Abstract
The importance of transforming growth factor beta-activated kinase 1 (TAK1) to cell survival has been demonstrated in many studies. TAK1 regulates signalling cascades, the NF-κB pathway and the mitogen-activated protein kinase (MAPK) pathway. TAK1 inhibitors can induce the apoptosis of cancerous cells, and irreversible inhibitors such as (5Z)-7-oxozeaenol are highly potent. However, they can react non-specifically with cysteine residues in proteins, which may have serious adverse effects. Reversible covalent inhibitors have been suggested as alternatives. We synthesised imidazopyridine derivatives as novel TAK1 inhibitors, which have 2-cyanoacrylamide moiety that can form reversible covalent bonding. A derivative with 2-cyano-3-(6-methylpyridin-2-yl)acrylamide (13h) exhibited potent TAK1 inhibitory activity with an IC50 of 27 nM. It showed a reversible reaction with β-mercaptoethanol, which supports its potential as a reversible covalent inhibitor.
Introduction
Transforming growth factor beta-activated kinase 1 (TAK1), also known as mitogen-activated protein kinase kinase kinase 7 (MAP3K7) or MEK kinase 7 (MEKK7), is a serine/threonine kinase encoded by MAP3K7 gene. Since it was first found to be activated by transforming growth factor beta (TGFβ) and bone morphologic protein (BMP)Citation1, TAK1 has been reported to mediate signal transduction for the regulation of cell proliferation and apoptosis pathwaysCitation2. TAK1 is activated by various exogenous stimuli, including interleukin-1 (IL-1), lipopolysaccharide (LPS), tumour necrosis factor alpha (TNFα), and TGFβCitation3,Citation4. TNFα has critical roles in signalling pathways for both cell survival and deathCitation5–7.
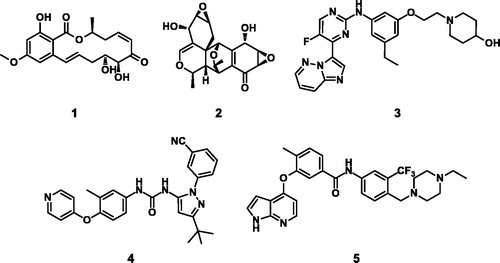
Because TAK1 is a key signalling element that is required for cell survival and death in TNF α signalling, it has emerged as a potential therapeutic target for cancer and inflammatory diseaseCitation8–10. In TNFα stimulated breast cancer cells, inhibition of TAK1 causes apoptosis by switching from NFκB pro-survival signalling to induction of effector caspasesCitation11. In-vivo studies have provided evidence of a strong relationship between TAK1 and various malignancies, including pancreatic cancerCitation12, colon cancerCitation13, and breast cancerCitation14. A number of small molecules have been reported to inhibit TAK1 (). (5Z)-7-Oxozeaenol (5Z7O, 1)Citation15 and epoxyquinol B (EPQB, 2)Citation16 are compounds derived from fungi which possess a resorcylic lactone and an epoxide, respectively. Imidazo[1,2-b]pyridazine (3) was developed as a reversible type I inhibitor. It fits into an active DFG-in conformation with TAK1Citation17. Pyrazole urea (4)Citation18 and 1H-pyrrolo[2,3-b]pyridine (5)Citation19 have been reported as Type II inhibitors which bind to TAK1 in the inactive DFG-out confirmation.
5Z7O is a potent irreversible TAK1 inhibitorCitation15, although it is also a promiscuous kinase inhibitor. The cis-enone of 5Z7O has off-target effects because it forms covalent bonds with reactive cysteine residuesCitation20. Acrylamide Michael acceptors irreversibly bind to nucleophiles such as cysteine under physiological conditionsCitation21. Michael acceptors that undergo dual activation by electron-withdrawing groups form reversible covalent bonds by increasing the α-carbon acidity of covalent adductsCitation22. Converting an irreversible warhead to a reversible warhead can limit off-target binding and increase the probability of binding the target siteCitation23.
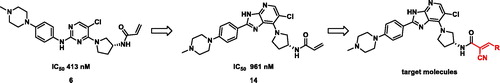
In this study, we designed imidazopyridines with 2-cyanoacrylamide moiety for reversible covalent TAK1 inhibition. The screening of our in-house chemical library led to identification of a pyrimidine compound (6) with an IC50 of 413 nM against TAK1. To identify novel scaffold as TAK1 inhibitors, imidazopyridine scaffold was designed based on a bioisosteric replacement strategy. The imidazopyridine 14 showed retained activity although its IC50 was approximately twice that of the pyrimidine (6). Target molecules were designed for reversible covalent chemistry by replacing the acrylamide moiety with various 2-cyanoacrylamide moieties ().
Materials and methods
Chemistry
Unless otherwise noted, all reagents and solvents were purchased from a commercial vendor and used without further purification. Reactions were monitored via thin-layer chromatography (TLC) using Merck TLC silica gel 60 F254 250 µm plates. Flash column chromatography was performed using ZEOprep 60 silica gel (Zeochem, 40–63 µm) and a CombiFlash system (Teledyne ISCO) loaded with pre-packed silica gel flash column cartridges (Welux™). 1H and 13C NMR spectra were obtained using a Resonance ECZ 600R NMR spectrometer (JEOL). 1H NMR spectra were collected at 600 MHz, and 13C spectra were collected at 150 MHz using tetramethylsilane (TMS) as an internal standard. Chemical shifts are reported in parts per million (ppm, δ) downfield of TMS, and the coupling constant (J) is reported in hertz (Hz). Splitting patterns are reported with the following abbreviations: s, singlet; d, doublet; t, triplet; q, quartette; p, pentet; dd, doublet of doublets; dt, doublet of triplets; td, triplet of doublets; m, multiplet; br, broad signal. High-resolution mass spectrometry (HRMS) was performed using a Q-Exactive MS (ThermoScientific) coupled with an Ultimate 3000 LC system (Dionex). A ThermoScientific Hypersil GOLD C18 column (2.1 mm × 50 mm, 1.9 µm) was used for separation.
