?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We here report a study on the activation of the ι-class bacterial CA from Burkholderia territorii (BteCAι). This protein was recently characterised as a zinc-dependent enzyme that shows a significant catalytic activity (kcat 3.0 × 105 s−1) for the physiological reaction of CO2 hydration to bicarbonate and protons. Some amino acids and amines, among which some proteinogenic derivatives as well as histamine, dopamine and serotonin, showed efficient activating properties towards BteCAι, with activation constants in the range 3.9–13.3 µM. L-Phe, L-Asn, L-Glu, and some pyridyl-alkylamines, showed a weaker activating effect towards BteCAι, with KA values ranging between 18.4 µM and 45.6 µM. Nowadays, no information is available on active site architecture, metal ion coordination and catalytic mechanism of members of the ι-group of CAs, and this study represents another contribution towards a better understanding of this still uncharacterised class of enzymes.
1. Introduction
Enzyme activation implies that a chemical compound binding to an enzyme favourably affects the corresponding catalysed reaction rateCitation1. Among the activators, worth mentioning are ions, small organic molecules (amines and amino acids, but also other derivatives), as well as peptides, proteins, and lipidsCitation1,Citation2. Enzyme activation is classified as essential and non-essential. In the first case, the enzymatic reaction occurs only when the activator is present; in the second case, the catalysed reaction takes place with or without the activatorCitation3,Citation4. Enzymatic reactions using ATP as substrate, such as those catalysed by kinases, are an excellent example of processes undergoing enzyme activationCitation5. The suitable substrate for these biocatalysts is the complex formed by ATP and Mg2+ (the ion acting as activator), and the reaction does not take place when Mg2+ is absent and ATP is presentCitation5. In contrast, an elegant and well-described example of non-essential activation is represented by the superfamily of carbonic anhydrases (CAs, EC 4.2.1.1), which are widely investigated by us and others as drug targetsCitation6–13. These enzymes are involved in the catalysis of a pivotal physiological reaction, the reversible hydration of carbon dioxide to bicarbonate and protonsCitation10,Citation13–18. Members of the CA superfamily are grouped into eight classes (α, β, γ, δ, ζ, η, θ and ι) according to their structural characteristics, and are distributed in all living organisms, starting from microorganisms to multicellular plants/animalsCitation13–17. For example, mammalian genomes encode only for numerous isoforms of the α-CA class and accomplish specialised functions in various tissues and organsCitation19–23. In plants, α- and β-CAs have an essential role in photosynthesis and biosynthetic reactions related to itCitation9. In simpler organisms, such as bacteria, Archaea and cyanobacteria, α-, β-, γ- and ι-CAs are present, which have a role in balancing the [CO2]/[HCO3-] ratio and the carbon dioxide fixationCitation9–11,Citation13,Citation18,Citation24. Marine diatoms encode for α-, δ-, ζ-, θ- and ι-CAs, which are involved in carbon dioxide fixation and metabolismCitation25–27. In addition to α- and β-forms, protozoan species also expressed η-CAs. These enzymes are involved in de novo purine/pyrimidine biosynthetic pathwaysCitation28. Finally, organisms of the fungal kingdom generally present enzymes of the β-class, which are present at least in one isoformCitation29–31. Fungal CO2-sensing is directly stimulated by HCO3−, which is produced in a CA-dependent mannerCitation31–34.
