaction curve. For IC50 determination, about 8 concentrations of the synthesised compounds were freshly prepared by three-fold serial dilutions DMSO and the assay buffer such that following the addition of the inhibitors, DMSO concentration would equal to 0.2%. and GraphPad Prism 5 software was used to calculate the IC50 values.
Computational studies
Docking procedure was performed via Autodock Tools (1.5.6). The crystallographic structure of human caspase-3 complexed with isatin sulphonamide (PDB ID: 1GFW) were retrieved from the Protein Data Bank. The co-crystallized ligand and water molecules were eliminated and the protein was converted to the pdbqt format using Autodock Tools (1.5.6). Compounds structures were drawn and 3 D-optimized using Marvin Sketch 15.8.10, 2015, ChemAxon (http://www.chemaxon.com), then converted to pdbqt by Autodock Tools. Each docking system were completed by 50 runs and the grid box parameters were set as follows: size_x = 50; size_y = 50; size_z = 50; centred on co-ligand’s position in PDB complex. Other parameters of Autodock search by the Lamarckian genetic algorithm (LGA) were left as default except population size and maximum number of evaluations which were changed to 100 and 1000000, respectively. Finally, interactions of the compounds were illustrated by discovery studio visualiser ver.4.5 to investigate their binding mode. Docking validation were confirmed through re-docking of 1GFW co-ligand into the receptor with the same docking parameters of the compounds.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 2005;258:479–517.
- Kemnitzer W, Kasibhatla S, Jiang S, et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 2. Structure-activity relationships of the 7- and 5-, 6-, 8-positions. Bioorg Med Chem Lett 2005;15:4745–51.
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 1999;68:383–424.
- Becker JW, Rotonda J, Soisson SM, et al. Reducing the peptidyl features of caspase-3 inhibitors: a structural analysis. J Med Chem 2004;47:2466–74.
- Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999;6:99–104.
- Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci 1997;22:299–306.
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998;281:1312–6.
- Lee D, Long SA, Adams JL, et al. Potent and selective nonpeptide inhibitors of caspases 3 and 7 inhibit apoptosis and maintain cell functionality. J Biol Chem 2000;275:16007–14.
- Rotonda J, Nicholson DW, Fazil KM, et al. The three-dimensional structure of apopain/CPP32, a key mediator of apoptosis. Nat Struct Biol 1996;3:619–25.
- Mittl PRE, Di Marco S, Krebs JF, et al. Structure of recombinant human CPP32 in complex with the tetrapeptide acetyl-Asp-Val-Ala-Asp fluoromethyl ketone. J Biol Chem 1997;272:6539–47.
- Ahmed FF, El-Hafeez AA, Abbas SH, et al. New 1,2,4-triazole-Chalcone hybrids induce Caspase-3 dependent apoptosis in A549 human lung adenocarcinoma cells. Eur J Med Chem 2018;151:705–22.
- Wu Z-R, Liu J, Li J-Y, et al. Synthesis and biological evaluation of hydroxycinnamic acid hydrazide derivatives as inducer of caspase-3. Eur J Med Chem 2014;85:778–83.
- Glória PMC, Coutinho I, Gonçalves LM, et al. Aspartic vinyl sulfones: inhibitors of a caspase-3-dependent pathway. Eur J Med Chem 2011;46:2141–6.
- Patel S, Modi P, Ranjan V, Chhabria M. Structure-based design, synthesis and evaluation of 2,4-diaminopyrimidine derivatives as novel caspase-1 inhibitors. Bioorg Chem 2018;78:258.
- Kassab AE, Hassan RA. Novel benzotriazole N-acylarylhydrazone hybrids: design, synthesis, anticancer activity, effects on cell cycle profile, caspase-3 mediated apoptosis and FAK inhibition. Bioorg Chem 2018;80:531–544.
- Brethon A, Chantalat L, Christin O, et al. New caspase-1 inhibitor by scaffold hopping into bio-inspired 3D-fragment space. Bioorg Med Chem Lett 2017;27:5373–5377.
- Patel S, Modi P, Chhabria M. Rational approach to identify newer caspase-1 inhibitors using pharmacophore based virtual screening, docking and molecular dynamic simulation studies. J Mol Graph Model 2018;81:106–115.
- Trond YJ, Hansen V. Isatin 1,2,3-triazoles as potent inhibitors against caspase-3. Bioorg Med Chem Lett 2011;21:1626–1629.
- Mou J, Wu S, Luo Z, et al. Structure-activity relationship study of a series of caspase inhibitors containing γ-amino acid moiety for treatment of cholestatic liver disease. Bioorg Med Chem Lett 2018;28:1874–1878.
- Ayoup MS, Wahby Y, Abdel-Hamid H, et al. Design, synthesis and biological evaluation of novel α-acyloxy carboxamides via Passerini reaction as caspase 3/7 activators. Eur J Med Chem 2019;168:340–356.
- Chu W, Zhang J, Zeng C, et al. N-benzylisatin sulfonamide analogues as potent caspase-3 inhibitors: synthesis, in vitro activity, and molecular modeling studies. J Med Chem 2005;48:7637–7647.