(R)-tert-butyl-(1-(2-amino-5-chloro-3-nitropyridin-4-yl)pyrrolidin-3-yl)carbamate (8)
2-Amino-4,5-dichloro-3-nitropyridine (208 mg, 1 mmol), (R)-3-(Boc-amino)pyrrolidine (186 mg, 1 mmol) and K2CO3 (276 mg, 2 mmol) were dissolved in MeCN (3 ml) and stirred at room temperature (RT) for 8 h. Excess water was added to the reaction mixture, and the mixture was stirred at RT for an additional 17 h. The reaction mixture was then filtered, and the filter cake was washed with water to obtain 8 (191 mg, 53%). 1H-NMR (600 MHz, DMSO-d6) δ 7.87 (s, 1H), 7.21 (d, J = 5.5 Hz, 1H), 6.88 (s, 2H), 4.02 (d, J = 5.5 Hz, 1H), 3.64 (m, 1H), 3.53 (m, 2H), 3.23 (m, 1H), 2.05 (m, 1H), 1.83 (m, 1H), 1.38 (s, 9H). 13C-NMR (150 MHz, DMSO-d6) δ 155.3, 152.7, 151.5, 146.0, 123.2, 107.7, 78.0, 56.2, 49.6, 49.3, 30.4, 28.2. HRMS (ESI) [M + H]+: m/z calcd. 358.1277. Found 358.1274.
(R)-tert-butyl (1-(2,3-diamino-5-chloropyridin-4-yl)pyrrolidin-3-yl)carbamate (9)
Compound 8 (179 mg, 0.5 mmol) and Fe powder (84 mg, 1.5 mmol) were dissolved in acetic acid (3 ml), and the mixture was stirred at 40 °C for 1 h. Saturated NaHCO3 was carefully added to the reaction mixture at 0 °C, and the mixture was extracted three times with ethyl acetate (EA). The organic layer was washed with brine, dried with Na2SO4, filtered and concentrated on a rotary evaporator. The concentrated mixture was purified via medium pressure liquid chromatography (MPLC) to obtain 9 (108 mg, 66%). 1H-NMR (300 MHz, CDCl3) δ 7.58 (s, 1H), 5.03 (m, 1H), 4.80 (m, 1H), 4.38 (m, 1H), 4.17 (bs, 2H), 3.85 (bs, 2H), 3.56 (m, 1H), 3.34 (m, 1H), 3.05 (m, 1H), 2.40 (m, 1H), 1.89 (m, 1H), 1.47 (s, 9H).
(R)-tert-butyl-(1-(6-chloro-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)carbamate (10)
Compound 9 (75 mg, 0.22 mmol), 4-(4-methylpiperazin-1-yl)benzaldehyde (45 mg, 0.22 mmol) and FeCl3 (1 mg, 0.007 mmol) were dissolved in dimethylformamide (DMF, 1 ml), and the mixture was stirred at 120 °C for 16 h. Water was added to the reaction mixture, and the mixture was extracted with dichloromethane (DCM) three times. The extract was washed with brine, dried with Na2SO4, filtered and concentrated on a rotary evaporator. The concentrated mixture was purified via MPLC to obtain 10 (38 mg, 32%). 1H-NMR (600 MHz, CDCl3) δ 8.00–7.96 (m, 3H), 7.01 (d, J = 8.3 Hz, 2H), 5.56 (s, 1H), 4.34–4.15 (m, 5H), 3.35 (s, 4H), 2.61 (s, 4H), 2.38 (s, 3H), 2.22 (t, J = 6.2 Hz, 1H), 2.03 (s, 1H), 1.74 (s, 1H), 1.46 (s, 9H), 1.26 (s, 8H). 13C-NMR (150 MHz, DMSO-d6) δ 155.0, 151.5, 148.7, 148.7, 144.2, 142.4, 127.0, 119.3, 114.2, 109.5, 77.5, 57.1, 54.1, 50.2, 50.0, 47.0, 45.3, 30.7, 28.0. HRMS (ESI) [M + H]+: m/z calcd. 512.2535. Found 512.2534.
(R)-1-(6-chloro-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-amine (11)
Compound 10 (35 mg, 0.09 mmol) was dissolved in DCM (0.5 ml). Trifluoroacetic acid (TFA, 0.5 ml) was slowly, and the mixture was stirred at RT for 1 h. Saturated NaHCO3 was added dropwise to the reaction mixture in addition to CHCl3/2-propanol (4:1). The organic layer was washed with saturated NaHCO3 and brine, dried with Na2SO4, filtered and concentrated on a rotary evaporator to obtain 11 (26 mg, 70%). 1H-NMR (300 MHz, DMSO-d6) δ 7.98 (d, 2H), 7.88 (s, 1H), 7.04 (d, 2H), 4.30 (m, 2H), 4.05 (m, 1H), 3.74 (m, 1H), 3.26 (m, 7H), 2.44 (m, 4H), 2.21 (s, 3H), 2.19 (m, 1H), 1.88 (m, 1H).
(R)-N-(1-(6-chloro-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyanoacetamide (12)
Compound 11 (1.62 g, 3.93 mmol), cyanoacetic acid (505 mg, 5.90 mmol), EDCI (1.13g, 5.90 mmol), HOBt (160 mg, 1.18 mmol), and DIPEA (2.1 ml, 11.79 mmol) were dissolved in DMF (30 ml). The mixture was stirred at RT for 16 h, then diluted with EA, washed with water and brine, dried with Na2SO4, filtered, and concentrated on a rotary evaporator. The concentrated mixture was purified via MPLC to obtain 12 (1.05 g, 56%). 1H-NMR (600 MHz, DMSO-d6) δ 8.64 (d, J = 5.5 Hz, 1H), 7.99 (d, J = 9.0 Hz, 2H), 7.89 (d, J = 4.8 Hz, 1H), 7.06 (d, J = 9.0 Hz, 2H), 4.34 (q, J = 4.8 Hz, 2H), 4.29–4.26 (m, 1H), 4.17–4.14 (m, 1H), 3.98 (d, J = 7.6 Hz, 1H), 3.65 (s, 2H), 3.31 (s, 6H), 2.60 (s, 4H), 2.33 (s, 3H), 2.15 (d, J = 6.9 Hz, 1H), 1.98–1.90 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 162.2, 151.6, 148.9, 148.9, 144.7, 142.3, 127.2, 127.0, 119.6, 116.2, 114.7, 109.2, 57.2, 54.0, 50.5, 49.3, 46.8, 30.7, 25.3. HRMS (ESI) [M + H]+: m/z calcd. 479.2069. Found 479.2068.
(R,E)-N-(1-(6-chloro-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-phenylacrylamide (13a)
Benzaldehyde (7 µl, 0.07 mmol) and piperidine (1 µl, 0.01 mmol) were added to a solution of 12 (35 mg, 0.07 mmol) in 2-propanol (1 ml). After stirring at 60 °C for 2 h, the reaction mixture was filtered, and the filtrate was purified via MPLC to obtain 13a (8 mg, 20%). 1H-NMR (600 MHz, DMSO-d6) δ 8.78 (d, J = 6.9 Hz, 1H), 8.14 (s, 1H), 7.99 (d, J = 9.0 Hz, 2H), 7.91 (s, 1H), 7.90–7.84 (m, 2H), 7.62–7.49 (m, 3H), 7.03 (d, J = 9.0 Hz, 2H), 4.50 (q, J = 5.5 Hz, 1H), 4.32 (q, J = 6.0 Hz, 1H), 4.29–4.19 (m, 3H), 3.24 (t, J = 4.8 Hz, 4H), 2.45 (t, J = 5.2 Hz, 4H), 2.28–2.16 (m, 4H), 2.09 (q, J = 6.0 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.66, 151.81, 150.34, 148.99, 148.93, 144.56, 142.53, 132.21, 131.93, 129.88, 129.17, 127.23, 127.13, 119.34, 116.27, 114.48, 109.66, 106.79, 56.49, 54.39, 50.61, 50.10, 47.12, 45.72, 30.67. HRMS (ESI) [M + H]+: m/z calcd. 567.2382. Found 567.2379.
