 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We report the first activation study of the β-class carbonic anhydrase (CA, EC 4.2.1.1) encoded in the genome of the protozoan pathogen Trichomonas vaginalis, TvaCA1. Among 24 amino acid and amine activators investigated, derivatives incorporating a second carboxylic moiety, such as L-Asp, L- and D-Glu, were devoid of activating effects up to concentrations of 50 µM within the assay system, whereas the corresponding compounds with a CONH2 moiety, i.e. L-Gln and L-Asn showed modest activating effects, with activation constants in the range of 26.9 − 32.5 µM. Moderate activation was observed with L- and D-DOPA, histamine, dopamine, serotonin, (2-Aminoethyl)pyridine/piperazine and morpholine (KA‘s ranging between 8.3 and 14.5 µM), while the best activators were L-and D-Trp, L-and D-Tyr and 4-amino-Phe, which showed KA‘s ranging between 3.0 and 5.1 µM. Understanding in detail the activation mechanism of β-CAs may be relevant for the design of enzyme activity modulators with potential clinical significance.
1. Introduction
Diseases provoked by protozoan pathogens are widespread and few effective agents for their treatment are availableCitation1–4. Furthermore, most of the drugs in clinical use are either dating back to the 50 s or the 60 s and are thus rather toxic and poorly effective, and/or extensive drug resistance has been developed to most of them in many places all over the world, creating thus pressure on the healthcare systems and a large number of casualtiesCitation1–4. This is particularly the case with malaria, caused by protozoans belonging to the genus Plasmodium, with five different species infecting humans, P. falciparum, P. vivax, P. ovale, P. malariae, and the zoonotic P. knowlesiCitation1, but also with other pathogens, such as Trypanosoma cruzi and T. brucei, provoking Chagas disease and African trypanosomiasis, respectivelyCitation2, various species of Leishmania, which provoke leishmaniasisCitation3, or Trichomonas vaginalis which is one of the most common such pathogens, to mention just few of them. Due to the lack of new drugs and the poor response to those available, alternative drug targets for fighting such diseases are constantly being looked for and proposedCitation1,Citation5,Citation6. Interesting novel strategies for drug development involve inhibition of carbonic anhydrases (CAs, EC 4.2.1.1) from pathogenic protozoansCitation1,Citation2,Citation5,Citation6. Indeed, it has been reported that strong anti-protozoan effect especially against T. cruzi as well as several Leishmania species can be achieved by inhibiting CAs with potent and in some cases specific CA inhibitors (CAIs)Citation1,Citation2,Citation5,Citation6. On the other hand, CA activators (CAAs) of such protozoan enzymes have been much less investigated, and in fact only two such reports are available in the literature. They include the activation study of the β-CA from L. donovani chagasi and Entamoeba histolytica, which were in fact reported by our groupsCitation7. CAAs started to be considered only recently for their potential clinical applicationsCitation8, and at least activation of the human CA (hCA) isoforms was demonstrated to be of interest for the modulation of emotional memory as well as the extinction of contextual fear memory, which opens relevant pharmacological applications for this class of compoundsCitation8.
Trichomonas vaginalis, the anaerobic protozoan responsible for the most frequent non-viral sexually transmitted disease in humansCitation9, has recently been investigated for the presence of CAs. Indeed, at least two such enzymes belonging to the β-CA class are present in its genome, TvaCA1Citation9,Citation10 and TvaCA2. Both the structure and catalytic properties of TvaCA1 have been characterised by X-ray crystallography and kinetic techniques, which showed it to be an efficient catalyst for the interconversion between CO2 and bicarbonate in the reaction which also generates protons. This reaction is probably an essential part of the molecular machinery involved in the pH regulation and metabolism of the parasiteCitation9. Furthermore, anion and sulphonamide inhibition studies of this enzyme were reportedCitation9,Citation10. Since humans do not have β-CAs in their genomes, but only α-class CA enzymesCitation11–13, some of which are well-known drug targets, modulation of TvaCA1 activity (and probably also the other isoform) might represent an interesting option for finding anti-protozoan agents with a novel mechanism of actionCitation1. Although activation of pathogenic CAs may be detrimental for the host organism, this phenomenon should also be investigated in detail. Importantly, many CAAs belong to the amine and amino acid classes and several of these compounds are endogenous and present in high concentrations in various tissues/cells, and thus may participate in the modulation of infection and virulence by the pathogenCitation14. Here we report the first activation study of the β-CA from T. vaginalis TvaCA1, with a series of amines and amino acids, many of which are naturally occurring compounds.