- Kopka K, Faust A, Keul P, et al. 5-Pyrrolidinylsulfonyl isatins as a potential tool for the molecular imaging of caspases in apoptosis. J Med Chem 2006;49:6704–6715.
- Lee D, Long SA, Murray JH, et al. Potent and selective nonpeptide inhibitors of caspases 3 and 7. J Med Chem 2001;44:2015–2026.
- Limpachayaporn P, Schafers M, Haufe G. Isatin sulfonamides: potent caspases-3 and -7 inhibitors, and promising PET and SPECT radiotracers for apoptosis imaging. Future Med Chem 2015;7:1173–1196.
- Krause-Heuer AM, Howell NR, Matesic L, et al. A new class of fluorinated 5-pyrrolidinylsulfonyl isatin caspase inhibitors for PET imaging of apoptosis. Med Chem Commun 2013;4:347–352.
- O’Brien T, Lee D. Prospects for caspase inhibitors. Mini Rev Med Chem 2004;4:153–165.
- Lakshmi PJ, Suneel Kumar BVS, Nayana RS, et al. Design, synthesis, and discovery of novel non-peptide inhibitor of caspase-3 using ligand based and structure based virtual screening approach. Bioorg Med Chem 2009;17:6040–6047.
- Morris GM, Huey R, Lindstrom W, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 2009;30:2785–2791.
- Ganesan R, Mittl PRE, Jelakovic S, Grütter MG. Extended substrate recognition in caspase-3 revealed by high resolution X-ray structure analysis. J Mol Biol 2006;359:1378–1388.
- Firoozpour L, Sadatnezhad K, Dehghani S, et al. An efficient piecewise linear model for predicting activity of caspase-3 inhibitors. DARU J Pharm Sci 2012;20:31–36.
- Firoozpour L, Edraki N, Nakhjiri M, et al. Cytotoxic activity evaluation and QSAR study of chromene-based chalcones. Arch. Pharm. Res 2012;35:2117–2125.
- Hassanzadeh H, Bahrami AR, Sadeghian H, et al. Cytotoxic and anticancer activities of an acridine derivative; 11-chloro-3-methyl-3H-imidazo[4,5-a]acridine on 5637 cells. Med Chem Res 2016;25:1852–1860.
- Mahdavi M, Davoodi J, Zali MR, Foroumadi A. Concomitant activation of caspase-9 and down-regulation of IAP proteins as a mechanism of apoptotic death in HepG2, T47D and HCT-116 cells upon exposure to a derivative from 4-aryl-4H-chromenes family. Biomed Pharmacother 2011;65:175–182.
- Aryapour H, Mahdavi M, Mohebbi SR, et al. Anti-proliferative and apoptotic effects of the derivatives from 4-aryl-4H-chromene family on human leukemia K562 cells. Arch Pharm Res 2012;35:1573–1582.
- Rahmani-Nezhad S, Safavi M, Pordeli M, et al. Synthesis, in vitro cytotoxicity and apoptosis inducing study of 2-aryl-3-nitro-2H-chromene derivatives as potent anti-breast cancer agents. Eur J Med Chem 2014;86:562–569.
- NazariTarhan H, Hosseinzadeh L, Aliabadi A, et al. Cytotoxic and apoptogenic properties of 2-phenylthiazole-4-carboxamide derivatives in human carcinoma cell lines. J Rep Pharm Sci 2012;1:1–6.
- Khoshneviszadeh M, Edraki N, Miri R, et al. QSAR Study of 4-Aryl-4H-chromenes as a new series of apoptosis inducers using different chemometric tools. Chem Biol Drug Des 2012;79:442–458.
- Zhang W, Ai J, Shi D, et al. Discovery of novel type II c-Met inhibitors based on BMS-777607. Eur J Med Chem 2014;80:254–266.
- Jin Y, Zhou Z-Y, Tian W, et al. 4′-Alkoxyl substitution enhancing the anti-mitotic effect of 5-(3′,4′,5′-substituted)anilino-4-hydroxy-8-nitroquinazolines as a novel class of anti-microtubule agents. Bioorg Med Chem Lett 2006;16:5864–5869.
- Yang S-K, Kang JS, Oelschlaeger P, Yang K-W. Azolylthioacetamide: a highly promising scaffold for the development of metallo-β-lactamase inhibitors. ACS Med Chem Lett 2015;6:455–460.
- Chiou CT, Lee W-C, Liao J-H, et al. Synthesis and evaluation of 3-ylideneoxindole acetamides as potent anticancer agents. Eur J Med Chem 2015;98:1–12.
- Guo X, Ma X, Yang Q, et al. Discovery of 1-aryloxyethyl piperazine derivatives as Kv1.5 potassium channel inhibitors (part I). Eur J Med Chem 2014;81:89–94.
- Dassault Systèmes BIOVIA. Discovery Studio Modeling Environment, Release 2017. San Diego: Dassault Systèmes; 2016.
- Limpachayaporn P, Schäfers M, Schober O, et al. Synthesis of new fluorinated, 2-substituted 5-pyrrolidinylsulfonyl isatin derivatives as caspase-3 and caspase-7 inhibitors: nonradioactive counterparts of putative PET-compatible apoptosis imaging agents. Bioorg Med Chem 2013;21:2025–2036.