(R,E)-N-(1-(6-chloro-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(4-methylthiazol-2-yl)acrylamide (13b)
Compound 13b was synthesised as described for 13a using 4-methylthiazole-2-carbaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.87 (d, J = 6.9 Hz, 1H), 8.25 (s, 1H), 7.98 (d, J = 9.0 Hz, 2H), 7.91 (s, 1H), 7.79 (s, 1H), 7.03 (d, J = 9.0 Hz, 2H), 4.49 (d, J = 5.5 Hz, 1H), 4.33–4.30 (m, 1H), 4.28–4.17 (m, 3H), 3.26 (s, 4H), 2.46 (s, 3H), 2.32–2.16 (m, 4H), 2.08 (d, J = 6.9 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 160.98, 158.09, 155.01, 151.74, 148.96, 148.92, 144.57, 142.53, 140.88, 127.22, 127.11, 121.71, 119.40, 115.48, 114.51, 109.63, 107.68, 56.46, 54.28, 50.59, 50.17, 47.01, 45.55, 30.63, 16.59. HRMS (ESI) [M + H]+: m/z calcd. 588.2055. Found 588.2048.
(R,E)-N-(1-(6-chloro-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(1-methyl-1H-imidazol-2-yl)acrylamide (13c)
Compound 13c was synthesised as described for 13a using 1-methyl-1H-imidazole-2-carbaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.63 (d, J = 6.9 Hz, 1H), 8.02–7.94 (2H), 7.94–7.83 (m, 2H), 7.51 (s, 1H), 7.29 (s, 1H), 7.04 (d, J = 9.0 Hz, 2H), 4.50 (q, J = 5.7 Hz, 1H), 4.39–4.15 (m, 4H), 3.80 (s, 3H), 3.26 (s, 4H), 2.48 (s, 4H), 2.30–2.16 (m, 4H), 2.10 (q, J = 5.7 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.96, 151.77, 148.98, 148.94, 144.58, 142.48, 140.11, 133.30, 131.31, 127.24, 127.15, 126.45, 119.41, 115.82, 114.52, 109.59, 104.09, 56.49, 54.31, 50.64, 50.05, 47.05, 45.60, 32.82, 30.63. HRMS (ESI) [M + H]+: m/z calcd. 571.2444. Found 571.2440.
(R,E)-N-(1-(6-chloro-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(1-methyl-1H-pyrazol-3-yl)acrylamide (13d)
Compound 13d was synthesised as described for 13a using 1-methyl-1H-pyrazole-3-carbaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.73 (d, J = 6.2 Hz, 1H), 8.08 (s, 1H), 7.98 (d, J = 9.0 Hz, 2H), 7.91 (d, J = 2.1 Hz, 1H), 7.90 (s, 1H), 7.03 (d, J = 9.0 Hz, 2H), 7.01 (d, J = 2.8 Hz, 1H), 4.47 (q, J = 5.5 Hz, 1H), 4.32 (q, J = 6.0 Hz, 1H), 4.29–4.14 (m, 3H), 3.94 (s, 3H), 3.25 (s, 4H), 2.46 (s, 4H), 2.29–2.14 (m, 4H), 2.09–2.07 (m, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.61, 151.80, 148.93, 144.59, 144.31, 142.50, 142.26, 133.44, 127.22, 127.12, 119.41, 116.18, 114.51, 109.51, 107.10, 105.02, 56.54, 54.38, 50.59, 50.00, 47.12, 45.70, 30.65. HRMS (ESI) [M + H]+: m/z calcd. 571.2444. Found 571.2439.
(R,E)-N-(1-(6-chloro-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(pyridin-2-yl)acrylamide (13e)
Compound 13e was synthesised as described for 13a using 2-pyridinecarboxaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.83 (d, J = 6.9 Hz, 1H), 8.80–8.70 (1H), 8.09 (s, 1H), 8.04–7.97 (2H), 7.96 (dd, J = 7.9, 1.7 Hz, 1H), 7.91 (s, 1H), 7.79 (d, J = 8.3 Hz, 1H), 7.53 (q, J = 4.1 Hz, 1H), 7.04 (t, J = 9.3 Hz, 2H), 4.50 (t, J = 5.5 Hz, 1H), 4.33 (q, J = 5.7 Hz, 1H), 4.30–4.18 (m, 2H), 3.24 (t, J = 4.8 Hz, 4H), 2.48–2.40 (m, 4H), 2.22 (d, J = 9.0 Hz, 4H), 2.09 (q, J = 5.7 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.62, 151.72, 150.00, 149.91, 148.83, 148.13, 144.48, 137.37, 127.13, 127.04, 126.83, 125.98, 119.25, 115.50, 114.39, 109.43, 62.96, 56.40, 54.30, 50.51, 49.99, 47.03, 45.63, 30.57. HRMS (ESI) [M + H]+: m/z calcd. 568.2334. Found 568.2352.
(R,E)-N-(1-(6-chloro-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3–(6-fluoropyridin-2-yl)acrylamide (13f)
Compound 13f was synthesised as described for 13a using 6-fluoropicolinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.87 (d, J = 6.2 Hz, 1H), 8.16 (q, J = 8.0 Hz, 1H), 8.05 (s, 1H), 7.98 (d, J = 9.0 Hz, 2H), 7.91 (s, 1H), 7.74 (dd, J = 7.6, 2.1 Hz, 1H), 7.37 (dd, J = 8.3, 2.1 Hz, 1H), 7.05–7.02 (m, 3H), 4.49 (t, J = 5.5 Hz, 1H), 4.32 (q, J = 5.7 Hz, 1H), 4.29–4.19 (m, 2H), 3.25 (t, J = 5.2 Hz, 4H), 2.47 (d, J = 4.1 Hz, 4H), 2.30–2.17 (m, 4H), 2.09 (dd, J = 7.2, 5.2 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.41, 161.08, 151.79, 149.00, 148.93, 148.33, 146.20, 144.56, 143.58, 143.52, 142.53, 127.23, 127.13, 125.27, 119.34, 115.03, 114.49, 110.49, 109.67, 56.45, 54.36, 50.62, 50.13, 47.07, 45.68, 30.66. HRMS (ESI) [M + H]+: m/z calcd. 586.2240. Found 586.2237.
