Abstract
Joining the global fight against Tuberculosis, the world's most deadly infectious disease, herein we present the design and synthesis of novel isatin-nicotinohydrazide hybrids (5a–m and 9a–c) as promising anti-tubercular and antibacterial agents. The anti-tubercular activity of the target hybrids was evaluated against drug-susceptible M. tuberculosis strain (ATCC 27294) where hybrids 5d, 5g and 5h were found to be as potent as INH with MIC = 0.24 µg/mL, also the activity was evaluated against Isoniazid/Streptomycin resistant M. tuberculosis (ATCC 35823) where compounds 5g and 5h showed excellent activity (MIC = 3.9 µg/mL). Moreover, the target hybrids were examined against six bronchitis causing-bacteria. Most derivatives exhibited excellent antibacterial activity. K. pneumonia emerged as the most sensitive strain with MIC range: 0.49–7.81 µg/mL. Furthermore, a molecular docking study has proposed DprE1 as a probable enzymatic target for herein reported isatin-nicotinohydrazide hybrids, and explored the binding interactions within the vicinity of DprE1 active site.
GRAPHICAL ABSTRACT
1. Introduction
Tuberculosis (TB) is considered to be the world’s top infectious killerCitation1. It is a complicated devastating disease that accounts for the death of 1.5 million individuals in 2018 as declared by WHOCitation1. About 23% of the world’s population is expected to be latent TB carriers with a high risk to develop the diseaseCitation2. The first-line drugs such as Isoniazid, Rifampicin, Ethambutol, and Streptomycin have shown significant activity against Mycobacterium tuberculosis however, the emergency of drug-resistant TB (DR-TB), especially multi-drug resistant TB (MDR-TB) is still a serious troubleCitation3. The emerged drug resistance can be considered as a global health crisis, where only one out of three accessing proper remedy among the patients falling ill in 2018Citation1.
Along with the emerged drug resistance, the development of secondary bacterial infection is considered to be a critical problemCitation4. Different bacterial strains have been isolated from sputum samples including: Haemophilus influenzae, Klebsiella pneumoniae, Streptococcus pneumoniae, and Moraxella catarrhalisCitation4,Citation5. The tubercular and the bacterial co-infection can lead to inadequate treatment causing serious complications such as pneumoniae, bronchial anthracofibrosis, and chronic airflow obstructionCitation5–8. Unfortunately, many virulent bacterial strains have developed bacterial resistance against most of the currently used antibiotics. The death rate due to bronchitis has been increased up to an alarming level recording about 0.7 million cases of death every year. Thus, there is a crucial need to design and synthesise new candidates that may help in the battle of the developed bacterial resistance towards the currently available anti-tubercular and antibacterial drugs.
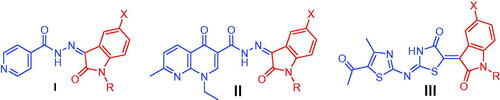
Isatin is a promising endogenous biologically active scaffold. It could be chemically modified to produce various heterocyclic compounds with different biological activitiesCitation9–14. Isatin based derivatives have revealed promising antibacterialCitation15–17 and anti-TB effectsCitation18,Citation19. Moreover, the nicotinohydrazide moiety has a large contribution to the field of medicinal chemistry. It has been incorporated in several active antimicrobialCitation20 and anti-tubercular agentsCitation21. In fact, the presence of a hydrazide group can be considered as a significant key for an optimum anti-tubercular activityCitation22. Recently, several research teams adopted molecular hybridisation techniques between isatin and different moieties for the design of new anti-mycobacterial agentsCitation23–26, such as isatin-INH hybrids I ()Citation25 and isatin-nalidixic acid hybrids II ()Citation26. Later, our research team has identified the N-substituted isatin-thiazolidinone hybrid III () as a promising antitubercular agent that produced an effective killing of M. aurum in infected macrophage model with broad-spectrum antimicrobial effect against sensitive and resistant bacterial strainsCitation27. SAR study revealed that N-benzylation or N-methylation of the 2-oxindole ring enhanced the activity by 2-4 folds as compared to the N-unsubstituted analogues.
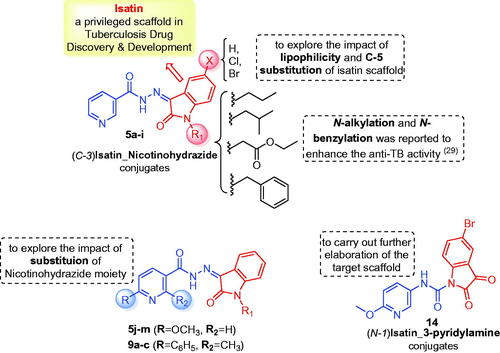
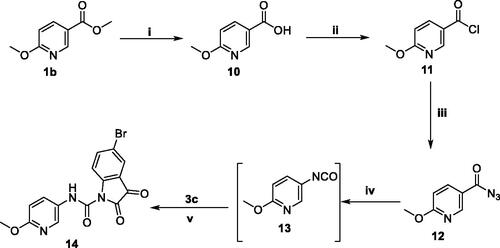
In view of the findings stated above and in a continuation of our previous work for the discovery of potential anti-tubercular and anti-microbial agentsCitation28, we decided to extend our research through conjugating different N-substituted isatin moieties with a nicotinohydrazide moiety to furnish target hybrids 5a-I (). In order to explore the impact of C-5 substitution, as well as to ensure different lipophilic environments that may be suitable for the mycobacterial activity, both 5-Cl and 5-Br substituents were grafted at isatin scaffold. As it was suggested that N-substitution of isatin scaffold could enhance the mycobacterial activityCitation27,Citation29, N-alkylation, or N-benzylation of the herein reported hybrids was performed (). Thereafter, the impact of C-2 and C-6 substitution of the nicotinohydrazide moiety the anti-tubercular and antibacterial activities were investigated through developing the two sets 5j–m and 9a–c (). Finally, an isatin-3-pyridylamine hybrid 14 was synthesised to carry out further elaboration of the target scaffold ().