The most extensively investigated CA activators (CAAs) belong to the compound groups of amines and amino acidsCitation2. The X-ray crystal structure of the human isoforms (hCA I and II) bound to activators, such as histamine, L-/D-histidine, L-/D-phenylalanine, D-tryptophan and others, allowed the comprehension of the activation mechanism and the structure-activity relationship governing itCitation2,Citation35–41. Contrary to most CA inhibitors (CAIs), such as anions and sulphonamides,Citation6,Citation9,Citation10,Citation42,Citation43 CAAs bind to molecular regions at the entrance of the enzyme active site enhancing the proton transfer processes between the Zn2+-bound water molecule and the reaction medium; this is accomplished by a supplementary pathway provided by the proton-shuttling moieties of the activatorCitation2,Citation44. As a result, CAAs increase the rate of the enzyme-catalysed process speeding up the proton transfer, which is the rate-determining step of the whole reaction, thus enhancing the catalytic efficacy of these enzymes (kcat up to 106 s−1)Citation2,Citation44. In the literature, the modulation of CAs activity through activators is less described than that by inhibitors; the latter is well documented for its relevance in the pharmacological fieldCitation20,Citation42,Citation45–48. Nevertheless, CAAs, such as D-phenylalanine and imidazole, have been recently proposed as neuroenhancement drugs possibly improving synaptic efficacy, spatial learning and memoryCitation2,Citation49. Moreover, it has been demonstrated that CA levels are significantly decreased in the brain of patients with Alzheimer’s disease (AD)Citation8,Citation50. These aspects corroborate the notion that CA modulators may have important applications in conditions in which individual learning and memory are impaired, such as aging or ADCitation8,Citation39,Citation50,Citation51. On the other hand, several CAIs (coumarins and their isosteres) were proved inhibiting CAs by occluding the entrance of the active site cavity; interestingly, their binding sites coincided with those observed for various CAAsCitation52. In this regard, it is evident that investigations on CAAs and studies on the structure-activity relationship governing their action could have the advantage to improve the general design of novel CAs modulators, which can mimic the activator binding mode but can have an opposite effect on the enzyme activity, as seen for coumarinsCitation52. This is an important issue also in another pharmacological context, since it has been shown that the interference with bacterial CA activity can impair the microorganism growth and virulence, making the CA inhibition an exciting approach to contrast the emergence of antibiotic resistance associated with many infectionsCitation53.
In analogy with what already done with other CAs classes from mammals and fungiCitation2,Citation4,Citation39,Citation44,Citation49,Citation51,Citation54,Citation55, we carried out an extensive study of the activation properties of amines and amino acids towards a member of the recently discovered group of ι-CAs. To this purpose, we investigated the effect of these CAAs on an enzyme identified in the genome of the non-pathogenic Gram-negative bacterium Burkholderia territorii recovered from groundwater samples (acronym BteCAι)Citation56. This study is the first characterisation of the activation of a CA belonging to the ι-class.
2. Materials and methods
2.1. Reagents
Amines and amino acid derivatives 1–24 were obtained from Sigma-Aldrich (Milan, Italy) at the highest purity commercially available.
2.2. Cloning, production and purification of BteCAι
The protocol already developed and described by usCitation57, involving enzyme cloning and expression in Escherichia coli, was here used to obtain a pure preparation of recombinant BteCAι. Briefly, The synthetic B. territorii gene encoding for the BteCAι was cloned into the expression vector pET100D-Topo/BteCAι and used to transform the Competent Escherichia coli BL21 (DE3) codon plus cells (Agilent). The cellular culture was induced with Isopropyl β-D-1-thiogalactopyranoside (IPTG) to overexpress the recombinant BteCAι. After the growth, the cells were harvested and disrupted by sonication. Cellular extract was purified using a nickel affinity column (His-Trap FF).
2.3. CA activation measurements
A Sx.18Mv-R Applied Photophysics (Oxford, UK) stopped-flow instrument was used to assay the CA-catalysed CO2 hydration activityCitation58. Phenol red (at a concentration of 0.2 mM) was used as an indicator, working at the absorbance maximum of 557 nm, with 10 mM HEPES, pH 7.5 (for α-CAs and BteCAι)Citation59,Citation60 or 10 mM TRIS, pH 8.3 (for β-CAs)Citation61–64 as buffers, containing 0.1 M NaClO4 (for maintaining constant ionic strength), following the CA-catalysed CO2 hydration reaction for a period of 10–100 s at 25 °C. The CO2 concentrations ranged from 1.7 to 17 mM to determine the kinetic parameters and activation constants. For each activator, at least six traces of the initial 5–10% of the reaction were used to determine the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of activators (at 0.1 mM concentration) were prepared in distilled-deionized water, and dilutions up to 1 nM were made thereafter with the assay buffer. Enzymes and activators were pre-incubated together for 15 min before the assay to allow the formation of the corresponding enzyme–activator complexes. The activation constant (KA) values, defined similarly to the inhibition constant counterparts, were obtained by considering the classical Michaelis–Menten equation (EquationEquation (1)(1)
(1) , which was fitted by the non-linear least squares method using PRISM 3, and represent the mean from at least three different determinations:
(1)
(1)
where [A]f is the free concentration of the activator.