2. Materials and methods
2.1. Chemistry
Compounds 1–24 are commercially available, highest purity reagents, from Sigma-Aldrich (Milan, Italy).
2.2. Enzymology
TvaCA1 was a recombinant enzyme obtained in-house as described earlierCitation9. Briefly, the TvaCA1 gene was identified from the Universal Protein Resource Database Uniprot (Protein entry: A2ENQ8). Gene synthesis and subcloning were performed by GeneArt (Thermo Fisher Scientific, Germany). TvaCA1 was expressed recombinantly in E. coli (OneShot® BL21 Star™ (DE3) Chemically Competent Cells, #C601003, Thermo Fisher Scientific, Finland). The recombinant protein was purified using Ni2+-NTA Agarose affinity chromatography resin (Macherey-Nagel GmbH Co., Germany). The 6xHis-tag was removed by thrombin (#RECOMT, Sigma-Aldrich, Finland) according to Thrombin CleanClive™ kit manual (Sigma-Aldrich, Finland), and the tag was separated from the core protein by Ni2+-NTA affinity chromatography.
2.3. Ca activity/activation measurements
An Sx.18Mv-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used to assay the catalytic activity of various CA isozymes for CO2 hydration reactionCitation15. Phenol red (at a concentration of 0.2 mM) was used as an indicator, working at the absorbance maximum of 557 nm, with 10 mM TRIS (pH 8.3, for β-CAs)Citation7 as buffers, 0.1 M NaClO4 (for maintaining constant ionic strength), following the CA-catalyzed CO2 hydration reaction for a period of 10 s at 25 °C. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each activator at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of activators (at 0.1 mM) were prepared in distilled-deionized water and dilutions down to 1 nM were made thereafter with the assay buffer. Enzyme (in the concentration range of 8–15 nM) and activator solutions were pre-incubated together for 15 min prior to assay, in order to allow for the formation of the enzyme–activator complexes. The activation constant (KA), defined similarly with the inhibition constant KI, can be obtained by considering the classical Michaelis–Menten equation (EquationEquation (1)(1)
(1) , which has been fitted by non-linear least squares by using PRISM 3:
(1)
(1)
where [A]f is the free concentration of activator.
Working at substrate concentrations considerably lower than KM ([S] ≪KM), and considering that [A]f can be represented in the form of the total concentration of the enzyme ([E]t) and activator ([A]t), the obtained competitive steady-state equation for determining the activation constant is given by EquationEquation (2)(2)
(2) :
(2)
(2)
where v0 represents the initial velocity of the enzyme-catalyzed reaction in the absence of activatorCitation16–19. This type of approach to measure enzyme-ligand interactions is in excellent agreement with recent results from native mass spectrometry measurementsCitation20.
3. Results and discussion
TvaCA1 is a β-CA that has an open active siteCitation21, meaning that the water molecule/zinc hydroxide acting as nucleophile in the catalytic cycle is coordinated to the metal ion.
As all β-CAs, TvaCA1 is a homodimer, possessing two long channel-like active sites in its molecule, as determined recently by X-ray crystallographic techniquesCitation9. Thus, this enzyme is rather different from the α-CAs present in the human host, which are generally monomeric enzymes with the zinc ion coordinated by three His residues and a water molecule, therefore possessing a rather ample active site where inhibitors and activators may bindCitation11–13. On the other hand, in TvaCA1 as in many β-CAs, the zinc ion is coordinated by two Cys residues, one His and one water molecule/hydroxide ionCitation9,Citation21–23. The rate-determining step for many CAs is the generation of the zinc hydroxide, nucleophilic species of the enzymeCitation11–13. In α-CAs, this proton transfer reaction from the water molecule coordinated to the zinc to the reaction medium, is assisted by a His residue placed in the middle of the active site cleft, i.e. His64 in most hCA isoformsCitation11–13. For β-class enzymes, the nature and position of the proton shuttling moiety are less well understood, being more complex than in the α-CAs. For example, recent X-ray crystallographic and mutagenesis studiesCitation22 allowed us to propose Asp309 as the proton shuttling residue for the β-CA PtLCIB3 of the diatom Phaeodactylum tricornutum, which differs substantially from the mechanism in α-CAs, for which a His residue, as mentioned above, has this role. It should be however mentioned that in another β-CA, the enzyme from Pisum sativum, the proton shuttle seems to be a Tyr residueCitation23. Thus, it is obvious that both the catalytic as well as the activation mechanisms of β-CAs might be more complex than for the well studied α-class enzymes, and investigating β-CAs activators might be relevant also from this viewpoint.