Herein seventeen new 2-oxindolin-3-ylidene-nicotinohydrazide derivatives (5a–m, 9a–c, and 14) were designed, synthesised, and evaluated for their anti-tubercular activity against drug-susceptible M. tuberculosis strain (ATCC 27294) and Isoniazid/Streptomycin resistant M. tuberculosis (ATCC 35823), together with the antibacterial effect against bronchitis causing-bacteria in an attempt to produce new candidates having potential activities with minimal bacterial resistance that may reduce the complications associated with the secondary bacterial infection.
Finally, molecular docking studies have been performed for the most active compounds into the active sites of two enzymes: flavoenzyme Decaprenylphosphoryl-β-D-Ribose 2′-Epimerase (DprE1) and enoyl-acyl carrier protein reductase (InhA) being critical enzymes in the metabolism and synthesis of M. tuberculosis cell wallCitation30,Citation31 to explore the possible mechanism of action of the new hybrids.
2. Results and discussion
2.1. Chemistry
The synthetic strategies adopted for the preparation of final compounds (5a–m, 9a–c and 14) were illustrated in Schemes 1–3. In the first scheme, different isatin derivatives 3a–c were alkylated with the appropriate alkyl halide in anhydrous DMF in the presence of K2CO3 as an acid binder to furnish the N-substituted indoline-2,3-diones 4a–i. On the other hand, methyl nicotinate 1a and methyl 6-methoxynicotinate 1b was refluxed with hydrazine hydrate in methanol to produce the key intermediate nicotinohydrazides 2a–b which subsequently reacted with N-substituted indoline-2,3-dione derivatives 4a–i in absolute ethanol containing a catalytic amount of glacial acetic acid to get the final compounds 5a–m (Scheme 1).
Scheme 1. Synthesis of target isatin hybrids 5a–m; (i) NH2NH2.H2O/methanol/reflux 4 h, (ii) R1-Br/DMF/KI (Cat.)/K2CO3/reflux 3 h, (iii) Ethanol absolute/drops glacial acetic acid (Cat.)/reflux 6 h.
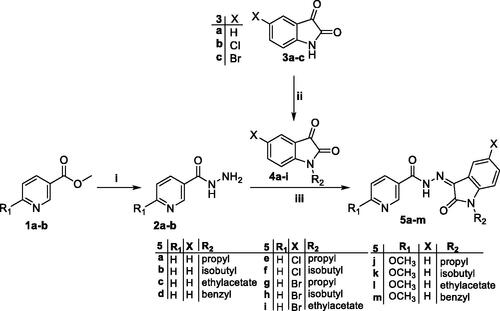
Scheme 2. Synthesis of target isatin hybrids 9a–c; (i) ethyl acetoacetate/NH4OAc/glacial acetic acid/reflux 6 h, (ii) NH2NH2.H2O/methanol/reflux 6 h, (iii) Ethanol absolute/drops glacial acetic acid (Cat.)/reflux 6 h.
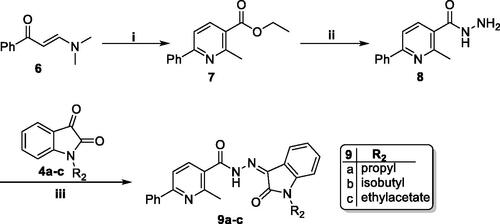
Scheme 3. Synthesis of target compound 14; (i) NaOH/MeOH/reflux 6 h, (ii) SOCl2/reflux 4 h, (iii) NaN3/acetone/stirring at R.T. 4 h, (iv) Dry toluene/reflux 1 h, (v) Dry toluene/reflux 5 h.
In the second scheme, 3-(dimethylamino)-1-phenylprop-2-en-1-one 6 was heated under reflux with ethyl acetoacetate and NH4OAc in glacial acetic acid to produce ethyl 2-methyl-6-phenylnicotinate 7 in which, the ester moiety was subjected to hydrazinolysis to produce the key intermediate 2-methyl-6-phenylnicotinohydrazide 8 which reacted with different N-substituted indoline-2,3-diones 4a–c to get target compounds 9a–c.
In the last scheme, methyl 6-methoxynicotinate 1b was refluxed with NaOH in MeOH to get the free 6-methoxynicotinic acid 10, which then undergone to chlorination via SOCl2 to furnish 6-methoxynicotinoyl chloride 11 where the chloride was subsequently substituted with an azide functionality to produce 6-methoxynicotinoyl azide 12. The azid derivative 12 was subjected to Curtius Rearrangement to get 5-isocyanato-2-methoxypyridine 13, that refluxed with 5-bromoindoline-2,3-dione 3c in dry toluene to furnish the final compound 14.
1H NMR spectra of the final compounds 5a–m and 9a–c were confirmed with the presence of a D2O exchangeable singlet signal attributable to the proton of the hydrazide linker at δ (13.31–13.86) ppm. Moreover, 1H NMR spectra for compounds 9a–c confirmed the presence of singlet signal in the aliphatic region for the CH3 group around δ 2.71 ppm, in addition to the extra aromatic protons for the 6-phenyl ring at a range of δ 7.44–7.57 ppm, whereas, spectra for compounds 5j–m and 14 confirmed the appearance of singlet signal for OCH3 groups at δ (3.82–3.97) ppm.