(R,E)-N-(1–(6-chloro-2–(4–(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-3-(6-chloropyridin-2-yl)-2-cyanoacrylamide (13g)
Compound 13g was synthesised as described for 13a using 6-chloropicolinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.88 (d, J = 6.9 Hz, 1H), 8.05 (s, 1H), 8.03 (t, J = 7.9 Hz, 1H), 7.98 (d, J = 9.0 Hz, 2H), 7.91 (s, 1H), 7.85–7.75 (1H), 7.66 (d, J = 7.6 Hz, 1H), 7.02 (d, J = 9.0 Hz, 2H), 4.50 (d, J = 5.5 Hz, 1H), 4.32 (q, J = 6.0 Hz, 1H), 4.24 (td, J = 7.4, 3.7 Hz, 2H), 3.25 (t, J = 4.8 Hz, 4H), 2.49–2.42 (4H), 2.23 (q, J = 7.1 Hz, 4H), 2.14–2.04 (m, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.37, 151.75, 150.53, 150.11, 148.98, 148.92, 146.17, 144.56, 142.52, 141.06, 127.23, 127.13, 126.80, 125.86, 119.36, 114.96, 114.49, 110.77, 109.67, 56.45, 54.31, 50.61, 50.12, 47.04, 45.60, 30.66. HRMS (ESI) [M + H]+: m/z calcd. 602.1945. Found 602.1943.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(6-methylpyridin-2-yl)acrylamide (13h)
Compound 13h was synthesised as described for 13a using 6-methylpicolinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.82 (d, J = 6.2 Hz, 1H), 8.04 (s, 1H), 7.99 (d, J = 9.0 Hz, 2H), 7.91 (s, 1H), 7.84 (t, J = 7.9 Hz, 1H), 7.61 (d, J = 7.6 Hz, 1H), 7.39 (d, J = 8.3 Hz, 1H), 7.03 (d, J = 9.0 Hz, 2H), 4.50 (q, J = 5.5 Hz, 1H), 4.33 (q, J = 5.7 Hz, 1H), 4.30–4.18 (m, 2H), 3.24 (t, J = 4.8 Hz, 4H), 2.46 (t, J = 4.8 Hz, 4H), 2.29–2.16 (m, 4H), 2.09 (q, J = 6.2 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.80, 158.49, 151.79, 149.32, 148.96, 148.16, 144.57, 142.51, 137.56, 127.22, 127.12, 125.67, 123.88, 119.37, 115.58, 114.49, 109.60, 109.35, 56.50, 54.36, 50.60, 50.05, 47.10, 45.69, 30.66, 23.71. HRMS (ESI) [M + H]+: m/z calcd. 582.2491. Found 582.2494.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(6-cyclopropylpyridin-2-yl)acrylamide (13i)
Compound 13i was synthesised as described for 13a using 6-cyclopropylpicolinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.76 (d, J = 6.9 Hz, 1H), 7.99 (s, 1H), 7.97 (s, 2H), 7.91 (s, 1H), 7.77 (t, J = 7.9 Hz, 1H), 7.48 (d, J = 7.6 Hz, 1H), 7.43 (d, J = 7.6 Hz, 1H), 7.03 (d, J = 9.0 Hz, 2H), 4.49 (d, J = 5.5 Hz, 1H), 4.32 (q, J = 6.0 Hz, 1H), 4.29–4.17 (m, 3H), 3.24 (t, J = 4.8 Hz, 4H), 2.48–2.40 (m, 4H), 2.28–2.18 (m, 4H), 2.18–2.11 (m, 1H), 2.08 (dd, J = 7.2, 5.2 Hz, 1H), 1.20–1.11 (m, 2H), 1.01–0.89 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 163.41, 161.90, 151.80, 149.34, 148.92, 148.32, 144.56, 142.51, 137.15, 127.22, 127.13, 124.68, 124.31, 119.34, 115.90, 114.48, 109.63, 108.99, 56.50, 54.39, 50.59, 50.02, 47.11, 45.71, 30.67, 17.11, 10.33. HRMS (ESI) [M + H]+: m/z calcd. 608.2648. Found 608.2641.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(5-methylpyridin-2-yl)acrylamide (13j)
Compound 13j was synthesised as described for 13a using 5-methylpicolinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.79 (d, J = 6.2 Hz, 1H), 8.60 (d, J = 1.4 Hz, 1H), 8.05 (s, 1H), 7.98 (d, J = 9.0 Hz, 2H), 7.91 (s, 1H), 7.77 (dd, J = 8.3, 1.4 Hz, 1H), 7.70 (d, J = 7.6 Hz, 1H), 7.03 (t, J = 8.3 Hz, 2H), 4.50 (q, J = 5.7 Hz, 1H), 4.33 (q, J = 6.0 Hz, 1H), 4.30–4.18 (m, 2H), 3.24 (t, J = 4.8 Hz, 4H), 2.47 (t, J = 4.8 Hz, 4H), 2.37 (s, 3H), 2.29–2.16 (m, 4H), 2.09 (q, J = 6.2 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.85, 151.78, 150.55, 148.93, 148.30, 147.47, 144.57, 142.52, 137.41, 136.36, 127.24, 127.14, 126.50, 119.38, 115.74, 114.50, 109.63, 108.43, 56.50, 54.36, 50.61, 50.07, 47.08, 45.67, 30.67, 18.17. HRMS (ESI) [M + H]+: m/z calcd. 582.2491. Found 582.2508.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(5-methoxypyridin-2-yl)acrylamide (13k)
Compound 13k was synthesised as described for 13a using 5-methoxypicolinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.71 (d, J = 6.2 Hz, 1H), 8.47 (d, J = 2.8 Hz, 1H), 8.05 (s, 1H), 7.99 (d, J = 8.3 Hz, 2H), 7.91 (s, 1H), 7.82 (d, J = 9.0 Hz, 1H), 7.52 (dd, J = 8.6, 3.1 Hz, 1H), 7.03 (d, J = 9.0 Hz, 2H), 4.50 (q, J = 5.7 Hz, 1H), 4.33 (q, J = 6.0 Hz, 1H), 4.30–4.18 (m, 3H), 3.93 (d, J = 18.6 Hz, 3H), 3.24 (t, J = 4.8 Hz, 4H), 2.46 (t, J = 4.5 Hz, 4H), 2.29–2.16 (m, 4H), 2.10 (q, J = 6.0 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 162.01, 156.96, 151.77, 148.97, 148.93, 147.86, 144.55, 142.51, 142.36, 138.80, 128.62, 127.23, 127.13, 120.20, 119.38, 116.01, 114.48, 109.62, 106.35, 56.50, 55.99, 54.35, 50.61, 50.05, 47.09, 45.67, 30.67. HRMS (ESI) [M + H]+: m/z calcd. 598.2440. Found 598.2434.