Working at substrate concentrations considerably lower than KM ([S] ≪KM), and considering that [A]f can be represented in the form of the total concentration of the enzyme ([E]t) and activator ([A]t), the obtained competitive steady-state equation for determining the activation constant value is given by EquationEquation (2)(2)
(2) :
(2)
(2)
where v0 represents the initial velocity of the enzyme-catalysed reaction in the absence of activatorCitation61–64. This type of approach to measuring enzyme-ligand interactions is in excellent agreement with recent results from native mass spectrometry measurementsCitation65.
3. Results and discussion
3.1. Validation of BteCAι activity
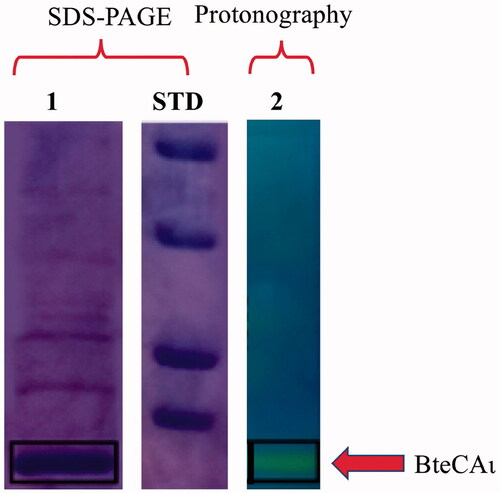
BteCAι was heterologously expressed in E. coli and purified as already reportedCitation57. Enzyme preparation homogeneity, purity and in gel hydratase activity were verified using sodium dodecyl-sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and protonography, respectively ().
Figure 1. Combined lanes of SDS-PAGE and protonography of BteCAι. Lane 1, purified recombinant BteCAι; Lane 2, protonogram showing the enzyme activity on the polyacrylamide gel; Lane STD, molecular markers, from the top: 50.0 kDa, 37.0 kDa, 25 kDa and 20 kDa. Boxes with continuous lines indicate the protein bands identifying BteCAι (calculated molecular mass of 19.0 kDa).
The CO2 hydratase activity and the kinetic constants of purified, recombinant BteCAι were determined using the stopped-flow technique. Comparative experiments with human CA isoform I (hCA I) and isoform II (hCA II) were perfomed to relate results to well-known enzyme species. BteCAι showed a high catalytic activity (kcat 3.0 × 105 s − 1) for the physiological reaction of CO2 hydration to bicarbonate and protons and, as expected, was inhibited by the sulphonamide acetazolamide (KI = 519 nM) ().
Table 1. BteCAι, hCAI and hCAII kinetic parameters for the catalysed CO2 hydration reaction.
Worth mentioning is the fact that purified, recombinant BteCAι displayed a kcat value of the same order of magnitude of hCA I, whereas the affinity for the substrate resulted higher than that of the two human isoforms (). Here, we underline that ι-CAs appear phylogenetically well separated from all the other bacterial CAs (α, β and γ), reinforcing the fact that these proteins were classified in a new CA classCitation66. It has been speculated that bacterial ι-CAs may derive from the modification of an ancestor gene, which they had in common with γ-CAs; the latter enzymes are so far considered the oldest class among all CAsCitation9,Citation10,Citation15,Citation18,Citation66. However, the hypothesis an ancestor gene common to ι- and γ-CAs needs to be validated by further work.
3.2. BtecAι activation profile
Purified, recombinant BteCAι was then used to determine the corresponding activation profile with amines and amino acids (compounds 1–24) reported in . Some of these compounds are biogenic amines or bioactive derivatives that have a well-known pharmacological activityCitation67. Resulting data provided original information on the activation profile of these CAAs with respect to a ι-CA class enzyme.
shows kinetic parameters of some CAs in the presence of the substrate, namely CO2 at a concentration of 15 mM, when experiments were performed in the absence or presence of the activator L-Trp (at 10 µM final concentration). As expected, under these experimental conditions L-Trp efficiently activated hCA I, hCA II and β-CA from Escherichia coli (EcoCAβ); these enzymes were here used as positive controls since corresponding kinetic data and their structures in complex with CAAs have already been describedCitation2,Citation68. Experiments originally demonstrated here that L-Trp can also exert an activation activity for a member of the ι-CA class. Indeed, the activator increased the kcat value of all these enzymes but did not influence the corresponding KM one (data not shown). Also in the case of ι-CAs, this proved that, when the enzyme/L-Trp complex is formed, the latter takes part in the proton transfer process, but the activator does not interfere with the binding of CO2 to the protein active site, since the value of KM remained unchanged notwithstanding the absence/presence of L-Trp ().