In addition, the propyl moiety in compounds (5a, e, g, j, and 9a) was confirmed with the appearance of two triplet signals for (–CH2CH3) and (N-CH2) at δ (0.89-0.91) and (3.70–3.75) ppm, respectively. Also, 1H NMR spectra for compounds (5b, f, h, k, and, 9b) displayed two doublet signals for (–CH(CH3)2) and (N-CH2) in the isobutyl moiety at δ (0.90-0.93) and (3.56-3.74) ppm, respectively, whereas, the ethyl acetate group of compounds (5c, i, l, and 9c) was represented by a triplet signal for (CO-CH2CH3), quartette signal for (CO–CH2CH3) as well as singlet signal for (N-CH2) at δ (1.20–1.22), (4.16–4.17) and (4.72–7.74) ppm, respectively. Furthermore, the N-benzyl substituted counterparts (5d and 5m) showed singlet signals around δ 5.02 ppm attributed to the benzylic (–CH2–C6H5) protons.
On the other hand, 13 C NMR spectra for hybrids 5a–m and 9a–c showed two signals resonating in the range δ (161.20–161.84) and (162.90–166.52) ppm attributable for the carbonyl carbons of the nicotinic hydrazide and indoline-2-one moieties, respectively. Moreover, compounds 5c, l, and 9c showed an extra signal for C = O group for the ester moiety at δ 167.86 ppm, whereas, compound 14 displayed the C = O signal for urea linker at δ 153.69 ppm, and two signals for indoline-2,3-dione moiety at δ 159.57 and 183.65 ppm. Additionally, 13 C NMR spectra for compounds 9a–c showed a signal corresponding to CH3 carbon at δ 23.88–27.24 ppm, while, spectra for compounds 5j–m and 14 displayed a signal corresponding to OCH3 carbon at δ (53.53–54.44) ppm.
Furthermore, the carbons of the N-propyl group in compounds (5a, e, g, k, and 9a) appeared as three signals resonating at δ (11.60–11.65 ppm), (20.81–20.87 ppm), and (41.28–41.48 ppm) for –CH2CH3, N-CH2CH2, and N-CH2, respectively, and the carbons of the N-isobutyl group in compounds (5f, l and 9b) appeared as three signals at a range of δ (20.36–20.45 ppm), (27.22–23.88 ppm) and (47.10–56.51 ppm) due to –CH(CH3)2–, –CH(CH3)2– and –N-CH2–, respectively. Finally, a signal attributable to the benzylic (–CH2–) carbon in the N-benzyl derivatives (5d and 5m) appeared around δ 43.10 ppm.
2.2. Biological evaluation
2.2.1. Anti-tubercular activity
All of the newly synthesised hybrids were evaluated for their anti-tubercular action towards M. tuberculosis (ATCC 27294) using the Microplate Alamar Blue Assay (MABA)Citation32. INH was used as the reference drug. The obtained results for the anti-mycobacterial activity were summarised in () and expressed as a minimum inhibitory concentration (MIC).
Table 1. MIC (µg/mL) for hybrids (5a–m, 9a–c, and 14) against M. tuberculosis (ATCC 27294) and Isoniazid/Streptomycin resistant M. tuberculosis (ATCC 35823), and LogP measurements for hybrids (5a–m, 9a–c, and 14).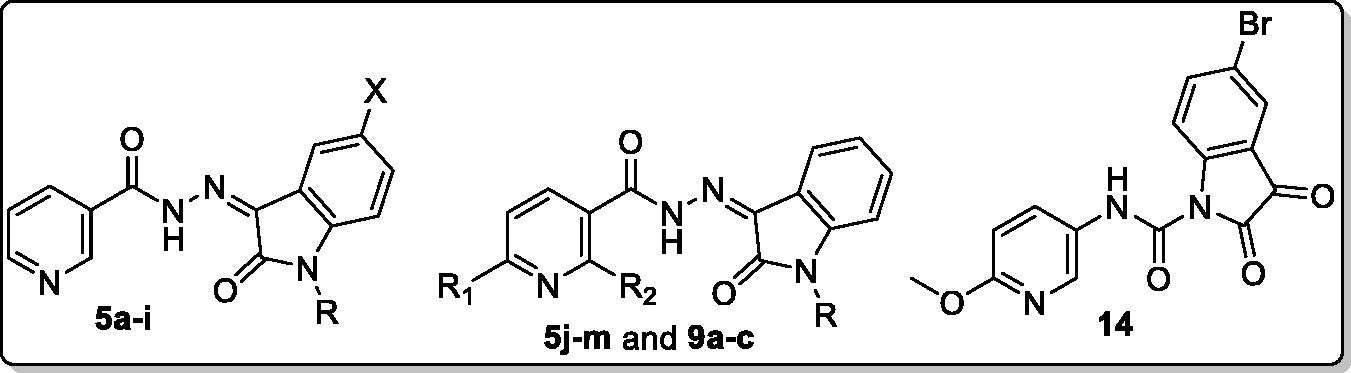
The results revealed that most tested compounds exerted potent to moderate anti-tubercular action with MIC range = 0.24-7.81 µg/mL. Compounds 5d, 5g, and 5h were found to be as potent as INH with MIC = 0.24 µg/mL. Compounds 5f, 5j, and 5k revealed potent activity with MIC range = 0.48–0.98 µg/mL, whereas derivatives 5a–5c, 5e, 5i, 5l, 5m, and 14 exhibited a moderate activity with MIC range = 1.95–7.81 µg/mL.