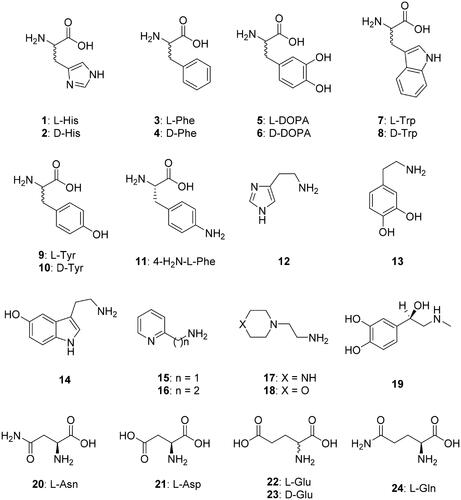
A panel of amino acid and amine derivatives of types 1–24 () were included in this study for investigating their activating properties against TvaCA1. These compounds were shown previously to act as CAAs against a range of CAs belonging to all known genetic CA families, including some α- and β-class enzymesCitation8,Citation14,Citation24.
Figure 1. Amino acids and amines 1–24 investigated as CAAs against TvaCA1. Some β-CAs have a so-called closed active site (at pH < 8.3), in which the fourth zinc ligand is an aspartate residue, and thus these enzymes are devoid of CO2 hydrase activityCitation21–23. However, at pH values > 8.3, the aspartate is involved in a hydrogen bond with an adjacent Arg residue, and an incoming water molecule/hydroxide ion replaces the aspartate as a zinc ligand, providing an open active site and thus a catalytically active enzymeCitation21–23.
We have first investigated whether the activators of TvaCA1 interfere with the binding of the substrate CO2 to the enzyme or whether they only contribute to the proton transfer processes, as for other CAs investigated so far for their activation. As seen from data of , L-Trp, at 10 µM, efficiently activates TvaCA1 (as well as other α- and β-CAs, such as hCA I and II or the β-CA from Escherichia coli, EcoCAβ) inducing a 6.2-times increase in the kcat of TvaCA1 but having no influence on KM, thus proving that the activator takes part in the proton transfer process within the enzyme-activator complex formed when the amino acid activator binds within the enzyme active site. However, this process appears not to interfere with the binding of CO2, since the value of KM is not changed ().
Table 1. Activation of hCA isozymes I, II, EcoCAβ and TvaCA1 with L-Trp, measured at 25 °CCitation15.
We have thereafter investigated the amino acids and amines 1–24 for their effects on the TvaCA1 activation, comparing this data with those for hCA I, II and EcoCAβ (). The following structure-activity relationship for the activation of the protozoan enzyme was possible to draw from the data of :
Table 2. Activation constants of hCA I, hCA II and the bacterial enzyme EcoCAβ (E. coli) and the protozoan TvaCA1 (T. vaginalis) with amino acids and amines 1–24, by a stopped-flow CO2 hydrase assayCitation15.
Amino acids incorporating a second carboxylic moiety, such as L-Asp, L- and D-Glu, were devoid of activating effects up to concentrations of 50 µM within the assay system, whereas the corresponding compounds with a CONH2 moiety, i.e, L-Gln and L-Asn showed modest activating effects, with activation constants in the range of 26.9–32.5 µM.
Moderate-weak TvaCA1 activation was also observed for the following amino acid derivatives: L-and D-His as well as L- and D-Phe, which showed KA‘s ranging between 16.3 and 24.5 µM.
A number of amino acid and amine derivatives investigated here acted as moderate-effective activators, with KA‘s ranging between 8.3 and 14.5 µM. They include: L- and D-DOPA, histamine, dopamine, serotonin, (2-Aminoethyl)pyridine/piperazine and morpholine (16–18), the aminomethyl derivative of pyridine 15, and L-adrenaline 19.
The most effective TvaCA1 activators were L-and D-Trp, L-and D-Tyr and 4-amino-Phe 11, which showed KA‘s ranging between 3.0 and 5.1 µM.
Small structural changes in the activator molecule have important consequences for the activation. For example, in the case of Phe, both the L- and D-enantiomers showed rather modest activating effects. The introduction of p-hydroxy moieties on the phenyl ring, as in L-and D-Tyr, led to a marked increase in the activating effects, but the introduction of a second phenolic OH moiety, as in L-and D-DOPA, diminished again the activating properties.