(R,E)-N-(1-(6-chloro-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3–(4-methylpyridin-2-yl)acrylamide (13l)
Compound 13l was synthesised as described for 13a using 4-methylpicolinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.81 (d, J = 6.9 Hz, 1H), 8.59 (d, J = 4.8 Hz, 1H), 8.01 (s, 1H), 7.99 (d, J = 8.3 Hz, 2H), 7.92 (s, 1H), 7.57 (s, 1H), 7.36 (d, J = 4.8 Hz, 1H), 7.03 (t, J = 8.3 Hz, 2H), 4.49 (t, J = 5.5 Hz, 1H), 4.36–4.18 (m, 3H), 3.24 (t, J = 4.8 Hz, 4H), 2.46 (t, J = 4.8 Hz, 4H), 2.35 (s, 3H), 2.29–2.16 (4H), 2.10 (q, J = 6.0 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.74, 151.80, 149.94, 149.73, 148.98, 148.93, 148.34, 148.19, 144.55, 142.56, 127.74, 127.43–127.16 (0 °C), 127.14, 126.70, 119.34, 115.60, 114.48, 109.73, 109.40, 56.46, 54.37, 50.58, 50.10, 47.09, 45.69, 30.65, 20.35. HRMS (ESI) [M + H]+: m/z calcd. 582.2491. Found 582.2503.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(pyridin-3-yl)acrylamide (13m)
Compound 13m was synthesised as described for 13a using nicotinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.92 (d, J = 2.1 Hz, 1H), 8.86 (d, J = 6.9 Hz, 1H), 8.73–8.66 (1H), 8.36–8.27 (1H), 8.23–8.14 (1H), 7.98 (d, J = 9.0 Hz, 2H), 7.92 (s, 1H), 7.59 (q, J = 4.4 Hz, 1H), 7.10–6.97 (m, 2H), 4.50 (q, J = 5.7 Hz, 1H), 4.37–4.18 (m, 3H), 3.25 (t, J = 4.8 Hz, 4H), 2.49 (d, J = 4.1 Hz, 4H), 2.23 (q, J = 6.9 Hz, 4H), 2.09 (q, J = 6.0 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.19, 152.24, 151.75, 151.03, 149.00, 148.93, 147.44, 144.55, 142.57, 135.81, 128.10, 127.23, 127.11, 124.05, 119.33, 115.89, 114.49, 109.73, 108.98, 56.41, 54.29, 50.65, 50.14, 47.02, 45.59, 30.66. HRMS (ESI) [M + H]+: m/z calcd. 568.2335. Found 568.2336.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(6-methylpyridin-3-yl)acrylamide (13n)
Compound 13n was synthesised as described for 13a using 6-methylnicotinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.80 (t, J = 6.2 Hz, 2H), 8.23 (dd, J = 8.3, 2.1 Hz, 1H), 8.14 (s, 1H), 7.98 (d, J = 9.0 Hz, 2H), 7.91 (s, 1H), 7.45 (d, J = 8.3 Hz, 1H), 7.02 (d, J = 9.0 Hz, 2H), 4.50 (d, J = 5.5 Hz, 1H), 4.35–4.26 (m, 2H), 4.24 (t, J = 6.9 Hz, 2H), 3.24 (d, J = 4.1 Hz, 4H), 2.59–2.52 (3H), 2.46 (d, J = 4.1 Hz, 4H), 2.22 (d, J = 13.8 Hz, 4H), 2.09 (dd, J = 6.9, 4.8 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 161.80, 161.37, 151.80, 150.92, 149.01, 148.93, 147.47, 144.55, 142.57, 135.78, 127.23, 127.12, 125.33, 123.46, 119.30, 116.10, 114.46, 109.74, 107.64, 56.42, 54.37, 50.64, 50.11, 47.09, 45.70, 30.68, 24.26. HRMS (ESI) [M + H]+: m/z calcd. 582.2491. Found 582.2490.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(2-methylpyridin-3-yl)acrylamide (13o)
Compound 13o was synthesised as described for 13a using 2-methylnicotinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.85 (d, J = 6.9 Hz, 1H), 8.55 (q, J = 2.1 Hz, 1H), 8.30 (s, 1H), 8.08 (d, J = 6.9 Hz, 1H), 7.99 (d, J = 9.0 Hz, 2H), 7.92 (s, 1H), 7.38 (q, J = 4.1 Hz, 1H), 7.02 (d, J = 9.0 Hz, 2H), 4.51 (t, J = 5.5 Hz, 1H), 4.27 (m, 3H), 3.24 (t, J = 4.8 Hz, 4H), 2.52 (s, 3H), 2.49–2.40 (m, 4H), 2.24 (t, J = 9.3 Hz, 4H), 2.12 (t, J = 5.9 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 160.98, 157.44, 151.79, 151.04, 149.04, 148.94, 148.05, 144.55, 142.59, 135.53, 127.25, 127.14, 127.03, 121.46, 119.33, 115.53, 114.48, 111.24, 109.80, 56.37, 54.35, 50.83–50.47 (0 °C), 50.16, 47.07, 45.67, 30.63, 22.69. HRMS (ESI) [M + H]+: m/z calcd. 582.2491. Found 582.2489.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(4-methylpyridin-3-yl)acrylamide (13p)
Compound 13p was synthesised as described for 13a using 4-methylnicotinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.85 (d, J = 6.9 Hz, 1H), 8.81–8.73 (1H), 8.53 (d, J = 4.8 Hz, 1H), 8.29 (s, 1H), 7.98 (d, J = 9.0 Hz, 2H), 7.92 (s, 1H), 7.38 (d, J = 4.8 Hz, 1H), 7.02 (d, J = 9.0 Hz, 2H), 4.51 (q, J = 5.5 Hz, 1H), 4.35–4.19 (m, 3H), 3.24 (d, J = 4.8 Hz, 4H), 2.46 (t, J = 4.8 Hz, 4H), 2.34 (s, 3H), 2.30–2.17 (m, 4H), 2.11 (q, J = 6.2 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 160.87, 151.81, 151.27, 149.03, 148.93, 147.98, 147.11, 146.92, 144.54, 142.56, 128.51, 127.23, 127.16–126.96 (0 °C), 125.31, 119.31, 115.65, 114.47, 111.21, 109.76, 56.36, 54.37, 50.65, 50.15, 47.08, 45.70, 30.62, 18.76. HRMS (ESI) [M + H]+: m/z calcd. 582.2491. Found 582.2486.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3-(pyridin-4-yl)acrylamide (13q)