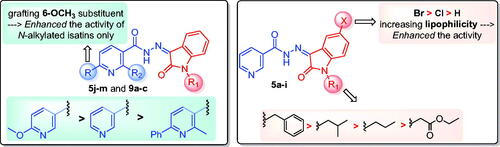
Concerning the structure–activity relationship, it was observed that the anti-tubercular activity of compounds 5a–5i was affected by two main factors; N-substitution and incorporation of halogen at position 5 of the oxindole ring. Regarding N-substitution it was shown that, compounds bearing isobutyl group: 5b, 5f, and 5h exhibited remarkable activity with MIC values = 1.95, 0.48, and 0.24 µg/mL, respectively. Similarly, compound 5d with N-benzyl moiety revealed a potent activity with MIC = 0.24 µg/mL. In contrast, compounds 5c and 5i carrying the N-ethylcarboxylate group were found to be the least active derivatives (MIC = 7.81 µg/mL). Moreover, the contribution of substitution at position 5 of oxindole ring on the activity was quite important. Incorporating 5-Br group in compounds 5g and 5h (MIC = 0.24 µg/mL) showed better activity than 5-Cl substituted compounds 5e and 5f (MIC = 0.48 and 3.9 µg/mL, respectively) and the unsubstituted congeners 5a–5c (MIC range = 3.9–7.81 µg/mL).
Then we turned our attention to study the impact of substitution at position 6 on the nicotinohydrazide moiety of the new hybrids. Comparing the anti-tubercular activity of 6-methoxy nicotinohydrazide derivatives 5j–5m against the unsubstituted counterparts 5a–5d, it was found that the introduction of 6-methoxy group in compounds 5j–5l (MIC = 0.98-3.9 µg/mL) enhanced the anti-tubercular activity by 2-4 fold compared to unsubstituted analogues 5a–5c (MIC range: 1.95–7.81 µg/mL) except for the benzyl derivatives 5d and 5m. Conversely, the introduction of 6-phenyl moiety to the nicotinohydrazide moiety as in compounds 9a–c (MIC = 15.63–31.25 µg/mL) led to a reduced anti-tubercular activity that may be due to steric factors.
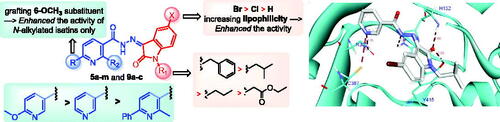
In conclusion, increasing the lipophilicity of the isatin scaffold via C-5 substitution with halogens (Br > Cl) was more advantageous for activity. Also, the anti-mycobacterial action of the target hybrids was decreased in the following order; 6-methoxy nicotinohydrazide > unsubstituted nicotinohydrazide > 2-methyl-6-phenyl nicotinohydrazide ().
2.2.2. Anti-tubercular activity towards Isoniazid and Streptomycin resistant M. tuberculosis (ATCC 35823)
The anti-tubercular activity against Isoniazid and Streptomycin resistant M. tuberculosis (ATCC 35823) was evaluated using MABACitation32. Investigating the results of the target compounds 5a–m, 9a–c, and 14 (), it was found that many of the tested derivatives exhibited potent to moderate activity with MIC range = 3.9-62.5 µg/mL. Compounds 5g and 5h with the best anti-tubercular effect against M. tuberculosis (ATCC 27294), showed excellent activity (MIC = 3.9 µg/mL) resistant M. tuberculosis strain (ATCC 35823), with more than 32 fold increase in the activity compared to INH and Streptomycin (MIC > 125 µg/mL). Moreover, compounds 5d, 5f and 5j elicited a good activity with MIC = 15.63 µg/mL, whereas derivatives 5b, 5k, and 14 produced a moderate activity with MIC = 62.5 µg/mL. Finally, compounds 5a and 5l were found to be least active showing MIC value = 125 µg/mL, while the 6-phenyl pyridine derivatives 9a–c were inactive with MIC >125 µg/mL.
2.2.3. Antibacterial activity against bronchitis causing-bacteria
The antibacterial activity for the synthesised compounds 5a–m, 9a–c, and 14 was evaluated against bronchitis causing-bacteria; Gram-negative Mycoplasma pneumoniae (ATCC 15531), K. pneumoniae (ATCC 43816), H. influenzae (ATCC 10211), M. catarrhalis (ATCC 25238), B. pertussis (ATCC 9340) and Gram-positive S. pneumoniae (ATCC 1659) using XTT Susceptibility Assay ()Citation33,Citation34. The results showed that, most of the evaluated compounds produced a significant antibacterial activity in comparison to the reference drug Azithromycin against the tested bacterial strains. The most sensitive strain towards the new hybrids was K. pneumoniae (ATCC 43816) with MIC range = 0.49–7.81 µg/mL, while M. pneumoniae (ATCC 15531) and M. catarrhalis (ATCC 25238) were the least sensitive strains MIC range = 0.98–125 µg/mL and MIC range = 3.9 to >125 µg/mL, respectively.
Table 2. MIC (µg/mL) for hybrids (5a–m, 9a–c, and 14) against bronchitis causing-bacteria as determined using XTT assay.