Table 2. Activation of BteCAι, hCA I, hCA II and EcoCAβ with L-Trp. Experiments were performed for the CO2 hydration reaction, at 25 °C, using a stopped-flow assayCitation58.
Compounds 1–24 were thereafter assayed dose-dependently for their interaction with BteCAι with the aim to assess the corresponding activation constant (KA) values (). Again, the activation data of hCA I and II were analysed and are here reported for comparison reasons. The following structure-activity relationship (SAR) data for the activation of BteCAι may be noted from the data reported in :
Table 3. Activation of BteCAι, hCA I and hCA II with amino acids and amines 1–24. Experiments were performed for the CO2 hydration reaction, at 25 °C, and performed by a stopped-flow assayCitation58.
Most of the tested activators showed an efficient and rather flat activating efficacy towards BteCAι, with KA values ranging between 3.9 and 13.3 µM. Both amino acids (1, 2, 4–11, 21, 23 and 24) as well as amines (12–14, 17–19) showed this type of behaviour, with basically poor SAR to be discussed due to the low range of activity variations. This condition is different from what observed for hCA I and hCA II activation, for which some activators also showed nanomolar activity, e.g., L-Phe, L- and D-Tyr (hCA I and hCA II), and L-adrenaline, L- and D-His (hCA I). For the enantiomeric pairs L-/D-His, L-/D-Phe, L-/D-Trp and L-/D-Tyr, the D-enantiomer was always a better activator than the corresponding L-one. Only for DOPA the reverse was true, with L-DOPA being almost 3 times a more efficient activator compared to the corresponding D-enantiomer.
Several compounds, among which L-Phe, the pyridyl-alkylamines 15 and 16, as well as L-Asn and L-Glu, showed rather weak CA activating effects against the ι-class enzyme, with KA values ranging between 18.4 and 45.6 µM. Also for the L-/D-Glu enantiomeric pair, the D-enantiomer was always a better activator than the corresponding L-one. When data of the L-Asp/L-Asn pair were evaluated, the amide derivative appeared a 5.42-fold weaker activator than the acid counterpart. These results highlight that rather small structural changes (even at the stereogenic center) in the assayed compounds can lead to rather important modifications of the corresponding CA activating properties.
As already anticipated above, the activation profile of the ι-class bacterial enzyme was very different from those of hCA I and hCA II.
4. Conclusions
In this study, we have reported an original analysis of the activation properties of various compounds towards the recently discovered group of ι-CAs. To this aim, we investigated the activation effect of amino acids and amines on BteCAι, which our group recently characterised as a zinc-dependent enzyme with a significant catalytic activity (kcat 3.0 × 105 s−1) for the physiological reaction of CO2 hydration to bicarbonate and protons. Some amino acids and amines, among which L-/D-His, D-Phe, L-/D-DOPA, L-/D-Trp, L-/D-Tyr, L-Asp, D-Glu, L-Gln, histamine, dopamine, serotonin, 1–(2-aminoethyl)-piperazine and others, showed efficient BteCAι activating properties, with activation constants ranging between 3.9 and 13.3 µM. Conversely, L-Phe, some pyridyl-alkylamines, L-Asn and L-Glu showed a weaker activating effect against this enzyme, with KA values ranging between 18.4 and 45.6 µM. Although no information is available on active site architecture, metal ion coordination and catalytic mechanism of members of the ι-group of CAs yet, the results of this study can add further information for a better understanding of this novel class of enzymes. Their rationalisation will be fully achieved when the X-ray crystal structure of BteCAι and of a BteCAι-CAA complex will be solved, and mechanistic considerations will be then finally elaborated according to a structural basis.