Compound 13q was synthesised as described for 13a using isonicotinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.94 (d, J = 6.2 Hz, 1H), 8.76 (q, J = 2.1 Hz, 1H), 8.13 (s, 1H), 8.08–7.94 (2H), 7.92 (s, 1H), 7.71 (d, J = 6.2 Hz, 1H), 7.04 (dd, J = 13.1, 9.0 Hz, 2H), 4.50 (t, J = 5.5 Hz, 1H), 4.38–4.20 (m, 3H), 3.26 (t, J = 4.8 Hz, 4H), 2.51 (s, 4H), 2.33–2.17 (m, 4H), 2.10 (t, J = 5.9 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 160.93, 151.73, 150.62, 150.14, 149.00, 148.93, 147.95, 144.57, 142.57, 139.07, 127.26, 127.12, 122.89, 122.82, 119.39, 115.32, 114.52, 111.45, 109.74, 56.42, 54.22, 50.63, 50.18, 46.95, 45.47, 30.66. HRMS (ESI) [M + H]+: m/z calcd. 568.2335. Found 568.2335.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-3-(2-chloropyridin-4-yl)-2-cyanoacrylamide (13r)
Compound 13r was synthesised as described for 13a using 2-chloroisonicotinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.96 (d, J = 6.2 Hz, 1H), 8.59 (d, J = 5.5 Hz, 1H), 8.11 (s, 1H), 7.97 (d, J = 8.3 Hz, 2H), 7.92 (s, 1H), 7.77 (s, 1H), 7.73 (t, J = 2.4 Hz, 1H), 7.02 (d, J = 9.0 Hz, 2H), 4.50 (q, J = 5.0 Hz, 1H), 4.33 (dd, J = 11.4, 3.8 Hz, 1H), 4.27 (q, J = 6.0 Hz, 1H), 4.22 (t, J = 6.9 Hz, 2H), 3.24 (s, 4H), 2.47 (s, 4H), 2.27–2.21 (m, 4H), 2.14–2.03 (m, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 160.56, 151.77, 151.04, 150.95, 149.05, 148.93, 146.44, 144.52, 142.75, 142.64, 127.25, 127.13, 123.53, 121.93, 119.26, 115.00, 114.46, 112.61, 109.96, 56.28, 54.31, 50.65, 50.25, 47.01, 45.61, 30.64. HRMS (ESI) [M + H]+: m/z calcd. 602.1945. Found 602.1937.
(R,E)-N-(1–(6-chloro-2–(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)pyrrolidin-3-yl)-2-cyano-3–(2-methoxypyridin-4-yl)acrylamide (13s)
Compound 13 s was synthesised as described for 13a using 2-methoxyisonicotinaldehyde as an aldehyde source. 1H-NMR (600 MHz, DMSO-d6) δ 8.91 (d, J = 6.9 Hz, 1H), 8.32 (t, J = 4.1 Hz, 1H), 8.07 (s, 1H), 7.98 (d, J = 9.0 Hz, 2H), 7.92 (s, 1H), 7.32 (d, J = 5.5 Hz, 1H), 7.14 (s, 1H), 7.02 (d, J = 9.0 Hz, 2H), 4.49 (d, J = 5.5 Hz, 1H), 4.29 (d, J = 4.8 Hz, 2H), 4.23 (t, J = 6.5 Hz, 2H), 3.89 (s, 3H), 3.24 (d, J = 4.1 Hz, 4H), 2.46 (d, J = 4.1 Hz, 4H), 2.25–2.16 (m, 4H), 2.14–2.03 (m, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 164.13, 160.91, 151.80, 149.02, 148.92, 148.04, 147.73, 144.54, 142.04, 127.23, 127.12, 119.29, 115.81, 115.29, 114.66–114.30 (0 °C), 111.41, 110.40, 109.79, 56.37, 54.35, 53.52, 50.78–50.45 (0 °C), 50.17, 47.07, 45.68, 30.64. HRMS (ESI) [M + H]+: m/z calcd. 598.2440. Found 598.2435.
(R)-N-(1–(5-chloro-2-((4–(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)pyrrolidin-3-yl)acrylamide (14)
Acryloyl chloride (4 µl, 0.05 mmol) was added to a solution of 11 (21 mg, 0.05 mmol) and Na2CO3 (16 mg, 0.15 mmol) in 1 ml aqueous THF (THF/H2O 3:1) at 0 °C, and the mixture was stirred at 0 °C for 2 h. The reaction mixture was extracted with DCM three times. The extract washed with brine, dried with Na2SO4 and concentrated on a rotary evaporator. The concentrate was then purified via MPLC to obtain 14 (11 mg, 47%). 1H-NMR (600 MHz, DMSO-d6) δ 9.00 (s, 1H), 8.42 (d, J = 6.9 Hz, 1H), 7.91 (s, 1H), 7.55 (d, J = 9.0 Hz, 2H), 6.84 (d, J = 9.0 Hz, 2H), 6.24 (dd, J = 17.2, 10.3 Hz, 1H), 6.13 (dd, J = 16.9, 2.4 Hz, 1H), 5.60 (dd, J = 10.3, 2.1 Hz, 1H), 4.39 (q, J = 5.0 Hz, 1H), 3.96 (q, J = 5.7 Hz, 1H), 3.90–3.75 (m, 2H), 3.67 (dd, J = 11.7, 3.4 Hz, 1H), 3.56–3.36 (1H), 3.09–2.98 (4H), 2.45 (t, J = 4.8 Hz, 4H), 2.21 (s, 3H), 2.12 (q, J = 6.2 Hz, 1H), 1.91 (t, J = 5.9 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6) δ 164.53, 157.44, 156.21, 145.76, 133.01, 131.44, 125.46, 119.75, 115.82, 102.08, 54.69, 54.13, 48.86, 48.51, 47.15, 45.69, 30.29. HRMS (ESI) [M + H]+: calcd. 442.2117. Found 442.2120.
HPLC analysis for reversible addition of BME to 13h
Phosphate-buffered saline (PBS) was prepared by mixing a solution of 91.2 mg monobasic potassium phosphate (KH2PO4) in 10 ml H2O and a solution of 116.7 mg dibasic potassium phosphate (K2HPO4) in 10 ml H2O. The 0.067 M phosphate solutions were mixed to obtain a pH 7.4 phosphate buffer. A solution of 48 mM β-mercaptoethanol (BME) in PBS (0.25 ml) was added to a solution of 13h (1 mg, ∼2 µmol) in dimethylsulphoxide (DMSO, 0.75 ml). Analysis of the reaction mixture after 30 min showed full conversion to the BME adduct. To determine whether the reaction between 13h and BME was reversible, the mixture was diluted 1:10 with PBS in DMSO and analysed via HPLC and mass spectra. The analysis was performed using a Waters HPLC system equipped with a 1525 pump, a PDA2998 detector, and a SunFire C18 column (4.6 × 150 mm, 5 μm). The eluent system consisted of 0.05% TFA in 8:2 to 1:9 water/acetonitrile.