Interestingly, compounds 5g and 5h showing best anti-tubercular activity with the minimal bacterial resistance against resistant-TB, revealed a broad-spectrum antibacterial action against most of the tested bronchitis causing-bacteria. They were found to be more potent than the reference drug against H. influenzae, M. catarrhalis, and B. pertussis (MIC = 0.24–3.9 µg/mL, Azithromycin MIC = 3.9–7.81 µg/mL). Additionally, they were equipotent to Azithromycin against M. pneumoniae, S. pneumoniae and K. pneumoniae (MIC = 1.95, 0.98, and 0.49 µg/mL, respectively).
Moreover, compound 5k showed MIC values (0.98-3.9 µg/mL) less than that expressed by Azithromycin (MIC = 1.95–7.81 µg/mL) against four bacterial strains: M. pneumoniae (ATCC 15531), H. influenza, M. catarrhalis (ATCC 25238) and B. pertussis (ATCC 9340). Also, compound 5j was as potent as the reference drug against H. influenzae, K. pneumoniae (ATCC 43816), M. catarrhalis (ATCC 25238), and B. pertussis (ATCC 9340). Furthermore, compounds 5f and 14 were two-fold more potent (with MIC = 1.95 µg/mL) than Azithromycin (MIC = 3.9 µg/mL) against H. influenza (ATCC 10211) and B. pertussis (ATCC 9340) and equipotent to Azithromycin against K. pneumoniae (ATCC 43816) with MIC = 0.49 µg/mL. Finally, derivatives 5a, 5b, and 5d produced the same MIC values = 0.49 µg/mL as that expressed by the standard drug against K. pneumoniae (ATCC 43816).
2.2.4. Cytotoxicity against non-tumorigenic lung fibroblast WI-38 cells
In order to assess the cytotoxic impact of hybrids 5g and 5h on normal human cells, an MTT cytotoxicity assay was carried out against non-tumorigenic WI-38 cells. The obtained results ascribed to hybrids 5g and 5h non-significant cytotoxic action with IC50 value = 60.95 and 54.01, respectively (), thus providing favourable selectivity indices (SI) equal to 15.6 and 13.8, respectively.
Table 3. In vitro cytotoxic effect for hybrids 5g and 5h towards non-tumorigenic WI-38 cells, and their Selectivity index (S.I.).
3. Molecular docking
Over the past decade, several druggable targets have been utilised in TB-drug discovery to develop new therapies in order to fight the global epidemic of TB. Most of these enzyme targets have an essential role in energy production, cell wall biosynthesis, and DNA replication, repair, and transcription. Examples of these enzymes are; Alanine racemase (Alr), Maltosyltransferase (GlgE), N-Acetylglucosamine-1-phosphate uridyltransferase (GlmU), Pantothenate synthetase (PS), Mtb ATP synthase subunit C (AtpE), Flavin-dependent thymidylate synthase (TS or ThyA), and flavoenzyme Decaprenylphosphoryl-β-D-Ribose 2′-Epimerase (DprE1)Citation30.
On account of their superior anti-mycobacterial activity, compounds 5g and 5h were selected for further in silico investigation to explore their possible molecular target. Two promising drug enzymes in M. tuberculosis were suggested as potential targets for the target compounds in this study; the flavoenzyme Decaprenylphosphoryl-β-D-Ribose 2′-Epimerase (DprE1) and the enoyl-acyl carrier protein reductase (InhA) enzymes. Both enzymes play an essential role in the metabolism of the cell wall and the synthesis of mycolic acid, respectivelyCitation30,Citation31. The selection of these two enzymes is based on a pilot docking study that explored the plausible binding interactions and energy scores for the prepared compounds with different enzyme targets.
The crystal structures of the two enzymes were downloaded from the protein data bank PDB ID: 4NCR for DprE1 and 2B35 for InhA. Despite, the importance of docking in providing a predicated binding mode and an account for a ligand strength, it remains a theoretical technique that needs proper validation by comparison with an experimental reference. Therefore, both co-crystallised ligands were re-docked into their corresponding enzymes. The calculated RMSD values between the co-crystallised and docked pose were 0.52 and 0.71 for DprE1 and InhA, orderly which indicates a valid and reliable docking protocol. Compound 5h has achieved −10.1 and −7.9 kcal/mole energy score for DprE1 and InhA, respectively, whereas, compound 5g has achieved −9.7 and −7.3 kcal/mole for DprE1 and InhA, respectively. Interestingly, both compounds 5g and 5h achieved a higher energy score than the co-crystalized ligand (−9.3 kcal/mole) in DprE1. These findings suggested that DprE1 may be a plausible target for the herein reported compounds.
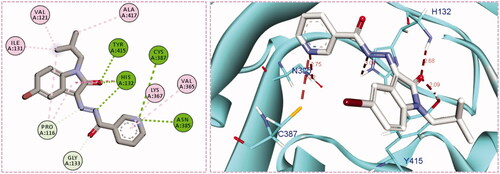
Inspection of the docking poses for the two examined compounds revealed their ability to fit perfectly in the active site of the DprE1 enzyme establishing different types of interactions ( and ). The oxindole ring in both compounds 5g and 5h was engaged in two H-bond interactions via its carbonyl oxygen (C = O) functionality with His-132 and Tyr-415 amino acids. Furthermore, the NH of the hydrazide linker and the nitrogen atom of the pyridine ring were involved in H-bond interactions with His-132 and Asn-385, respectively. Moreover, the nitrogen atom of the pyridine ring in compound 5h achieved an extra hydrogen bond interaction with Cys-387 which may explain its higher energy score than 5g. Worthy of note, the N-isobutyl group of compound 5h established several hydrophobic interactions with non-polar residues lining a hydrophobic pocket, such as Pro-116, Val-121, Ile-131, and Ala-417.