Acknowledgements
We are grateful to Valentina Brasiello and Giovanni Del Monaco for their technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Katsumata M. Influence of enzyme activators and inhibitors, present in an enzyme preparation, on the relations between reaction rate and enzyme concentration. J Theor Biol 1969;24:294–306.
- Supuran CT. Carbonic anhydrase activators. Future Med Chem 2018;10:561–73.
- Okamoto M, Hayashi K. Dynamic behavior of cyclic enzyme systems. J Theor Biol 1983;104:591–8.
- Vullo D, Del Prete S, Capasso C, Supuran CT. Carbonic anhydrase activators: activation of the beta-carbonic anhydrase from Malassezia globosa with amines and amino acids. Bioorg Med Chem Lett 2016;26:1381–5.
- London WP, Steck TL. Kinetics of enzyme reactions with interaction between a substrate and a (metal) modifier. Biochemistry 1969;8:1767–79.
- Nocentini A, Angeli A, Carta F, et al. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J Enzyme Inhib Med Chem 2021;36:561–80.
- Angeli A, Carta F, Nocentini A, et al. Carbonic anhydrase inhibitors targeting metabolism and tumor microenvironment. Metabolites 2020;10:412–32.
- Blandina P, Provensi G, Passsani MB, et al. Carbonic anhydrase modulation of emotional memory. Implications for the treatment of cognitive disorders. J Enzyme Inhib Med Chem 2020;35:1206–14.
- Supuran CT, Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin Ther Pat 2018;28:745–54.
- Supuran CT, Capasso C. An overview of the bacterial carbonic anhydrases. Metabolites 2017;7:56–74.
- Supuran CT, Capasso C. Carbonic anhydrase from Porphyromonas gingivalis as a drug target. Pathogens 2017;6:30–42.
- Capasso C, Supuran CT. Inhibition of bacterial carbonic anhydrases as a novel approach to escape drug resistance. Curr Top Med Chem 2017;17:1237–48.
- Capasso C, Supuran CT. An overview of the carbonic anhydrases from two pathogens of the oral cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr Top Med Chem 2016;16:2359–68.
- Annunziato G, Angeli A, D’Alba F, et al. Discovery of new potential anti-infective compounds based on carbonic anhydrase inhibitors by rational target-focused repurposing approaches. ChemMedChem 2016;11:1904–14.
- Ozensoy Guler O, Capasso C, Supuran CT. A magnificent enzyme superfamily: carbonic anhydrases, their purification and characterization. J Enzyme Inhib Med Chem 2016;31:689–94.
- Del Prete S, Vullo D, De Luca V, et al. Sulfonamide inhibition studies of the beta-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem 2016;24:1115–20.
- Del Prete S, De Luca V, De Simone G, et al. Cloning, expression and purification of the complete domain of the eta-carbonic anhydrase from Plasmodium falciparum. J Enzyme Inhib Med Chem 2016;31:54–9. doi:https://doi.org/10.1080/14756366.2016.1217856.
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- McKenna R, Supuran CT. Carbonic anhydrase inhibitors drug design. Subcell Biochem 2014;75:291–323.
- Neri D, Supuran CT. Interfering with ph regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Supuran CT. Carbonic anhydrases–an overview. Curr Pharm Des 2008;14:603–14.
- Capasso C, Supuran CT. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr Med Chem 2015;22:2130–9.
- Rogato A, Del Prete S, Nocentini A, et al. Phaeodactylum tricornutum as a model organism for testing the membrane penetrability of sulphonamide carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2019;34:510–8.
- Angeli A, Pinteala M, Maier SS, et al. Inhibition of alpha-, beta-, gamma-, delta-, zeta- and eta-class carbonic anhydrases from bacteria, fungi, algae, diatoms and protozoans with famotidine. J Enzyme Inhib Med Chem 2019;34:644–50.
- Berrino E, Bozdag M, Del Prete S, et al. Inhibition of alpha-, beta-, gamma-, and delta-carbonic anhydrases from bacteria and diatoms with n’-aryl-n-hydroxy-ureas. J Enzyme Inhib Med Chem 2018;33:1194–8.