Kinase assays
Kinase assays were performed by Invitrogen (now Thermo Fisher Scientific, Waltham, MA) or Reaction Biology Corp. (Malvern, PA). Inhibitory activity of compounds for TAK1 was evaluated using LanthaScreen® Eu Kinase Binding Assay (Invitrogen, Waltham, MA). The kinase profile of compound 13h was determined by the kinase HotSpot Profiling service of Reaction Biology Corporation. All assays were performed at Km for ATP.
Cell culture
MDA-MB-231 cells were obtained from the Korean Cell Line Bank. Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% foetal bovine serum and 1% penicillin/streptomycin at 37 °C with 5% CO2 under humidified atmosphere.
Caspase-3/7 assay
The assay was performed using Apo-ONE® Homogeneous Caspase-3/7 Assay kits (Catalogue No. G7790, Promega, Madison, WI). MDA-MB-231 cells in a concentration of 5000 cells/100 µl were seeded in each well of a black 96-well plate and starved after adhering to the plate. The cells were incubated for 24 h, and the serum-starved cells were treated with either Takinib or 13h in the presence or absence of TNFα (10 ng/ml). All samples and the control contained DMSO in a final concentration of 0.5%. After another 24 h of incubation, 100 µL of Apo-ONE® caspase-3/7 reagent was added to each well, and the cells were incubated at room temperature in darkness for 2 h. Fluorescence was then measured at 530 nm with excitation at 485 nm using a FlexStation 3 Multi-Mode microplate reader (Molecular Devices). Statistical analyses of the data included a one-way ANOVA, followed by Tukey’s multiple comparison test.
Results and discussion
Transforming the irreversible terminal acrylamide to 2-cyanoacrylamide was key for the synthesis of reversible derivatives. The synthetic route for the imidazopyridine derivatives is outlined in Scheme 1. Synthesis of target molecules commenced with nucleophilic addition of aminopyrrolidine to 7, which gave 8 in a moderate yield. The nitro compound (8) was reduced in the presence of Fe and acetic acid at 40 °C to yield 9, which was followed by ring closure with 4–(4-methylpiperazin-1-yl)benzaldehyde to give imidazopyridine 10Citation24. The Boc group was removed under acidic conditions, and a subsequent reaction with cyanoacetic acid provided the key intermediate (12). Target compounds 13a–s were obtained from 12 via Knoevenagel condensation with various aldehydes. Irreversible derivative 14 was synthesised by reacting 11 with acryloyl chloride at 0 °C.
Scheme 1. Reagents and conditions: (a) (R)-3-Boc-aminopyrrolidine, K2CO3, MeCN, RT, 17 h, 53%; (b) Fe powder, AcOH, 40 °C, 1 h, 66%; (c) 4–(4-Methylpiperazin-1-yl)benzaldehyde, FeCl3, DMF, 120 °C, 16 h, 32%; (d) TFA, DCM, RT, 1 h, 70%; (e) Cyanoacetic acid, EDCI, HOBt, DIPEA, DMF, RT, 16 h, 56%; (f) Aldehyde, piperidine, 2-propanol, 60 °C, 2 h, 20%; (g) Na2CO3, aqueous THF, acryloyl chloride, 0 °C, 2 h, 47%.
A structure-activity relationship (SAR) study was performed to optimise the R group on the 2-cyanoacrylamide moiety (). Phenyl derivative 13a had an IC50 of 385 nM, which was ∼2.5-fold lower than the IC50 of 14. Among the compounds with five-membered heterocycles (13b–d), the 4-methylthiazolyl derivative (13b) exhibited the highest potency. Conversion of the phenyl group (13a) to pyridine without a substituent afforded 13e, 13m, and 13q, which had activities that were 8-fold to 14-fold higher. Further SAR analysis was performed for substituted pyridine derivatives. The IC50 values of the 2-pyridinyl derivatives increased with additional bulky substituents on the aromatic ring (e.g. 13g, 13i, and 13k) whereas small substituents (e.g. 13f and 13h) maintained or improved potency. Interestingly, the potency of the derivatives with methyl substituents (13h, 13j, and 13l) was excellent regardless of the methyl position. Unlike the 2-pyridinyl derivatives, the position of the methyl substituent affected the activity of 3-pyridinyl derivatives (13n–p). Introducing a substituent to the 4-pyridinyl group resulted in lower activity (13q–s). Covalent docking studies of the 13h and 14 with C174 of the TAK1 kinase domain were conducted. The free energies of binding were estimated to be −9.65 kcal/mol and −7.05 kcal/mol for 13h and 14, respectively. Imidazopyridine of both compounds formed two hydrogen bonds with A107 in the hinge region. The pyridinyl group of 13h occupied the back pocket of TAK1 and formed a hydrogen bonding with D175. These results correspond to the TAK1 inhibitory activity of 13h and 14 (). A representative imidazopyridine derivative (13h) had an IC50 of 27 nM for TAK1, but inhibition of other MAP kinases by 13h (1 µM) was as low as 10–15% (). Although limited, the kinase profile indicated that 13h was selective for TAK1 over other MAP kinases and BRAF.
Figure 3. Predicted binding mode of 13h with C174 of TAK1 kinase domain (PDB: 4L52)Citation25. Imidazopyridine core interacts with hinge region of TAK1 and pyridinyl nitrogen forms a hydrogen bond with D175. The estimated free energy of binding was found to be −9.65 kcal/mol and −7.05 kcal/mol for 13h and 14, respectively. Covalent docking study was performed using Autodock via flexible side chain methodCitation26. The figure was visualised using Discovery Studio 2020 Visualiser.
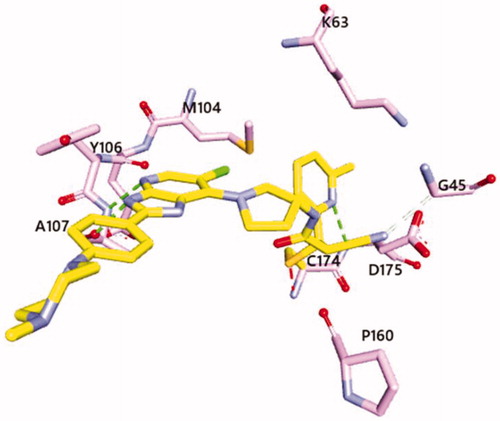
Table 1. TAK1 enzymatic assay with imidazopyridine derivatives.