Figure 4. The 2D diagram and 3D representation for compound 5g displaying its interactions with the DprE1 binding site.
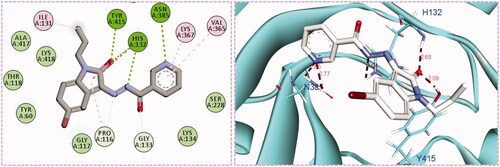
Figure 5. The 2D diagram and 3D representation for compound 5h displaying its interactions with the DprE1 binding site.
The 2 D diagrams for interactions of compounds 5g and 5h in InhA active site (Figure S1), and the 3 D illustrations of the superimposition for the docking poses and the co-crystalized ligands in DprE1 and InhA active sites (Figure S2), as well as the detailed interactions achieved by compounds 5g and 5h within the DprE1 binding site (Table S3) were provided in the Supporting Materials.
4. Conclusion
In this work, different sets of isatin-nicotinohydrazide hybrids (5a–m, 9a–c) and isatin-3-pyridylamine hybrid 14 were designed and synthesised. The anti-mycobacterial activity towards M. tuberculosis (ATCC 27294), as well as towards the INH and Streptomycin resistant M. tuberculosis (ATCC 35823), were evaluated for the seventeen newly synthesised 2-oxindolin-3-ylidene-nicotinohydrazide derivatives. The obtained results identified compounds 5g and 5h as the most potent anti-tubercular agents in this study (MIC = 0.24 µg/mL), with minimal mycobacterial resistance (MIC = 3.9 µg/mL). Furthermore, all herein reported hybrids were examined for their antibacterial activity against six bronchitis causing-bacteria; M. pneumoniae, H. influenzae, K. pneumoniae, M. catarrhalis, B. pertussis, and S. pneumonia. Most hybrids exerted significant antibacterial activity in comparison to the reference drug Azithromycin against the tested bacterial strains. The most sensitive strain was K. pneumoniae with MIC range = 0.49–7.81 µg/mL. Interestingly, compounds 5g and 5h displayed a broad-spectrum antibacterial activity against most of the tested bronchitis causing-bacteria. Moreover, an in silico molecular modelling study has proposed DprE1 as a potential enzyme target for herein reported isatin-nicotinohydrazide hybrids. Compounds 5g and 5h were able to fit perfectly in the active site of the DprE1 enzyme achieving a higher energy score (−9.7 and −10.1 kcal/mole, respectively) than the co-crystalized ligand (−9.3 kcal/mole). The oxindole ring was engaged in two H-bond interactions via its carbonyl oxygen (C = O) functionality with His-132 and Tyr-415 amino acids, whereas, the NH of the hydrazide linker and the nitrogen atom of the pyridine ring were involved in H-bond interactions with His-132 and Asn-385, respectively. In addition, the N-isobutyl group of compound 5h established several hydrophobic interactions with non-polar residues lining a hydrophobic pocket.
5. Experimental
5.1. Chemistry
5.1.1. General
I.R. spectra have been recorded on Schimadzu FT-IR 8400S spectrophotometer. NMR spectra were recorded by Bruker-Avance 400 spectrometer. 13 C NMR spectra were run at 100 MHz in deuterated dimethylsulphoxide (DMSO-d6). Chemical shifts (δH) are reported relative to the solvent (DMSO-d6). NMR analyses were carried out at the NMR Unit, Faculty of Pharmacy, Mansoura University, Mansoura, Egypt, whereas IR analyses were carried out at the Faculty of Science, Mansoura University, Mansoura, Egypt. Elemental analyses were carried out at the Regional Centre for Microbiology and Biotechnology, Al-Azhar University, Cairo, Egypt. Compounds 2a,bCitation35, 4a–iCitation36,Citation37, 8Citation38, and 11Citation39, were prepared as described previously.
5.1.2. Synthesis of target compounds 5a–m and 9a–c
In a round-bottom flask, the appropriate nicotinohydrazide derivative 2a–b or 8 (2.5 mmol) were added to an equivalent amount of N-substituted indoline-2,3-dione derivatives 4a–i in 15 ml of glacial acetic acid. The previous mixture was heated under reflux with TLC monitoring, after complete consummation of starting materials, the reaction flask was cooled to room temperature. The formed precipitate was collected under vacuum, washed with hexane, cold water and recrystallized from dioxane/methanol mixture to furnish final compounds 5a–m and 9a–c, respectively.
5.1.3. Synthesis of target compound 14
6-Methoxynicotinoyl azide 12 (0.3 g, 1.6 mmol) was dissolved in anhydrous toluene (20 ml) which then heated under reflux for 1 h. To the previous solution, an equivalent amount of 5-bromoisatin was added and the reaction mixture was refluxed for 5 h. The produced precipitate was filtrated while hot washed with hexane and recrystallized from acetonitrile to furnish compound 14.
Full characterisation details (NMR, IR, and elemental analysis) for the target hybrids (5a–m, 9a–c, and 14) have been provided in the Supporting Materials.
5.2. Biological evaluation
5.2.1. Anti-tubercular activity
Microplate Alamar blue assay (MABA)Citation32 has been utilised to determine MICs for all herein prepared hybrids (5a–m, 9a–c, and 14) against M. tuberculosis ATCC 27294 (Isoniazid-susceptible strain) and Mycobacterium tuberculosis ATCC 35823 (Isoniazid and Streptomycin resistant strain). The detailed procedures were presented in the supporting materials.