The activation profile of TvaCA1 with compounds 1–24 is quite different from those of other enzymes, such as hCA I and II or EcoCAβ, but no TvaCA1-selective activators were detected so far.
4. Conclusions
We report the first activation study of the β-class CA encoded in the genome of the protozoan pathogen T. vaginalis, TvaCA1. In a series of 24 amino acid and amine activators, derivatives incorporating a second carboxylic moiety, such as L-Asp, L- and D-Glu, were devoid of activating effects up to concentrations of 50 µM within the assay system, whereas the corresponding compounds with a CONH2 moiety, i.e. L-Gln and L-Asn showed modest activating effects, with activation constants in the range of 26.9 − 32.5 µM. Moderate activation has been observed with L- and D-DOPA, histamine, dopamine, serotonin, (2-Aminoethyl)pyridine/piperazine and morpholine (KA’s ranging between 8.3 and 14.5 µM), whereas the best activators were L-and D-Trp, L-and D-Tyr and 4-amino-Phe, which showed KA’s ranging between 3.0 and 5.1 µM. Understanding in detail the activation mechanism of β-CAs may be relevant for the design of enzyme activity modulators with potential clinical significance.
Acknowledgement
We acknowledge the infrastructure support from Biocenter Finland.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- (a) D’Ambrosio K, Supuran CT, De Simone G. Are carbonic anhydrases suitable targets to fight protozoan parasitic diseases? Curr Med Chem 2018;25:5266–78. (b) Capasso C, Supuran CT. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin Ther Targets 2015;19:1689–704. (c) Krungkrai J, Supuran CT. The alpha-carbonic anhydrase from the malaria parasite and its inhibition. Curr Pharm Des 2008;14:631–40. (d) Packard RM. The origins of antimalarial-drug resistance. N Engl J Med 2014;371:397–9.
- (a) Vermelho AB, Rodrigues GC, Supuran CT. Why hasn’t there been more progress in new Chagas disease drug discovery? Expert Opin Drug Discov 2020;15:145–58. (b) Pan P, Vermelho AB, Capaci Rodrigues G, et al. Cloning, characterization, and sulfonamide and thiol inhibition studies of an α-carbonic anhydrase from Trypanosoma cruzi, the causative agent of Chagas disease. J Med Chem 2013;56:1761–71.
- (a) Garcia AR, Oliveira DMP, Claudia F Amaral A, et al. Leishmania infantum arginase: biochemical characterization and inhibition by naturally occurring phenolic substances. J Enzyme Inhib Med Chem 2019;34:1100–9. (b) Syrjanen L, Vermelho AB, Rodrigues Ide A, et al. Cloning, characterization, and inhibition studies of a β-carbonic anhydrase from Leishmania donovani chagasi, the protozoan parasite responsible for leishmaniasis. J Med Chem 2013;56:7372–81.
- (a) Küng E, Fürnkranz U, Walochnik J. Chemotherapeutic options for the treatment of human trichomoniasis. Int J Antimicrob Agents 2019;53:116–27. (b) Alessio C, Nyirjesy P. Management of resistant trichomoniasis. Curr Infect Dis Rep 2019;21:31.
- (a) Angeli A, Etxebeste-Mitxeltorena M, Sanmartín C, et al. Tellurides bearing sulfonamides as novel inhibitors of leishmanial carbonic anhydrase with potent antileishmanial activity. J Med Chem 2020;63:4306–14. (b) Nocentini A, Osman SM, Almeida IA, et al. Appraisal of anti-protozoan activity of nitroaromatic benzenesulfonamides inhibiting carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani. J Enzyme Inhib Med Chem 2019;34:1164–71. (c) da Silva Cardoso V, Vermelho AB, Ricci Junior E, et al. Antileishmanial activity of sulphonamide nanoemulsions targeting the β-carbonic anhydrase from Leishmania species. J Enzyme Inhib Med Chem 2018;33:850–7.
- (a) Vermelho AB, da Silva Cardoso V, Ricci Junior E, et al. Nanoemulsions of sulfonamide carbonic anhydrase inhibitors strongly inhibit the growth of Trypanosoma cruzi. J Enzyme Inhib Med Chem 2018;33:139–46. (b) Vermelho AB, Capaci GR, Rodrigues IA, et al. Carbonic anhydrases from Trypanosoma and Leishmania as anti-protozoan drug targets. Bioorg Med Chem 2017;25:1543–55. (c) Nocentini A, Cadoni R, Dumy P, et al. Carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani chagasi are inhibited by benzoxaboroles. J Enzyme Inhib Med Chem 2018;33:286–9.