Table 2. Kinase profile of 13h (1 µM).
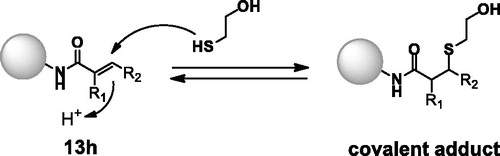
To evaluate the reversible covalent properties of the described compounds, we reacted 13h with β-mercaptoethanol (BME) and determined the reversibility of covalent adduct formation using a previously reported method (Scheme 2)Citation22.
The reaction between 13h and BME generated a covalent adduct, which was identified via high-resolution mass spectrometry (HRMS, Figure S1). The BME adduct mixture was diluted 10-fold to confirm that adduct formation was reversible. After dilution, the BME adduct gradually reverted to 13h (Figure S1).
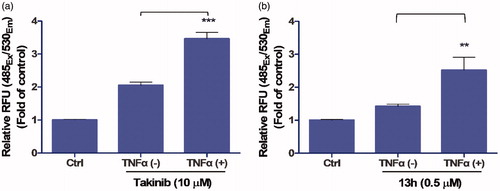
TAK1 inhibition has been shown to induce the apoptosis of TNFα-stimulated breast cancer cellsCitation11. To assess its activity in a cell-based model, the caspase-3/7 activity of 13h was measured in MDA-MB-231 cells. Takinib, a potent TAK1 inhibitorCitation11, was used as a positive control. Like Takinib, 13h (0.5 µM) induced significant caspase activation in the presence of TNFα, indicating that 13h strongly inhibited TAK1 in the cells ().
Conclusions
We discovered potent imidazopyridine TAK1 inhibitors derived from 2-cyanoacrylamide-bearing pyrimidine derivatives. The introduction of the phenyl group into 2-cyanoacrylamide moiety led to increased activity. Among substituents of 2-cyanoacrylamide, pyridines exhibited better activity than the phenyl group or 5-membered heterocycles. These data indicated that aryl group of 2-cyanoacrylamide should provide a contribution to the interaction with TAK1. We postulate that they will act as reversible covalent TAK1 inhibitors based on the reversible reaction between 13h and BME. Our results may contribute to the identification of novel kinase inhibitors or reversible covalent inhibitors.
Supplemental Material
Download PDF (4.8 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yamaguchi K, Shirakabe K, Shibuya H, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 1995;270:2008–11.
- Wullaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes. Biochem Pharmacol 2006;72:1090–101.
- Takaesu G, Ninomiya-Tsuji J, Kishida S, et al. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of TAK1 by inducing TAB2 translocation in the IL-1 signaling pathway. Mol Cell Biol 2001;21:2475–84.
- Jurida L, Soelch J, Bartkuhn M, et al. The activation of IL-1-induced enhancers depends on TAK1 kinase activity and NF-κB p65. Cell Rep 2015;10:726–39.
- Gupta S. A decision between life and death during TNF-alpha-induced signaling. J Clin Immunol 2002;22:185–94.
- Van Herreweghe F, Festjens N, Declercq W, Vandenabeele P. Tumor necrosis factor-mediated cell death: to break or to burst, that’s the question. Cell Mol Life Sci 2010;67:1567–79.
- Stojanov S, McDermott MF. The tumour necrosis factor receptor-associated periodic syndrome: current concepts. Expert Rev Mol Med 2005;7:1–18.
- Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci 2012;33:522–30.
- Josephs SF, Ichim TE, Prince SM, et al. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med 2018;16:242.
- Wajant H. The role of TNF in cancer. Results Probl Cell Differ 2009;49:1–15.
- Totzke J, Gurbani D, Raphemot R, et al. Takinib, a selective TAK1 inhibitor, broadens the therapeutic efficacy of TNF-α inhibition for cancer and autoimmune disease. Cell Chem Biol 2017;24:1029–39.
- Melisi D, Xia Q, Paradiso G, et al. Modulation of pancreatic cancer chemoresistance by inhibition of TAK1. J Natl Cancer Inst 2011;103:1190–204.
- Takahashi H, Jin C, Rajabi H, et al. MUC1-C activates the TAK1 inflammatory pathway in colon cancer. Oncogene 2015;34:5187–97.
- Iriondo O, Liu Y, Lee G, et al. TAK1 mediates microenvironment-triggered autocrine signals and promotes triple-negative breast cancer lung metastasis. Nat Commun 2018;9:1994.
- Ninomiya-Tsuji J, Kajino T, Ono K, et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 2003;278:18485–90.
- Kamiyama H, Usui T, Sakurai H, et al. Epoxyquinol B, a naturally occurring pentaketide dimer, inhibits NF-kappaB signaling by crosslinking TAK1. Biosci Biotechnol Biochem 2008;72:1894–900.
- Buglio D, Palakurthi S, Byth K, et al. Essential role of TAK1 in regulating mantle cell lymphoma survival. Blood 2012;120:347–55.
- Kilty I, Green MP, Bell AS, et al. TAK1 inhibition in the DFG-out conformation. Chem Biol Drug Des 2013;82:500–5.
- Tan L, Nomanbhoy T, Gurbani D, et al. Discovery of type II inhibitors of TGFβ-activated kinase 1 (TAK1) and mitogen-activated protein kinase kinase kinase kinase 2 (MAP4K2). J Med Chem 2015;58:183–96.
- Schirmer A, Kennedy J, Murli S, et al. Targeted covalent inactivation of protein kinases by resorcylic acid lactone polyketides. Proc Natl Acad Sci USA 2006;103:4234–9.
- Wissner A, Overbeek E, Reich MF, et al. Synthesis and structure-activity relationships of 6,7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2). J Med Chem 2003;46:49–63.
- Serafimova IM, Pufall MA, Krishnan S, et al. Reversible targeting of noncatalytic cysteines with chemically tuned electrophiles. Nat Chem Biol 2012;8:471–6.
- Bandyopadhyay A, Gao J. Targeting biomolecules with reversible covalent chemistry. Curr Opin Chem Biol 2016;34:110–6.
- Ha TH, Kim HS, Lee KI, et al. Novel imidazopyridine derivatives as a tyrosine kinase inhibitor, Patent WO2013100631A1, South Korea; 2013.
- Hornberger KR, Berger DM, Crew AP, et al. Discovery and optimization of 7-aminofuro[2,3-c]pyridine inhibitors of TAK1. Bioorg Med Chem Lett 2013;23:4517–22.
- Bianco G, Forli S, Goodsell DS, Olson AJ. Covalent docking using autodock: two-point attractor and flexible side chain methods. Protein Sci 2016;25:295–301.