5.2.2. XTT Susceptibility Assay for the antibacterial activity
A colorimetric broth microdilution method was utilised to determine the MIC for the tested hybrids following the XTT [2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide]-reduction assayCitation33,Citation34. MICs for the tested hybrids was assessed against bacteria causing bronchitis; Gram-negative bacteria: H. influenzae ATCC 10211, M. pneumoniae ATCC 15531, K. pneumoniae ATCC 43816, M. catarrhalis ATCC 25238, and Bordetella pertussis ATCC 9340, as well as S. pneumoniae ATCC 1659, representing Gram-positive bacterium, that obtained from the American type culture collection. The detailed experimental procedures have been added in the supporting materials.
5.2.3. Cytotoxicity assay
The MTT assayCitation40 was used to assess the cytotoxic impact of hybrids 5g and 5h towards the normal human lung WI-38 cells as reported earlierCitation41.
5.3. Molecular docking
Aiming to define the molecular target for the best two lead compounds, the crystal structures of both DprE1 and InhA enzymes of TB were downloaded from the Protein data bank ID: 4NCR and 2B35, respectivelyCitation42,Citation43. Vina Autodock software was implemented in the current work to conduct the docking studies. The program demands both the ligand and the receptor in the pdbqt format, for that, M.G.L tools were used to prepare the two enzymes and the ligands into the right formatCitation44. Also, the active sites were determined by generating a grid box sized 24 × 24 × 24 Å surrounding the binding domain of each co-crystallised ligand with its corresponding enzyme (centre_x = 17.3, centre_y = −20 and centre_z = 3.4 for 4ncr and centre_x = 17, centre_y = 12.5 and centre_z = 10.5 for 2b35). Docking validity and reliability were ensured by re-docking each co-crystallised ligands with their corresponding enzyme, then calculating the RMSD between the co-crystallised pose and the docked pose. Discovery studio 4.5 visualiser was employed in the analysis of docking results as well as in the evaluation of best candidates based on binding affinity score and interaction with receptorCitation45.
Supplemental Material
Download PDF (2 MB)Acknowledgement
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
References
- World Health Organization. Global Tuberculosis Report, WHO. Geneva, Switzerland: WHO; 2019.
- World Health Organization. Global Tuberculosis Report, WHO. Geneva, Switzerland: WHO; 2018.
- Coninx R, Mathieu C, Debacker M, et al. First-line tuberculosis therapy and drug-resistant Mycobacterium tuberculosis in prisons. Lancet 1999;353:969–73.
- Langbang A, Deka N, Rahman H, Kalita D. A study on bacterial pathogens causing secondary infections in patients suffering from tuberculosis and their pattern of antibiotic sensitivity. Int J Curr Microbiol App Sci 2016;5:197–203.
- Wei M, Zhao Y, Qian Z, et al. Pneumonia caused by Mycobacterium tuberculosis. Microb Infect 2020;22:278–84.
- Arora A, Krishnaswamy UM, Moideen RP, Padmaja MS. Tubercular and bacterial coinfection: a case series. Lung India 2015;32:172–5.
- Byrne L, Marais BJ, Mitnick CD, et al. Chronic airflow obstruction after successful treatment of multidrug-resistant tuberculosis. ERJ Open Res 2017;3:00026.
- Kunal S, Shah A. The concomitant occurrence of pulmonary tuberculosis with bronchial anthracofibrosis. Indian J Tuberc 2017;64:5–9.
- Kidwai M, Jahan A, Mishra NK. Isatins: a diversity orientated biological profile. Med Chem 2014;4:451–68.
- Singh G, Fong J, Liu Y, et al. Synthesis and preliminary antimicrobial analysis of isatin-ferrocene and isatin-ferrocenyl chalcone conjugates. ACS Omega 2018;3:5808–13.
- Medvedev A, Buneeva O, Glover V. Biological targets for isatin and its analogues: implications for therapy. Biol: Targets Ther 2007;1:151–62.
- Rane RA, Karunanidhi S, Jain K, et al. A recent perspective on discovery and development of diverse therapeutic agents inspired from isatin alkaloids. Curr Top Med Chem 2016;16:1262–89.
- Pandeya SN, Smitha S, Jyoti M, Sridhar SK. Biological activities of isatin and its derivatives. Acta Pharm 2005;55:27–46.
- Tehrani KHME, Hashemi M, Hassan M, et al. Synthesis and antibacterial activity of Schiff bases of 5-substituted isatins. Chin Chem Lett 2016;27:221–5.
- Guo H. Isatin derivatives and their anti-bacterial activities. Eur J Med Chem 2019;164:678–88.
- Thanh ND, Giang NTK, Quyen TH, et al. Synthesis and evaluation of in vivo antioxidant, in vitro antibacterial, MRSA and antifungal activity of novel substituted isatin N-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)thiosemicarbazones. Eur J Med Chem 2016;123:532–43.
- Wang R, Yin X, Zhang Y, Yan W. Design, synthesis and antimicrobial evaluation of propylene-tethered ciprofloxacin-isatin hybrids. Eur J Med Chem 2018;156:580–6.
- Xu Y, Guan J, Xu Z, Zhao S. Design, synthesis and in vitro anti-mycobacterial activities of homonuclear and heteronuclear bis-isatin derivatives. Fitoterapia 2018;127:383–6.
- Akhaja TN, Raval JP. Design, synthesis, in vitro evaluation of tetrahydropyrimidine–isatin hybrids as potential antibacterial, antifungal and anti-tubercular agents. Chin Chem Lett 2012;23:446–9.