- Angeli A, Del Prete S, Alasmary FAS, et al. The first activation studies of the eta-carbonic anhydrase from the malaria parasite Plasmodium falciparum with amines and amino acids. Bioorg Chem 2018;80:94–8.
- Mogensen EG, Janbon G, Chaloupka J, et al. Cryptococcus neoformans senses CO2 through the carbonic anhydrase can2 and the adenylyl cyclase cac1. Eukaryot Cell 2006;5:103–11.
- Schlicker C, Hall RA, Vullo D, et al. Structure and inhibition of the CO2-sensing carbonic anhydrase can2 from the pathogenic fungus Cryptococcus neoformans. J Mol Biol 2009;385:1207–20.
- Klengel T, Liang WJ, Chaloupka J, Ruoff C, et al. Fungal adenylyl cyclase integrates CO2 sensing with camp signaling and virulence. Curr Biol 2005;15:2021–6.
- Uno I, Matsumoto K, Hirata A, Ishikawa T. Outer plaque assembly and spore encapsulation are defective during sporulation of adenylate cyclase-deficient mutants of saccharomyces cerevisiae. J Cell Biol 1985;100:1854–62.
- Chang JC, Oude-Elferink RP. Role of the bicarbonate-responsive soluble adenylyl cyclase in ph sensing and metabolic regulation. Front Physiol 2014;5:42.
- Ohkuni K, Hayashi M, Yamashita I. Bicarbonate-mediated social communication stimulates meiosis and sporulation of saccharomyces cerevisiae. Yeast 1998;14:623–31.
- Briganti F, Mangani S, Orioli P, et al. Carbonic anhydrase activators: X-ray crystallographic and spectroscopic investigations for the interaction of isozymes i and ii with histamine. Biochemistry 1997;36:10384–92.
- Temperini C, Scozzafava A, Vullo D, Supuran CT. Carbonic anhydrase activators. Activation of isozymes i, ii, iv, va, vii, and xiv with l- and d-histidine and crystallographic analysis of their adducts with isoform ii: engineering proton-transfer processes within the active site of an enzyme. Chemistry 2006;12:7057–66.
- Temperini C, Scozzafava A, Puccetti L, Supuran CT. Carbonic anhydrase activators: X-ray crystal structure of the adduct of human isozyme ii with l-histidine as a platform for the design of stronger activators. Bioorg Med Chem Lett 2005;15:5136–41.
- Bhatt A, Mondal UK, Supuran CT, et al. Crystal structure of carbonic anhydrase ii in complex with an activating ligand: implications in neuronal function. Mol Neurobiol 2018;55:7431–7.
- Licsandru E, Tanc M, Kocsis I, et al. A class of carbonic anhydrase i - selective activators. J Enzyme Inhib Med Chem 2017;32:37–46.
- Supuran CT. Carbonic anhydrase inhibition/activation: trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr Pharm Des 2010;16:3233–45.
- Temperini C, Scozzafava A, Supuran CT. Carbonic anhydrase activation and the drug design. Curr Pharm Des 2008;14:708–15.
- Carta F, Dumy P, Supuran CT, Winum JY. Multivalent carbonic anhydrases inhibitors. Int J Mol Sci 2019;20:5352–65.
- Aspatwar A, Winum JY, Carta F, et al. Carbonic anhydrase inhibitors as novel drugs against mycobacterial beta-carbonic anhydrases: an update on in vitro and in vivo studies. Molecules 2018;23:2911–24.
- Angeli A, Chiaramonte N, Manetti D, et al. Investigation of piperazines as human carbonic anhydrase i, ii, iv and vii activators. J Enzyme Inhib Med Chem 2018;33:303–8.
- Supuran CT. Carbonic anhydrase inhibitors: an editorial. Expert Opin Ther Pat 2013;23:677–9.
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74.
- Supuran CT. Experimental carbonic anhydrase inhibitors for the treatment of hypoxic tumors. J Exp Pharmacol 2020;12:603–17.
- Angeli A, Carta F, Nocentini A, et al. Response to perspectives on the classical enzyme carbonic anhydrase and the search for inhibitors. Biophys J 2021;120:178–81.