- (a) Bua S, Haapanen S, Kuuslahti M, et al. Activation studies of the β-carbonic anhydrase from the pathogenic protozoan Entamoeba histolytica with amino acids and amines. Metabolites 2019;9:26. (b) Angeli A, Donald WA, Parkkila S, Supuran CT. Activation studies with amines and amino acids of the β-carbonic anhydrase from the pathogenic protozoan Leishmania donovani chagasi. Bioorg Chem 2018;78:406–10.
- (a) Supuran CT. Carbonic anhydrase activators. Future Med Chem 2018;10:561–73. (b) Blandina P, Provensi G, Passsani MB, et al. Carbonic anhydrase modulation of emotional memory. Implications for the treatment of cognitive disorders. J Enzyme Inhib Med Chem 2020;35:1206–14. (c) Schmidt SD, Costa A, Rani B, et al. The role of carbonic anhydrases in extinction of contextual fear memory. Proc Natl Acad Sci USA 2020;117:16000–8.
- (a) Urbański LJ, Di Fiore A, Azizi L, et al. Biochemical and structural characterisation of a protozoan beta-carbonic anhydrase from Trichomonas vaginalis. J Enzyme Inhib Med Chem 2020;35:1292–9. (b) Urbański LJ, Angeli A, Hytönen VP, et al. Inhibition of the newly discovered β-carbonic anhydrase from the protozoan pathogen Trichomonas vaginalis with inorganic anions and small molecules. J Inorg Biochem 2020;213:111274.
- Urbański LJ, Angeli A, Hytönen VP, et al. Inhibition of the β-carbonic anhydrase from the protozoan pathogen Trichomonas vaginalis with sulphonamides. J Enzyme Inhib Med Chem 2021;36:329–34.
- (a) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. (b) Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016; 473:2023–32. (c) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- (a) Supuran CT. Carbonic anhydrases and metabolism. Metabolites 2018;8:25. (b) Supuran CT. Exploring the multiple binding modes of inhibitors to carbonic anhydrases for novel drug discovery. Expert Opin Drug Discov 2020;15:671–86. (c) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60. (d) Mishra CB, Tiwari M, Supuran CT. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: where are we today? Med Res Rev 2020;40:2485–565.
- (a) Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68. (b) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88. (c) Nocentini A, Supuran CT. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin Drug Discov 2019;14:1175–97. (d) Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72.
- (a) Supuran CT, Capasso C. Antibacterial carbonic anhydrase inhibitors: an update on the recent literature. Expert Opin Ther Pat 2020;30:963–82. (b) Nocentini A, Del Prete S, Mastrolorenzo MD, et al. Activation studies of the β-carbonic anhydrases from Escherichia coli with amino acids and amines. J Enzyme Inhib Med Chem 2020;35:1379–86. (c) Lotlikar SR, Kayastha BB, Vullo D, et al. Pseudomonas aeruginosa β-carbonic anhydrase, psCA1, is required for calcium deposition and contributes to virulence. Cell Calcium 2019;84:102080.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- (a) Del Prete S, Bua S, Supuran CT, Capasso C. Escherichia coliγ-carbonic anhydrase: characterisation and effects of simple aromatic/heterocyclic sulphonamide inhibitors. J Enzyme Inhib Med Chem 2020;35:1545–54. (b) Del Prete S, De Luca V, Bua S, et al. The effect of substituted benzene-sulfonamides and clinically licensed drugs on the catalytic activity of CynT2, a carbonic anhydrase crucial for Escherichia coli life cycle. Int J Mol Sci 2020;21:4175. (c) Angeli A, Ferraroni M, Pinteala M, et al. Crystal structure of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Burkholderia pseudomallei. Molecules 2020; 25:2269.
- (a) Del Prete S, Nocentini A, Supuran CT, Capasso C. Bacterial ι-carbonic anhydrase: a new active class of carbonic anhydrase identified in the genome of the Gram-negative bacterium Burkholderia territorii. J Enzyme Inhib Med Chem 2020;35:1060–8. (b) Gitto R, De Luca L, Mancuso F, et al. Seeking new approach for therapeutic treatment of cholera disease via inhibition of bacterial carbonic anhydrases: experimental and theoretical studies for sixteen benzenesulfonamide derivatives. J Enzyme Inhib Med Chem 2019;34:1186–92. (c) Angeli A, Pinteala M, Maier SS, et al. Inhibition of α-, β-, γ-, δ-, ζ- and η-class carbonic anhydrases from bacteria, fungi, algae, diatoms and protozoans with famotidine. J Enzyme Inhib Med Chem 2019;34:644–50. (d) Supuran CT, Nicolae A, Popescu A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;31:431–8.