- Han YJ, Wang L, Li QB, Xue LW. Synthesis, crystal structure, and antibacterial activity of oxovanadium (V) complexes derived from N'-[1-(5-fluoro-2-hydroxyphenyl)methylidene]nicotinohydrazide and N'-(5-fluoro-2-hydroxy benzyli dene)-2-hydroxynaphthylhydrazide. Russ J Coord Chem 2017;43:612–8.
- Soliman DH, Eldehna WM, Ghabbour HA, et al. Novel 6-phenylnicotinohydrazide derivatives: design, synthesis and biological evaluation as a novel class of antitubercular and antimicrobial agents. Biol Pharm Bull 2017;40:1883–93.
- Castelo-Branco FS, de Lima EC, Domingos JLdO, et al. New hydrazides derivatives of isoniazid against Mycobacterium tuberculosis: higher potency and lower hepatocytotoxicity. Eur J Med Chem 2018;146:529–40.
- Gao F, Yang H, Lu T, et al. Design, synthesis and anti-mycobacterial activity evaluation of benzofuran-isatin hybrids. Eur J Med Chem 2018;159:277–81.
- Chen R, Zhang H, Ma T, et al. Ciprofloxacin-1,2,3-triazole-isatin hybrids tethered via amide: design, synthesis, and in vitro anti-mycobacterial activity evaluation. Bioorg Med Chem Lett 2019;29:2635–7.
- Aboul-Fadl T, Mohammed FAH, Hassan EAS. Synthesis, antitubercular activity and pharmacokinetic studies of some schiff bases derived from 1-alkylisatin and isonicotinic acid hydrazide (inh). Arch Pharm Res 2003;26:778–84.
- Aboul-Fadl T, Bin-Jubair FAS, Aboul-Wafa O. Schiff bases of indoline-2,3-dione (isatin) derivatives and nalidixic acid carbohydrazide, synthesis, antitubercular activity and pharmacophoric model building. Eur J Med Chem 2010;45:4578–86.
- Abo-Ashour MF, Eldehna WM, George RF, et al. Novel indole-thiazolidinone conjugates: design, synthesis and whole-cell phenotypic evaluation as a novel class of antimicrobial agents. Eur J Med Chem 2018;160:49–60.
- Abo-Ashour MF, Eldehna WM, George RF, et al. Synthesis and biological evaluation of 2-aminothiazole-thiazolidinone conjugates as potential antitubercular agents. Future Med Chem 2018;10:1405–19.
- Karali N, Gürsoy A, Kandemirli F, et al. Synthesis and structure-antituberculosis activity relationship of 1H-indole-2,3-dione derivatives. Bioorg Med Chem 2007;15:5888–904.
- Borsari C, Ferrari S, Venturelli A, Costi MP. Target-based approaches for the discovery of new antimycobacterial drugs. Drug Discov Today 2017;22:492–502.
- Rožman K, Sosič I, Fernandez R, et al. A new ‘golden age’ for the antitubercular target InhA. Drug Discov Today 2017;22:492–502.
- Hassan NW, Saudi MN, Abdel-Ghany YS, et al. Novel pyrazine based anti-tubercular agents: design, synthesis, biological evaluation and in silico studies. Bioorg Chem 2020;96:103610.
- Tunney MM, Ramage G, Field TR, et al. Rapid colorimetric assay for antimicrobial susceptibility testing of Pseudomonas aeruginosa. Antimicrob Agents Chemother 2004;48:1879–81.
- Fathallah N, Raafat MM, Issa MY, et al. Bio-guided fractionation of prenylated benzaldehyde derivatives as potent antimicrobial and antibiofilm from Ammi majus L. fruits-associated Aspergillus amstelodami. Molecules 2019;24:4118–24.
- Finger GC, Starr LD, Roe A, Link WJ. Aromatic fluorine compounds. X. The 2, 3-and 2, 6-difluoropyridines. J Org Chem 1962;27:3965–8.
- Eldehna WM, Fares M, Ibrahim HS, et al. Synthesis and cytotoxic activity of biphenylurea derivatives containing indolin-2-one moieties. Molecules 2016;21:762.
- Attia MI, Eldehna WM, Afifi SA, et al. New hydrazonoindolin-2-ones: synthesis, exploration of the possible anti-proliferative mechanism of action and encapsulation into PLGA microspheres. PLoS One 2017;12:e0181241.
- Eldehna WM, Hassan GS, Al-Rashood ST, et al. Synthesis and in vitro anticancer activity of certain novel 1-(2-methyl-6-arylpyridin-3-yl)-3-phenylureas as apoptosis-inducing agents. J Enzyme Inhib Med Chem 2019;34:322–32.
- Luk K-C, So S-S, Zhang J, Zhang Z. Oxindole derivatives, Patent 7576082, USA; 2009.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
- Ismail RS, Abou-Seri SM, Eldehna WM, et al. Novel series of 6-(2-substitutedacetamido)-4-anilinoquinazolines as EGFR-ERK signal transduction inhibitors in MCF-7 breast cancer cells. Eur J Med Chem 2018;155:782–96.
- Makarov V, Lechartier B, Zhang M, et al. Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol Med 2014;6:372–83.
- Sullivan TJ, Truglio JJ, Boyne ME, et al. High affinity InhA inhibitors with activity against drug-resistant strains of Mycobacterium tuberculosis. ACS Chem Biol 2006;1:43–53.
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31:455–61.
- https://3dsbiovia.com/resource-center/downloads/