- Nocentini A, Cuffaro D, Ciccone L, et al. Activation of carbonic anhydrases from human brain by amino alcohol oxime ethers: towards human carbonic anhydrase vii selective activators. J Enzyme Inhib Med Chem 2021;36:48–57.
- Provensi G, Carta F, Nocentini A, et al. A new kid on the block? Carbonic anhydrases as possible new targets in alzheimer’s disease. Int J Mol Sci 2019;20:4724–40.
- Mollica A, Macedonio G, Stefanucci A, et al. Five- and six-membered nitrogen-containing compounds as selective carbonic anhydrase activators. Molecules 2017;22:2178–90.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- Kaur J, Cao X, Abutaleb NS, et al. Optimization of acetazolamide-based scaffold as potent inhibitors of vancomycin-resistant enterococcus. J Med Chem 2020;63:9540–62.
- Tanini D, Capperucci A, Supuran CT, Angeli A. Sulfur, selenium and tellurium containing amines act as effective carbonic anhydrase activators. Bioorg Chem 2019;87:516–22.
- Innocenti A, Hall RA, Scozzafava A, et al. Carbonic anhydrase activators: activation of the beta-carbonic anhydrases from the pathogenic fungi Candida albicans and Cryptococcus neoformans with amines and amino acids. Bioorg Med Chem 2010;18:1034–7.
- De Smet B, Mayo M, Peeters C, et al. Burkholderia stagnalis sp. Nov. And Burkholderia territorii sp. Nov., two novel Burkholderia cepacia complex species from environmental and human sources. Int J Syst Evol Microbiol 2015;65:2265–71.
- De Luca V, Petreni A, Nocentini A, et al. Effect of sulfonamides and their structurally related derivatives on the activity of iota-carbonic anhydrase from Burkholderia territorii. Int J Mol Sci 2021;22:571–83.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes b and c. J Biol Chem 1971;246:2561–73.
- Vullo D, Innocenti A, Nishimori I, et al. Carbonic anhydrase activators: activation of the human isoforms vii (cytosolic) and xiv (transmembrane) with amino acids and amines. Bioorg Med Chem Lett 2007;17:4107–12.
- Innocenti A, Hilvo M, Parkkila S, et al. Carbonic anhydrase activators. Activation of the membrane-associated isoform xv with amino acids and amines. Bioorg Med Chem Lett 2009;19:3430–3.
- Angeli A, Del Prete S, Pinteala M, et al. The first activation study of the beta-carbonic anhydrases from the pathogenic bacteria Brucella suis and Francisella tularensis with amines and amino acids. J Enzyme Inhib Med Chem 2019;34:1178–85.
- Stefanucci A, Angeli A, Dimmito MP, et al. Activation of beta- and gamma-carbonic anhydrases from pathogenic bacteria with tripeptides. J Enzyme Inhib Med Chem 2018;33:945–50.
- Bua S, Haapanen S, Kuuslahti M, et al. Activation studies of the beta-carbonic anhydrase from the pathogenic protozoan Entamoeba histolytica with amino acids and amines. Metabolites 2019;9:26–33.
- Angeli A, Donald WA, Parkkila S, Supuran CT. Activation studies with amines and amino acids of the beta-carbonic anhydrase from the pathogenic protozoan Leishmania donovani chagasi. Bioorg Chem 2018;78:406–10.
- Nguyen GTH, Tran TN, Podgorski MN, et al. Nanoscale ion emitters in native mass spectrometry for measuring ligand-protein binding affinities. ACS Cent Sci 2019;5:308–18.
- Del Prete S, Nocentini A, Supuran CT, Capasso C. Bacterial iota-carbonic anhydrase: a new active class of carbonic anhydrase identified in the genome of the gram-negative bacterium Burkholderia territorii. J Enzyme Inhib Med Chem 2020;35:1060–8.
- Provensi G, Nocentini A, Passani MB, et al. Activation of carbonic anhydrase isoforms involved in modulation of emotional memory and cognitive disorders with histamine agonists, antagonists and derivatives. J Enzyme Inhib Med Chem 2021;36:719–26.
- Nocentini A, Del Prete S, Mastrolorenzo MD, et al. Activation studies of the beta-carbonic anhydrases from Escherichia coli with amino acids and amines. J Enzyme Inhib Med Chem 2020;35:1379–86.