- (a) Angeli A, Del Prete S, Alasmary FAS, et al. The first activation studies of the η-carbonic anhydrase from the malaria parasite Plasmodium falciparum with amines and amino acids. Bioorg Chem 2018;80:94–8. (b) Angeli A, Buonanno M, Donald WA, et al. The zinc – but not cadmium – containing ζ-carbonic from the diatom Thalassiosira weissflogii is potently activated by amines and amino acids. Bioorg Chem 2018;80:261–5. (c) Angeli A, Del Prete S, Osman SM, et al. Activation studies of the γ-carbonic anhydrases from the antarctic marine bacteria Pseudoalteromonas haloplanktis and Colwellia psychrerythraea with amino acids and amines. Marine Drugs 2019;17:238–46. (d) Angeli A, Alasmary FAS, Del Prete S, et al. The first activation study of a δ-carbonic anhydrase: TweCAδ from the diatom Thalassiosira weissflogii is effectively activated by amines and amino acids. J Enzyme Inhib Med Chem 2018;33:680–5.
- (a) Vullo D, Del Prete S, Osman SM, et al. Burkholderia pseudomallei γ-carbonic anhydrase is strongly activated by amino acids and amines. Bioorg Med Chem Lett 2017;27:77–80. (b) Vullo D, Del Prete S, Osman SM, et al. Comparison of the amine/amino acid activation profiles of the β- and γ-carbonic anhydrases from the pathogenic bacterium Burkholderia pseudomallei. J Enzyme Inhib Med Chem 2018;33:25–30. (c) Angeli A, Del Prete S, Osman SM, et al. Activation studies with amines and amino acids of the β-carbonic anhydrase encoded by the Rv3273 gene from the pathogenic bacterium Mycobacterium tuberculosis. J Enzyme Inhib Med Chem 2018;33:364–9.
- (a) Nguyen GTH, Tran TN, Podgorski MN, et al. Nanoscale ion emitters in native mass spectrometry for measuring ligand-protein binding affinities. ACS Cent Sci 2019;5:308–18. (b) Nguyen GTH, Nocentini A, Angeli A, et al. Perfluoroalkyl substances of significant environmental concern can strongly inhibit human carbonic anhydrase isozymes. Anal Chem 2020;92:4614–22.
- Covarrubias AS, Bergfors T, Jones TA, et al. Structural mechanics of the pH-dependent activity of beta-carbonic anhydrase from Mycobacterium tuberculosis. J Biol Chem 2006; 281:4993–9.
- Jin S, Vullo D, Bua S, et al. Structural and biochemical characterization of novel carbonic anhydrases from Phaeodactylum tricornutum. Acta Crystallogr D Struct Biol 2020;76:676–86.
- Kimber MS, Pai EF. The active site architecture of Pisum sativum beta-carbonic anhydrase is a mirror image of that of alpha-carbonic anhydrases. Embo J 2000;19:1407–18.
- (a) Briganti F, Mangani S, Orioli P, et al. Carbonic anhydrase activators: x-ray crystallographic and spectroscopic investigations for the interaction of isozymes I and II with histamine. Biochemistry 1997;36:10384–92. (b) Temperini C, Scozzafava A, Vullo D, et al. Carbonic anhydrase activators. Activation of isozymes I, II, IV, VA, VII, and XIV with l- and d-histidine and crystallographic analysis of their adducts with isoform II: engineering proton-transfer processes within the active site of an enzyme. Chemistry 2006;12:7057–66. (c) Temperini C, Scozzafava A, Vullo D, et al. Carbonic anhydrase activators. Activation of isoforms I, II, IV, VA, VII, and XIV with L- and D-phenylalanine and crystallographic analysis of their adducts with isozyme II: stereospecific recognition within the active site of an enzyme and its consequences for the drug design. J Med Chem 2006;49:3019–27. (d) Akocak S, Supuran CT. Activation of α-, β-, γ- δ-, ζ- and η- class of carbonic anhydrases with amines and amino acids: a review. J Enzyme Inhib Med Chem 2019;34:1652–9.
